Introduction:
One-anastomosis gastric bypass (OAGB) complication, such as leakage, can be dangerous and should be managed properly, yet little data exist in the literature regarding the management of leaks after OAGB, and there are no guidelines to date.
Methods:
The authors performed a systematic review and meta-analysis of the literature and 46 studies, examining 44 318 patients were included.
Results:
There were 410 leaks reported in 44 318 patients of OAGB published in the literature, which represents a prevalence of 1% of leaks after OAGB. The surgical strategy was very variable among all the different studies; 62.1% of patients with leaks had to undergo another surgery due to the leak. The most commonly performed procedure was peritoneal washout and drainage (with or without T-tube placement) in 30.8% of patients, followed by conversion to Roux-en-Y gastric bypass in 9.6% of patients. Medical treatment with antibiotics, with or without total parenteral nutrition alone, was conducted in 13.6% of patients. Among the patients with the leak, the mortality rate related to the leak was 1.95%, and the mortality due to the leak in the population of OAGB was 0.02%.
Conclusion:
The management of leaks following OAGB requires a multidisciplinary approach. OAGB is a safe operation with a low leak risk rate, and the leaks can be managed successfully if detected in a timely fashion.
Keywords: bariatric, leak, one-anastomosis gastric bypass, weight loss
Introduction
Highlights
Four hundred ten leaks were reported out of 44 318 cases of one-anastomosis gastric bypass (OAGB) published in the literature, which represents a prevalence of 1% of leaks after OAGB.
In all, 62.1% of patients with leaks had to undergo another surgery due to a leak after OAGB.
Medical treatment alone was conducted in 13.6% of patients.
The mortality rate related to leaks was 1.95% and the mortality due to leaks in the general population of OAGB was 0.02%.
A growing number of surgeons are performing one-anastomosis gastric bypass (OAGB) around the world. OAGB is an International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO) recognized bariatric surgical procedure since 2018 and has recently been endorsed by the American Society for Metabolic & Bariatric Surgery (ASMBS)1,2. OAGB is also the third most common bariatric operation after sleeve and Roux-en-Y gastric bypass (RYGB). This procedure has gained popularity during the last decade as it combines several advantages; a relatively short operating time duration and learning curve compared to RYGB, a high efficiency in the treatment of obesity and its associated medical problems, and a simple possibility of reversal to normal anatomy3.
Despite these advantages, OAGB complications such as leakage can be dangerous and should be managed properly. The leak rate after OAGB has been reported in less than 1% of patients, but leak diagnosis should be done as soon as possible to prevent diffuse peritonitis and subsequent sepsis4. Different approaches for both diagnosis and treatment of leak after OAGB exists and should be selected depending on the patient’s hemodynamic condition, surgeon’s experience, and many other factors.
Only little data exist in the literature regarding the management of leaks after OAGB, and to date, there are no published guidelines. The aim of this systematic review and meta-analysis is to give an update on the different strategies available and to guide surgeons for optimal management of leaks following OAGB.
Materials and methods
This work has been reported in line with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) criteria5. We also assessed the level of compliance with AMSTAR (A MeaSurement Tool to Assess systematic Reviews) 2 in this work6. This systematic review and meta-analysis were registered in the Prospective Register of Systematic Reviews (PROSPERO) (# CRD42021247913) and researchregistry.com (#researchregistry8285).
Search
A systematic review of the literature was made by searching through PubMed, Embase, and Scopus databases by 1 January 2022. We identified all articles describing the occurrence of leaks after OAGB using keywords: ‘one anastomosis gastric bypass’ or ‘one-anastomosis gastric bypass’ or ‘OAGB’ or ‘Single anastomosis’ or ‘Omega loop’ or ‘mini gastric-bypass’ or ‘mini gastric bypass’ or ‘MGB’ AND ‘leak’ or ‘peritonitis’ or ‘perforation’ or ‘abscess’ or ‘collection’ or ‘fistula’ or ‘complication’ or ‘reoperation’ or ‘sepsis’ or ‘septic’ or ‘conversion’ or ‘revision’. The references of the articles were manually reviewed for additional relevant papers. Duplicate studies were removed. We did not take into account if OAGB was a primary or a revisional surgery for the analysis, as this specificity was not always well described in articles.
Statistical analysis
The main measure of the effect/effect size was prevalence (ratio of cases to the total population). Cochrane’s test (Q test) (showing significant heterogeneity in the meta-analysis) and I 2 (showing the amount of heterogeneity, ranging from 0 to 100%.) were used to assess the heterogeneity among the studies. The random-effects model was used for the continuous and frequency outcome under study. Random-effects meta-analysis was performed to estimate the main index, which was the pooled prevalence, at the 95% CI. A forest plot was used to present the pooled prevalence. Publication bias was assessed using Begg’s test. The analysis was performed using Stats version 13. Averages of quantitative variables were only reported according to the articles. In the meta-analysis process, we weighted each study by N (sample size). For descriptive purposes, tables and figures were used.
Data extraction
Data on the included articles, including author, year, type of study, patients’ numbers (F/M), age, follow-up, mortality, leak management, limb size, BMI, and complications such as leakage, perforation, and peritonitis were retrieved by two independent investigators. The differences observed in this process were corrected by a third investigator independent from the other two. The Newcastle–Ottawa Scale was used for the qualitative assessment of studies7.
Results
A total of 46 studies examining 44 318 patients were included in this meta-analysis (Fig. 1).
Figure 1.
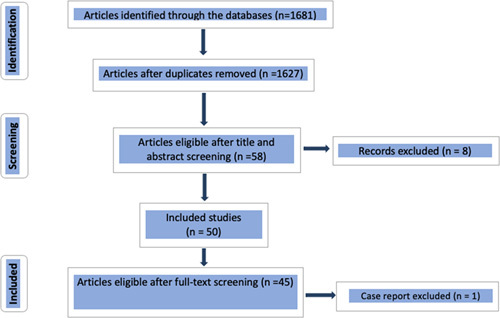
Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
A total of 415 articles were found in PubMed, 1238 in Embase, and 28 in Scopus. Some articles were found twice in separate databases. Among these articles, after the first screening, we only retained 58 articles. Non-English articles were then excluded, as well as articles that were not relevant to our search. PRISMA guidelines were followed for systematic review.
Study characteristics of the patients included in the meta-analysis are presented in Table 1.
Table 1.
Main characteristics of the included studies in the systematic review.
