Dear Editor,
Yellow fever (YF) is a mosquito-borne virus infection manifested as an ailment range from fever, headache, joint pain, body ache, epigastric pain, nausea, vomiting, exhaustion to fatal renal failure, and severe liver disease with bleeding and jaundice which causes yellowish skin, hence named “yellow fever. The causative agent of YF, yellow fever virus (YFV), is an enveloped plus-sense RNA virus of the genus Flavivirus like West Nile, Dengue, Japanese encephalitis, Zika, and St. Louis viruses. The virus is transmitted to human and nonhuman primates by infected mosquitoes1. Clinical manifestations of YFV infection are shown in Figure 1.
Figure 1.
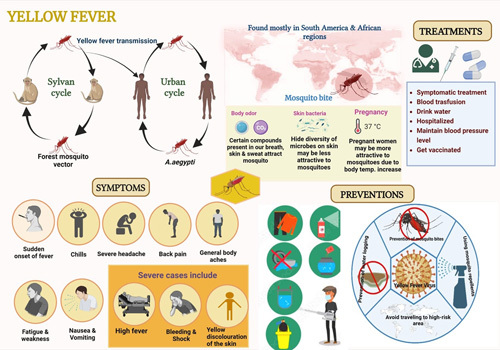
Schematic representation of Transmission, symptoms prevention and treatment of yellow fever virus. Figure produced using Biorendor.
YF is endemic in subtropical and tropical parts of Africa and Central and South America. Phylogeographic evidence indicated that the YF was originated in Africa and spread to the Americas through the slave trade in the 15th–16th centuries. Central America’s subtropical climate with vector flourish made it a good place for YFV to spread to its non-native niches, like the coastal towns of eastern America. There are two main lineages of YFV. The first lineage comprises four genotypes, two each from Western Africa and South America. The differences between the Western Africa and South America genotypes are thought to emerge about 47 decades ago. The second lineage is made up of three viruses from Central or East Africa. Transmission of the YFV occurs in three distinct environments/cycles: the sylvatic (jungle), the intermediate (savannah), and the urban. The sylvatic (jungle) transmission occurs between nonhuman primates (such as monkeys) and mosquito species living in the forest’s upper levels, that is, Aedes spp. in Africa and Haemagogus/Sabethes spp. in South America. People who come into contact with monkeys, such as tourists or people working in the jungle, are at risk of contracting the virus because infected mosquitoes carry the infection from monkey to person. The intermediate cycle was first found in Africa and some parts of South America when people move into areas like the African savannahs or the Amazon jungles. The viruses are spread from mosquitoes to humans living or working in rainforest fringe areas in Africa. The YFV is carried by sylvatic mosquitoes and spreads through the population, causing localized outbreaks in rural areas. YFV can spread either way in this cycle, monkey-to-human or human-to-human by the bite of a mosquito. Typically, a human infected in the bush or savannah will travel to a city and spread the infection there. In the urban cycle, people are the primary hosts and Aedes aegypti is the primary vector. During urban epidemics, the R0 of YF could be 5–7. The 2015–2016 Angola epidemic, there were 4347 suspected cases, 884 confirmed cases, and 377 deaths, demonstrating the threat of YF urban epidemics.
The incubation period of YF is 3–6 days after the mosquito bite. This is followed by flu-like symptoms and 1–2 days relapse period. After a relapse, 20–60% of infected subjects progress to a severe stage including hemorrhagic fever, jaundice, thrombocytopenia, and liver and kidney problems which may result in widespread organ failure, vascular disease, and death. Pathogenesis of YFV in human is viscerotropic. The inoculated virus in the skin infects dendritic cells which migrate to lymph nodes and spread. The virus replicates in the liver causing apoptosis and lytic necrosis in hepatic cells, which combined with steatosis lead to severe liver damage. Severe damage can occur to heart, kidneys, thymus, and spleen. More research is needed to get a complete picture of how tissue-specific tropism affects YFV replication.
