Supplemental Digital Content is Available in the Text.
Keywords: Body mass index, ispaghula, obese, overweight, psyllium, weight
ABSTRACT
Background:
Psyllium is a natural, predominantly soluble fiber that forms a viscous gel when hydrated and is not digested or fermented. In the small intestine, psyllium gel increases chyme viscosity, slowing the degradation and absorption of nutrients. Psyllium has a significant effect in patients with metabolic syndrome and type-2 diabetes on glycemic control, while lowering serum cholesterol in hypercholesterolemic patients. Some randomized controlled studies have shown that psyllium also facilitates weight loss in overweight and obese participants.
Objectives:
A comprehensive review and meta-analysis assessing psyllium's impact on body weight, body mass index (BMI), and waist circumference in overweight and obese participants.
Data sources:
A comprehensive search was performed (Medline, Scopus, Cochrane Database) through March 21, 2022, using search terms to identify randomized, controlled, clinical studies designed to assess weight loss in overweight and obese participants over at least 2 months. Data were analyzed using the inverse variance method with random effects models.
Conclusions:
Six studies meeting inclusion criteria were identified (total n = 354). The meta-analysis showed that psyllium, dosed just before meals (mean dose 10.8 g/day, mean duration 4.8 months), was effective for decreasing body weight (MD = −2.1 kg [95% confidence interval [CI]: −2.6 to −1.6]; p < .001), BMI (MD = −0.8 kg/m2 [95% CI: −1.0 to −0.6]; p < .001) and waist circumference (MD = −2.2 cm [95% CI: −2.9 to −1.4]; p < .001) in overweight and obese populations.
Implications for practice:
Gel-forming nonfermented psyllium fiber, dosed just before meals, is effective in facilitating weight loss in overweight and obese participants.
The Centers for Disease Control and Prevention (CDC) defines “overweight” as a body mass index (BMI) of 25 to <30 kg/m2 and “obese” as a BMI of ≥30.0 kg/m2 (CDC Defining Adult Overweight & Obesity, 2022c). Most adults in the United States (aged 20 years and older) are overweight/obese (73.6%; 2017–2018) (CDC Obesity and Overweight, 2022d). When obesity is considered separately, the prevalence is 41.9% of the US adult population (2017–2018) (CDC Adult Obesity Facts, 2022b). Epidemiologic data suggest that dietary fiber intake is inversely associated with body weight (Anderson et al., 2009). However, it is important to note that statistical associations do not establish causation. Epidemiologic data cannot establish that the fiber component of a high-fiber diet is the causative agent for any observed change in body weight. Furthermore, recommendations to adhere to a high-fiber diet, which are based on a statistical association between a high-fiber diet and a reduced risk of cardiovascular disease, have not led to a decrease in the prevalence of obesity in the United States (Institute of Medicine Food and Nutrition Board, 2002). From 1999 to 2000 through 2017–2020, the prevalence of obesity increased from 30.5% to 41.9%, and the prevalence of severe obesity increased from 4.7% to 9.2% (CDC Adult Obesity Facts, 2022b). Childhood obesity is also a serious problem in the United States, with a 19.7% prevalence of obesity in children and adolescents aged 2–19 years (14.7 million children and adolescents, 2017–2020) (CDC Childhood Obesity Facts, 2022a). Obesity increases the risk of serious health consequences, including all-cause mortality, hypertension, dyslipidemia, type-2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, many types of cancers, low quality of life, clinical depression, anxiety, body pain, and difficulty with physical functioning (CDC Health Effects of Overweight and Obesity, 2022e). Modest weight loss (e.g., 5–10%) can lead to significant improvement in health outcomes (Brown et al., 2021).
To establish whether fiber plays a direct/causative role in weight loss, isolated fibers must be assessed in randomized controlled clinical studies that are adequately designed to assess weight loss. Adequate study design includes duration (e.g., multiple months), dose (e.g., 10 g/day in divided doses taken with meals), appropriate population (overweight/obese), and a negative control. Studies that take steps to maintain subject weight constant, as often done in cholesterol-lowering research where weight loss is considered a confounding factor, should be excluded from consideration (McRorie, Gibb, et al., 2021). Although subjective measures of satiety/hunger and single meal measures of energy intake might be considered suggestive of a potential for weight loss, only well-controlled, multimonth, clinical studies that directly assess weight loss can establish clinical efficacy. The objective of this comprehensive review and meta-analysis was to assess the clinical efficacy of psyllium, a natural nonfermented gel-forming fiber supplement, on weight loss in overweight and obese participants.
Methods
Data sources
A comprehensive search was performed using Medline, Scopus, and Cochrane Central Register of Controlled Trials. The original searches were performed on March 15–18, 2021, and updated on March 21, 2022. Reference lists of identified studies and reviews were manually searched for additional studies. Key search terms included psyllium, ispaghula, weight, body mass index, overweight, and obese.
Study eligibility
Publications identified by searches were screened for inclusion based on the following study design criteria: randomized, concurrent negative control, overweight/obese participants, treatment of at least 2 months in duration, psyllium alone (not in combination with other actives), at least 7 g/day in divided doses before/with meals, and consistent background diet throughout the study. Studies designed to assess the cholesterol-lowering effects of psyllium in hypercholesterolemia were excluded because by design, these studies attempt to maintain a stable body weight throughout the study to remove weight loss as a confounding factor for cholesterol lowering (McRorie, et al., 2021). For example, “…if the weight varied by > 1 kg, the energy intake was modified.”(Sola et al., 2010). Also, “The study protocol specified that participants should attempt to maintain a stable body weight throughout the trial.” (Davidson et al., 1998). Study designs were independently evaluated for inclusion by two investigators (R.D.G., J.W.M.).