| First author, year, reference | Study type | Mean follow-up, (range) | N | Female% (N) | Mean age (years) (range) | Mean BMI, kg/m2 (range) | Primary or revision | Length BP limb8 | Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| Scavone et al., 20209 | Retro | 60 months | 953 | 71.7% (684) | 41.8 | 49.4 | Both | 180–240 | 100±16 (primary) 118±22 (revision) |
| Bashah et al., 202010 | Retro | 3.8±1.4 years | 49 | 85.7% (42) | 37.83±9.36 | 43.6±7.4 | Revision | 150–200 | N/A |
| Lessing et al., 202011 | Retro | 2 years | 57 | 63.1% (36) | 47.7±10.8 | 42.8±7.0 | Revision | N/A | N/A |
| Neuberg et al., 202012 | Retro | 92 months (76–111) | 163 | N/A | 41±11.4 | 41.2±6.5 | Both | 150 | N/A |
| Liagre et al., 201913 | Retro | 90 days | 2780 | 85% (39) | 45 (26–64) | 41.5 (31–55) | Both | N/A | N/A |
| Sohrabi Maralani et al., 202114 | Retro | 5 years | N/A | N/A | 39.73±11.50 | 44.79±6.07 | N/A | N/A | N/A |
| Debs et al., 202015 | Retro | 55 months (8–144) | 77 | 81.8% (63) | 45.3±14.8 | 40.1 (29–57) | Revision only | 150 | 42.0±8.0 |
| Younis et al., 202016 | Retro | 6 months | 9 | 44.4% (4) | 41±11 (23–57) | 44±8 | Both | N/A | N/A |
| Musella et al., 201717 | Retro | 5 years | 2678 | 70.4% (1885) | 42.2±3.8 | 45.39±3.63 | Both | 165–260 | 86.6±36.5 (primary) 109.3±24.8 (revision) |
| Lessing et al., 201718 | Retro | 12 months | 407 | 62.4% (254) | 4 1.8±1 2.05 | 41.7±5.77 | Both | 200 | N/A |
| Nevo et al., 202119 | Retro | 21 months | 21 | 76% (16) | 43.2±12.1 | 39.7±5.9 | Revision only | 200 | N/A |
| Musella et al., 201920 | Retro | 20.8 months (6–156) | 196 | N/A | 46.1±10.5 | 45.1±7 | Revision only | 226 | N/A |
| Noun et al., 201821 | Prosp | 12 months | 21 | 52.3% (11) | 39±12 (18–65) | 42.9±6.5 | Revision only | N/A | N/A |
| Nagliati et al., 201922 | Prosp | 2 years | 8 | N/A | N/A | N/A | Both | N/A | N/A |
| Poublon et al., 202023 | Retro | 3 years | 185 | 75.5% (139) | 46±9.0 | 40.9 (36–45) | Revision only | 150–250 | 72 (56–95) |
| Meydan et al., 201724 | Retro | 6 months | 154 | 72.1% (111) | 47.06 | 41.76 | Both | 150–200 | N/A |
| Bolckmans et al., 201925 | Prosp | 9 years | 526 | 89.3% (25) | N/A | N/A | Primary only | 200 | N/A |
| Alkhalifah et al., 201826 | Retro | 10 years | 1731 | 70% (1212) | 33.8±10.4 | 40.4±7.7 | Primary only | 150–250 | 124.6±38.8 |
| Chansaenroj et al., 201727 | Retro | N/A | 26 | 61.5% (16) | 35.9±8.8 | 39.3±8.9 | Revision only | N/A | 180.2±58.7 |
| Apers et al., 201828 | Prosp | 3 years | 287 | 85.4% (245) | 44 (19–69) | 42 (32–76) | Primary only | 150–250 | 50 (25-120) |
| Almalki et al., 201829 | Retro | 5 years | 81 | 74% (60) | 38.7±9.8 | 37.8±9.6 | Revision only | N/A | 167.7±55.8 |
| Genser et al., 201630 | Retro | 8 years and 9 months | 2321 | N/A | 41 (26–63) | N/A | Both | N/A | N/A |
| de la Cruz et al., 202031 | Retro | 3 years | 42 | N/A | N/A | 43.4±9.2 | Revision only | 200 | N/A |
| Parmar et al., 201832 | Retro | 6 months to 12 years | 12 807 | N/A | 41.2 | 46.6 | N/A | N/A | 123±39 |
| Soong et al., 201933 | Retro | 12 months | 940 | 62.3% (586) | 40.6 | 40 | Primary only | 400 | 142 |
| Navarrete et al., 201834 | Prosp | 12 months | 100 | 64% (64) | 40.5±12.4 | 44.8±12.1 | Primary only | N/A | 69±4.62 |
| Lo et al., 202035 | Retro | 12 months | 73 | 61% (39) | 40.8 | 42.5 | Primary only | N/A | 117 |
| Parmar et al., 202036 | Retro | 32.7 months (6–84) | 376 | 67.7% (254) | 44.3 | 29.2 | N/A | 120 | 89.5 (49–150) |
| Khalaj et al., 202037 | Retro | 12 months | 548 | 85% (457) | 39.5 | 46 | Primary only | 160–200 | 71.8 |
| Salama et al., 201638 | Prosp | 12 months | 39 | N/A | 38.7 | 39.7 | Revision only | 180 | 145±29 (125–235) |
| Taha et al. 201739 | Retro | 6–36 months | 1520 | 62.7% (953) | 37.2±11.4 | 46.8±6.6 | Both | 150–300 | 57 |
| AlSabah et al., 201840 | Retro | 12 months | 31 | 89.7% (28) | 41.4±10.2 | 42.6±5.8 | Revision only | 175–200 | 118.2±53.1 |
| Pujol Rafols et al., 201841 | Retro | 12–60 months | 191 | 89.5% (171) | 40.6±11.2 | 39.8±6.9 | Revision only | 150–250 | N/A |
| Beaupel et al., 20174 | Retro | 24.5 (4–108) | 17 | 76.5% (13) | 48 (23–62) | 51 (38–70) | Both | 150–200 | N/A |
| Carbajo et al., 200542 | Retro | 2 years | 209 | 82% (172) | 41 (14–66) | 48 (39–86) | Both | 200 | 93 (70–150) |
| Noun et al., 201243 | Retro | 60 months | 1000 | 66.1% (661) | 33.15 | 42.5 | Both | 150 | 89 (primary) 144 (revision) |
| Piazza et al., 201544 | Retro | 5 years | 48 | 82% | 38 | 43.4 | Revision only | 180–240 | N/A |
| Chevallier et al., 201545 | Retro | 7 years | 1000 | 71.2% (712) | 41.8 | 45.7 | Both | 200 | N/A |
| Ghosh et al., 201746 | Retro | 12 months | 74 | 91% (67) | 48.3 | 46 | Revision only | 150 | 72.7 |
| Abdallah et al., 202247 | Retro | 12 months | 80 | 77.5% (62) | 41 | 50.9 | Primary only | 200 (170–300) | N/A |
| Plamper et al., 201748 | Retro | N/A | 169 | 71.6% (121) | N/A | 54.1% | Primary only | N/A | N/A |
| Parmar et al., 201649 | Retro | 2 years | 125 | 68.8% (86) | 45 | 48.1 | Primary only | N/A | 92.4 |
| Bruzzi et al., 201550 | Retro | 5 years | 126 | 79% (99) | 50±10 | 47 | Revision only | N/A | 110 |
| Disse et al., 201451 | Retro | 21.4 months | 20 | N/A | 49.5 | 40.1 | Primary only | N/A | 105 |
| Johnson et al., 20078 | Retro | N/A | 32 | N/A | N/A | N/A | Primary only | N/A | N/A |
| Beargeat et al., 201752 | Retro | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Piazza et al., 201153 | Retro | 2 | 197 | 75% (148) | 37.9 | 52.9 | N/A | N/A | 120 |
| Noun et al., 200754 | Retro | N/A | 126 | N/A | N/A | N/A | N/A | N/A | N/A |
| Kular et al., 201455 | Retro | 6 years | 1054 | 67.5% (712 ) | 38.4 | 43.2 | N/A | 200 | 52 |
| Musella et al., 201456 | Retro | 12–60 months | 974 | 51.2% (499) | 39.4 | 48±4.58 | Both | 224.6±23.2 | 95±51.6 |
| Wang et al., 200557 | Retro | Up to 36 months | 423 | 79% (336) | 30.8 | 44.2 | Both | 200 | 95±41.5 |
| Docimo et al., 202258 | Retro | 1 month | 279 | 81.7% (228) | 46.1 (11.04) | 44.5±7.7 | Primary | N/A | N/A |
| Rayman et al., 202159 | Retro review | 25.5 months (8–60) | 144 | 74.3% (107) | 42.4±10.5 | 40.6±5.9 | Revision only | N/A | N/A |
| Rutledge and Walsh, 200560 | Prosp | 38.7 months (1.0–74.4) | 2410 | 85% (2049) | 39 (14–78) | 46±7 (34–74) | Both | N/A | 37.5 |
| Almuhanna et al., 202161 | Retro | N/A | 2223 | 70.2% (1560) | 35.3±11.4 (14–71 ) | 40.2±11.9 | N/A | N/A | N/A |
| Goel et al., 202162 | Retro | 3 years | 3187 | 53.8% (1712) | 43.3±12.2 | 44.5±7.9 | Both | N/A | N/A |
| Garcia-Caballero et al., 200563 | Case report | N/A | 1 | 1 | 36 | 43 | Primary only | N/A | N/A |
BP limb, biliopancreatic limb, Retro, retrospective study ; Prosp, prospective study.
