Background:
Various regional analgesia techniques are used to reduce postoperative pain in patients undergoing lumbar spine surgery. Traditionally, wound infiltration (WI) with local anesthetics has been widely used by surgeons. Recently, other regional analgesia techniques, such as the erector spinae plane block (ESPB) and thoracolumbar interfascial plane (TLIP) block, are being used for multimodal analgesia. The authors aimed to determine the relative efficacy of these using a network meta-analysis.
Materials and methods:
The authors searched PubMed, EMBASE, the Cochrane Controlled Library, and Google Scholar databases to identify all randomized controlled trials that compared the analgesic efficacy of the following interventions: ESPB, TLIP block, WI technique, and controls. The primary endpoint was postoperative opioid consumption during the first 24 hours after surgery, while the pain score, estimated postoperatively at three different time periods, was the secondary objective.
Results:
The authors included 34 randomized controlled trials with data from 2365 patients. TLIP showed the greatest reduction in opioid consumption compared to controls [mean difference (MD) =−15.0 mg; 95% CI: −18.8 to −11.2]. In pain scores, TLIP had the greatest effect during all time periods compared to controls (MD=−1.9 in early, −1.4 in middle, −0.9 in late). The injection level of ESPB was different in each study. When only surgical site injection of ESPB was included in the network meta-analysis, there was no difference compared with TLIP (MD=1.0 mg; 95% CI: −3.6 to 5.6).
Conclusions:
TLIP showed the greatest analgesic efficacy after lumbar spine surgery, in terms of postoperative opioid consumption and pain scores, while ESPB and WI are also alternative analgesic options for these surgeries. However, further studies are needed to determine the optimal method of providing regional analgesia after lumbar spine surgery.
Keywords: erector spinae plane block, lumbar spine surgery, nerve block, network meta-analysis, postoperative pain, thoracolumbar interfascial plane block
Introduction
Highlights
Various regional analgesia techniques are used to reduce postoperative pain in patients undergoing lumbar spine surgery.
We employed a network meta-analysis to assess the postoperative analgesic efficacy of erector spinae plane block, thoracolumbar interfascial plane (TLIP) block, wound infiltration, and controls.
TLIP block showed the greatest analgesic efficacy after lumbar spine surgery.
The erector spinae plane block performed at the surgical level showed similar efficacy to the TLIP block.
Lumbar spine surgery is a commonly performed orthopedic or neurosurgical procedure associated with moderate-to-severe postoperative pain1. Timely and adequate pain management after spinal surgery is important for early ambulation and improving functional outcomes. There are several types of surgery, such as laminectomy, decompression, fusion, and discectomy, depending on the type and invasiveness of the disease2. The intensity of postoperative pain is dependent on various nociceptive and neuropathic pain mechanisms3, which come into play in response to mechanical irritation, compression, or postoperative inflammation in the related anatomical structures.
Traditionally, wound infiltration (WI) with local anesthetics has been widely used by surgeons to manage postoperative pain following lumbar spine surgery4. The method is simple, safe, and may reduce the use of opioids, additional complications during perioperative periods, the duration of hospitalization, and costs. However, the clinical significance of these advantages was small and limited to the immediate postoperative period4.
Recently, other regional analgesia techniques, such as the erector spinae plane block (ESPB)5–7 and thoracolumbar interfascial plane (TLIP) block8–10, are being used for multimodal analgesia for lumbar spine surgery. These techniques target the dorsal rami of the spinal nerves to anesthetize the posterior midline area.
Although many studies have reported the efficacy of these regional analgesia techniques and compared their effectiveness in spine surgery, the relative efficacy of these techniques has not been compared using network meta-analysis (NMA). NMA is a statistical technique for estimating the effect size of several studies with multiple interventions or treatments. The indirect comparisons of different groups that have never been directly compared are possible through a third or another comparator. If multiple treatment groups are to be compared at the same time, a mixed treatment comparison can be performed using both direct and indirect comparison studies. Thus, NMA identifies the most superior group and estimate a relative ranking11. Therefore, we identified and reviewed all articles that have investigated the effects of various methods of postoperative analgesia in lumbar spine surgery and used NMA to rank these methods according to their effectiveness.
Our primary outcome was opioid consumption during the first 24 hours after surgery, and we evaluated pain severity at three different postoperative periods, namely early, middle, and late, as the secondary outcome.
Materials and methods
This study was conducted in accordance with the recommended guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis12 and registered with the International Prospective Register of Systematic Reviews (PROSPERO, CRD42022309271).
Data source and search strategy
A literature search was conducted independently by two authors to identify eligible studies for this systematic review and meta-analysis. The databases searched were PubMed, EMBASE, and the Cochrane Library. Medical Subject Heading and text terms were combined and followed by Boolean logical operators. The language was limited to English, and an exhaustive search was conducted using the following Medical Subject Heading terms: [{(“Lumbar spine” OR “Spinal stenosis OR Spondylolisthesis) AND (Decompression OR “Surgical Procedures, Operative”)} AND {(“Thoracolumbar interfascial plane block” OR TLIP OR “Erector spinae plane block” OR ESPB) AND (“Anesthesia, Local” OR “Local anesthetic infiltration”)}]”. The primary search was conducted in January 2022, and an additional search was conducted on 28 February 2022 during the revision to include more recent studies. The reference lists of selected articles were searched manually. Full search strategies for individual data are provided in Figure 1.
Figure 1.