YF outbreak database was made and put together in two parts, one for Africa and another for the rest of the world2. From 1985 to 2012, there were 95% of all YF cases in the Americas: 54% in Peru, 18% in Bolivia, 16% in Brazil, and 7% in Colombia. Other places where YF can be spread are Argentina, Ecuador, French Guiana, Guyana, Panama, Paraguay, Suriname, Trinidad and Tobago, and Venezuela3. In 2013, 23 YF cases were reported to PAHO/WHO including 15 deaths in Peru and Colombia (case fatality rate 65.2%). In 2016, the first YF cases were found in Asia; the patients were from an Angola outbreak4. Spread of YFV has changed in the last 20 years, as shown by recent large outbreaks in Brazil in 2016–2019, and Angola in 2015–2016 and Democratic Republic of Congo (DRC) in 2016, which were previously thought to be low-risk areas. In 2017 and 2018, YF cases were reported from Europe (one case each in France, the Netherlands, Germany, Romania, and Switzerland)5. Expanding YF endemic regions in Brazil led to a reassessment of YF endemicity in this country. Five new regions were added in 2000. Information about outbreaks of YF in South America was gathered. Usually, YF reports for both Africa and America came from places like the WHO weekly epidemiological record, disease outbreak news from the WHO, the WHO YF surveillance database (YFSD), the Brazilian Ministry of Health, and the Pan-American Health Organization (PAHO). About 200,000 people worldwide get YF annually and ∼30,000 deaths6. In Africa, 151 confirmed YF cases were reported in 2021 by Cameroon, the Central African Republic, Chad, Côte d’Ivoire, Gabon, Ghana, Nigeria, DRC, and the Republic of the Congo7. From January 1 to August 26, 2022, 10 countries – Cameroon, Central African Republic, Chad, Côte d’Ivoire, DRC, Ghana, Kenya, Niger, Nigeria, and Republic of the Congo – reported additional 123 YF cases, bringing the total to 274 cases8. Six of these countries are still reporting confirmed YF cases with ongoing transmission in 2022, while Côte d’Ivoire and Nigeria have reported probable cases9. Gabon has not reported any new cases since 2021. From January 1 to August 26, 2022, 33 confirmed cases of YF were reported from eight African countries, that is, Central African Republic (33%, 11 cases), Cameroon (24%, eight cases), DRC (13%, four cases), Kenya (9%, three cases), Chad (6%, two cases), the Republic of the Congo (6%, two cases), Uganda (6%, two cases), and Ghana (3%, one case)10. Most cases were reported in the fourth quarter of 2021. From January 1, 2021 to August 26, 2022, about 33% of all confirmed cases happened in Ghana. From 184 confirmed cases in Ghana, 73% were under 30 years old, and the ratio of men to women was 1 : 211. These African outbreaks have been well linked to climate models based on rain, temperature, and ability to survive the winter supporting the idea that this vector-borne disease12.
YFV can be diagnosed through the patient’s travel history and the likely contact with vectors that carry the virus, physical exam and clinical manifestations, and the results of diagnostic lab tests including serology and PCRs13. However, the lab tests usually need highly trained personnel and specialized equipment which are lacking in resource-limited areas.
There is no specific drug to treat YF. Infection prevention can be done by using insect repellent, wearing long-sleeved shirts and pants, and getting vaccinated (see Fig. 1). In the mid-1900s, the spread of A. aegypti mosquito in cities was successfully controlled in the United States and Francophone Africa. However, since the end of the 20th century, YF has come back in both areas. At least 200,000 infections and 30,000 deaths were reported worldwide in 199014. YF is now found in 47 countries in Africa and Central and South Americas. The WHO estimates that 200,000 severe cases and up to 60,000 deaths happen every year15. YF’s death rate in epidemics ranged from 5 to 50%. In 2018, YF was responsible for about 109,000 severe infections and 51,000 deaths2. Massive outbreaks of YFV in Brazil (2016–2018), DRC, Angola, and Uganda (2016), and Nigeria (2019) indicate that the disease has re-emerged16,17.
Since 1980, the YF vaccine has been used and regulated. One vaccine shot protects people for life. The goal of the YF vaccine is to stop the spread of the YFV from humans-to-mosquitoes-to-humans within a local community by greater than 80%. All modern YF vaccines are made of the 17D seed strain subculture: 17DD (passage 195), 17D-204 (passage 204), and 17D-213, which was made from 17D-20418. CIP guidelines suggests that a booster dose should be given every 10 years. The side effects of YFV are usually mild and may show up in 10 days. This includes hypersensitivity reactions, neurotropic diseases, and viscerotropic diseases with symptoms like headache, malaise, myalgia, and fever19. One way to improve the effectiveness of vaccinations in high-risk areas is to implement and strengthen coverage rates at younger ages to reach the right level of population immunity. Mass immunization campaigns to prevent YF give long-lasting immunity in a short time, even though the population is proliferating and there are gaps in immunization11. International Health Regulations suggests that travelers from endemic countries must show proof that they have been vaccinated against YF. This protects their health and stops unvaccinated people from spreading the disease to other places20. To prevent the spread and outbreak of YF around the world, the WHO program to eliminate yellow fever epidemics recommends that all travelers, 9 months and older, get vaccinated to stop the spread of epidemics, especially in South America and Africa11. The eliminate yellow fever epidemics program plans to use fractionated doses for the vaccination before 2026. Most YFV infected people either show no symptoms or experience only mild symptoms before making a full recovery. It usually takes 3–6 days after infection for symptoms to appear in those who are susceptible. Those who have just returned from or been living in an infected region and have begun to experience any of the YF symptoms should seek medical attention immediately. Infected individuals are ‘viraemic’ (infectious to mosquitoes) for up to 5 days before the beginning of fever and for up to 2 days afterward.