Study characteristics regarding the management of leaks are presented in Table 2.
Table 2.
Leak-related data of the included studies in the meta-analysis.
| First author, year, reference | Leak rate | Diagnosis | Time after OAGB | Leak management | Reoperation (due to leak) | Death after leak |
|---|---|---|---|---|---|---|
| Scavone et al., 20209 | 5 of 953 (0.5%) | CT scan Oral contrast series |
First week | N/A | N/A | 0% |
| Bashah et al., 202010 | 1 of 49 (2%) | N/A | ‘shortly’ | Surgery: conversion to RYGB | 1 (100%) | 0% |
| Lessing et al., 202011 | 2 of 57 (3.51%) | N/A | N/A | N/A | N/A | 0% |
| Neuberg et al., 202012 | 1 of 163 (0.61%) | N/A | ‘early’ | N/A | N/A | 0% |
| Liagre et al., 201913 | 46 of 2780 (1.7%) | Oral CT scan Endoscopic findings Intraoperative |
10 days (1–42) |
Medical (N=9): fasting, total parenteral nutrition, and antimicrobial therapy Interventional/endoscopy (N=23): percutaneous drainage and/or endoscopy Surgery: laparoscopy: washout and drainage (+T-tube placement in 5 cases) (N=13); conversion to RYGB (N=1) |
14 (30%) | 0% |
| Sohrabi Maralani et al., 202114 | 1 of 805 (0.1%) | N/A | N/A | N/A | 1 (100%) | 100% (1) |
| Debs et al., 202015 | 1 of 77 (1.3%) | N/A | N/A | Surgery (N=1): Kehr tube and drainage | 1 (100%) | 0% |
| Younis et al., 202016 | N/A | CT scan | Less than 4 weeks | Interventional/endoscopy: all had fully covered stents (N=9) Surgery: laparotomy RYGB conversion (N=2) Laparotomy after 2 weeks of treatment due to stent migration and ileum perforation |
2 (22%) | 11% (1) |
| Musella et al., 201717 | 13 of 2251 (0.6%) | N/A | N/A | Surgery depending on the leak site
Anastomotic leak (N=5): -laparoscopic revision/Braun anastomosis (N=2) -laparoscopic repair (N=1) -laparoscopic reversal surgery (N=1) -conservative treatment/laparotomy (N=1) Gastric pouch leak (N=7): -laparoscopic repair (N=5) -conservative treatment (N=1) -revision/laparotomy (N=1) Gastric remnant leak: -laparoscopic repair (N=1) |
11 of 13 (84.6%) | 1 (7.7%) |
| Lessing et al., 201718 | 7 of 407 (1.7%) | N/A | 6.5 days (2–14) | Medical: fasting, total parenteral nutrition, and antimicrobial therapy (N=3) Surgery: laparoscopic drainage (N=3), laparoscopic drainage after failed percutaneous drainage (N=1) |
3 of 407 (0.73%) | 0% |
| Nevo et al., 202119 | 1 of 21 (4.7%) | N/A | N/A | Interventional (N=1): percutaneous drainage | 0% | 0% |
| Musella et al., 201920 | 1 of 196 (0.5%) | N/A | N/A | N/A | N/A | N/A |
| Nagliati et al., 201922 | 1 of 8 (12.5%) | Intraoperative | 1 day | Surgery (N=1): no details | 1 (12.5%) | N/A |
| Poublon et al., 202023 | 1 of 185 (0.5%) | N/A | N/A | N/A | N/A | 0% |
| Meydan et al., 201724 | 1 of 154 (0.65%) | Clinical presentation: septic shock | 4 days | Surgery (N=1): laparoscopic conversion to RYGB | 1 (100%) | N/A |
| Bolckmans et al., 201925 | 5 of 526 (0.95%) | N/A | N/A | Surgery (N=5): laparoscopic conversion to RYGB | 5 (100%) | N/A |
| Alkhalifah et al., 201826 | 20 of 1731 (1.15%) | N/A | N/A | N/A | N/A | N/A |
| Chansaenroj et al., 201727 | 2 of 26 (7.7%) | N/A | N/A | Surgery (N=2): laparoscopic exploration, repair and drainage | 2 (100%) | 0% |
| Apers et al., 201828 | 4 of 287 (1.4%) | N/A | N/A | Medical (N=2): feeding tube Surgery (N=2): laparoscopy (no details) |
2 (50%) | N/A |
| Almalki et al., 201829 | 5 of 81 (6.2%) | N/A | N/A | N/A | N/A | N/A |
| Genser et al., 201630 | 35 of 2321 (1.5%) | Systematic oral contrast series (N=4) Oral CT scan (N=4) Intraoperative (N=27) |
9 days (97%) (0–28) |
Surgery (N=35): all had washout and drainage: -laparoscopy (N=33) -laparotomy (N=2) Interventional/endoscopy (N=2): in addition to surgery in patients with large staple lines breakdown needing endoscopic stenting |
35 (100%) | 0% |
| de la Cruz et al., 202031 | 1 of 42 (2.4%) | N/A | N/A | Surgery (N=1): laparoscopy (no details) | 1 (100%) | N/A |
| Parmar and Mahawar, 201832 | 123 of 12 807 (0.96%) | N/A | N/A | N/A | N/A | N/A |
| Soong et al., 201933 | 5 of 940 (0.5%) | N/A | N/A | N/A | N/A | N/A |
| Parmar et al., 202036 | 1 of 376 (0.3%) | <30 days | N/A | Surgery: conversion to RYGB | 1 (100%) | N/A |
| Khalaj et al., 202037 | 3 of 548 (0.5%) | Oral CT scan | <30 days | Interventional (N=2): drainage and intravenous antibiotics Surgery (N=1): urgent peritoneal lavage and antimicrobial therapy |
1 (33%) | 1 (0.18%) |
| Salama and Sabry, 201638 | 1 of 39 (2.6%) | N/A | 2 days | Surgery (N=1): direct suture of the injured bowel | 1 (100%) | 0 |
| Taha et al., 201739 | 2 of 1520 (0.1%) | N/A | 2 days | Surgery (N=2): -conversion to RYGB (N=1) -repair of the defect (N=1) |
2 (100%) | 0 |
| AlSabah et al., 201840 | 2 of 31 (6.45%) | CT scan | N/A | Interventional/endoscopy (N=2): -stent (N=1) -percutaneous drainage (N=1) |
0% | 0% |
| Pujol Rafols et al., 201841 | 5 of 191 (2.6%) | N/A | N/A | N/A | N/A | 0% |
| Beaupel et al., 20174 | 10 of 1430: study conducted among 17 patients with leakage after OAGB – but 10 had undergone an initial OAGB in the center, which leads to a leak rate of 0.7% (10/1430) |
Oral CT scan (88% ) Intraoperative |