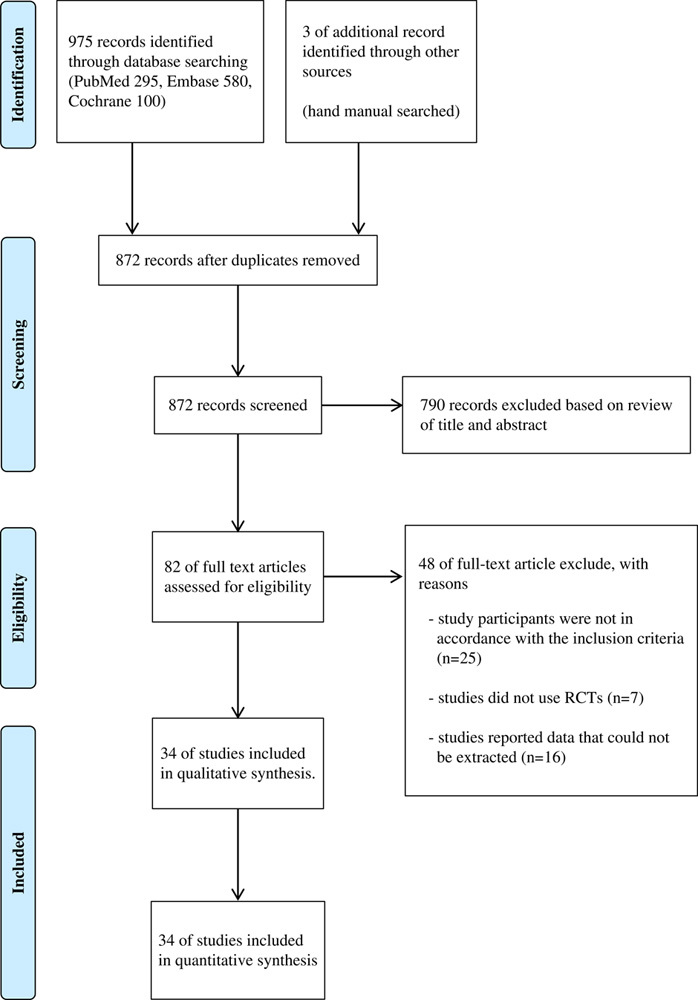
Study flow diagram. RCT, randomized controlled trial.
Inclusion and exclusion criteria
Studies were deemed eligible if they were randomized controlled trials (RCTs), published in English, and reported postoperative pain scores in both experimental and control groups, or outcomes as pain scores and quantity of opioids consumed. Non-RCTs (quasiexperimental design), abstracts, conference proceedings, unpublished grey literature, and review studies were excluded. Among regional analgesia techniques, studies that used continuous block by catheterization and adjuvants were excluded.
Review procedure
Study selection involved six steps. First, two investigators imported the titles and abstracts of identified articles into a reference management software (EndNote 20; Clarivate) and performed a preliminary review. Second, duplicate articles were identified and eliminated using the reference management software. Third, they independently reviewed all imported studies and excluded those that did not conform to the inclusion criteria, such as study design, participants, type of intervention, or comparisons. Fourth, three investigators independently reviewed all the titles and abstracts for relevance. Fifth, we retrieved the full text of the papers that met all the inclusion criteria for data extraction and linked multiple reports of the same study. Lastly, the finalized studies were confirmed and coded for analysis by two investigators. The coding sheets were independently checked for accuracy by investigators not involved in the review process.
Data extraction
Information from the included articles was independently extracted by two reviewers, and each selection was reviewed twice by both reviewers together. To evaluate the outcomes in individual studies, pain scores and opioid consumption were determined for each group, and the mean and SD were obtained. Median and interquartile ranges, as approximations of mean and SD, were determined using an estimation method proposed by Wan et al. 13. When outcome data were available only as a graph, a virtual ruler was used to extract the value by matching the interval between the basic unit of the plot and the ruler. Effect sizes and standard errors were calculated. Additional data, including location, sample size, characteristics of individual study populations, and intervention designs, were extracted using a predesigned data extraction table.
Outcome definitions
The primary outcome was cumulative opioid consumption during the first 24 hours after surgery. All opioids were converted to equianalgesic intravenous (i.v.) morphine doses (i.v. morphine 1 mg=i.v. fentanyl 10 µg=i.v. sufentanil 2 µg=i.v. tramadol 10 mg=i.v. pethidine 7.5 mg)14,15. The secondary outcome was a pain score assessed at three time periods during the first 24 hours after surgery, namely, early (up to 6 h), middle (6–18 h), and late (18–24 h). When multiple data points were available for each time period, pain scores closest to 1 h for early, 12 h for middle, and 24 h for late were used. Pain scores determined using visual analog scales (VASs) were converted to a 0–10 analog scale to permit statistical evaluation.
Data synthesis and statistical analysis
A random-effects NMA within a frequentist framework was performed using R software, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria), and the ‘netmeta’ package for frequentist NMA16,17. A network plot was constructed to evaluate both direct and indirect comparisons of network structure using data from all included studies. Heterogeneity was evaluated using the I 2 statistic. The Q-statistic, based on the full design-by-treatment interaction random-effects model, was calculated to evaluate global inconsistencies18. Local inconsistencies between direct and indirect effects were evaluated using the net splitting technique. If the P value of the net splitting was below 0.05, we presumed a significant disagreement (inconsistency) between the direct and indirect estimates. Net split results were visualized as forest plots, with a direct evidence plot showing the percentage of direct and indirect evidence used for each estimated comparison. A mean path length greater than 2 indicated that a comparison estimate should be interpreted with caution. In addition, a net heat plot was constructed to ascertain the importance of each comparison and detect any inconsistencies in the design. A network league table and forest plot were obtained to evaluate the results of the comparisons between interventions. Outcomes are presented as mean differences (MDs) with a 95% CI. To rank the analgesic interventions according to their efficacy, we used the P scores, which are based solely on the point estimates and SEs of the network estimates19. In addition, the resampling method with 100,000 simulations is used to calculate the surface under the cumulative ranking curves (SUCRA) for frequentist NMA. The P score and SUCRA ranged from 0 to 1, where, statistically, 1 indicated the best and 0 the worst. Any potential publication bias was assessed using comparison-adjusted funnel plots and Egger’s test. To enhance the applicability of the study findings, we used Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (Table 4) to evaluate the evidence level of the included outcomes. We rated the quality of the evidence as very low, low, moderate, and high. The ratings depended on the presence of risk in five areas: methodological quality, directness of evidence, heterogeneity, precision of effect estimates, and publication bias. The GRADE approach appraises the quality of a body of evidence for each outcome based on five domains: (1) risk of bias of the included studies (methodological quality), (2) inconsistency (i.e. heterogeneity), (3) indirectness (relevance to the review question), (4) imprecision (i.e. confidence intervals), and (5) risk of publication bias20.
