Background:
Studies evaluating sex differences in colorectal cancer (CRC) tumor microenvironment are limited, and no previous study has focused on rectal cancer patients’ constitutive immune surveillance mechanisms. The authors aimed to assess gender-related differences in the immune microenvironment of rectal cancer patients.
Methods:
A systematic review and meta-analysis were conducted up to 31 May 2021, including studies focusing on gender-related differences in the CRC tumor microenvironment. Data on the mutational profile of rectal cancer were extracted from the Cancer Genome Atlas (TCGA). A subanalysis of the two IMMUNOREACT trials (NCT04915326 and NCT04917263) was performed, aiming to detect gender-related differences in the immune microenvironment of the healthy mucosa in patients with early (IMMUNOREACT 1 cohort) and locally advanced rectal cancer following neoadjuvant therapy (IMMUNOREACT 2 cohort). In the retrospective IMMUNOREACT 1 cohort (therapy naive), the authors enrolled 442 patients (177 female and 265 male), while in the retrospective IMMUNOREACT 2 cohort (patients who had neoadjuvant therapy), we enrolled 264 patients (80 female and 184 male). In the prospective IMMUNOREACT 1 cohort (therapy naive), the authors enrolled 72 patients (26 female and 46 male), while in the prospective IMMUNOREACT 2 cohort (patients who had neoadjuvant therapy), the authors enrolled 105 patients (42 female and 63 male).
Results:
Seven studies reported PD-L1 expression in the CRC microenvironment, but no significant difference could be identified between the sexes. In the TGCA series, mutations of SYNE1 and RYR2 were significantly more frequent in male patients with rectal cancer. In the IMMUNOREACT 1 cohort, male patients had a higher expression of epithelial cells expressing HLA class I, while female patients had a higher number of activated CD4+Th1 cells. Female patients in the IMMUNOREACT 2 cohort showed a higher infiltration of epithelial cells expressing CD86 and activated cytotoxic T cells (P=0.01).
Conclusions:
Male patients have more frequent oncogene mutations associated with a lower expression of T-cell activation genes. In the healthy mucosa of female patients, more Th1 cells and cytotoxic T cells suggest a potentially better immune response to the tumor. Sex should be considered when defining the treatment strategy for rectal cancer patients or designing prognostic scores.
Keywords: gender-related difference, immune surveillance mechanisms, rectal cancer, tumor microenvironment
Highlights
Currently, few studies address the gender-related difference in the tumor microenvironment.
SYNE 1 and RYR2 mutations in rectal cancer are more frequent in males, modeling it unfavorably.
A high number of CD4+ Th1 cells shows active immune surveillance in women with early rectal cancer.
Women who had neoadjuvant therapy showed a more functional interaction between epithelium and T cells.
Sex should be considered when defining the management of rectal cancer patients.
Introduction
Colorectal cancer (CRC) represents a major cause of cancer-related death worldwide, accounting for ~147 950 new cases and 53 200 deaths in the United States in 2020. Of these, ~43 340 cases were due to rectal cancer1. The prognosis and treatment of rectal cancer are strictly dependent on the stage of the disease, according to the American Joint Committee on Cancer’s tumor-node-metastasis (TNM) staging system2. Nevertheless, reliable prognostic criteria, which can help predict tumor behavior and aggressiveness, thus guiding therapeutic choices, are still lacking.
The role of the immune microenvironment in the prognosis of CRC has been widely investigated, and some authors have specifically focused on the role of the immune response in predicting the survival of rectal cancer patients3. Recently, Däster et al.4 suggested that, in early rectal cancer, the levels of CD8+ T-cell infiltration could help in predicting nodal involvement, thus critically influencing patients’ treatment possibilities. Moreover, the cell density of stromal Foxp3(+), a marker of Treg (regulator T cells) lymphocytes, is strongly associated with tumor regression grade and recurrence-free survival in patients with locally advanced rectal cancer (LARC, i.e. staged T3–T4 or node-positive)5.
Sex is an important biological determinant of the immune response to cancer, and some differences between sexes have been reported regarding rectal cancer, which is significantly more common in male patients, with a reported male-to-female incidence rate ratio of 1.621. Women with CRC have also been reported to be diagnosed at a more advanced stage and in an emergency, but they have better survival rates compared to their male counterpart6. Some studies analyzed the impact of female hormones on the tumoral microenvironment in murine cancer models: estrogens were identified as regulators of a prometastatic immune microenvironment in the liver7, while estradiol (E2) has been found to considerably increase programmed death ligand 1 (PD-L1) protein expression in endometrial and breast cancer8. The binding of PD-L1 to its receptor, programmed cell death protein 1 (PD-1), inhibits the activations of tumor-infiltrating lymphocytes, allowing for tumor cells to escape immunosurveillance9. The role of PD-L1 in defining the prognosis of CRC is still debated, although, in most studies, PD-L1 expression has been associated with poor clinical outcomes10. Other mutations, influencing the tumor microenvironment (TME) and modulating patients’ immune responses, have been described in the literature11,12. Nevertheless, despite some convincing results, studies evaluating sex differences in the context of the tumoral microenvironment in CRC are limited. Moreover, no previous study focused on the constitutive immunosurveillance mechanism of patients with rectal cancer and, even less, on the potential differences between sexes in terms of the rectal mucosa immune system. Thus, the aim of the present study (IMMUNOREACT 5) was to assess the differences in the rectal mucosa immune microenvironment between male and female patients and their relation with clinicopathological characteristics and the outcome of rectal cancer.