4 days (1–28) | Surgery (N=14): -conversion to RYGB (N=4): leak of the GT or the GJA: conversion was performed lavage, drainage, and treatment of the perforation (T-tube intubation N=2, suture N=1, anastomosis resection and refection N=1) |
14 (100%) | 0% |
| Carbajo et al., 200542 | 4 of 209 (1.9%) | Oral contrast series | 1 day | Medical: conservative management (no details) | 0% | 0% |
| Noun et al., 201243 | 5 of 1000 (0.5%) | Oral contrast series | 1 week (2 leaks) 2 weeks (3 leaks) |
Medical/interventional: -cutaneous fistula that healed with conservative management more than 2 weeks after surgery (N=3) -percutaneous drainage (N=3) Surgery: suturing of the GT and drainage (N=1) -conversion to RYGB after failed percutaneous drainage (N=1) |
2 (40%) | 0% |
| Chevallier et al., 201545 | 6 of 1000 (0.6%) | N/A | ‘early’ | Surgery (N=6) (no details) | 6 (100%) | 0% |
| Ghosh et al., 201746 | 1 of 74 (1.35%) | N/A | ‘early’ | Interventional (N=1): percutaneous drainage | 0% | 0% |
| Plamper et al., 201748 | 1 of 169 (0.6%) | N/A | ‘early’ | N/A | N/A | 0% |
| Bruzzi et al., 201550 | 1 of 126 (0.79%) | Intraoperative | N/A | Surgery (laparotomy), no details | 1 (100%) | 0% |
| Johnson et al., 20078 | N/A | N/A | N/A | Surgery (N=3): -conversion to RYGB (N=2) |
3 (100%) | 0% |
| Kular et al., 201455 | 2 of 1054 (0.2%) | N/A | 2 | Surgery (laparotomy), repair (no details) | 2 (100%) | 0% |
| Musella et al., 201456 | 10 of 974 (1%) | N/A | 1–12 days | Surgery (N=6) (no detail) | 6 (60%) | 1 (0.001%) |
| Wang et al., 200557 | 9 of 423 (2.1%) | N/A | N/A | Medical (N=6): total parenteral nutrition for minor leakage (N=6) Surgery (N=3): reoperation for drainage |
3 (33%) | 1 (0.23%) |
| Docimo et al., 202258 | 3 of 279 (1.1%) | N/A | N/A | Surgery (N=1) (no details) | 1 (33%) | N/A |
| Rayman et al., 202159 | 2 of 144 (1.4%) | N/A | N/A | N/A | N/A | N/A |
| Rutledge and Walsh, 200560 | 26 of 2410 (1.1%) | Intraoperative | N/A | Surgery: (no details) Laparoscopic re-exploration and repair | N/A | No |
| Almuhanna et al., 202161 | 19 of 2223 (0.85%) | N/A | N/A | N/A | N/A | 2 (0.09%) |
| Goel et al., 202162 | 7 of 3187 (0.2%) | CT scan Oral contrast series Ultrasounds |
N/A | Medical/interventional: pigtail, drainage Surgery: laparoscopy (no details) |
N/A | No |
| Garcia-Caballero et al., 200563 | 1 case report | Oral contrast series | N/A | Medical/interventional: total parenteral nutrition, endoscopic fibrin glue | N/A | No |
CT scan, computed tomography scan; oral CT scan, orally ingested computed tomography scan; GJ anastomosis, gastrojejunal anastomosis; GT, gastric tube; reoperation, number of patients with a leak who needed a reoperation and percentage; OAGB, one-anastomosis gastric bypass; RYGB, Roux-en-Y gastric bypass.
Descriptive characteristics
Table 3 shows a mean procedure time of 98.45±35.93 min, a mean age of 41.34±4.21 years, a mean BMI of 43.7±4.2 kg/m2, and a median hospital stay of 3.63 days.
Table 3.
Mean and SD of main quantitative variables.
| Variable | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|
| Procedure time, min | 38 | 180 | 98.45 | 35.93 |
| Mean age, year | 31 | 50 | 41.34 | 4.21 |
| BMI, kg/m2 | 29 | 54 | 43.67 | 4.19 |
| Hospital stay, day, median (interquartile range) | 3.63 (2–5.53) |
Regarding the time of leak after OAGB, we found a rate of ‘acute’ leaks (within 7 days) of 33.3% (N=6) and ‘early’ leaks (1–6 weeks) of 66.7% (N=12), while there are no reports about ‘late’ leaks (6–12 weeks) (N=0).
Leak prevalence
There were 410 leaks reported in a total of 44 318 cases of OAGB published in the literature. Hence, the pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 1% (or 0.01 with 73.75% I 2), that is one out of every 100 surgeries of OAGB experience leakage (Fig. 2).
Figure 2.
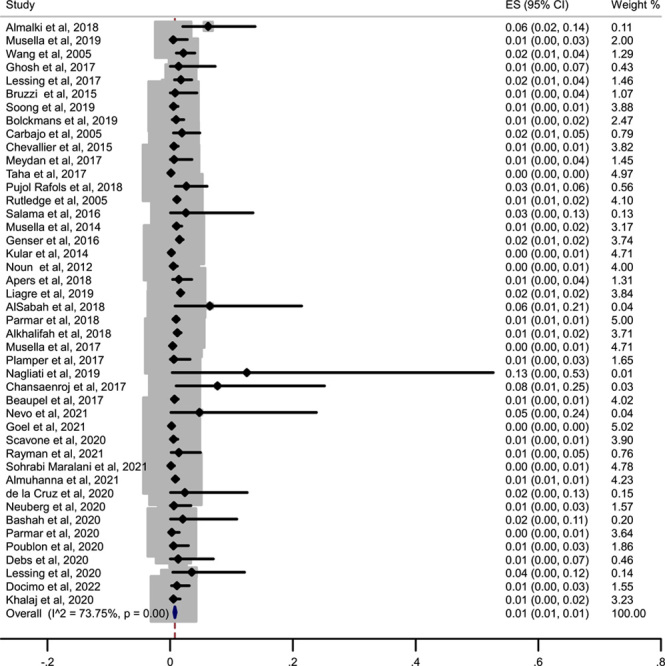
Forest plot showing the prevalence of leaks among the different studies included in the meta-analysis. ES, effect size.
Two studies, including Johnson et al.8 and Younis et al.16, were deleted from leak prevalence analysis because they reported leak as a reason for revision surgery and endoscopic management among their revision and endoscopic procedures, not amongst all revisional OAGB patients.
We did not find a ‘cutoff’ year regarding the leak rate; the leak rate was stable, for instance, there were no more leaks before or after a precise year, and the leak rate did not drop after a precise year. This was to understand whether the leak rate was more in the earlier years when probably more surgeons were in their learning curve of this operation.
Leak prevalence across primary and revision studies
The pooled estimation of a meta-analysis of prevalence studies reported a prevalence of 1% (or 0.01 with 2.98% I 2) for revision studies and 1% (or 0.01 with 0% I 2) for primary studies, that is one out of every 100 surgeries of OAGB experience leakage in the two types of studies. The following figure shows a nonsignificant difference between the two types of studies (primary vs. revision/secondary studies) (P=0.57), but visually and clinically, the prevalence of leak is higher in the revision studies, and we can see the range of 1–8% prevalence in the revision studies while in the primary studies, there is only 1% prevalence of leak (Fig. 3).