Table 4.
P-scores and ranking of the included techniques.
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Opioid consumption | TLIP: 0.9965 | ESPB: 0.5875 | WI: 0.4161 | Control: 0.0000 |
| Early postoperative period (up to 6 h) pain score | TLIP: 0.9333 | ESPB: 0.6753 | WI: 0.3871 | Control: 0.0042 |
| Middle postoperative period (6–18 h) pain score | TLIP: 0.9923 | ESPB: 0.6114 | WI: 0.3813 | Control: 0.0150 |
| Late postoperative period (18–24 h) pain score | TLIP: 0.9369 | WI: 0.6737 | ESPB: 0.3889 | Control: 0.0005 |
ESPB, erector spinae plane block; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Results
Baseline characteristics of the included studies
The literature screening process and results are shown in Figure 1. The screening sequence of the Preferred Reporting Items for Systematic Review and Meta-Analysis 2009 flow diagram, which compared the analgesic efficacy of TLIP block, ESPB WI, and controls (no block), identified 34 studies21–54, corresponding to a total of 2365 patients.
In total 978 records were obtained from the initial literature search. Based on full-text examination, 48 records were excluded for various reasons: 25 studies were not in accordance with the inclusion criteria, 7 studies were not RCTs, and 16 studies reported data that could not be extracted (Fig. 1). Table 1 lists the characteristics of the included studies, while Table 2 provides data on the number of included studies and enrolled patients sorted by outcome. The raw data of this NMA is provided as Supplementary Materials 1, Supplemental Digital Content 1, http://links.lww.com/JS9/A98.
Table 1.
Characteristics of included studies.
| References | Year | Country | Surgery | Group (n) | Block level | Local anesthetics | Block timing | Opioid data | Nonopioid multimodal analgesia protocol | Pain score data form (early, middle, late period) (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| Milligan et al.21 | 1993 | Ireland | Elective lumbar discectomy | Control (30) WI (30) | 10 ml into the wound, 5 ml laterally into the erector spini muscle, 5 ml subcutaneously along both margins of the wound | 20 ml of 0.5% bupivacaine | Before wound closure | Morphine | NA | Table (1,8,24) |
| Mack et al.22 | 2001 | USA | Single level, unilateral microscopic lumbar discectomy | Control (10) WI (10) |
Into the wound | 15 ml of 0.25% bupivacaine | Before wound closure | Morphine | NA | NA |
| Mirzai et al.23 | 2002 | Turkey | Lumbar disc surgery for single-level unilateral herniated nucleus pulposus | Control (22) WI (22) |
Paravertebral muscles and subcutaneous tissue | 20 ml of 0.25% bupivacaine | During wound closure | Meperidine, NA for 24 h data | NA | Plot (1,12,NA) |
| Yörükoğlu et al.24 | 2005 | Turkey | Elective surgery for lumbar disc disease within 3 hours | Control (20) WI (20) |
Paraspinal muscle and skin | 30 ml of 0.25% bupivacaine | Before wound closure | Meperidine | Naproxen sodium tablets (75 mg) as required | Plot (0.5,12,24) |
| Ersayli et al.25 | 2006 | Turkey | Scheduled first unilateral lumbar discectomy | Control (15) WI (15) |
Musculus multifidi near the operated level | 30 ml of 0.25% bupivacaine | Before wound closure | Morphine | NA | Table (1,8,24) |
| Esmail et al.26 | 2008 | Iran | Surgery for one level lumbar intervertebral disc herniation | Control (83) WI (83) |
Subcutaneous tissue | 20 ml of 2% lidocaine with 1/500 000 epinephrine | Before incision | Morphine (intramuscular) | NA | Table (6,12,24) |
| Gurbet et al.27 | 2008 | Turkey | Surgery for unilateral lumbar disc herniation | Control (19) WI (19) |
Musculus multifidi near the operated level | 30 ml of 0.25% levobupivacaine | Before wound closure | Morphine | Diclofenac (75 mg) as rescue analgesic | NA |
| Ozyilmaz et al.28 | 2012 | Turkey | Elective single space lumbar discectomy | Control (20) WI (20) |
Over the incision line on the paravertebral muscles and cutaneous and subcutaneous tissue | 20 ml of 0.75% levobupivacaine | Before wound closure | Pethidine | Diclofenac (75 mg) as rescue analgesic | Plot (1,12,24) |
| Mohta et al.33 | 2019 | India | Tubercular spine surgery | Control (16) WI (16) |
Wound | 0.375% ropivacaine 3 mg/kg with adrenaline 5 μg/ml and dexmedetomidine 1 μg/kg in a total volume of 0.8 ml/kg | Before wound closure | Morphine | Diclofenac (1 mg/kg) or tramadol (1 mg/kg) at the time of wound closure | Plot (0.5,8,24) |
| Kraiwattanapong et al.39 | 2020 | Thailand | One or two levels of lumbar spinous process splitting laminectomy due to degenerative lumbar spinal stenosis | Control (26) WI (23) |
Wound (30 ml) and 20 ml for paraspinal muscle bilaterally | Total volume 50 ml of levobupivacaine 100 mg, morphine 5 mg, ketorolac tromethamine 30 mg, epinephrine 0.25 mg and normal saline | Unknown | Morphine | Pregabalin (75 mg) at bedtime postoperatively | Plot (0,12,24) |
| Ahiskalioglu et al.29 | 2018 | Turkey | Scheduled for 2 or 3 level posterior lumbar instrumentation surgery | Control (20) TLIP (20) |
L3, modified plane | 20 ml of 0.25% bupivacaine | Unknown | Fentanyl | Non specified supplementary analgesia | Plot (1,12,24) |