Methods
Study design
The present study (IMMUNOREACT 5) aimed to assess the differences in the immune microenvironment in the rectal mucosa between male and female patients with rectal cancer. We designed the study in three different steps. First, we performed a systematic review on immune surveillance-related gene expression in male and female rectal cancer patients; second, we evaluated the different mutations in rectal cancer in male and female patients; and third, we evaluated the difference in terms of immune surveillance gene expression in the healthy rectal mucosa surrounding rectal cancer in the patients enrolled in the IMMUNOlogical microenvironment in Rectal Adenocarcinoma Treatment (IMMUNOREACT) trial. The work has been reported in line with the STROCSS, Supplemental Digital Content 1, http://links.lww.com/JS9/A51 criteria13 and it was registered at the Research Registry with the unique identifying number: researchregistry8499 (https://www.researchregistry.com/browse-the-registry#home/registrationdetails/637b729fba31a1002144c8eb/).
The IMMUNOREACT protocol was approved by the ethical committee of the coordinating center (CESC code 4448/AO/20) and each of the collaborating centers. The two arms of the trial (i.e. IMMUNOREACT 1 and IMMUNOREACT 2) are registered at clinicaltrials.gov (NCT04915326 and NCT04917263, respectively). All the consecutively enrolled patients gave their written informed consent to be enrolled in the study. The study was conducted according to Helsinki’s declaration principles and it received funding from AIRC under IG 2019 – ID. 23381 project to MS.
SYSTEMATIC REVIEW: search methods
A systematic review was conducted according to the Preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines14. Institutional Review Board approval was not required. We included studies focusing on the following issues: assessing tumoral microenvironment in the surgical specimen of patients with histologically confirmed colorectal or rectal cancer; the correlation between tumoral microenvironment and clinicopathological features and/or outcome and prognosis; sex differences among tumoral microenvironment and their association with survival outcome and/or clinical and pathological aspects.
An electronic search for relevant publications in English literature up to 31 May 2021 was performed by three independent reviewers (M.C., G.S., M.S.) using Medline/PubMed, Embase, Scopus, and the Cochrane Database of Systematic Reviews. The search headings are reported in Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A52. A manual cross-reference search of the eligible papers was performed to identify additional relevant articles. All titles were initially screened, and appropriate abstracts were reviewed. Each of the relevant publication reference sections and Google Scholar were also screened for other applicable publications. Study selection is shown in Figure 1.
Figure 1.
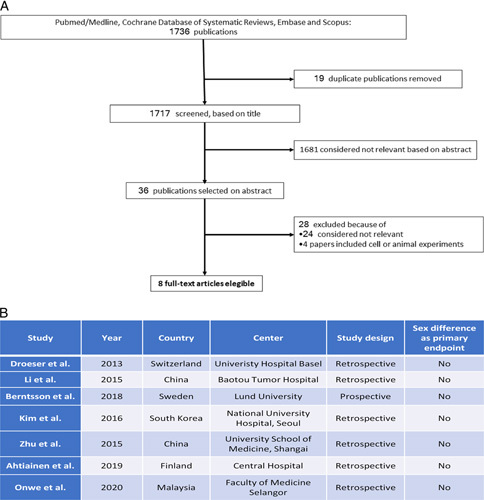
(A) PRISMA flowchart describing the different steps of the studies selection. (B) Selected studies characteristics.
External cohort
Data on the mutational profile of rectal adenocarcinoma were extracted from the Cancer Genome Atlas (TCGA) deposited public database, available at: https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations 15. This data set contains mutation and gene expression profiles of rectal cancer in different settings. The PanCancer study encompassed 11 286 tumor samples from 33 cancer types, for which molecular data were available from at least one of the five assay platforms. Of these, 9759 had complete data for four platforms: aneuploidy and mutations, DNA methylation, mRNA, and miRNA. RPPA protein data were available for a subset of samples (7858). We selected the data set to include patients with rectum adenocarcinoma (READ). Original data were plotted on a novel database and analyzed.
IMMUNOREACT: study design
The IMMUNOlogical microenvironment in Rectal Adenocarcinoma Treatment (IMMUNOREACT) study was designed to answer the following questions: (IMMUNOREACT 1) is it possible to predict the presence of nodal metastasis in patients with early rectal cancer? (IMMUNOREACT 2) is it possible to identify, among LARC, those with sustained complete response to neoadjuvant chemotherapy/radiotherapy?
The study started in 2019 and is currently ongoing in nine Italian centers (secondary or tertiary center in North-Central Eastern Italy). Inclusion criteria for the first aim of the study (IMMUNOREACT 1) were: patients who underwent a surgical procedure for T1 and T2 rectal carcinoma, with a minimal number of 10 retrieved lymph nodes in case of anterior resection or Miles abdominal perineal resection, with full availability of clinical records, and at least 1 year of follow up. Inclusion criteria for the second aim of the study (IMMUNOREACT 2) were: patients with locally advanced (cT3-4 and/or N+, TNM stage II–III) low and medium rectal cancer (<11 cm) or low rectum adenocarcinoma cT less than or equal to 2, at risk for abdominoperineal amputation, undergoing neoadjuvant therapy including long course radiotherapy (45 Gy) and fluoropyrimidine-based regimens, treated at least 6 weeks after the end of the neoadjuvant therapy and with full availability of clinical records at least 1 year of follow up. The study was articulated into a retrospective and exploratory step (step A) and a prospective validation step (step B). The first step (step A) was conducted on formalin-fixed paraffin-embedded (FFPE) slides retrieved from the pathology archives, testing a panel of molecular markers exploring the immune reaction to cancer (i.e. antigen-presenting cells and T lymphocytes activation). The second step (step B) was conducted on fresh tissue samples obtained from normal rectal mucosa proximal (3–15 cm) to cancer or to the site of previous cancer (in the case of a complete response to chemo radio therapy) at the time of surgery.
