Since the emergence of coronavirus disease-2019 (COVID-19), many subvariants have emerged from the mother Omicron variant, such as the BA.4, BA.5, and the recent BA.2.751,2. Despite the reports of COVID-19 cases and deaths will have decreased globally by the end of 2022, there are new coronavirus omicron subvariants that are technically expected to release a new wave of infections across many parts of the world as the recent XBB, which is a new rapidly emerging omicron subvariant that has first been detected in August 2022 in India and Singapore then rapidly detected in 35 different countries with 1.3% global prevalence by October 2022 (Fig. 1). Despite its rapid transmission rate, the subvariant uses the same transmission methods as the omicron variant, which are droplet and contamination transmission, so experts believe that it is the most immune-evasive subvariant of omicron, with the highest risk of reinfection, according to World Health Organization (WHO) data3. In Singapore, XBB increased from 22 to 54% of regional cases in a single week, outpacing the dominating BA.5 and BA.2.75 strains from the previous week4; similarly, in Bangladesh, where XBB represented 85% of COVID cases within only weeks of initial detection, this indicates the unforeseen transmissibility of this subvariant5,6.
Figure 1.
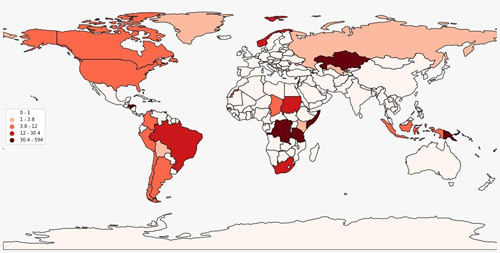
A map showing the geographical distribution of XBB subvariant according to total variant sequences as reported by GISAID.3
The genome structure of XBB subvariant
The XBB is a recombinant of BA.2.75 and BA.2.10.1 omicron subvariants in which the two subvariants infect the same cell allowing the exchange of genetic material of both, producing an entirely new hybrid subvariant, as identified by the recent updates of epidemiological week 40 from the Global Initiative on Sharing Avian Influenza Data (GISAID)3. XBB subvariant has at least seven new mutations from the original virus in the spike protein binding site. The recently detected spike mutations are BA.2+S: V83A, S:H146Q, S: Y144-, S: Q183E, S: G252V, S: V213E, S: G339H, S: L368I, S: R346T, S: V445P, S: N460K, S: G446S, S: F490S and S: F486S7. The XBB genome even showed multiple sublineage divisions as the XBB.1 and XBB.2, and XBB.3 as identified by the COVID-spectrum gene identifier, but these sublineages did not yet show a particularly warning spread as the original XBB 8,9 (Fig. 2).
Figure 2.
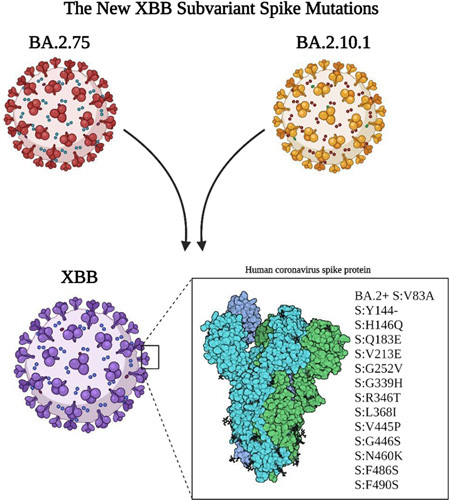
The spike mutations of the new XBB subvariant according to WHO.7
One small mutation in the XBB spike protein generated the XBB.1 sublineage that was first discovered in the United States on September 15; GISAID statistics showed that it accounts for about 0.26% of cases that have undergone genetic sequencing in 15 days. Also, the data from GISAID revealed that XBB.1 has two subline divisions, XBB.1.1 with ORF1a P309L mutation and XBB.1.2 with an additional S640F spike mutation, and both can be found in Denmark and Singapore. The XBB.2 sublineage has a specific D253G spike mutation and could be found in Japan, India, and Australia. The XBB.3 sublineage has ORF1b 11988V mutation, and like the XBB.1, it has other division forms as the XBB 3.1 with Q677R spike mutation and was first detected in Singapore and India. This is the initial data about XBB spike mutations, as reported by experts so far10 (Fig. 3).