| Ammar et al.30 | 2018 | Egypt | Single or multiple level lumbar discectomy | Control (35) TLIP (35) |
L3, original plane | 20 ml of 0.25% bupivacaine, 20 ml of 1% lidocaine | After induction | Morphine | Intravenous paracetamol (1 g) every 6 h | Plot (2,12,24) |
| Chen et al.31 | 2019 | China | Lumbosacral spine fusion surgery | Control (30) TLIP (30) |
L3, original plane | 30 ml of 0.375% ropivacaine | After induction | Sufentanil | Intravenous flurbiprofen (50 mg) at end of surgery | Plot (1,12,24) |
| Ozmen et al.34 | 2019 | Turkey | Elective single-level herniated lumbar disc surgery | Control (40) TLIP (40) |
L3, modified plane | 40 ml of 0.25% bupivacaine | Before induction | Fentanyl | Dexketoprofen (50 mg) at near end of surgery and postoperative 12 h | Plot (1,12,24) |
| Eltaher et al.42 | 2021 | Egypt | Elective spine surgery (discectomy, laminectomy, and spinal fixation) | Control (30) TLIP (30) |
L3, original plane | 40 ml of 0.25% bupivacaine | Unknown | Morphine | Intravenous paracetamol (1 g) every 8 h | Table (2,12,24) |
| El Ghamry et al.32 | 2019 | Egypt | Elective posterior lumbar interbody fusion due to double level lumbar spondylolisthesis (L3–L5) | Control (30) ESPB (30) |
L3 | 40 ml of 0.25% bupivacaine | Before induction | Morphine | Intravenous paracetamol (1 g) every 6 h, Ketorolac loading (30 mg) and every 8 h (15 mg) | Plot (2,12,24) |
| Yayik et al.35 | 2019 | Turkey | One or two-level open lumbar decompression surgery | Control (30) ESPB (30) |
L3 | 40 ml of 0.25% bupivacaine | Before induction | Tramadol | Ibuprofen (400 mg) every 12 h | Table (2,12,24) |
| Eskin et al.38 | 2020 | Turkey | Elective lumbar decompression surgery for one or two vertebral levels | Control (40) ESPB (40) |
The vertebra in the middle of the incision line | 40 ml of 0.25% bupivacaine | After surgery | Tramadol | Intravenous paracetamol 10 mg/kg, tenoxicam 10 mg After induction, Intravenous paracetamol (1 g) every 8 h, Dexketoprofen (50 mg) every 24 h | Table (2,12,24) |
| Singh et al.40 | 2020 | India | Elective lumbar spine surgery (prolapsed lumbar intervertebral disk, lumbar stenosis, or laminectomy) | Control (20) ESPB (20) |
T10 | 40 ml of 0.5% bupivacaine | Before induction | Morphine | Intravenous diclofenac (1.5 mg/kg) every 8 h | Table (0,12,24) |
| Zhang et al.41 | 2020 | China | Open posterior lumbar decompression surgery (prolapsed lumbar intervertebral disk, lumbar stenosis) | Control (30) ESPB (30) |
T12 | 50 ml of 0.3% ropivacaine | Before induction | Morphine | NA | Table (NA, NA,24) |
| Finnerty et al.43 | 2021 | Ireland | Open thoracolumbar vertebral decompression for degenerative stenosis or trauma at two or more levels, with or without fusion | Control (30) ESPB (30) |
Mid-point of the planned incision | 40 ml of 0.25% levobupivacaine | After induction | Oxycodone | Intravenous paracetamol (1 g) and dexketoprofen (50 mg) after induction, Intravenous paracetamol (1 g) every 6 h, Oral ibuprofen (400 mg) every 8 h | Plot (0,12,24) |
| Goel et al.44 | 2021 | India | Elective single-level transforaminal lumbar interbody fusion surgery | Control (50) ESPB (51) |
Surgical level | 40 ml of 0.25% bupivacaine | After induction | Fentanyl | Intravenous paracetamol (1 g) and ketorolac (30 mg) after induction, Intravenous paracetamol (1 g) every 6 h, ketorolac (30 mg) every 8 h, and pregabalin (75 mg) once a day | Table (2,12,24) |
| Jin et al.45 | 2021 | China | Elective two-level or three-level lumbar laminoplasty for lumbar spinal stenosis | Control(32) ESPB(30) |
Vertebral levels of the surgery | 40 ml of 0.375% ropivacaine | After induction | Oral morphine milligram equivalent | Intravenous parecoxib (40 mg and intramuscular pethidine (50 mg) as rescue analgesic | Plot (1,12,24) |
| Wahdan et al.46 | 2021 | Egypt | Lumbar spine procedures on any two levels between L1 and L5 (discectomy, laminectomy, and fixation) | Control (70) ESPB (70) | Operating level | 40 ml of 0.25% levobupivacaine | After induction | Morphine | Intravenous ketorolac (30 mg) every 8 h | Plot (0,12,24) |
| Yeşiltaş et al.48 | 2021 | Turkey | Posterior spinal instrumentation and fusion for spondylolisthesis | Control (28) ESPB (28) |
Freehand ESPB technique directly by the surgical team under vision | 20 ml (1:1) mixture solution of 0.25% bupivacaine and 1.0% lidocaine 0.25% bupivacaine and 1.0% lidocaine | End of surgery | Morphine | Paracetamol every 8 h | Table (1,12,24) |
| Yörükoğlu et al.49 | 2021 | Turkey | Elective single-level lumbar microdiscectomy | Control (26) ESPB (28) |
Surgical level | 40 ml of 0.25% bupivacaine | Before surgery | Morphine | Tramadol (100 mg) and paracetamol (1 g) at end of surgery, Intravenous tenoxicam (20 mg) as recue analgesic | Table (1,12,24) |
| Yu et al.50 | 2021 | China | Posterior internal fixation for single-level lumbar fracture | Control (40) ESPB (40) |
Fractured lumbar vertebra | 60 ml of 0.25% bupivacaine | After induction | Sufentanil | Flurbiprofen included in patient controlled analgesia | Table (6,12,24) |