Histopathology
Histopathological examination of all resected specimens consisted of evaluation of tumor stage, residual tumor, grading, and number of lymph nodes involved. The specimens were fixed in 10% formaldehyde and set in paraffin. The lymph nodes were counted and assessed by a pathologist. Nodal status (N0, N1) was evaluated in accordance with the 8th edition of the TNM classification, but for the purpose of this study, the number of metastatic lymph nodes and their site were also analyzed16. Tumor lymphomonocytic infiltrate was classified as high grade, low grade, or absent.
Immunohistochemistry
In the retrospective step of the study a panel of immunohistochemistry (IHC) markers, similar to that adopted by Pagès et al.17 in their seminal study, (CD3 (pan T-cell marker), CD4 (T-helper marker), CD8 (cytotoxic marker), CD8beta (marker of cytotoxic activation), Tbet (T-bet: T-box expressed in T cells, a Th1 marker), FoxP3 (forkhead box P3, a Treg marker), and PD-L1 (programmed death ligand 1) of immune surveillance are tested on the healthy rectal mucosa according to the concept of the field cancerization.
Tissue microarray construction was performed after selecting two healthy rectal mucosa areas from two separate FFPE blocks. Then two tissue cores (1 mm diameter) were punched out of these areas using the Tissue ArrayerMinicore 3 (Alphelys, Plaisir, France), as previously described18. IHC analyses were performed using standard procedures, and the resulting sections were evaluated by a single pathologist in a blinded fashion. Immunocomplexes were detected using the Dako Real Envision System Peroxidase and 3-3′ di-aminobenzidine tetrahydrochloride cromogen as a substrate (Dako, Glostrup, Denmark) in FFPE sections. IHC staining was performed using monoclonal antibodies for MLH1 (clone ES05, 1 : 100; Dako), PMS2 (clone EP51, 1 : 100; Dako), MSH2 (clone FE11, 1 : 100; Dako), MSH6 (clone EP49, 1 : 100; Dako), PD-L1 (clone 22C3, 1 : 50; Dako), CD80 (clone 37711, 1 : 40; R&D Systems Inc.), CD8 (clone C8/144B, 1 : 200; Dako). The sections were lightly counterstained with hematoxylin and it was performed automatically. Furthermore, for all the IHC markers (CD3, CD4, CD8, CD8beta, Tbet, FoxP3, and PD-L1) the absolute number of positive cells was obtained by considering the mean number of positive cells observed in 5 high-power field (×40). CD8/CD4, CD8/CD3, CD8beta/CD4, and CD8beta/CD3 ratio were calculated. To assess the T-cytotoxic and activated T-cytotoxic ratio, frequencies of patients with high Tbet/CD4 and FoxP3/CD4 ratios were compared with those of patients with low ratios.
Flow cytometry
Prospectively collected rectal mucosa samples, washed in Hank’s balanced salt solution containing 10 mmol/l dithiothreitol, were then digested to obtain single-cell suspensions. Freshly isolated cells (105) were stained in PBS/2% FBS with appropriate combinations of fluorescein isothiocyanate- and peroxidase-conjugated antibodies. Single-cell suspensions were subjected to flow cytometry to determine the proportion of epithelial cells (Cytokeratin 20, Cyt-20+) acting as antigen-presenting cells (expressing CD80, CD86, CD40, HLA ABC, or HLA DR) and the proportion of activated CD8+T cells (CD8+positive for CD28, CD38, or CD69), inhibitory T cells (CD3+CTLA-4+), activated CD4+Th cells (CD4+CD25+) and of activated T regulators (CD4+CD25+FoxP3+).
Statistical analysis
For primary analyses, we considered the expression of PD-L1 between sexes. Using random effects model meta-analyses, we calculated the overall frequency of expression of PD-L1 with a 95% CI by pooling the individual frequencies of at least two primary studies. Forest plots were used to assess statistical heterogeneity, and the I 2 statistic was calculated (0–40%, not important; 30–60%, moderate heterogeneity; 50–90%, substantial heterogeneity; 75–100%, considerable heterogeneity). The meta-analyses were conducted using Review Manager 5.2. Statistical analysis of the TCGA and IMMUNOREACT datasets was performed using R 4.0 (R Foundation for Statistical Computing)19. Descriptive statistics were reported as mean (SE) for continuous variables and percentages (absolute numbers) for categorical variables. Fishers’ test was used to compare dichotomic variables and the Mann–Whitney U-test for comparisons of continuous variables. Survival analysis was performed with the Kaplan–Meier method, and the Cox F-test was used to compare the overall survival between the two groups. Set the standardized effect size at 0.80, a probability of type I error (alpha) at 0.05 and a probability of type II (beta) error at 0.20 the minimal sample size was 25 participants per group. Univariable and multivariable Cox proportional hazard models were used to assess the correlation between sex and the expression of immunological gatekeepers in healthy rectal mucosa, and a P value less than 0.05 was considered statistically significant.
Results
PD-L1 expression in rectal cancer females and male patients
To assess the differences in the tumor immune microenvironment between sexes, tumor expression of PD-L1 in tumors from females and males investigated. Six studies compared PD-L1 expression in male and female CRC patients20–25, while one study reported only on MSI-H CRC patients26. No significant difference could be identified between sexes, with male patients having only a slightly lower probability to have PD-L1 positive tumors (odds ratio=0.95, 95% CI=0.80–1.14 fixed+random model) (Figs 2a, 3b). These findings suggest that currently there is insufficient evidence about the difference between sexes in term of immune microenvironment in rectal cancer.