Figure 3.
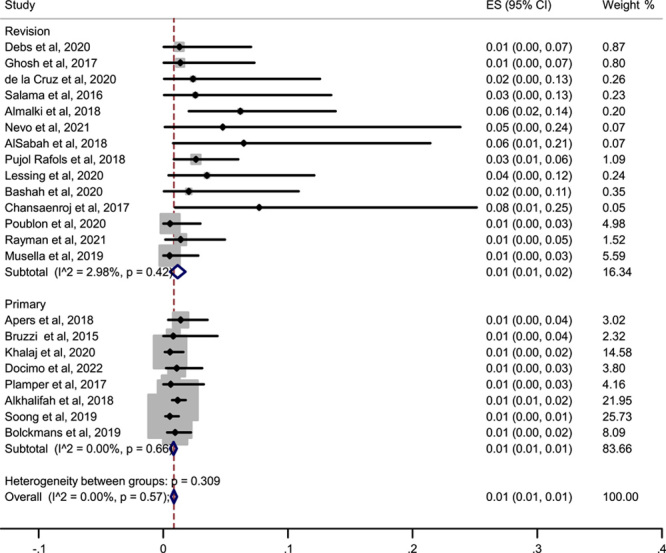
Forest plot showing the prevalence of leak across only primary and revision studies. ES, effect size.
Leak diagnosis
Computed tomography scan (CT scan) with or without oral contrast was the most commonly used technique to diagnose leaks, as it was used in 47% of studies. Diagnostic laparoscopy (intraoperative finding) was done in 32% of cases. The use of upper gastrointestinal oral contrast series was reported in 21% of studies, ‘endoscopy’ and ‘clinical presentation’ were reported in 15% of studies (Fig. 4).
Figure 4.
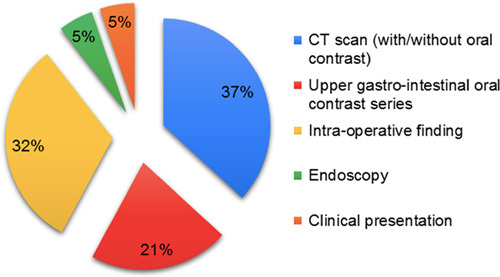
Diagnostic approach of leak after OAGB. CT, computed tomography; OAGB, one-anastomosis gastric bypass.
Leak management
Several treatment options were reported: medical treatment only, percutaneous drainage, endoscopic treatment using stent or pigtails, or glue and surgical treatment. Very few articles justified the choice-making process and the decisions of one option or the other.
Among the 410 leaks reported, clear numbers and statistics regarding precisely how leaks were treated were available in only 198 patients.
Surgical management
The surgical strategy was very variable among all the different studies.
Among these 198 patients, 123 (62.1%) of them had to undergo another surgery because of a leak. The most commonly used procedure was a peritoneal washout and drainage (with or without T-tube placement) in 61 (30.8%) patients, followed by conversion to RYGB in 19 (9.6%) patients. Other surgical options include repair of the anastomosis and drainage in 14 (7.1%) patients and surgical reversal of the OAGB in 1 patient. No details were given regarding the kind of surgery performed in 32 (16.2%) patients.
Conservative management
Medical treatment with antibiotics with or without total parenteral nutrition alone was conducted in 27 (13.6%) patients. In addition to medical treatment, percutaneous drainage was reported in 9 (4.6%) patients. Endoscopic treatment without surgery was the chosen option in 33 (16.7%) patients.
Mortality
In this meta-analysis, out of 410 with a leak, 8 patients died: the mortality rate related to the leak was 1.95%. Hence, the mortality due to leaks was 8 out of 44 318 patients (0.02%).
Discussion
This study gives an updated insight into the state of the literature, with an average rate of leaks of 1%, and there is no statistically significant difference in leak rates between primary and revisional OAGB in the presence of an experienced surgical team. The occurrence of a leak often leads to another surgical procedure, as roughly 60% of patients actually require a surgical exploration.
In this review, leak diagnosis was most often made after an oral contrast CT scan. It is important to keep in mind that a leak following an OAGB is an emergency, and therefore patients’ clinical presentation should always be taken into account before any radiological or complementary diagnostic exam. When a leak is suspected, no further investigations preceding a surgical exploration should be performed if the patient is unstable and/or shows signs of severe sepsis. A tachycardia over 120 beats per minute (bpm) in the first postoperative days is a strong element to schedule a surgical exploration without any delay or further examination64 to decrease mortality and subsequent morbidities. Caiazzo et al.65 showed that in most cases, mortality after bariatric surgery is the consequence of delays in the management of leaks, resulting in the constitution of diffuse peritonitis. This ‘surgical’ attitude was also the one adopted by Genser et al.30, who recommend an ‘aggressive’ management of leaks, systemically involving a surgical exploration when a leak is suspected in order to obtain a rapid recovery and a decreased risk of mortality, at the cost of increased morbidity. In all cases, surgery must always be adapted to the clinical situation; therefore, in the presence of septic shock with the need for catecholamines, the procedure should be as quick as possible, and a simple lavage and drainage can be performed in such critical patients. The addition of a feeding jejunostomy in the efferent limb can be an interesting option in complicated situations in order to avoid long-lasting parenteral nutrition.
Most leaks following OAGB cannot be assimilated to leaks occurring after laparoscopic sleeve gastrectomy or RYGB. Indeed, leaks after OAGB raise concerns, as the most common leak site is the gastrojejunal anastomosis (GJA). Unlike after GJA leaks following RYGB, where the leak stays isolated from the bile, after OAGB, the GJA leak is a high-flow leak, exposed to a strong concentration of bile flowing from an afferent limb to the leak site. This is why in such a situation, especially if the surgery is recent and when the leak episode is well tolerated, many bariatric surgeons recommend directly converting to the RYGB condition in order to isolate the bile flow from the GJA. If the leak is not of the GJA but still takes place in the lower part of the gastric pouch, conversion to the RYGB by dividing the pouch above the leak site is also a good and safe option.
Conversion to RYGB exhibits good results in the literature. The IFSO Worldwide One Anastomosis Gastric Bypass Survey showed that conversion of OAGB to RYGB for leak management is the most common bariatric surgical procedure among bariatric surgeons66. Blockmans et al.25 reported control of the sepsis and a complete treatment of the leak in five out of five early leaks after OAGB. Similar good results were observed by Poghosyan et al.67 and Beaupel et al.4, who also experimented with uncomplicated conversion to RYGB in the treatment of leaks.
In the case of conversion to RYGB, it has been suggested that the gastric pouch could be shortened to avoid the fashion of a big or/and broad gastric pouch that causes acid reflux and anastomotic ulcers68. Conversion to RYGB is a procedure requiring high surgical skills and, therefore, cannot always be performed by all general surgeons during an emergency. This should be done within expert units by experienced surgeons.
If the leak is located at the top of the gastric pouch, below the cardia (proximal staple line), conversion to RYGB should be avoided as it rarely allows a sufficient gastric length to fashion a new gastric pouch. We suggest in such a situation simply place a surgical drain near the leak orifice if surgery is performed and/or proceed to an endoscopic placement of a gastric stent or pigtail, depending on the leak size.