| Zhang et al.51 | 2021 | China | Lumbar spine surgery | Control (29) ESPB (30) |
T10 | 50 ml of 0.3% ropivacaine | Before induction | Morphine | Flurbiprofen (50 mg), dezocine (0.1–0.2 mg/kg) and dexmedetomidine (0.3 μg/kg) | Table (NA, NA,24) |
| Zhang et al.52 | 2021 | China | Primary open posterior lumbar spinal fusion surgery | Control (30) ESPB (30) |
L3 | 40 ml of 0.4% ropivacaine | Before surgery | Sufentanil | Flurbiprofen (1.5 mg/kg) loading at end of surgery and continuous infusion (6 mg/h) | Plot (4,12,24) |
| Zhu et al.53 | 2021 | China | Posterior lumbar fusion surgery | Control (20) ESPB (20) |
L2 | 40 ml of 0.375% ropivacaine | Before surgery | Oxycodone | Flurbiprofen (50 mg) before the end of surgery | Table (0.5,12,24) |
| Asar et al.54 | 2022 | Turkey | Elective spinal surgery with instrumentation involving single or multilevels in the lumbar or thoracic regions | Control (35) ESPB (35) |
T10 | Total 40 ml volume consisting of 20 ml of 0.5% bupivacaine, 10 ml of 2% lidocaine, and 10 ml of 0.9% NaCl | End of surgery | Total morphine equivalent dose | Intravenous paracetamol (1 g) and tramadol (1 mg/kg) before the end of surgery | Plot (1,12,24) |
| Ekinci et al.37 | 2020 | Turkey | Single-level lumbar disc surgery | WI (30) TLIP (30) |
Surgery site (WI) L3 (TLIP), modified plane | 20 ml of 0.5% bupivacaine (WI) 40 ml of 0.25% bupivacaine (TLIP) | After induction | Fentanyl | Intravenous paracetamol (1 g) every 8 h | Table (2,8,24) |
| Ciftci et al.36 | 2020 | Turkey | Single-level lumbar discectomy and hemilaminectomy surgery | Control (30) TLIP (30) ESPB (30) |
L3, modified plane | 40 ml of 0.25% bupivacaine | After induction | Fentanyl | Intravenous paracetamol (1 g) every 6 h | Table (2,8,24) |
| Wang et al.47 | 2021 | China | Lumbar spine fusion surgery | Control (100) TLIP (102) ESPB (102) |
L3 (TLIP), original plane T12 (ESPB) | 30 ml of 0.375% ropivacaine | After induction | Sufentanil, NA for 24 h data | Flurbiprofen included in patient controlled analgesia | Plot (1,12,24) |
ESPB, erector spinae plane block; NA, not applicable; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Table 2.
Model, heterogeneity, consistency test, and GRADE quality of evidence assessment for primary and secondary outcomes.
| Consistency test | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Number of studies | Number of patients | Number of pairwise comparison | Number of designs | I 2 (%) | Global P value | Local P value | Quality of the evidence (GRADE) | Comments |
| Opioid consumption | 32 | 2017 | 34 | 5 | 96.8 | 0.095 | WI vs. control (0.020) and TLIP vs. WI 0.020) significant, other comparisons are insignificant | ⊕⊕⊕⊖Moderate quality | Pooled meta-analysis of included studies suggests that compared to control probably reduces opioid consumption |
| Early postoperative period (up to 6 h) pain score | 30 | 2188 | 34 | 5 | 97.3 | 0.472 | All comparisons are insignificant | ⊕⊕⊖⊖Low quality | Downgraded for concerns related to inconsistency and indirectness bias |
| Middle postoperative period (6–18 h) pain score | 30 | 2188 | 34 | 5 | 95.4 | 0.888 | All comparisons are insignificant | ⊕⊕⊖⊖Low quality | Downgraded for concerns related to inconsistency and publication bias |
| Late postoperative period (18–24 h) pain score | 31 | 2263 | 34 | 5 | 91.7 | 0.342 | ESPB vs. control (0.046) significant, other comparisons are insignificant | ⊕⊕⊖⊖Low quality | Downgraded for concerns related to imprecision, inconsistency, and publication bias |
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate.
Global inconsistency based on the full design-by-treatment interaction random-effects model18; local inconsistency based on difference between direct and indirect estimates by net splitting technique.
ESPB, erector spinae plane block; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; I 2, Higgins’ I 2; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Methodological quality and risk of bias
Individual studies were assessed using the Cochrane Collaboration’s Risk of Bias (ROB) tool20 and ranked according to a low/high/unclear grading scale (Fig. 2). The ROB assessment was performed in Reviewer Manager (5.4 version). The overall quality of the 34 studies included was moderate. Included articles with clear explanations of random sequence generation and allocation concealment had a low risk of bias, whereas those without explanations had a high risk or were unclear. Some studies showed possible bias in patient selection and methodology, with 75% showing an unclear or high risk of bias in performance concealment and 80% in blinding of outcome assessment. Importantly, no significant publication bias (Egger’s regression test, P>0.05) was evident in any of the included studies (page 16 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102). A comparison-adjusted funnel plot yielded a visually symmetric plot for both opioid consumption and pain scores at all three time periods studied (page 16 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102). The quality of evidence was rated as very low to low, as per the GRADE system (Table 2).