Figure 2.
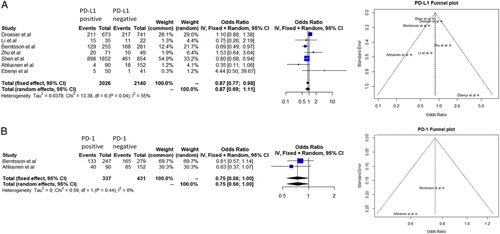
(A) Forrest plot describes the relationship between PD-L1 expression and sex, respectively. Funnel plot describes the risk of bias of the meta-analysis. (B) Forrest plots describes the relationship between PD-1 expression and sex, respectively. Funnel plot describes the risk of bias of the meta-analysis. PD-L1 and PD-1 expression have been dichotomized in the different studies but the cutoff values are not homogeneous.
Figure 3.
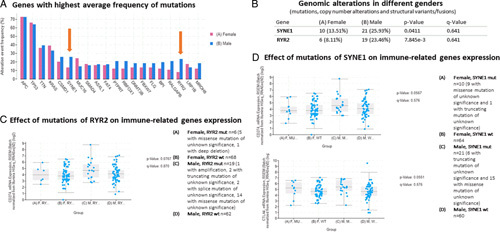
Analysis on the Cancer Genome Atlas (TCGA) panCancer Atlas (2018) rectal cancer series (READ). (A) Genes with the highest average frequency of mutations in rectal cancer patients. (B) Among these genes, two, SYNE1 and RYR2, have a higher frequency of mutation in male patients. (C) Effect of mutations of RYR2 on PD-L1 mRNA expression: PD-L1 mRNA expression is higher in male patients with mutated RYR2. (D) Effect of mutations of SYNE1 on PD-L1 and CTLA-4 mRNA expression. PD-L1 and CTLA-4 mRNA levels are higher in male patients with mutated SYNE1.
Mutational analysis in females and male patients with rectal cancer
Therefore, to assess the differences in the mutational rate between sexes, the rate of the most frequently mutated genes was compared in females and males within the TGCA rectal cancer series. No difference was observed in terms of antigen-presenting cells, TP53, TTN, KRAS, CSMD1, MUC16, SMAD4, ASXL1, FAT4, PTPRT, RBFOX1, DMNT3B, FBXW7, FLG, BPI, RALGAP8, LRP1B, and MROH8 (Fig. 3a). On the contrary, as shown in Figure 3b, mutations of SYNE1 and RYR2 were significantly more frequent in male patients with rectal cancer than in female ones (P=0.04 and <0.001, respectively). Both of these genes have immunological implications, and we observed that male patients with RYR2 mutations had higher PD-L1 expression (P=0.05) (Fig. 3c). Moreover, among patients with SYNE1 mutations, either male or female patients had a higher expression of CTLA-4 (P=0.05) while only male patients had a higher expression of PD-L1 (P=0.05) (Fig. 3d). These findings suggest that the most commonly mutated genes in males are associated with a higher expression of immune checkpoint genes, leading to a weaker immune microenvironment in rectal cancer in male patients.
Differences in the mucosal immune microenvironment of male versus female rectal cancer patients at basal condition
Then, to investigate the role of the constitutive immune surveillance mechanisms in patients with rectal cancer, we performed IHC and FACS analysis in the ‘healthy’ rectal mucosa surrounding the cancer. In the retrospective IMMUNOREACT 1 cohort (therapy naive), we enrolled 442 patients (177 female and 265 male), while in the prospective IMMUNOREACT 1 cohort (therapy naive), we enrolled 72 patients (26 female and 46 male). The patient’s characteristics are shown in Supplementary Table 1a–b, Supplemental Digital Content 2, http://links.lww.com/JS9/A52. The mean fluorescence intensity (MFI) for epithelial cells (CK+cells, expressing Cytokeratin 20, Cyt-20+) acting as antigen-presenting cells (expressing HLA ABC) and the proportion of activated CD4+Th1-cell (T-bet+) in patients with T1–T2 rectal cancer who had not received neoadjuvant therapy (i.e. IMMUNOREACT 1 cohort) are depicted in Figure 4. Male patients had a higher expression of CK+HLA-ABC+cells, expressed as MFI, compared to their female counterparts (P=0.018, Figs 4.1a). Nevertheless, female patients had a higher number of activated CD4+Th1-cell (T-bet+, P=0.05, Fig. 4.1b). However, as shown in Supplementary Figure 1, Supplemental Digital Content 3, http://links.lww.com/JS9/A53 the expression of PD-L1 in patients with T1–T2 rectal cancer tended to be higher in female patients compared to male patients (P=0.08). Finally, as shown in Figure 4.1c, in therapy-naive rectal cancer patients females tended to have a better overall survival (P=0.07). These findings suggest that, in therapy-naive rectal cancer in males, there are more antigens (possibly tumor neoantigens, we do not have data about this) to be presented. However, female patients show a more favorable immune microenvironment with a higher Th1-cell rate than male, patients and these findings are supported by the better overall survival of these patients.
Figure 4.