Data extraction and assessment of bias
Two reviewers separately extracted data from identified articles. Data extracted included population studied, sample size, subject demographics (sex, age, BMI), study design, treatment duration, blinding, dose, treatment comparator, administration form, background diet, funding source, and country. Efficacy data were extracted for each treatment group in one of the following formats: 1) baseline and end-of-treatment mean and standard error (SE) or SD and 2) change from baseline mean and SE, SD, or 95% confidence interval (CI). In some cases, data were available in graph but not table format, in which case, relevant data were extracted from figures with Un-Scan-It Graph Digitizing Software Version 7.0 for Windows. In some cases, authors were contacted for additional information.
The Cochrane Risk of Bias Tool (The Cochrane Collaboration, 2020) was used to assess the following five standard domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding (performance bias), incomplete outcome data (attrition bias), and selective outcome reporting (reporting bias). Each domain was considered “low risk of bias” when the outcome was unlikely to be affected, “unclear risk of bias” when insufficient information was provided to make judgment, and “high risk of bias” when the outcome was likely affected.
Data management and statistical analysis
Summary body weight, BMI, and waist circumference data were extracted from each publication. When baseline and postbaseline treatment means and corresponding SDs were available, the baseline to postbaseline correlation was approximated from the reported treatment-comparison p values. For Pal et al. (2011), it was observed that postbaseline weight and BMI means were lower compared with baseline in all treatment groups, yet waist circumference means were all higher than baseline. An inquiry was made to the first author to reconcile this unlikely finding, and she reported that the reported waist circumference change from baseline magnitudes were correct but that their signs were inadvertently reversed (i.e., on average, waist circumference decreased postbaseline).
The standard inverse-variance method with random-effects model was used to perform meta-analysis calculations for each end point. Results were reported as mean difference (MD) with 95% CI and interpreted as statistically significant at p < 5%. Interstudy heterogeneity was assessed and quantified with the Cochrane Q-statistic and I2 (West et al., 2013). Publication bias for body weight, BMI, or waist circumference was not assessed with the funnel plot asymmetry test because in each instance, there were fewer than 10 studies available. Comprehensive Meta-Analysis software version 2.2.064 was used to make meta-analysis calculations. Forest plots were created with SAS/GRAPH software running on SAS Enterprise Guide, version 7.15 HF9 for Windows 10.
Results
Search results
The results of the literature review are summarized in Figure 1. A total of 2,496 citations were identified in the initial search, with 48 reviewed in full. A total of six articles satisfied the design criteria and were included in the final meta-analysis. Body weight and BMI were reported in all six articles (n = 354) (Abutair et al., 2016; Akbarzadeh et al., 2016; Cicero et al., 2010; Pal et al., 2011, 2016; Soltanian & Janghorbani, 2019), whereas waist circumference was reported in just five articles (n = 303) (Abutair et al., 2016; Akbarzadeh et al., 2016; Cicero et al., 2010; Pal et al., 2011, 2016).
Figure 1.
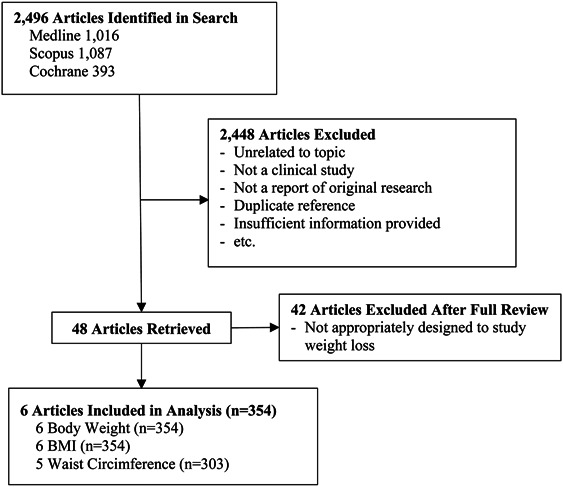
Summary of the literature search for the effect of psyllium fiber on body weight, BMI, and waist circumference.
Meta-analysis population characteristics
The characteristics of the six clinical studies included in the meta-analysis are summarized in Supplemental Digital Content 1, http://links.lww.com/JAANP/A211. Studies were conducted in four different countries and included a total of 354 participants. Females comprised 57% of the entire population. Mean age ranged from >35 to 58 years. Abutair, et al. (2016) reported that subject age was >35 years, but not the mean age. All study populations were either overweight or obese, with mean BMI ranging from 28.7 to 33.9 kg/m2. All studies followed a randomized, controlled, parallel-group design and evaluated psyllium doses ranging from 7 to 15 g/day for periods of 2–12 months. One study was double blind, four were single blind, and in one study participants were not blinded.
Results of the Cochrane Risk of Bias assessment are summarized in Supplemental Digital Content 2, http://links.lww.com/JAANP/A212. In no case was the risk of bias considered to be high. For sequence generation, blinding, and incomplete outcome data, the percentage of studies judged to be low risk of bias was 50%, 17%, and 67%, respectively. The risk of bias was unclear for all studies regarding allocation concealment and selective outcome reporting.