Figure 2.
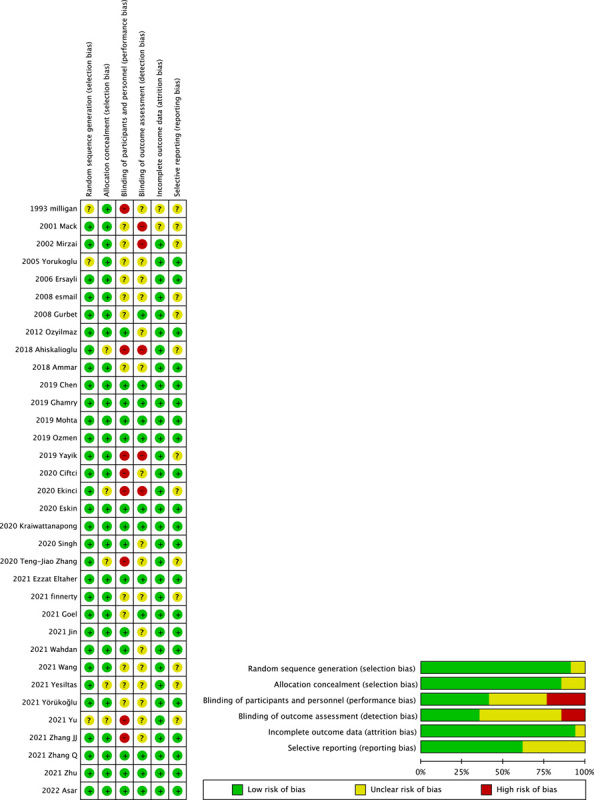
Assessment of risk of bias for included studies. The overall quality of the included. studies were deemed satisfactory.
Heterogeneity and consistency test
The results of the I 2 and Q statistics (based on the full design-by-treatment interaction random-effects model) indicated that a random-effects model may be suitable for revealing any inconsistency or heterogeneity in our network model (Table 2). Furthermore, the colored background of the net heat plot implied that a random-effects model may be appropriate for our data (pages 12 and 13 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102). The direct evidence plot (pages 6 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102) and the forest plot of the net splitting results (pages 14 and 15 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102) were used to evaluate local inconsistency.
Efficacy outcomes (NMA)
Of the included studies, 32 RCTs21,22,24–46,48–54 had reported on opioid consumption, while 3021,23–26,28–40,42–50,52–54, 3021,23–26,28–40,42–50,52–54, and 3121,24–26,28–54 RCTs had provided data on pain scores for the early, middle, and late periods, respectively.
The network between the ESPB and controls was greater than that between other techniques, followed by that between the WI and TLIP blocks. Compared to the controls, as shown in Figure 3A, TLIP blocks showed the greatest analgesic effect as opioid consumption was the least (MD=−15.0 mg; 95% CI: −18.8 to −11.2), followed by ESPB (MD=−9.7 mg; 95% CI: −12.1 to −7.4), WI (MD=−8.3 mg; 95% CI: −11.6 to −5.0). Even compared with ESPB (MD=5.3 mg; 95% CI: 1.0–9.6) and WI (MD=6.7 mg; 95% CI: 2.0–11.4), TLIP blocks showed significant reduction in opioid consumption (Fig. 3B).
Figure 3.
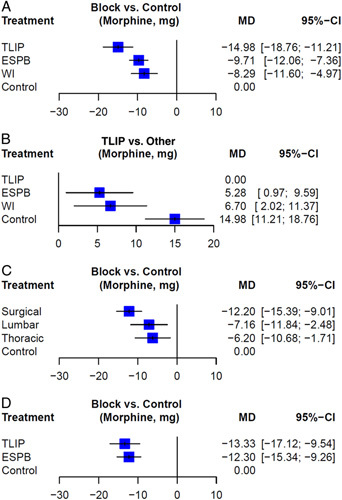
Forest plots for network meta-analysis. (A) Opioid consumption in the first 24 h compared with control. (B) Compared with thoracolumbar interfascial plane (TLIP). (C) Erector spinae plane block (ESPB) by injection level. (D) Only surgical site injection of ESPB included. Mean difference and 95% CI are shown.
Next, compared to controls, pain scores were lowest after TLIP during the early period (MD=−1.9, 95% CI: −2.7 to −1.1), followed by ESPB and WI (Fig. 4A). In the middle and late period, TLIP blocks and ESPB showed superior analgesic effects over controls in reducing the pain score, whereas WI did not have a significant effect (Fig. 4B, C). Local inconsistency between the WI and controls was significant in opioid consumption (Table 2). Table 3 shows the network league table, which provides both direct comparison and full model results.
Figure 4.
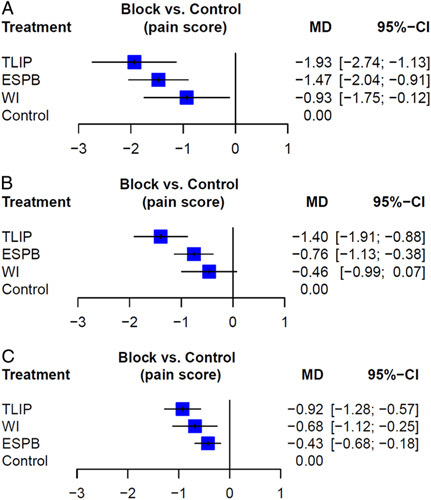
Forest plots for pain score (A) early postoperative period (up to 6 h). (B) Middle postoperative period (6–18 h). (C) Late postoperative period (18–24 h). Mean difference (MD) and 95% CI are shown. ESPB, erector spinae plane block; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Table 3.