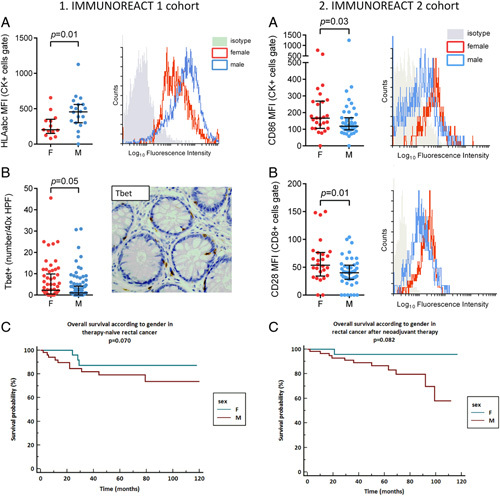
Analysis of the IMMUNOREACT 1 and 2 cohort. (4.1A) The proportion of epithelial cells acting as antigen-presenting cells (CK+HLA ABC+) is higher males in therapy naive rectal cancer series. (4.1B) The proportion of activated CD4+Th1-cell (T-bet+) is higher in females in therapy naïve rectal cancer series. (4.1C) Overall survival tends to be better in females in therapy naive rectal cancer series. (4.2A) The proportion of epithelial cells acting as antigen-presenting cells (CK+CD86+) is higher in female patients who had neoadjuvant therapy. (4.2B) The proportion of activated CD8+T cells (CD8+CD28+) is higher in female patients who had neoadjuvant therapy. (4.2C) Overall survival tends to be better in females patients who had neoadjuvant therapy.
Differences in the mucosal immune microenvironment of male versus female rectal cancer patients after neoadjuvant therapy
Finally, to investigate the effect of neoadjuvant radiochemotherapy on the constitutive immune surveillance mechanisms within the rectal mucosa of patients with rectal cancer, we performed IHC and FACS analyses. In the retrospective IMMUNOREACT 2 cohort (patients who had neoadjuvant therapy), we enrolled 264 patients (80 female and 184 male), while in the prospective IMMUNOREACT 2 cohort (patients who had neoadjuvant therapy), we enrolled 105 patients (42 female and 63 male). The patient’s characteristics are shown in Supplementary Table 2a–b, Supplemental Digital Content 2, http://links.lww.com/JS9/A52. In the IMMUNOREACT 2 cohort, including patients with rectal cancer who had neoadjuvant therapy, the MFI for epithelial cells (CK+cells, expressing Cytokeratin 20, Cyt-20+) acting as antigen-presenting cells (expressing CD86), and for activated CD8+T cells (positive for CD28) in patients with LARC who received neoadjuvant therapy is shown in Figures 4.2a and 4.2b, respectively. Female patients had a higher MFI for CK+CD86+cells and for CD8+CD28+cells, compared to their male counterparts (P=0.03 and 0.01, respectively). Nevertheless, male patients tended to have a higher number of CD8+cells at IHC (P=0.073, Supplementary Fig. 2a, Supplemental Digital Content 4, http://links.lww.com/JS9/A54). The infiltration of activated T regulators (FoxP3+), was not significantly different in the two sexes (P=0.107, Supplementary Fig. 2b, Supplemental Digital Content 4, http://links.lww.com/JS9/A54). Finally, as shown in Figure 4.2c, in rectal cancer patients, females who underwent neoadjuvant therapy tended to have better overall survival (P=0.08). These findings suggest that, in female patients with rectal cancer who underwent neoadjuvant therapy, there is a higher activation of epithelial cells acting as antigen-presenting cells and a corresponding activation of cytotoxic T cells than in males. These data confirm that female patients show a more favorable immune microenvironment than male patients, which can justify a tendency toward better overall survival.
Discussion
The prognostic role of the immune TME in CRC has been assessed in many studies27,28. Recently, the association between sex and clinical outcome has also been inquired about, based on the findings of some large studies showing some differences between men and women concerning the pharmacokinetics and pharmacodynamics of anticancer drugs, drug toxicity, and tumor biology. However, the sex difference in terms of immune response to rectal cancer was scarcely addressed. Since the effect of hormonal status on the immune response to cancer know, this could be a crucial point in the management of these patients. Our study aims to fill this relevant gap in the current literature by analyzing all the available evidence concerning sex differences in the TME of rectal cancer patients and analyzing immune surveillance-related gene expression in the healthy rectal mucosa surrounding cancer as a result of the IMMUNOREACT project. This project was designed to study the immune microenvironment of healthy mucosa surrounding rectal cancer, with the hypothesis that it retains a relevant trace of the cross-talk between tumor cells and the immune system.
In the first step of the study, we performed a systematic review of immune surveillance-related gene expression in rectal cancer. The main result of our systematic review and meta-analysis of the literature is the lack of reliable results concerning the influence of sex on the expression of immune surveillance-related genes in rectal cancer, mainly due to the paucity of available studies or the lack of stratification for sex in the presentation of the molecular results26. In particular, all the studies that could be selected were only focused on PD-L1 expression in rectal cancer, while no study showed the sex difference in terms of T cells subpopulations within the TME. Thus, the main conclusion of this systematic review is that, in the current literature, the data are not sufficient to draw any conclusions about the role of gender in the local immune response to rectal cancer.
Therefore, in the second step of the study, we aimed to identify mutations that could be responsible for the differences observed in the TME of rectal cancer in both sexes. In the TGCA rectal cancer series, we found that mutations of SYNE1 and RYR2 were significantly more frequent in male patients than in female ones. Mutations of RYR2 are associated with a higher expression of PD-L1 in breast cancer patients11. Besides, RYR2 represents per se a powerful immunomodulator, and its mutation has been found to favor the infiltration of CD8+T cells, activated memory CD4+T cells, and macrophages29. In the TCGA series, we observed that male patients with RYR2 mutations had a higher PD-L1 expression, and, among patients with SYNE1 mutations, either male or female patients had a higher expression of CTLA-4 while only male patients had a higher expression of PD-L1. These differences seem to explain why male patients, who more frequently have mutations in SYNE1 and RYR2, have a worse prognosis than female patients. Higher expression of PD-L1 and CTLA-4 can produce impairment of the immune surveillance mechanisms, which can directly impact on patient’s survival probabilities.