Effect of psyllium on body weight
The mean effect of psyllium fiber on body weight relative to negative control is shown in Figure 2A. In five of the six clinical studies, a statistically significant reduction in mean weight was reported for psyllium relative to control. The overall mean effect is highly statistically significant (MD = −2.1 kg [95% CI: −2.6 to −1.6]; p < .001). There was no indication of interstudy heterogeneity (I2 = 0%, p = .67), evidenced by the consistency of results across studies and overlap in CIs.
Figure 2.
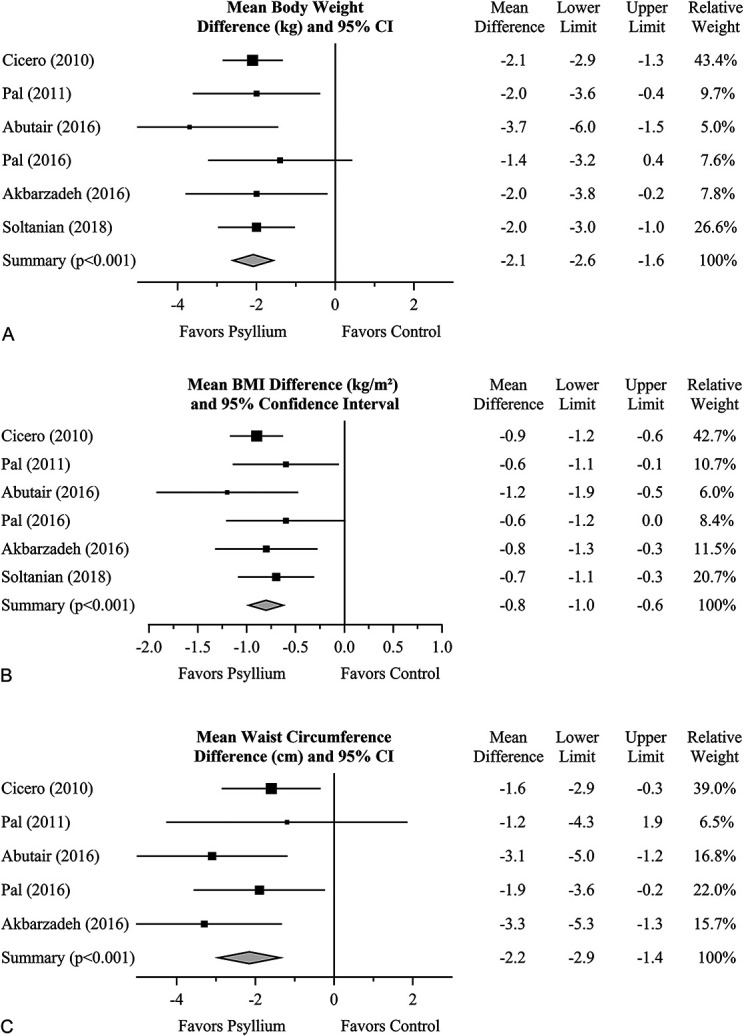
The effect of psyllium fiber on body weight (A), BMI (B), and waist circumference (C) in overweight and obese individuals relative to negative control. Squares represent the mean treatment difference with 95% CI. Diamonds represent the overall effect size and 95% CI, using the generic inverse-variance random-effects model. As mentioned earlier, the sign of the Pal et al. (2011) result shown here for waist circumference is opposite that reported in the publication; this corrects an error found through correspondence with the first author. CI = confidence interval; BMI = body mass index.
Effect of psyllium on body mass index
The mean effect of psyllium fiber on BMI relative to negative control is shown in Figure 2B. Not surprisingly, results essentially mirror those of body weight. In five of the six clinical studies, a statistically significant reduction in mean BMI was reported for psyllium relative to control. The overall mean effect is highly statistically significant (MD = −0.8 kg/m2 [95% CI: −1.0 to −0.6]; p < .001). There was no evidence of interstudy heterogeneity (I2 = 0%, p = .71).
Effect of psyllium on waist circumference
The mean effect of psyllium fiber on waist circumference relative to negative control is shown in Figure 2C. The overall mean effect is highly statistically significant (MD = −2.2 cm [95% CI: −2.9 to −1.4]; p < .001). There was no indication of interstudy heterogeneity (I2 = 0%, p = .49).
Discussion
The epidemic of obesity
According to the CDC, obesity is an epidemic in the United States (CDC Adult Obesity Facts, 2022b). Nearly three of every four adults are overweight or obese (73.6%; 2017–2018), with approximately 4 of 10 qualifying as obese (BMI ≥30 kg/m2) (CDC Adult Obesity Facts, 2022b; CDC Obesity and Overweight, 2022d). Obesity increases the risk of serious health consequences, including all-cause mortality, hypertension, dyslipidemia, type-2 diabetes, coronary heart disease, stroke, gallbladder disease, osteoarthritis, sleep apnea, many types of cancers, low quality of life, clinical depression, anxiety, body pain, and difficulty with physical functioning (CDC Health Effects of Overweight and Obesity, 2022e). It is important that a relatively small decrease in body weight, such as 5–10%, can decrease health risks/improve overall health (Brown et al., 2021).