Figure 3.
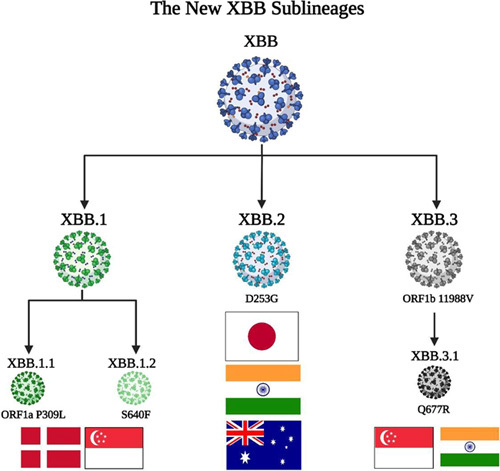
A brief of XBB sublineages.
The clinical picture of the XBB subvariant
Generally, the Omicron variants’ patients are commonly presented with prodromal symptoms, such as pyrexia, cough, and malaise. In addition, hypogeusia, anosmia, pharyngitis, skin rashes, and headaches also were reported11,12. BA.1 and BA.2 subvariants’ predominant forms reveal features like tiredness, fever, runny nose, and cough. At the same time, severe throat aches and general weakness were determined in patients with BA.4 and BA.5 subvariants1. Regarding the clinical diagnosis and available data of the XBB subvariant, most cases in India and Singapore demonstrated the same clinical consequences of previous Omicron sublineages but with discrepancies in some symptoms3. According to WHO, the probable effect of the XBB subvariant is profoundly impacted by the regional immune geography. However, these outcomes were gathered from sentinel countries and could not be completely pervasive in an alternative area. Accordingly, laboratory-based efforts are necessary to promptly create such assessments with global intelligibility3.
Immune reactions and expected response to BA.5 vaccine
The current BA.5 vaccination boosters and herd immunity may not offer adequate coverage against infection with the new subvariants. In a study examining the neutralizing activity against six recently emerging Omicron subvariants (BQ.1.1, BF.7, BA.5, BA.2.75.2, BA.4.6, and XBB.1) using serum from people who received four doses of parental mRNA vaccination or a BA.5-bivalent booster, XBB.1 showed the highest amount of immune evasion, indicating the possibility of these new subvariants unseating the dominant BA.5 in circulation. However, the BA.5-bivalent booster produces superior neutralization against the recently developed XBB subvariant than the primary mRNA vaccination13,14.
There are higher neutralization reactions against the new omicron subvariants, including XBB, among people with a history of COVID-19 infection after vaccination with the BA.5-bivalent vaccine14. However, Reinfections mainly affected people who had contracted the disease before Omicron. There is currently no evidence in favor of escape from recent immunological reactions brought on by other Omicron lineages. Also, the regional immunological landscape, which is influenced by the magnitude and timing of prior Omicron waves as well as the COVID-19 vaccination coverage, appears to be a key factor in determining whether the increased immune escape of XBB is sufficient to drive new infection waves3.
Although the XBB variant developed its way of evading the immune system by acquiring mutations outside the spike RBD, robust immunity is developed by people formerly infected by BA.2 and BA.5 variants. Moreover, getting infected with the recent BQ.1 lineage offers double immunity, as it may somewhat protect XBB variants.15
However, the high transmissibility and immune evasion recorded of XBB subvariant that have the potential to establish a new worldwide wave of COVID-19 infection, there are no yet sufficient strong evidence data about the new subvariant spread, especially in the crude developing countries that lack both the vaccinations coverage and the surveillance of new COVID-19 infections. Further studies are recommended to report the neutralizing effect of the current vaccines with the new XBB subvariant.
Provenance and peer review
Not commissioned, internally peer reviewed.
Ethical approval
NA
Sources of funding
NA
Author’s contribution
All authors have contributed to writing and reviewing the manuscript.
Conflicts of interest
All authors declare no conflict of interest.
Guarantor
Nour Shaheen.
Footnotes
Nour Shaheen: ORCID: https://orcid.org/0000-0001-8663-4044.
The data in this correspondence article is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 12 January 2023
Contributor Information
Mahmoud T. Hefnawy, Email: Mahmoudhefnawy77@gmail.com.