In the third step of the study, we studied the microenvironment of the healthy mucosa surrounding rectal cancer to analyze the constitutive immune microenvironment within the rectal mucosa, which could explain the different behavior of rectal cancer in male and female patients. In therapy-naive patients, we observed that women had a higher number of activated lymphocytes in the healthy mucosa surrounding rectal cancer. Female patients had a higher number of activated Th1 cells infiltrating the healthy mucosa surrounding the tumor. Moreover, these patients have less HLA class I expressed on their epithelial cells, suggesting lower antigen expression from these cells compared to male patients. In women, the higher number of activated CD4+Th1 cells is a marker of active immune surveillance mechanisms, and they are activated by HLA class II molecules that were not different in the two sexes30–32. On the other hand, a higher expression of PD-L1 in women could be linked to higher levels of circulating estrogens; the correlation between estrogens and PD-L1 is well known, and it has been confirmed in other tumors33,34. In CRC, high E2, free E2, and free testosterone have been associated with a higher risk of mortality among female patients35. However, the number of female patients under 50 years old is pretty low thus, the mere immunological and mutational mechanisms are more likely.
In patients with rectal cancer who had neoadjuvant therapy, epithelial cells acting as antigen-presenting cells (expressing CD86) and activated CD8+T cells (positive for CD28) were higher in female patients compared to their male counterparts. These data showed that, after the neoadjuvant therapy, in the healthy mucosa surrounding the cancer, the interplay between epithelial cells and T cells was more functional in women with rectal cancer than in men with the same condition30. This primary cross-talk may play a relevant role in the immune response to cancer after neoadjuvant therapy.
Our study has some limitations, and the first of them is that the studies included in our meta-analysis show a great deal of heterogeneity; particularly, the cutoff for defining patients as PD-L1 positive or negative is not clearly described, thus limiting the interpretability of the results, and no information about the T cells subpopulations was provided. On the other hand, in the IMMUNOREACT trial, we considered a relatively small number of immune-related genes, so that some pathways of activation of the immune system could not be so easily recognized. Nevertheless, our trial first analyzed the immune microenvironment of healthy mucosa surrounding rectal cancer and should be considered the first step in a line of research analyzing the role of constitutive mutations of immunomodulating genes in defining the natural history of rectal cancer in male and female patients.
In conclusion, we observed that the frequency of SYNE 1 and RYR2 mutations in rectal cancer is more frequent in males than females, and these mutations can have a role in modeling the immune microenvironment of the adenocarcinoma unfavorably. Moreover, in women with therapy naive rectal cancer, a constitutively higher number of activated CD4+Th1 cells in the healthy mucosa shows active immune surveillance mechanisms. Furthermore, women who had neoadjuvant therapy showed a more functional interaction between epithelial cells and T cells in the healthy mucosa surrounding cancer. As shown in Figure 5, these findings can explain why women have a better outcome than male patients with rectal cancer and are particularly relevant since several trials are now ongoing testing immune checkpoint inhibitors in CRC, calling for reliable predictors of therapy response36. Moreover, the analysis of TME has been proposed to predict response to chemotherapy in CRC patients, and the immunoscore is currently being tested as a potential predictor of response to neoadjuvant chemoradiotherapy in LARC37. Based on the results of our study and as proposed by other authors6, sex should be considered when defining the treatment strategy of rectal cancer patients or designing prognostic scores, acknowledging the significant differences in epidemiology, cancer biology, and response to therapy.
Figure 5.
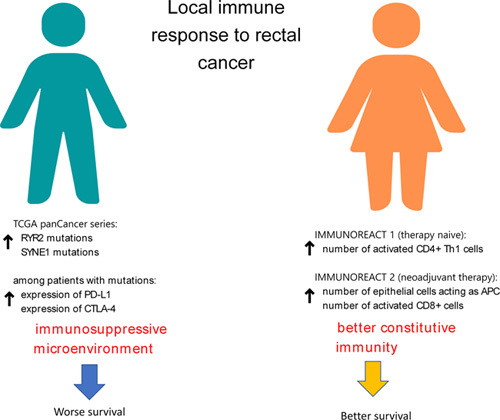
Difference in local immune response against rectal cancer between males and females. These difference may justify a tendency of a better outcome in female patients with rectal cancer.
Ethical approval
The IMMUNOREACT protocol was approved by the Ethical Committee of the coordinating center (Azienda Università Ospedale di Padova; CESC code 4448/AO/20) and of each of the collaborating centers.
Sources of funding
The funding source (AIRC under IG 2019) had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The project is funded by AIRC Investigator Grant 2019 Id. 23381 to Marco Scarpa (P.I.).
Author contribution
S.G.: writing the manuscript, study analysis, data collection. F.M.: study analysis, data collection, writing the manuscript. C.G.: study design, data collection, writing the manuscript. S.M., C.V., F.S., C.F., D.C.B., B.R.: study design, data analysis, revising the manuscript for relevant contribution. S.N., K.A., A.I., C.M., D.S.O., R.C., S.A., V.C., M.F., F.L., B.F., B.S., B.G., G.V., D.S.L., S.R., M.M., P.A., C.I., S.T., D.T.A.P., Z.V., P.P., F.B., A.S., P.G., R.A., M.R., B.G., G.S., R.C., P.G., C.C., Z.M., P.A., A.M., I.M.: study design, data collection, revising the manuscript for relevant contribution. C.I., P.S., S.M.: study design, data analysis, writing the manuscript.