Meta-analysis findings
This systematic review and meta-analysis of six, randomized, controlled, clinical studies was designed to evaluate the effects of psyllium on body weight, BMI, and waist circumference in overweight and obese participants (Abutair et al., 2016; Akbarzadeh et al., 2016; Cicero et al., 2010; Pal et al., 2011, 2016; Soltanian & Janghorbani, 2019). The three meta-analyses showed that divided doses of psyllium (before meals, 7–15 g/day, mean 10.8 g/day; duration 2–12 months, mean 4.8 months) resulted in statistically significant reductions in body weight (−2.1 kg, p < .001), BMI (−0.8 kg/m2, p < .001), and waist circumference (−2.2 cm, p < .001). These meta-analyses strengthen the existing clinical evidence that psyllium, dosed before meals as a dietary supplement, provides an effective modality for reducing body weight, BMI, and waist circumference in overweight and obese populations. An average weight loss of 2.1 kg over an average of 4.8 months translates to −0.44 kg/month. Over a 12-month period, this would translate to a weight loss of 5.3 kg, which is 6.1% of the average body weight of the participants in the six clinical studies (86.6 kg). This weight loss falls within the 5–10% weight loss range shown to improve overall health (Brown et al., 2021).
Apparent contradiction of other meta-analysis findings
Two previous meta-analyses concluded that psyllium had no significant effect on weight loss, but both online publications had significant methodological flaws (Mofrad et al., 2020; Xiao et al., 2020). As discussed in the methods section, a meta-analysis of the effects of a specific fiber on weight loss should focus on studies with 1) a sufficient dose of the specific fiber (e.g., 10–15 g/day for psyllium) delivered just before/with meals; 2) a sufficient duration of treatment for significant weight loss to occur (e.g., multiple months); 3) an overweight/obese population (significant weight loss does not occur in normal or underweight participants with fiber consumption); and 4) does not preclude the potential for weight loss to occur as part of the study design (e.g., cholesterol-lowering studies typically specify that weight loss is a confounding factor and is controlled/minimized) (McRorie et al., 2021). In the Xiao et al. (2020) meta-analysis of weight, three studies (Abutair et al., 2016; Soltanian & Janghorbani, 2019; Anderson, et al., 1999) were included. Soltanian was incorrectly cited with the author's first name, “Noureddin,” instead of the last name “Soltanian.” More importantly, the treatment effect in that study showed statistically significant weight loss with psyllium, yet Xiao et al. (2020) reported weight gain for the study in the meta-analysis. In addition, Anderson et al. (1999) conducted a cholesterol-lowering study designed to maintain a stable body weight, which may have minimized observed weight loss (“During the dietary stabilization phase, participants received instruction on a traditional weight-maintaining diabetes exchange diet ….”). These findings undermine the credibility of the body weight meta-analysis results reported by Xiao et al., 2020.
The second meta-analysis by Mofrad et al. (2020) included 23 published studies, but most of the studies did not meet the above criteria for inclusion in a weight loss meta-analysis. For example, three studies (Hylander & Rӧssner, 1983; Wolever et al., 1994; Vuksan et al., 2008) had a treatment duration of only 2–3 weeks, minimizing any potential to observe significant weight loss. One study (Ricklefs-Johnson et al., 2017) compared high-dose flaxseed (28 g/day) to a lower dose of psyllium (9 g/day) without a negative control. Mofrad et al. (2020) presented this disparate comparison as “weight gain” for psyllium in the forest plot. However, a review of the original study data showed that psyllium 9 g/day resulted in weight loss versus baseline. Seven of the publications included were cholesterol-lowering studies, which consider weight loss a confounding factor to be avoided to show that the fiber, not weight loss, yielded the observed changes in serum cholesterol concentration. Most of the cholesterol-lowering publications provided statements in the methods section that a stable weight was maintained throughout the study (e.g., Sola et al., 2010 “…if the weight varied by > 1 kg, the energy intake was modified.”). Removing the publications with inappropriate study designs from the Mofrad et al. (2020) and Xiao et al. (2020) meta-analyses, as was done in the current meta-analysis, showed significant weight loss for psyllium.
Psyllium weight loss mechanism of action
Despite decades of research, the cause of the obesity epidemic in the United States remains open to debate. The conventional mantra, “energy balance,” asserts that all calories are equal, and obesity is a matter of balancing “calories in versus calories out.” As concluded in a 2017 publication entitled “Obesity Pathogenesis: An Endocrine Society Scientific Statement,” “a calorie is a calorie,” and “A major area of emphasis is the science of energy homeostasis, the biological process that maintains weight stability by actively matching energy intake to energy expenditure over time.” (Schwartz et al., 2017). The Endocrine Society also acknowledged, “However, growing evidence indicates that obesity pathogenesis involves processes far more complex than the passive accumulation of excess calories. It is this complexity that lies at the heart of why obesity is so difficult to treat.” (Schwartz et al., 2017).
A competing theory asserts that it is not the number of calories consumed, but the composition of the food (e.g., 100 calories of spinach produce a completely different metabolic response than 100 calories of bacon or 100 calories of candy) (Fung, 2016). The fact that the inflection point of the US obesity epidemic coincides with the US Federal Government's publication of The Dietary Goals for the United States in 1977 (Select Committee on Nutrition and Human Needs, 1977) is often cited as supportive evidence. The government's dietary guidelines, built on the “calories in versus calories out” theory, caused an overhaul of the American diet away from fat and toward refined carbohydrates. According to the “insulin imbalance” model, increased consumption of refined carbohydrates results in elevated insulin levels, which then drive calories into adipose tissue (Fung, 2016). Therefore, obesity is not a problem of caloric imbalance but rather hormonal imbalance, where the primary hormone is insulin. Furthermore, chronic hyperinsulinemia fueled by chronic overconsumption of sugar and refined carbohydrates results in insulin resistance in liver and muscle tissues. Insulin resistance forces the pancreas to drive insulin levels even higher, thus accelerating the body's slide toward obesity (Bikman, 2020; Fung, 2016). This poses a question: which model of weight gain, “calories in versus calories out” or “insulin imbalance,” best explains the weight loss observed in the current meta-analysis?