Endoscopic management of a leak can play an important role in suitable conditions and at the right time to prevent a second surgical approach. The value of endoscopy in the treatment of leaks, alone or combined with surgery, is now indubitable but data regarding specifically endoscopic management of leaks after OAGB are still scarce in the literature. Liagre et al. proposed endoscopy for patients with failure of medical treatment alone and/or in association with percutaneous drainage of an abscess with a leak orifice clearly identified on a CT scan and/or in the presence of digestive fluid leaking through the abdominal drain left in place after surgical exploration. They also suggested that if the leak orifice on the digestive side was less than 1 cm in diameter, a double pigtail drain could be used to obtain an intraluminal drainage of the collection13. Endoscopic treatment can be chosen for leaks occurring after the first postoperative week in patients with no major signs of sepsis. In 2020, Younis et al. reported a median time between surgery and endoscopy of 12 days. In their study, fully covered stents were placed for a median duration of 26 days. All patients with anastomotic leaks had a favorable outcome, whereas this treatment succeeded in only one patient with a staple line leak. Despite this attitude, two patients developed a late fistula needing additional drainage procedures (including pigtail), and another patient had an emergency laparotomy due to a stent migration with perforation of the ileum16. Endoscopy can also be a second-line treatment, as was described by Beaupel et al.4 in their 2017 study when they used stents for two patients in second intention as a treatment of persistent leaks after surgical treatment, obtaining closure of the leak orifice within 4 weeks.
Endoscopic stenting can also add to surgical procedures in large gastric tube staple line failure to decrease the gastric tube content spillage and accelerate the recovery time, as has been reported by Gesner et al.30, although it was not recommended by them because of the risk of stent migration that may lead to obstruction and perforation.
The recent experts’ consensus about patient selection in OAGB recommended this procedure as a suitable revisional procedure for weight regain after primary restrictive bariatric procedures2. This systematic review and meta-analysis also confirms the results of two previously published meta-analyses about the safety of revisional OAGB in the subject of leakage, which is the most common major complication after revisional OAGB69,70.
A suggested algorithm regarding the management of patients with a suspicion of the leak, according to the included papers in this meta-analysis, is shown in Figure 5.
Figure 5.
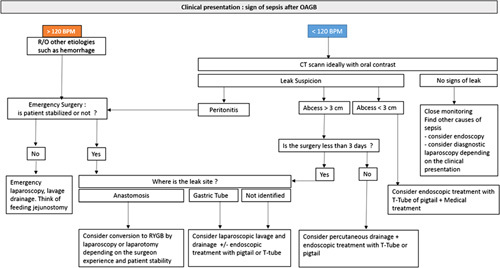
Suggested algorithm regarding the management of patients with a suspicion of the leak. CT, computed tomography; R/O, rule out; RYGB, Roux-en-Y gastric bypass.
Despite our efforts, this review has several weaknesses. Most of the studies included in this review did not mention or elaborate on how precisely leaks were managed. We could only extract this data from less than half of the reviewed articles. Therefore, there is probably a publication bias in this aspect. The statistical validity of such pooling of heterogeneous data cannot be perfect. This work is only meant to be indicative for surgeons and is here to guide them, as every case is unique. Despite all these shortcomings, this is a significant paper documenting how OAGB leaks are managed today by a significant number of surgical groups from around the world.
Conclusion
The management of leaks following OAGB requires a multidisciplinary team approach. OAGB is a safe operation with a low leak rate, and the leaks can be managed successfully if detected in a timely fashion. There is no significant difference between leak incidence after primary and revisional OAGB and correct surgical technique, and increasing the surgical team experience can decrease the leak rates after OAGB. With the increasing popularity of this technique, it is a necessity that the management of leaks following OAGB is clarified, and this question should be addressed in international guidelines in the near future.
Ethical approval
Not applicable (review of the literature).
Sources of funding
No funding.
Author contribution
M.K.: conceived the idea for the topic, data gathering, consulting, and writing; R.K.: consulting and reviewing; R.V.: statistics and methodology; C.P.: data gathering, consulting, and reviewing; A.H.D.: data gathering, consulting, and reviewing. S.S.S.: data gathering, consulting, and reviewing; M.B.: organization leadership, data gathering, consulting, and writing.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO.
Unique identifying number or registration ID: CRD42021247913.
Hyperlink to your specific registration (must be publicly accessible and will be checked): CRD42021247913.
Guarantor
Dr Marine Benois.
Data availability statement
Data regarding this article are easy to verify as all relevant articles are cited and available online.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 10 April 2023
Contributor Information
Mohammad Kermansaravi, Email: mkermansaravi@yahoo.com.
Radwan Kassir, Email: radwankassir42@hotmail.fr.
Rohollah Valizadeh, Email: rohvali4@gmail.com.
Chetan Parmar, Email: drcparmar@gmail.com.
Amir Hossein Davarpanah Jazi, Email: davarpanahjazi@gmail.com.
Shahab Shahabi Shahmiri, Email: shshahabi@yahoo.fr.
Marine Benois, Email: marinebenois@gmail.com.
References
- 1. De Luca M, Tie T, Ooi G, et al. Mini gastric bypass-one anastomosis gastric bypass (MGB-OAGB)-IFSO position statement. Obes Surg 2018;28:1188–1206. [DOI] [PubMed] [Google Scholar]
- 2. Kermansaravi M, Parmar C, Chiappetta S, et al. Patient selection in one anastomosis/mini gastric bypass – an expert modified Delphi consensus. Obes Surg 2022;32:2512–2524. [DOI] [PubMed] [Google Scholar]
- 3. Kermansaravi M, Shahmiri SS, Davarpanah Jazi AH, et al. Reversal to normal anatomy after one-anastomosis/mini gastric bypass, indications and results: a systematic review and meta-analysis. Surg Obes Relat Dis 2021;17:1489–1496. [DOI] [PubMed] [Google Scholar]
- 4. Beaupel N, Bruzzi M, Voron T, et al. Management of acute intra-abdominal sepsis caused by leakage after one anastomosis gastric bypass. Surg Obes Relat Dis 2017;13:1297–1305. [DOI] [PubMed] [Google Scholar]
- 5. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 8. Johnson WH, Fernanadez AZ, Farrell TM, et al. Surgical revision of loop (“mini”) gastric bypass procedure: multicenter review of complications and conversions to Roux-en-Y gastric bypass. Surg Obes Relat Dis 2007;3:37–41. [DOI] [PubMed] [Google Scholar]
- 9. Scavone G, Caltabiano DC, Gulino F, et al. Laparoscopic mini/one anastomosis gastric bypass: anatomic features, imaging, efficacy and postoperative complications. Updates Surg 2020;72:493–502. [DOI] [PubMed] [Google Scholar]