Conflicts of interest disclosure
None.
Research registration unique identifying number (UIN)
1. Name of the registry: clinicaltrials.gov and Research Registry.
2. Unique identifying number or registration ID: NCT04915326 and researchregistry8499.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT04915326 and https://www.researchregistry.com/browse-the-registry#home/?view_2_search=Scarpa&view_2_page=1
Guarantor
Marco Scarpa
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data sharing
Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and supplementary materials), along with the IMMUNOREACT study protocol, will be made available beginning 9 months and ending 36 months following the article’s publication to researchers who provide a methodologically sound proposal. Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement.
Supplementary Material
Footnotes
G.S., M.F., S.P., and M.S. equally contributed to this study.
IMMUNOREACT Study Group: Luca Saadeh, Dario Parini, Daniela Prando, Beatrice Salmaso, Gianluca Buzzi, Loretta Di Cristoforo, Ylenia Camilla Spolverato, Chiara Cipollari, Salvatore Candioli, Laura Gavagna, Giulia Pozza, Mario Godina, Isabella Mondi, Licia Laurino, Monica Ortenzi, Mario Guerrieri, Giovanni Tagliente, Monica Tomassi, Umberto Tedeschi.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 16 February 2023
Contributor Information
Matteo Fassan, Email: matteo.fassan@unipd.it.
Giulia Capelli, Email: giulia.capelli1990@gmail.com.
Melania Scarpa, Email: melania.scarpa@iov.veneto.it.
Silvia Negro, Email: silvia.negrosn01@gmail.com.
Valentina Chiminazzo, Email: valentina.chiminazzo@studenti.unipd.it.
Andromachi Kotsafti, Email: andromachi.kotsafti@iov.veneto.it.
Imerio Angriman, Email: imerio.angriman@unipd.it.
Michela Campi, Email: michela.campi@gmail.com.
Ottavia De Simoni, Email: ottavia.desimoni@gmail.com.
Cesare Ruffolo, Email: cesare.ruffolo@aopd.vneto.it.
Stepanyan Astghik, Email: stepanyan.astgh@gmail.com.
Chiara Vignotto, Email: chiara.vignotto@aodp.veneto.it.
Federico Scognamiglio, Email: federico.scognamiglio@aopd.veneto.it.
Giulia Becherucci, Email: giulia.becherucci@gmail.com.
Giorgio Rivella, Email: giorgio.rivella@unipd.it.
Francesco Marchegiani, Email: marchegiani.fra@gmail.com.
Luca Facci, Email: lucafacci1@gmail.com.
Francesca Bergamo, Email: francesca.bergamo@iov.veneto.it.
Stefano Brignola, Email: stefano.brignola@studenti.unipd.it.
Gianluca Businello, Email: glc.businello@gmail.com.
Vincenza Guzzardo, Email: vincenza.guzzardo@unipd.it.
Luca Dal Santo, Email: lucas1186dalsanto@gmail.com.
Roberta Salmaso, Email: roberta.salmaso@unipd.it.
Marco Massani, Email: marco.massani@aulss2.veneto.it.
Anna Pozza, Email: annapozza@yahoo.it.
Ivana Cataldo, Email: ivana.cataldo@aulss2.veneto.it.
Tommaso Stecca, Email: tomaz86@gmail.com.
Angelo Paolo Dei Tos, Email: aneglop.deitos@unipd.it.
Vittorina Zagonel, Email: vittorina.zagonel@iov.veneto.it.
Pierluigi Pilati, Email: pilati.pierluigi@iov.veneto.it.
Boris Franzato, Email: boris.franzato@iov.veneto.it.
Antonio Scapinello, Email: scapinello.antonio@iov.veneto.it.
Giovanni Pirozzolo, Email: giovanni.pirozzolo@gmail.com.
Alfonso Recordare, Email: alf.recordare@gmail.com.
Roberto Merenda, Email: roberto.merenda@aulss3.veneto.it.
Giovanni Bordignon, Email: giobordi@gmail.com.
Silvio Guerriero, Email: silguerri@gmail.com.
Chiara Romiti, Email: chiara.romiti@sanita.marche.it.
Giuseppe Portale, Email: portale.giuseppe@libero.it.
Chiara Cipollari, Email: chiara.cipollari@aulss6.veneto.it.
Maurizio Zizzo, Email: maurizio.zizzo@asmn.re.it.
Andrea Porzionato, Email: andrea.porzionato@iov.veneto.it.
Marco Agostini, Email: marco.agostini@iov.veneto.it.
Francesco Cavallin, Email: cescocava@libero.it.
Romeo Bardini, Email: romeo.bardini@unipd.it.
Isacco Maretto, Email: isacco.maretto@aopd.veneto.it.
Ignazio Castagliuolo, Email: ignazio.castagliuolo@unipd.it.
Salvatore Pucciarelli, Email: puc@unipd.it.
Marco Scarpa, Email: marcoscarpa@yahoo.it.