All six of the studies were randomized and designed to balance caloric intake across treatment groups. However, psyllium does provide a modest satiety effect (Brum et al., 2016), and this could have resulted in small unobserved reductions in caloric intake for psyllium treatment groups. However, it should be noted that two clinical studies assessed the effects of psyllium on macronutrient and micronutrient absorption and found that psyllium had no significant effect on either (Kawasakia et al., 2010; Pal et al., 2022). Therefore, the satiety effect of psyllium might help explain some of the weight loss observed for psyllium, but it seems an unlikely explanation for the entire effect observed in this meta-analysis.
All participants in the current meta-analysis were overweight/obese and, per the “insulin imbalance” model, they should have experienced some degree from insulin resistance. Two of the six studies were comprised patients with type-2 diabetes (Abutair et al., 2016; Soltanian & Janghorbani, 2019), whereas one study assessed a population with metabolic syndrome (Cicero et al., 2010). Psyllium has been shown to provide significant glycemic benefits in patients with compromised glycemic control. A meta-analysis of patients with type-2 diabetes found that psyllium lowered fasting glucose by 37 mg/dl (p < .001) and HbA1c by 0.97% (p = .048) on average compared with placebo (Gibb et al., 2015). Taken together, it is plausible that the mechanism by which psyllium caused a reduction in body weight was primarily a decrease in serum insulin concentration and insulin resistance. As further evidence, a statistically significant (p ≤ .01) reduction in fasting insulin and insulin resistance was reported in two of the studies assessed in the current meta-analyses (Abutair et al., 2016; Cicero et al., 2010). A reduction in insulin resistance has also been proposed as the mechanism of action by which psyllium lowers blood pressure (Schulman & Zhou, 2009). All things considered, the most plausible evidence-based explanation for the weight loss observed in this meta-analysis for psyllium is a combination of reduced insulin resistance through improved glycemic control and caloric reduction from increased satiety, the former likely being more substantial than the latter.
Hierarchy of evidence-based scientific research
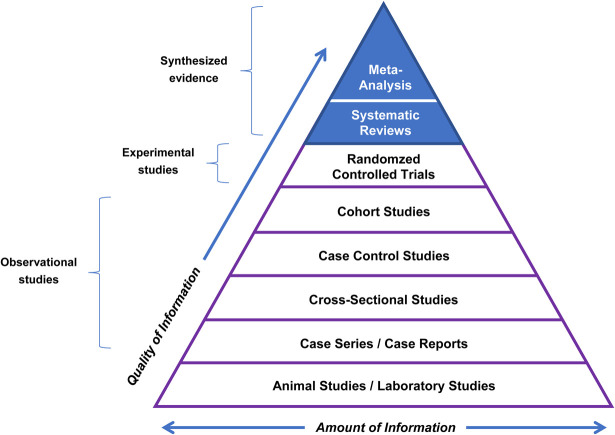
Evidence-based clinical practice has a hierarchy of scientific evidence. Meta-analyses are the highest order of clinical evidence (Figure 3; Duke University, 2022). The lowest order is nonhuman studies (animal studies and in vitro studies), whereas the next four levels are observational studies (not randomized or placebo controlled), which cannot establish cause and effect (Figure 3). The third highest level is composed of prospective, randomized, controlled clinical studies, the gold standard for individual clinical studies. The top two levels are considered “synthesized evidence,” combining available randomized/controlled clinical studies into a systemic review or meta-analysis of randomized, controlled, clinical studies. Individual randomized controlled clinical studies can vary significantly in their outcomes, so it is important to rigorously/statistically evaluate the totality of clinical evidence from randomized controlled clinical studies. A statistically significant outcome in meta-analysis of randomized, controlled, clinical studies supports a conclusion of “clinically proven.”
Figure 3.
Hierarchy of scientific evidence for evidence-based clinical practice (reprinted with permission from Duke University Medical Center Library and Archives34). Meta-analyses of randomized, controlled, clinical studies represent the highest order of clinical evidence. A statistically significant outcome supports a conclusion of “clinically proven.”
Figure 3 shows the hierarchy of scientific evidence for evidence-based clinical practice (reprinted with permission from Duke University Medical Center Library and Archives (2022)). Meta-analyses of randomized controlled clinical studies represent the highest order of clinical evidence. A statistically significant outcome supports a conclusion of “clinically proven.”
Health benefits of psyllium
In addition to the current meta-analysis showing that psyllium provided significant reductions in body weight, BMI, and waist circumference, other published meta-analyses have shown psyllium to be clinically proven for additional health benefits (Table 1). These include decreasing fasting blood glucose and HbA1c in patients with metabolic syndrome and type 2 diabetes (Gibb et al., 2015), lowering elevated low-density lipoprotein (LDL) and total cholesterol in patients with hypercholesterolemia (Jovanovski et al., 2018), providing a cholesterol-lowering benefit equivalent to doubling the dose of a statin drug in patients already being treated for hypercholesterolemia with a statin drug (Brum et al., 2018) and lowering blood pressure in patients with hypertension (Khan et al., 2018). A recent meta-analysis also showed that nonfermenting gel-forming psyllium is more effective than wheat bran for increasing stool output in patients with chronic idiopathic constipation (McRorie et al., 2020). The water-holding capacity of the psyllium gel acts as a stool normalizer, softening hard stool in constipation, firming loose/liquid stools in diarrhea, and normalizing stool form/reducing symptoms in irritable bowel syndrome (IBS) (McRorie et al., 2020). Psyllium is the only isolated fiber recommended for treatment of IBS by the American College of Gastroenterology (Ford et al., 2018) and chronic idiopathic constipation by the American Gastroenterological Association (Bharucha et al., 2013).