- 10. Bashah M, Aleter A, Baazaoui J, et al. Single anastomosis duodeno-ileostomy (SADI-S) versus one anastomosis gastric bypass (OAGB-MGB) as revisional procedures for patients with weight recidivism after sleeve gastrectomy: a comparative analysis of efficacy and outcomes. Obes Surg 2020;30:4715–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lessing Y, Nevo N, Pencovich N, et al. One anastomosis gastric bypass as a revisional procedure after failed laparoscopic adjustable gastric banding. Obes Surg 2020;30:3296–3300. [DOI] [PubMed] [Google Scholar]
- 12. Neuberg M, Blanchet MC, Gignoux B, et al. Long-term outcomes after one-anastomosis gastric bypass (OAGB) in morbidly obese patients. Obes Surg 2020;30:1379–1384. [DOI] [PubMed] [Google Scholar]
- 13. Liagre A, Queralto M, Juglard G, et al. Multidisciplinary management of leaks after one-anastomosis gastric bypass in a single-center series of 2780 consecutive patients. Obes Surg 2019;29:1452–1461. [DOI] [PubMed] [Google Scholar]
- 14. Sohrabi Maralani M, Azadnajafabad S, Elyasinia F, et al. Postoperative outcomes and advantages of hand-sewn laparoscopic one-anastomosis gastric bypass: experience on 805 patients. Obes Surg 2021;31:627–633. [DOI] [PubMed] [Google Scholar]
- 15. Debs T, Petrucciani N, Kassir R, et al. Laparoscopic conversion of sleeve gastrectomy to one anastomosis gastric bypass for weight loss failure: mid-term results. Obes Surg 2020;30:2259–2265. [DOI] [PubMed] [Google Scholar]
- 16. Younis F, Shnell M, Gluck N, et al. Endoscopic treatment of early leaks and strictures after laparoscopic one anastomosis gastric bypass. BMC Surg 2020;20:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musella M, Susa A, Manno E, et al. Complications following the mini/one anastomosis gastric bypass (MGB/OAGB): a multi-institutional survey on 2678 patients with a mid-term (5 years) follow-up. Obes Surg 2017;27:2956–2967. [DOI] [PubMed] [Google Scholar]
- 18. Lessing Y, Pencovich N, Khatib M, et al. One-anastomosis gastric bypass: first 407 patients in 1 year. Obes Surg 2017;27:2583–2589. [DOI] [PubMed] [Google Scholar]
- 19. Nevo N, Lessing Y, Abu-Abeid S, et al. Roux-en-Y gastric bypass versus one anastomosis gastric bypass as a preferred revisional bariatric surgery after a failed silastic ring vertical gastroplasty. Obes Surg 2021;31:654–658. [DOI] [PubMed] [Google Scholar]
- 20. Musella M, Bruni V, Greco F, et al. Conversion from laparoscopic adjustable gastric banding (LAGB) and laparoscopic sleeve gastrectomy (LSG) to one anastomosis gastric bypass (OAGB): preliminary data from a multicenter retrospective study. Surg Obes Relat Dis 2019;15:1332–1339. [DOI] [PubMed] [Google Scholar]
- 21. Noun R, Slim R, Chakhtoura G, et al. Resectional one anastomosis gastric bypass/mini gastric bypass as a novel option for revision of restrictive procedures: preliminary results. J Obes 2018;2018:4049136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nagliati C, Troian M, Pennisi D, et al. Enhanced recovery after bariatric surgery: 202 consecutive patients in an Italian bariatric center. Obes Surg 2019;29:3133–3141. [DOI] [PubMed] [Google Scholar]
- 23. Poublon N, Chidi I, Bethlehem M, et al. One anastomosis gastric bypass versus Roux-en-Y gastric bypass, remedy for insufficient weight loss and weight regain after failed restrictive bariatric surgery. Obes Surg 2020;30:3287–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meydan C, Raziel A, Sakran N, et al. Single anastomosis gastric bypass-comparative short-term outcome study of conversional and primary procedures. Obes Surg 2017;27:432–438. [DOI] [PubMed] [Google Scholar]
- 25. Bolckmans R, Arman G, Himpens J. Efficiency and risks of laparoscopic conversion of omega anastomosis gastric bypass to Roux-en-Y gastric bypass. Surg Endosc 2019;33:2572–2582. [DOI] [PubMed] [Google Scholar]
- 26. Alkhalifah N, Lee WJ, Hai TC, et al. 15-year experience of laparoscopic single anastomosis (mini-)gastric bypass: comparison with other bariatric procedures. Surg Endosc 2018;32:3024–3031. [DOI] [PubMed] [Google Scholar]
- 27. Chansaenroj P, Aung L, Lee WJ, et al. Revision procedures after failed adjustable gastric banding: comparison of efficacy and safety. Obes Surg 2017;27:2861–2867. [DOI] [PubMed] [Google Scholar]
- 28. Apers J, Wijkmans R, Totte E, et al. Implementation of mini gastric bypass in the Netherlands: early and midterm results from a high-volume unit. Surg Endosc 2018;32:3949–3955. [DOI] [PubMed] [Google Scholar]
- 29. Almalki OM, Lee WJ, Chen JC, et al. Revisional gastric bypass for failed restrictive procedures: comparison of single-anastomosis (mini-) and Roux-en-Y gastric bypass. Obes Surg 2018;28:970–975. [DOI] [PubMed] [Google Scholar]
- 30. Genser L, Carandina S, Tabbara M, et al. Presentation and surgical management of leaks after mini-gastric bypass for morbid obesity. Surg Obes Relat Dis 2016;12:305–312. [DOI] [PubMed] [Google Scholar]
- 31. de la Cruz M, Büsing M, Dukovska R, et al. Short- to medium-term results of single-anastomosis duodeno-ileal bypass compared with one-anastomosis gastric bypass for weight recidivism after laparoscopic sleeve gastrectomy. Surg Obes Relat Dis 2020;16:1060–1066. [DOI] [PubMed] [Google Scholar]
- 32. Parmar CD, Mahawar KK. One anastomosis (mini) gastric bypass is now an established bariatric procedure: a systematic review of 12,807 patients. Obes Surg 2018;28:2956–2967. [DOI] [PubMed] [Google Scholar]
- 33. Soong TC, Almalki OM, Lee WJ, et al. Measuring the small bowel length may decrease the incidence of malnutrition after laparoscopic one-anastomosis gastric bypass with tailored bypass limb. Surg Obes Relat Dis 2019;15:1712–1718. [DOI] [PubMed] [Google Scholar]
- 34. Navarrete S, Leyba JL, Ll SN, et al. Results of the comparative study of 200 cases: one anastomosis gastric bypass versus Roux-en-Y gastric bypass. Obes Surg 2018;28:2597–2602. [DOI] [PubMed] [Google Scholar]
- 35. Lo HC. The learning curve of one anastomosis gastric bypass and its impact as a preceding procedure to Roux-en Y gastric bypass: initial experience of one hundred and five consecutive cases. BMC Surg 2020;20:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parmar CD, Zakeri R, Mahawar K. A systematic review of one anastomosis/mini gastric bypass as a metabolic operation for patients with body mass index ≤35 kg/m(2). Obes Surg 2020;30:725–735. [DOI] [PubMed] [Google Scholar]
- 37. Khalaj A, Mousapour P, Motamedi MAK, et al. Comparing the efficacy and safety of Roux-en-Y gastric bypass with one-anastomosis gastric bypass with a biliopancreatic limb of 200 or 160 cm: 1-year results of the Tehran Obesity Treatment Study (TOTS). Obes Surg 2020;30:3528–3535. [DOI] [PubMed] [Google Scholar]
- 38. Salama TM, Sabry K. Redo surgery after failed open VBG: laparoscopic minigastric bypass versus laparoscopic Roux en Y gastric bypass – which is better? Minim Invasive Surg 2016;2016:8737519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taha O, Abdelaal M, Abozeid M, et al. Outcomes of omega loop gastric bypass, 6-years experience of 1520 cases. Obes Surg 2017;27:1952–1960. [DOI] [PubMed] [Google Scholar]