References
- 1. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020;70:145–64. [DOI] [PubMed] [Google Scholar]
- 2. AJCC/UICC. 2017. TNM Classification of Malignant Tumours, 8th ed. Wiley Blackwell. [Google Scholar]
- 3. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- 4. Däster S, Eppenberger-Castori S, Hirt C, et al. High frequency of CD8 positive lymphocyte infiltration correlates with lack of lymph node involvement in early rectal cancer. Dis Markers 2014;2014:792183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCoy MJ, Hemmings C, Miller TJ, et al. Low stromal Foxp3+regulatory T-cell density is associated with complete response to neoadjuvant chemoradiotherapy in rectal cancer. Br J Cancer 2015;113:1677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baggio G, Corsini A, Floreani A, et al. Gender medicine: a task for the third millennium. Clin Chem Lab Med 2013;51:713–27. [DOI] [PubMed] [Google Scholar]
- 7. Milette S, Hashimoto M, Perrino S, et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat Commun 2019;10:5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schalper KA, Velcheti V, Carvajal D, et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcomes in breast carcinomas. Clin Cancer Res 2014;20:2773–82. [DOI] [PubMed] [Google Scholar]
- 9. Jomrich G, Silberhumer GR, Marian B, et al. Programmed death-ligand 1 expression in rectal cancer. Eur Surg 2016;48:352–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shen Z, Gu L, Mao D, Chen M, Jin R. Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis. World J Surg Oncol 2019;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cimas FJ, Manzano A, Baliu-Piqué M, et al. Genomic mapping identifies mutations in RYR2 and AHNAK as associated with favorable outcome in basal-like breast tumors expressing PD1/PD-L1. Cancers (Basel) 2020;12:2243. doi: 10.3390/cancers12082243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li CH, Prokopec SD, Sun RX, et al. Sex differences in oncogenic mutational processes. Nat Commun 2020;11:4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathew G, Agha R. for the STROCSS Group. STROCSS 2021: Strengthening the Reporting of cohort, cross-sectional and case-control studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Brit Med J 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. TCGA Study Abbreviations. Accessed 30 June 2022. https://gdc.cancer.gov/resources-tcga-users/tcga-code-tables/tcga-study-abbreviations.
- 16. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 17. Pagès F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med 2005;353:2654–66. [DOI] [PubMed] [Google Scholar]
- 18. Cappellesso R, Fassan M, Hanspeter E, et al. HER2 status in gastroesophageal cancer: a tissue microarray study of 1040 cases. Hum Pathol 2015;46:665–72. [DOI] [PubMed] [Google Scholar]
- 19. Team RC. 2020 R: A Language and Environment for Statistical Computing. Accessed 30 June 2022. https://R-project.org/
- 20. Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013;49:2233–42. [DOI] [PubMed] [Google Scholar]
- 21. Li XF, Liu XF, Yang YY, et al. Correlation study of Bcl-2, B7-H1, EGFR, VEGF, and colorectal cancer. Am J Cancer Res 2015;5:2277–84. [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu H, Qin H, Huang Z, et al. Clinical significance of programmed death ligand-1 (PD-L1) in colorectal serrated adenocarcinoma. Int J Clin Exp Pathol 2015;8:9351–99. [PMC free article] [PubMed] [Google Scholar]
- 23. Berntsson J, Eberhard J, Nodin B, et al. Expression of programmed cell death protein 1 (PD-1) and its ligand PD-L1 in colorectal cancer: relationship with sidedness and prognosis. Oncoimmunology 2018;7:e1465165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahtiainen M, Wirta EV, Kuopio T, et al. Combined prognostic value of CD274 (PD-L1)/PDCDI (PD-1) expression and immune cell infiltration in colorectal cancer as per mismatch repair status. Mod Pathol 2019;32:866–83. [DOI] [PubMed] [Google Scholar]
- 25. Onwe EE, A GF, Abdullah M, et al. Predictive potential of PD-L1, TYMS, and DCC expressions in treatment outcome of colorectal carcinoma. Cancer Biol Adv Treat 2020;1292:97–112. [DOI] [PubMed] [Google Scholar]
- 26. Kim JH, Park HE, Cho NY, et al. Characterization of PD-L1-positive subsets of microsatellite-unstable colorectal cancers. Br J Cancer 2016;115:490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009;15:6412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer 2013;109:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu Z, Xiang L, Wang R, et al. Bioinformatic analysis of immune significance of RYR2 mutation in breast cancer. BioMed Res Int 2021;2021:8072796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scarpa M, Brun P, Scarpa M, et al. CD80-CD28 signaling controls the progression of inflammatory colorectal carcinogenesis. Oncotarget 2015;6:20058–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marchiori C, Scarpa M, Kotsafti A, et al. Epithelial CD80 promotes immune surveillance of colonic preneoplastic lesions and its expression is increased by oxidative stress through STAT3 in colon cancer cells. J Exp Clin Cancer Res 2019;38:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scarpa M, Marchiori C, Scarpa M, et al. CD80 expression is upregulated by TP53 activation in human cancer epithelial cells. Oncoimmunology 2021;10:1907912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mo Z, Liu J, Zhang Q, et al. Expression of PD-1, PD-L1, and PD-L2 is associated with differentiation status and histological type of endometrial cancer. Oncol Lett 2016;12:944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang L, Huang F, Mei J, et al. Posttranscriptional control of PD-L1 expression by 17β-estradiol via PI3K/Akt signaling pathway in ERα-positive cancer cell lines. Int J Gynecol Cancer 2017;27:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yang W, Giovannucci EL, Hankinson SE, et al. Endogenous sex hormones and colorectal cancer survival among men and women. Int J Cancer 2020;147:920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yaghoubi N, Soltani A, Ghazvini K, et al. PD-1/ PD-L1 blockade is a novel treatment for colorectal cancer. Biomed Pharmacother 2019;110:312–8. [DOI] [PubMed] [Google Scholar]
- 37. Kirilovsky A, Sissy CE, Zeitoun G, et al. The ‘Immunoscore’ in rectal cancer: could we search for quality beyond the quantity of life? Oncotarget 2022;13:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