Table 1.
Summary of clinically proven health benefits for Psyllium
| Health Benefit | Treatment Population | Meta-Analysis Reference |
| Weight loss | Overweight/obese | Current study |
| Improved glycemic control | Metabolic syndrome, type-2 diabetes mellitus, prediabetes | Gibb et al., 2015 |
| Cholesterol lowering | Hypercholesterolemia | Jovanovski et al., 2018 |
| Lower blood pressure | Hypertension | Khan et al., 2018 |
| Increased stool output/softer stools | Chronic idiopathic constipation | McRorie et al., 2020 |
| Normalize stool form/decrease symptoms | Irritable bowel syndrome |
Ford et al., 2008
Moayyedi et al., 2014 |
A limitation on this meta-analysis is the relatively small number of clinical studies. Although the meta-analysis included data from three continents, a broader representation from other populations, including the United States, would have provided a more robust picture of psyllium efficacy. In addition, four of the six studies were just 2–3 months in duration and thus afford limited information on the long-term weight loss potential of psyllium fiber in overweight and obese individuals. Finally, the majority of Cochrane Risk of Bias assessments (Supplemental Digital Content 2, http://links.lww.com/JAANP/A212) resulted in “unclear risk of bias” owing to the lack of complete information provided in published results.
Conclusions
In conclusion, the present meta-analysis showed that psyllium, consumed as a supplement in divided doses just before meals, is clinically proven to decrease body weight, BMI, and waist circumference in overweight/obese participants. Additional meta-analyses support clinically proven benefits of psyllium fiber in improving glycemic control in metabolic syndrome and type 2 diabetes, lowering LDL and total cholesterol in hyperlipidemia and patients being treated with a statin, decreasing blood pressure in hypertension, increasing stool output, softening hard stool in chronic constipation, and decreasing symptoms/normalizing stool form in IBS. Psyllium is the only isolated fiber recommended for the treatment of IBS by the American College of Gastroenterology and chronic idiopathic constipation by the American Gastroenterological Association.
Supplementary Material
Footnotes
Competing interests: The authors are employees of Proctor and Gamble, the manufacturer of Metamucil, a psyllium-containing product.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaanp.com).
Data sharing: All data used to conduct the reported meta-analysis are shared in this article.
Authors' contributions: J. W. McRorie conceived the idea of conducting the meta-analysis. R. D. Gibb conducted the meta-analysis. J. W. McRorie and R. D. Gibb wrote the manuscript and had primary responsibility for final content. All authors read and approved the final manuscript.
Contributor Information
Kyle J. Sloan, Email: sloan.kj@pg.com.
Johnson W. McRorie, Jr, Email: mcroriejw54@gmail.com.
References
- Abutair A. Naser I. A., & Hamed A. (2016). Soluble fibers from psyllium improve glycemic response and body weight among diabetes type 2 patients (randomized control trial). Nutrition Journal, 15(1), 86–87. 10.1186/s12937-016-0207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarzadeh Z. Nourian M. Askari G., & Maracy M. (2016). The effect of Psyllium on body composition measurements and liver enzymes in overweight or obese adults with nonalcoholic fatty liver disease (NAFLD). International Journal of Advanced Biotechnology Research, 7(3), 1545–1554. https://www.researchgate.net/publication/290527713_The_effect_of_psyllium_on_anthropometric_measurements_and_liver_enzymes_in_overweight_or_obese_adults_with_nonalcoholic_fatty_liver_disease_NAFLD [Google Scholar]
- Anderson J. Allgood L. Turner J. Oeltgen P., & Daggy B. (1999). Effects of psyllium on glucose and serum lipid responses in men with type 2 diabetes and hypercholesterolemia. The American Journal of Clinical Nutrition, 70(4), 466–473. 10.1093/ajcn/70.4.466 [DOI] [PubMed] [Google Scholar]
- Anderson J. Baird P. Davis R. H. Jr Ferreri S. Knudtson M. Koraym A. Waters V., & Williams C. (2009). Health benefits of dietary fiber. Nutrition Reviews, 67(4), 188–205. 10.1111/j.1753-4887.2009.00189.x [DOI] [PubMed] [Google Scholar]
- Bharucha A. Pemberton J., & Locke G. (2013). American gastroenterological association technical review on constipation. Gastroenterology, 144(1), 218–238. 10.1053/j.gastro.2012.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikman B. (2020). Why we get sick: The hidden epidemic at the root of most chronic disease: And how to fight it. BenBella Books. [Google Scholar]
- Brown J. Buscemi J. Milsom V. Malcolm R., & O'Neil P. (2016). Effects on cardiovascular risk factors of weight losses limited to 5–10%. Translational Behavioral Medicine, 6(3), 339–346. 10.1007/s13142-015-0353-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brum J. Gibb R. Peters J., & Mattes R. (2016). Satiety effects of psyllium in healthy volunteers. Appetite, 105, 27–36. 10.1016/j.appet.2016.04.041 [DOI] [PubMed] [Google Scholar]