In conclusion, avoiding getting bitten by mosquitoes is the most efficient strategy to protect oneself from becoming infected with the YFV. YF vaccines have been around for more than 80 years, and they are safe and work well. Most people are protected for life by a single dose. Figure 1 shows the possible preventions against YFV. The vaccine is a live version of the virus that has been attenuated and is given as a single shot. People older than 9 months who live or travel to places in Africa and South America where the YFV is a risk should get a vaccine. Some countries prohibit entry of YF unvaccinated people. There are vaccination requirements and suggestions for certain countries (https://www.cdc.gov/yellowfever/vaccine/index.html). The bite of a mosquito can occur at any time of the day or night. Before going on a trip, make sure to use bug repellent, dress in long-sleeved shirts and slacks, treat clothing and gear, and get vaccinated.
Ethical approval
The authors declare no involvement of animal studies or human participants in the study as it is a compiled letter article.
Sources of funding
No funding received.
Author contribution
S.M., I.P., and K.D.: designed the study. S.M., I.P., and S.K.: made the first draft. T.S., S.L.N., A.G., T.B.E., and W.C.: updated the manuscript. K.D.: reviewed the final draft. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
None.
Guarantor
Dr Kuldeep Dhama, Dr Wanpen Chaicumpa, and Dr Sumira Malik.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 16 February 2023
Sumira Malik and Ishan pandey contributed equally.
Contributor Information
Sumira Malik, Email: smalik@rnc.amity.edu.
Ishan pandey, Email: ishan.pandey411@gmail.com.
Shristi Kishore, Email: shristi.kishore@student.amity.edu.
T. Sundarrajan, Email: chemistrysundar@gmail.com.
Shachindra L. Nargund, Email: slnargund@nargund.edu.in.
Arbinda Ghosh, Email: dra.ghosh@gauhati.ac.in.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd.
Wanpen Chaicumpa, Email: wanpen.cha@mahidol.ac.th.
Kuldeep Dhama, Email: kdhama@rediffmail.com.
References
- 1. Gianchecchi E, Cianchi V, Torelli A, et al. Yellow fever: origin, epidemiology, preventive strategies and future prospects. Vaccines (Basel) 2022;10:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gunthorpe KAM, Jean K, Cibrelus L, et al. Quantifying model evidence for yellow fever transmission routes in Africa. PLoS Comput Biol 2019;15:e1007355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jean K, Hamlet A, Benzler J, et al. Eliminating yellow fever epidemics in Africa: vaccine demand forecast and impact modelling. PLoS Negl Trop Dis 2020;14:e0008304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cracknell Daniels B, Gaythorpe K, Imai N, et al. Yellow fever in Asia-a risk analysis. J Travel Med 2021;28:015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jácome R, Carrasco-Hernández R, Campillo-Balderas JA, et al. A yellow flag on the horizon: the looming threat of yellow fever to North America. Int J Infect Dis 2019;87:143–50. [DOI] [PubMed] [Google Scholar]
- 6. WHO. Managing Yellow fever epidemics. Technical document. January 2019.
- 7. WHO. Yellow Fever – West and Central Africa. December 2021.
- 8. Nwaiwu AU, Musekiwa A, Tamuzi JL, et al. The incidence and mortality of yellow fever in Africa: a systematic review and meta-analysis. BMC Infect Dis 2021;21:1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization Regional Office for Africa. Weekly bulletins on outbreaks and other emergencies. Week 17: April 2022.
- 10. NaTHNaC. Yellow Fever Zone, Countries with risk of yellow fever transmission. May 2021.
- 11. WHO. Eliminate yellow fever epidemics (EYE) strategy 2017–2026. 2021. [PubMed]
- 12. Haslwanter D, Lasso G, Wec AZ, et al. Genotype-specific features reduce the susceptibility of South American yellow fever virus strains to vaccine-induced antibodies. Cell Host Microbe 2022;30:248–59.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO. Yellow fever. 2019. Accessed May 7, 2019. https://www.who.int/news-room/fact-sheets/detail/yellow-fever.
- 14. Aliaga-Samanez A, Real R, Segura M, et al. Yellow fever surveillance suggests zoonotic and anthroponotic emergent potential. Commun Biol 2022;5:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen LH, Wilson ME. Yellow fever control: current epidemiology and vaccination strategies. Trop Dis Travel Med Vaccines 2020;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunha MS, da Costa AC, de Azevedo Fernandes NCC, et al. Epizootics due to Yellow Fever Virus in São Paulo State, Brazil: viral dissemination to new areas (2016-2017). Sci Rep 2019;9:5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdullahi IN, Anka AU, Emeribe AU, et al. The interplay between environmental factors, vector competence and vaccine immunodynamics as possible explanation of the 2019 yellow fever re-emergence in Nigeria. New Microbes New Infect 2021;41:100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferreira CC, Campi-Azevedo AC, Peruhype-Magalhāes V, et al. The 17D-204 and 17DD yellow fever vaccines: an overview of major similarities and subtle differences. Expert Rev Vaccines 2018;17:79–90. [DOI] [PubMed] [Google Scholar]
- 19. Monath TP, Nichols R, Archambault WT, et al. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg 2002;66:533–41. [DOI] [PubMed] [Google Scholar]
- 20. Barrett ADT. The reemergence of yellow fever. Science 2018;361:847–88. [DOI] [PubMed] [Google Scholar]