Network league table for all the interventions for opioid consumption, pain score at early (up to 6 h), middle (6–18 h), and late (18–24 h) during the first 24 h postoperative period.
| Opioid consumption | |||
| Control | 9.50 (7.13, 11.87) | 13.52 (9.32, 17.72) | 9.62 (6.12, 13.12) |
| 9.71 (7.36, 12.06) | ESPB | 0.00 (−9.21, 9.21) | — |
| 14.98 (11.21, 18.76) | 5.28 (0.97, 9.59) | TLIP | −16.53 (−26.02 −7.04) |
| 8.29 (4.97, 11.60) | −1.42 (−5.46, 2.63) | −6.70 (−11.37, −2.02) | WI |
| Pain score: early postoperative period (up to 6 h) | |||
| Control | 1.48 (0.90, 2.05) | 1.63 (0.76, 2.51) | 1.14 (0.27, 2.00) |
| 1.47 (0.91, 2.04) | ESPB | 0.75 (−0.96, 2.47) | — |
| 1.93 (1.13, 2.74) | 0.46 (−0.47, 1.40) | TLIP | −2.40 (−4.65; −0.15) |
| 0.93 (0.12, 1.75) | −0.54 (−1.53, 0.45) | −1.00 (−2.07, 0.07) | WI |
| Pain score: middle postoperative period (6–18 h) | |||
| Control | 0.73 (0.35, 1.11) | 1.37 (0.80, 1.94) | 0.50 (−0.07, 1.07) |
| 0.76 (0.38, 1.13) | ESPB | 0.36 (−0.73, 1.45) | — |
| 1.40 (0.88, 1.91) | 0.64 (0.04, 1.24) | TLIP | −1.20 (−2.66, 0.26) |
| 0.46 (−0.07, 0.99) | −0.30 (−0.94, 0.35) | −0.93 (−1.63, −0.24) | WI |
| Pain score: late postoperative period (18–24 h) | |||
| Control | 0.39 (0.13, 0.64) | 0.95 (0.56, 1.34) | 0.70 (0.22, 1.17) |
| 0.43 (0.18, 0.68) | ESPB | 0.05 (−0.65, 0.76) | — |
| 0.92 (0.57, 1.28) | 0.50 (0.09, 0.90) | TLIP | −0.30 (−1.30, 0.70) |
| 0.68 (0.25, 1.12) | 0.25 (−0.24, 0.75) | −0.24 (−0.76, 0.28) | WI |
Estimates are presented as mean differences (95% CI). Mean differences below 0 favor the column intervention and mean differences above 0 favor the row intervention.
The upper triangle displays only the pooled effect size for the direct comparisons available in our network. No direct comparison is expressed in an empty field. The lower triangle contains the estimated effect sizes for each comparison, even if only indirect evidence was available.
ESPB, erector spinae plane block; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Ranking hierarchy
Table 4 shows the P scores for analgesic efficacy and ranking of the five groups. The TLIP block was ranked first for 24-h opioid consumption (0.997) and pain scores of all periods (0.933, 0.992, and 0.936). ESPB emerged second in all outcomes except for the late period pain score. WI ranked second in the pain score of the late period. The results of SUCRAs were similar to the P scores (pages 10 of Supplementary Materials 2–5, Supplemental Digital Content 2, http://links.lww.com/JS9/A99; Supplemental Digital Content 3, http://links.lww.com/JS9/A100; Supplemental Digital Content 4, http://links.lww.com/JS9/A101; Supplemental Digital Content 5, http://links.lww.com/JS9/A102). Figure 5 shows the cumulative probability curves for each outcome.
Figure 5.
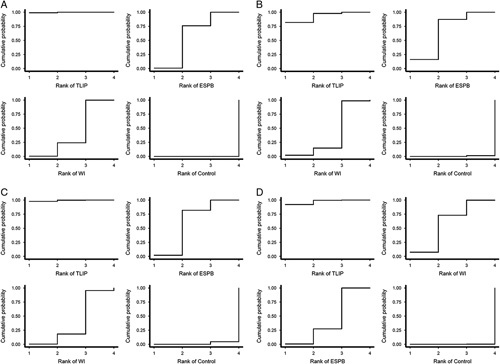
Cumulative probability curves of (A) opioid consumption (B) early postoperative period (up to 6 h), (C) middle postoperative period (6–18 h), and (D) late postoperative period (18–24 h). ESPB, erector spinae plane block; TLIP, thoracolumbar interfascial plane; WI, wound infiltration.
Exploratory subgroup analysis (opioid consumption)
The injection level of ESPB differed in each study. Additional analysis was performed by dividing the patients into fixed thoracic and lumbar levels, and surgical site levels. All three approach levels had significantly lower opioid consumption compared to the controls, but the effect size was the largest when injected according to the surgical level (MD=−12.2 mg; 95% CI: −15.4 to −9.0). There was no statistical difference between the surgical site level and lumbar level injection (MD=−5.0 mg; 95% CI: −10.7 to 0.6), but a significant difference was detected between the surgical site level and thoracic level injection (MD=−6.0 mg; 95% CI: −11.5 to −0.5) (Fig. 3C, page 17 of Supplementary Material 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A99).
The TLIP block was divided into medial planes, as in the original method, between the multifidus and longissimus, and lateral planes, as in the modified method, between the longissimus and the iliocostalis, according to the injection plane. Both planes showed a significant difference compared to the controls, and there was no difference according to the method employed (lateral plane: MD=−16.5 mg; 95% CI: −22.2 to −10.9; medial plane: MD=−10.9 mg; 95% CI: −15.0 to −6.8) (page 21 of Supplementary Materials 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A99).
Finally, when only surgical site injection of ESPB was included in NMA, there was no difference compared with TLIP block (MD=1.0 mg; 95% CI: −3.6 to 5.6) (Fig. 3D, page 24 of Supplementary Materials 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A99).
Discussion
Multiple regional analgesia techniques are used in clinical settings to improve postoperative pain management in lumbar spinal surgery, and our NMA not only demonstrated the potential benefits of these but also ranked them according to their efficacy55. When compared to systemic analgesia, all three regional analgesia techniques significantly reduced cumulative opioid consumption during the first 24 hafter surgery, and the TLIP block showed remarkable effectiveness in reducing opioid consumption.