Nour Shaheen, Email: nourshaheen40@gmail.com.
Omar A. Abdelwahabd, Email: omar3240109@gmail.com.
Mariam Tarek Desouki, Email: mariamdesouki362@gmail.com.
Almoatazbellah Attalla, Email: Moataz.anwar1811@gmail.com.
Abdurthman Khaity, Email: abdulrhman.marwan.khaity@gmail.com.
Ala’ A. Rababah, Email: aarababah@hotmail.com.
Abdelrahman Mohamed, Email: abdelrahman-elgharib@hotmail.com.
Youssef Soliman, Email: youssefrefaat138@gmail.com.
Rehab A. Diab, Email: rehab_diab97research@outlook.com.
Asmaa Elganady, Email: Asmaa.elganady@gmail.com.
Samar A. Amer, Email: Dr_samar11@yahoo.com.
References
- 1. Shaheen N, Mohamed A, Attalla A, et al. Could the new BA.2.75 sub-variant cause the emergence of a global epidemic of COVID-19? A scoping review. Infect Drug Resist 2022;15:6317–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shaheen N, Mohamed A, Soliman Y, et al. Could the new BA.2.75 sub-variant lead to another COVID-19 wave in the world? - Correspondence. Int J Surg 2022;105:106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. TAG-VE statement on Omicron sublineages BQ.1 and XBB. Accessed November 19, 2022. https://www.who.int/news/item/27-10-2022-tag-ve-statement-on-omicron-sublineages-bq.1-and-xbb .
- 4. MOH | News Highlights. Accessed November 19, 2022. https://www.moh.gov.sg/news-highlights/details/update-on-covid-19-situation-and-measures-to-protect-healthcare-capacity .
- 5. What do we know about new COVID-19 Omicron subvariants XBB and BQ.1 spreading in Australia? ABC News. Accessed November 19, 2022. https://www.abc.net.au/news/health/2022-11-10/covid-omicron-xbb-bq1-subvariants-sublineages-wave-infections/101617744 .
- 6. icddr, b - News. Accessed November 19, 2022. https://www.icddrb.org/news-and-events/news?id=897&task=view .
- 7. Tracking SARS-CoV-2 variants. Accessed November 19, 2022. https://www.who.int/activities/tracking-SARS-CoV-2-variants .
- 8. Chen C, Nadeau S, Yared M, et al. CoV-spectrum: analysis of globally shared SARS-CoV-2 data to identify and characterize new variants. Bioinformatics 2022;38:1735–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong C. Subvariant ‘soup’ may drive wave. New Sci (1956) 2022;256:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GREAT NEWS! SARS-CoV-2 XBB recombinant variant spawning newer sub-lineages such as XBB.1, XBB.1.1, XBB1.2, XBB.2, XBB.3, XBB.3.1, XBB.4 and XBB.5!. Thailand Medical News. Accessed November 19, 2022. https://www.thailandmedical.news/news/great-news-sars-cov-2-xbb-recombinant-variant-spawning-newer-sub-lineages-such-as-xbb-1,-xbb-1-1,-xbb1-2,-xbb-2,-xbb-3,-xbb-3-1,-xbb-4-and-xbb-5 .
- 11. Setiabudi D, Sribudiani Y, Hermawan K, et al. The Omicron variant of concern: the genomics, diagnostics, and clinical characteristics in children. Front Pediatr 20222;10:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bazargan M, Elahi R, Esmaeilzadeh A. OMICRON: virology, immunopathogenesis, and laboratory diagnosis. J Gene Med 2022;24:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubin R. How immune-evasive omicron offspring and a lack of mitigation measures could shape a COVID-19 winter wave. JAMA. November 16, 2022. Accessed November 19, 2022. https://jamanetwork.com/journals/jama/fullarticle/2798913 .
- 14. Kurhade C, Zou J, Xia H, et al. Low neutralization of SARS-CoV-2 Omicron BA. 2.75. 2, BQ. 1.1, and XBB. 1 by 4 doses of parental mRNA vaccine or a BA. 5-bivalent booster. Biorxiv 2022;2022. [DOI] [PubMed] [Google Scholar]
- 15. Callaway E. COVID ‘variant soup’ is making winter surges hard to predict. Nature 2022;611:213–214. [DOI] [PubMed] [Google Scholar]