- Brum J. Ramsey D. McRorie J. Bauer B., & Kopecky S. (2018). Meta-analysis of usefulness of psyllium fiber as adjuvant anti-lipid therapy to enhance cholesterol lowering efficacy of statins. The American Journal of Cardiology, 122(7), 1169–1174. 10.1016/j.amjcard.2018.06.040 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2022a). Childhood Obesity Facts. https://www.cdc.gov/obesity/data/childhood.html [Google Scholar]
- Center for Disease Control and Prevention. (2022b). Adult Obesity Facts. https://www.cdc.gov/obesity/data/adult.html [Google Scholar]
- Centers for Disease Control and Prevention. (2022c). Defining Adult Overweight & Obesity. https://www.cdc.gov/obesity/adult/defining.html [Google Scholar]
- Center for Disease Control and Prevention. (2022d). Obesity and Overweight. https://www.cdc.gov/nchs/fastats/obesity-overweight.htm [Google Scholar]
- Center for Disease Control and Prevention. (2022e). Health Effects of Overweight and Obesity. https://www.cdc.gov/healthyweight/effects/index.html [Google Scholar]
- Cicero A. Derosa G. Bove M. Imola F. Borghi C., & Gaddi A. (2010). Psyllium improves dyslipidaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet. Mediterranean Journal of Nutrition and Metabolism, 3(1), 47–54. 10.1007/s12349-009-0056-1 [DOI] [Google Scholar]
- Darooghegi Mofrad M. Mozaffari H. Mousavi S. Sheikhi A., & Milajerdi A. (2020). The effects of psyllium supplementation on body weight, body mass index and waist circumference in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Critical Reviews in Food Science and Nutrition, 60(5), 859–872. 10.1080/10408398.2018.1553140 [DOI] [PubMed] [Google Scholar]
- Davidson M. Maki K. Kong J. Dugan L. Torri S. Hall H. Drennan K. Anderson S. Fulgoni V. Saldanha L., & Olson B. (1998). Long-term effects of consuming foods containing psyllium seed husk on serum lipids in subjects with hypercholesterolemia. The American Journal of Clinical Nutrition, 67(3), 367–376. 10.1093/ajcn/67.3.367 [DOI] [PubMed] [Google Scholar]
- Duke University Medical Center Library and Archives. (2022 November 22). Evidence-Based Practice: Study Design. https://guides.mclibrary.duke.edu/ebm/studydesign [Google Scholar]
- Ford A. Moayyedi P. Chey W. Harris L. Lacy B. Saito Y., & Quigley E. (2018). American College of Gastroenterology monograph on management of irritable bowel syndrome. American Journal of Gastroenterology, 113, 1–18. 10.1038/s41395-018-0084-x [DOI] [PubMed] [Google Scholar]
- Ford A. Talley N. Spiegel B. Foxx-Orenstein A. Schiller L. Quigley E., & Moayyedi P. (2008). Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: Systematic review and meta-analysis. Bmj: British Medical Journal, 337(nov13 2), a2313. 10.1136/bmj.a2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J. (2016). The Obesity code - Unlocking the Secrets of Weight Loss. Greystone Books. [Google Scholar]
- Gibb R. McRorie J. Russell D. Hasselblad V., & D'Alessio D. (2015). Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. The American Journal of Clinical Nutrition, 102(6), 1604–1614. 10.3945/ajcn.115.106989 [DOI] [PubMed] [Google Scholar]
- Hylander B., & Rӧssner S. (2009). Effects of dietary fiber intake before meals on weight loss and hunger in a weight-reducing club. Advances in Medical Sciences, 213(3), 217–220. 10.1111/j.0954-6820.1983.tb03720.x [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, Food and Nutrition Board. (2002). Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids Cholesterol, Protein and Amino Acids. The National Academies Press. [Google Scholar]
- Jovanovski E. Yashpal S. Komishon A. Zurbau A. Blanco Mejia S. Ho H. V. T. Li D. Sievenpiper J. Duvnjak L., & Vuksan V. (2018). Effect of psyllium (Plantago ovata) fiber on LDL cholesterol and alternative lipid targets, non-HDL cholesterol and apolipoprotein B: A systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition, 108(5), 922–932. 10.1093/ajcn/nqy115 [DOI] [PubMed] [Google Scholar]
- Kawasaki N. Urashima M. Odaira H. Noro T., & Suzuki Y. (2010). Effects of gelatinization of enteral nutrients on human gastric emptying. Gastroenterology Research, 3(3), 106–111. 10.4021/gr2010.06.213w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K. Jovanovski E. Ho H. Marques A. Zurbau A. Mejia S. Sievenpiper J., & Vuksan V. (2018). The effect of viscous soluble fiber on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD, 28(1), 3–13. 10.1016/j.numecd.2017.09.007 [DOI] [PubMed] [Google Scholar]
- McRorie J. Fahey G. Gibb R., & Chey W. (2020). Laxative effects of wheat bran and psyllium: Resolving enduring misconceptions about fiber in treatment guidelines for chronic idiopathic constipation. Journal of the American Association of Nurse Practitioners, 32(1), 15–23. 10.1097/jxx.0000000000000346 [DOI] [PubMed] [Google Scholar]
- McRorie J. Gibb R. Sloan K., & McKeown N. (2021). Psyllium: The gel-forming nonfermented isolated fiber that delivers multiple fiber-related health benefits. Nutrition Today, 56(4), 169–182. 10.1097/NT.0000000000000489 [DOI] [Google Scholar]