- 40. AlSabah S, Al Haddad E, Al-Subaie S, et al. Short-term results of revisional single-anastomosis gastric bypass after sleeve gastrectomy for weight regain. Obes Surg 2018;28:2197–2202. [DOI] [PubMed] [Google Scholar]
- 41. Pujol Rafols J, Al Abbas AI, Devriendt S, et al. Roux-en-Y gastric bypass, sleeve gastrectomy, or one anastomosis gastric bypass as rescue therapy after failed adjustable gastric banding: a multicenter comparative study. Surg Obes Relat Dis 2018;14:1659–1666. [DOI] [PubMed] [Google Scholar]
- 42. Carbajo M, García-Caballero M, Toledano M, et al. One-anastomosis gastric bypass by laparoscopy: results of the first 209 patients. Obes Surg 2005;15:398–404. [DOI] [PubMed] [Google Scholar]
- 43. Noun R, Skaff J, Riachi E, et al. One thousand consecutive mini-gastric bypass: short- and long-term outcome. Obes Surg 2012;22:697–703. [DOI] [PubMed] [Google Scholar]
- 44. Piazza L, Di Stefano C, Ferrara F, et al. Revision of failed primary adjustable gastric banding to mini-gastric bypass: results in 48 consecutive patients. Updates Surg 2015;67:433–437. [DOI] [PubMed] [Google Scholar]
- 45. Chevallier JM, Arman GA, Guenzi M, et al. One thousand single anastomosis (omega loop) gastric bypasses to treat morbid obesity in a 7-year period: outcomes show few complications and good efficacy. Obes Surg 2015;25:951–958. [DOI] [PubMed] [Google Scholar]
- 46. Ghosh S, Bui TL, Skinner CE, et al. A 12-month review of revisional single anastomosis gastric bypass for complicated laparoscopic adjustable gastric banding for body mass index over 35. Obes Surg 2017;27:3048–3054. [DOI] [PubMed] [Google Scholar]
- 47. Abdallah E, Emile SH, Zakaria M, et al. One-anastomosis gastric bypass (OAGB) with fixed bypass of the proximal two meters versus tailored bypass of the proximal one-third of small bowel: short-term outcomes. Surg Endosc 2022;36:328–335. [DOI] [PubMed] [Google Scholar]
- 48. Plamper A, Lingohr P, Nadal J, et al. Comparison of mini-gastric bypass with sleeve gastrectomy in a mainly super-obese patient group: first results. Surg Endosc 2017;31:1156–1162. [DOI] [PubMed] [Google Scholar]
- 49. Parmar CD, Mahawar KK, Boyle M, et al. Mini gastric bypass: first report of 125 consecutive cases from United Kingdom. Clinic Obes 2016;6:61–67. [DOI] [PubMed] [Google Scholar]
- 50. Bruzzi M, Rau C, Voron T, et al. Single anastomosis or mini-gastric bypass: long-term results and quality of life after a 5-year follow-up. Surg Obes Relat Dis 2015;11:321–326. [DOI] [PubMed] [Google Scholar]
- 51. Disse E, Pasquer A, Espalieu P, et al. Greater weight loss with the omega loop bypass compared to the Roux-en-Y gastric bypass: a comparative study. Obes Surg 2014;24:841–846. [DOI] [PubMed] [Google Scholar]
- 52. Bergeat D, Lechaux D, Ghaina A, et al. Postoperative Outcomes of Laparoscopic Bariatric Surgery in Older Obese Patients: a Matched Case-Control Study. Obes Surg 2017;27:1414–1422. [DOI] [PubMed] [Google Scholar]
- 53. Piazza L, Ferrara F, Leanza S, et al. Laparoscopic mini-gastric bypass: short-term single-institute experience. Updates Surg 2011;63:239–242. [DOI] [PubMed] [Google Scholar]
- 54. Noun R, Riachi E, Zeidan S, et al. Mini-gastric bypass by mini-laparotomy: a cost-effective alternative in the laparoscopic era. Obes Surg 2007;17:1482–1486. [DOI] [PubMed] [Google Scholar]
- 55. Kular KS, Manchanda N, Rutledge R. A 6-year experience with 1,054 mini-gastric bypasses-first study from Indian subcontinent. Obes Surg 2014;24:1430–1435. [DOI] [PubMed] [Google Scholar]
- 56. Musella M, Susa A, Greco F, et al. The laparoscopic mini-gastric bypass: the Italian experience: outcomes from 974 consecutive cases in a multicenter review. Surg Endosc 2014;28:156–163. [DOI] [PubMed] [Google Scholar]
- 57. Wang W, Wei PL, Lee YC, et al. Short-term results of laparoscopic mini-gastric bypass. Obes Surg 2005;15:648–654. [DOI] [PubMed] [Google Scholar]
- 58. Docimo S, Yang J, Zhang X, et al. One anastomosis gastric bypass versus Roux-en-Y gastric bypass: a 30-day follow-up review. Surg Endosc 2022;36:498–503. [DOI] [PubMed] [Google Scholar]
- 59. Rayman S, Assaf D, Azran C, et al. Sleeve gastrectomy failure – revision to laparoscopic one-anastomosis gastric bypass or Roux-n-Y gastric bypass: a multicenter study. Obes Surg 2021;31:2927–2934. [DOI] [PubMed] [Google Scholar]
- 60. Rutledge R, Walsh TR. Continued excellent results with the mini-gastric bypass: six-year study in 2,410 patients. Obes Surg 2005;15:1304–1308. [DOI] [PubMed] [Google Scholar]
- 61. Almuhanna M, Soong TC, Lee WJ, et al. Twenty years’ experience of laparoscopic 1-anastomosis gastric bypass: surgical risk and long-term results. Surg Obes Relat Dis 2021;17:968–975. [DOI] [PubMed] [Google Scholar]
- 62. Goel R, Nasta AM, Goel M, et al. Complications after bariatric surgery: a multicentric study of 11,568 patients from Indian bariatric surgery outcomes reporting group. J Minim Access Surg 2021;17:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garcia-Caballero M, Carbajo M, Martinez-Moreno JM, et al. Drain erosion and gastro-jejunal fistula after one-anastomosis gastric bypass: endoscopic occlusion by fibrin sealant. Obes Surg 2005;15:719–722. [DOI] [PubMed] [Google Scholar]
- 64. Kassir R, Debs T, Blanc P, et al. Complications of bariatric surgery: presentation and emergency management. Int J Surg 2016;27:77–81. [DOI] [PubMed] [Google Scholar]
- 65. Caiazzo R, Baud G, Clément G, et al. Impact of centralized management of bariatric surgery complications on 90-day mortality. Ann Surg 2018;268:831–837. [DOI] [PubMed] [Google Scholar]
- 66. Haddad A, Bashir A, Fobi M, et al. The IFSO Worldwide One Anastomosis Gastric Bypass Survey: techniques and outcomes? Obes Surg 2021;31:1411–1421. [DOI] [PubMed] [Google Scholar]
- 67. Poghosyan T, Caille C, Moszkowicz D, et al. Roux-en-Y gastric bypass for the treatment of severe complications after omega-loop gastric bypass. Surg Obes Relat Dis 2017;13:988–994. [DOI] [PubMed] [Google Scholar]
- 68. Tarhini A, Rives-Lange C, Jannot AS, et al. One-anastomosis gastric bypass revision for gastroesophageal reflux disease: long versus short biliopancreatic limb Roux-en-Y gastric bypass. Obes Surg 2022;32:970–978. [DOI] [PubMed] [Google Scholar]
- 69. Kermansaravi M, Shahmiri SS, DavarpanahJazi AH, et al. One anastomosis/mini-gastric bypass (OAGB/MGB) as revisional surgery following primary restrictive bariatric procedures: a systematic review and meta-analysis. Obes Surg 2021;31:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parmar CD, Gan J, Stier C, et al. One anastomosis/mini gastric bypass (OAGB-MGB) as revisional bariatric surgery after failed primary adjustable gastric band (LAGB) and sleeve gastrectomy (SG): a systematic review of 1075 patients. Int J Surg 2020;81:32–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data regarding this article are easy to verify as all relevant articles are cited and available online.