- Moayyedi P. Quigley E. Lacy B. Lembo A. Saito Y. Schiller L. Soffer E. Spiegel B., & Ford A. (2014). The effect of fiber supplementation on irritable bowel syndrome: A systematic review and meta-analysis. American Journal of Gastroenterology, 109(9), 1367–1374. 10.1038/ajg.2014.195 [DOI] [PubMed] [Google Scholar]
- Pal S. Ho S. Gahler R., & Wood S. (2016). Effect on body weight and composition in overweight/obese Australian adults over 12 months consumption of two different types of fibre supplementation in a randomized trial. Nutrition & Metabolism, 13(1), 82–91. 10.1186/s12986-016-0141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S. Khossousi A. Binns C. Dhaliwal S., & Ellis V. (2011). The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin and other metabolic syndrome risk factors in overweight and obese individuals. British Journal of Nutrition, 105(1), 90–100. 10.1017/s0007114510003132 [DOI] [PubMed] [Google Scholar]
- Pal S. McKay J. Fo S. Jane M. Gahler R., & Wood S. (2022). Micronutrient status of individuals with overweight and obesity following 3 months' supplementation with PolyGlycopleX (PGX®) or psyllium: A randomized controlled trial. BMC Nutrition, 42(8), 1–11. 10.1186/s40795-022-00534-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs-Johnson K. Johnston C., & Sweazea K. (2017). Ground flaxseed increased nitric oxide levels in adults with type 2 diabetes: A randomized comparative effectiveness study of supplemental flaxseed and psyllium fiber. Obesity Medicine, 5, 16–24. 10.1016/j.obmed.2017.01.002 [DOI] [Google Scholar]
- Schulman I., & Zhou M. (2009). Vascular insulin resistance: A potential link between cardiovascular and metabolic diseases. Current Hypertension Reports, 11(1), 48–55. 10.1007/s11906-009-0010-0 [DOI] [PubMed] [Google Scholar]
- Schwartz M. Seeley R. Zeltser L. Drewnowski A. Ravussin E. Redman L., & Leibel R. (2017). Obesity pathogenesis: An endocrine society scientific statement. Endocrine Reviews, 38(4), 267–296. 10.1210/er.2017-00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Select Committee on Nutrition and Human Needs, United States Senate. (1977). Dietary Goals for the United States. [20402 Stock No. 052-070-03913-2]. U.S. Washington, D.C. Government Printing Office. [Google Scholar]
- Sola R. Bruckert E. Valls R. Narejos S. Luque X. Castro-Cabezas M. Domenech G. Torres F. Heras M. Farres X. Vaquer J. V. Martínez J. M. Almaraz M. C., & Anguera A. (2010). Soluble fibre (Plantago ovata husk) reduces plasma low-density lipoprotein (LDL) cholesterol, triglycerides, insulin, oxidised LDL and systolic blood pressure in hypercholesterolaemic patients: A randomised trial. Atherosclerosis, 211(2), 630–637. 10.1016/j.atherosclerosis.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Noureddin S. Mohsen J., & Payman A. (2018). Effects of psyllium vs. placebo on constipation, weight, glycemia, and lipids: A randomized trial in patients with type 2 diabetes and chronic constipation. Complementary therapies in medicine, 40, 1-7. 10.1016/j.ctim.2018.07.004 [DOI] [PubMed] [Google Scholar]
- Soltanian N., & Janghorbani M. (2019). Effect of flaxseed or psyllium vs. placebo on management of constipation, weight, glycemia, and lipids: A randomized trial in constipated patients with type 2 diabetes. Clinical Nutrition ESPEN, 29, 41–48. 10.1016/j.clnesp.2018.11.002 [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2020). RoB2: A Revised Risk-of-bias Tool for Randomized Trials. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials [Google Scholar]
- Vuksan V. Jenkins A. Jenkins D. Rogovik A. Sievenpiper J., & Jovanovski E. (2008). Using cereal to increase dietary fiber intake to the recommended level and the effect of fiber on bowel function in healthy persons consuming North American diets. The American Journal of Clinical Nutrition, 88(5), 1256–1262. 10.3945/ajcn.2008.25956 [DOI] [PubMed] [Google Scholar]
- West S. L. Gartlehner G. Mansfield A. J. Poole C. Tant E. Lenfestey N. Lux L. J. Amoozegar J. Morton SC. Carey T. C. Viswanathan M., & Lohr K. N. (2013 May 17). Comparative Effectiveness Review Methods: Clinical Heterogeneity. U. S. Department of Health and Human Services, Agency for Healthcare Research and Quality. https://www.ncbi.nlm.nih.gov/books/NBK53317/table/ch3.t2/ [PubMed] [Google Scholar]
- Wolever T. Jenkins D. Mueller S. Patten R. Relle L. Boctor D. Ransom T. Chao E. McMillan K., & Fulgoni V. (1994). Psyllium reduces blood lipids in men and women with hyperlipidemia. The American Journal of the Medical Sciences, 307(4), 269–273. 10.1097/00000441-199404000-00005 [DOI] [PubMed] [Google Scholar]
- Xiao Z. Chen H. Zhang Y. Deng H. Wang K. Bhagavathula A. Almuhairi S. Ryan P. Rahmani J. Dang M. Kontogiannis V. Vick A., & Wei Y. (2020). The effect of psyllium consumption on weight, body mass index, lipid profile, and glucose metabolism in diabetic patients: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytotherapy Research, 34(6), 1237–1247. 10.1002/ptr.6609 [DOI] [PubMed] [Google Scholar]