The TLIP block used to block the dorsal rami of the thoracolumbar nerves was first described by Hand et al. 9 in 2015. The plane in the original technique is close to the surgical incision site, between the multifidus and longissmus; therefore, the process is modified by injecting between the longissimus and iliocostalis into the lateral plane10. Several studies report injecting into the original medial plane30,31,42,47, while modified planes have also been used in some studies29,34,36,37. In one study that directly compared two planes, a modified TLIP block had a shorter performance time, a higher success rate for a one-time block, and a similar analgesic effect compared with the classic TLIP block56. In our exploratory analysis, there was no difference in opioid consumption between the two planes when comparing indirect effects. More well-planned RCTs are required to clarify this issue further.
Although both techniques target the dorsal rami, and TLIP block is a slightly more superficial block than ESPB, TLIP block showed a statistically superior effect compared to ESPB in our analysis. Three reasons can be inferred from these results. First, the level of the injection is more important in the ESPB than in the TLIP block. Second, when ESPB is performed at the lumbar level, the injection point may vary according to the relatively large transverse processes. Third, the TLIP block is more suitable for multilevel analgesia because it is relatively easy to hydrodissect compared to the ESPB.
We performed a subgroup analysis of ESPB to determine the reason for the superiority of the TLIP block. ESPB may have different effects, depending on the injection level. Several studies injected at a fixed thoracic level40,41,47,51,54, or fixed lumbar level32,35,52,53. However, most surgeries perform the injection at the surgical level corresponding to the largest effect size38,43–46,48–50. In addition, injection at the surgical site level reduced opioid consumption compared to that at the fixed thoracic level, and this was statistically significant. It is thought that the results showed a smaller effect size for ESPB than for TLIP block due to injection level differences between the studies of ESPB. In this context, when only the surgical site injection of ESPB was included in the NMA, there was no difference compared with the TLIP block. In the studies on the TLIP blocks, injection was performed at the level of L-3, the location at which it was performed in all studies and as performed in the original paper. A direct comparison of ESPB and TLIP block was conducted by Wang et al. 47. It was not included in our analysis because the amount of opioid consumption 24 h postoperatively was unknown, but it can be seen that there is no difference between the two groups in terms of the number of PCA injections. Additionally, according to Ciftci et al.36, TLIP block and ESPB showed similar opioid consumption. Since the results of our analysis depend on indirect comparisons, more studies with direct comparisons are needed.
In a cadaveric study about ESPB, a fourth lumbar ESP injection had limited craniocaudal spread compared to injection in the thoracic region.57,58. In other words, the lumbar ESPB is localized at the injection level, and therefore it may be important to perform the injection according to the surgical level. The distance between the spinal process line and the neurovascular bundles is determined by the vertebral level, from 29 mm at L1 to 75 mm at L359. Therefore, the location of the injection on the large and long lumbar transverse process, determines whether the dorsal ramus is blocked or not.
The interfascial plane blocks are highly dependent on sufficient volumes of local anesthetics to spread between the muscle layers and fascial planes. The TLIP block is a plane block that separates two muscles, and such planes are easier to hydrodissect than planes between bone and muscle. Therefore, the TLIP block may be better in terms of multilevel hydrodissection.
All three techniques compared in our study showed superior results compared to the controls. However, statistically significant differences are not always clinically significant. A difference of 10 mg or more in parenteral morphine60 and a change of 10 mm on a 100 mm VAS are commonly accepted as clinically significant61. In both these respects, only TLIP block is suitable for lumbar spine surgery. However, even a small difference can have a different clinical meaning depending on the grade of the pain score at which it is effective. In our opinion, a change of ~1–2 points in pain scores in patients who had initially experienced moderate-to-severe pain should be considered a clinically significant difference; hence, a change in pain score from initial values of 4–5 points to less than 3 was defined as a clinically significant difference. Furthermore, a score of less than 33 points on the 100-point VAS scale is accepted as a state of well-controlled pain in clinical settings61.
Recently, two procedure-specific postoperative pain management (PROSPECT) guidelines for spine surgery have been published. TLIP block and ESPB have not yet been mentioned in laminectomy62 and are not recommended in complex spine surgery due to limited procedure-specific evidence63. They searched for studies published until 31 March 2020, for laminectomy, and April 2020, for complex spine surgery. Since many of the studies included in our NMA were published later, it seems that they were not properly reflected. Additionally, in a recently published NMA by Bae et al.64 including studies published until January 2021, fascial plane block (no distinction between TLIP and ESPB) showed no effect in reducing opioid usage at postoperative 24 h. However, only five studies were included in their analysis, and in addition, one of them focused on cervical surgery. In our study, the NMA included many studies published after 2021. Further, the distinction between TLIP block and ESPB is a differentiating point of our study.
This study has several potential limitations. First, the included studies were highly heterogeneous. Despite including only RCTs with patients who underwent lumbar spinal surgery, the concentration of local anesthetics, technical details, and nonopioid multimodal analgesia were not consistent. In addition, there are so many different types of lumbar spinal surgery. Multilevel open surgery and single-level scope surgery will understandably have different pain pathophysiology. Although most of the studies were performed in elective surgery, the information on revision surgery was not known. Therefore, further analysis is required to validate our findings with more elaborate evidence. Second, the time points at which pain scores were measured were not consistent among the RCTs and were not presented as accurate values. To reduce any bias, we divided the time period into three intervals and used the values corresponding to each interval as representative values. Lastly, ESPB and TLIP are currently developing techniques that may lead to possible publication bias. Moreover, it may be too early to draw conclusions from this analysis alone.
Conclusions
The results of the NMA reported here are significant because a comparison of regional analgesic techniques based on their efficacy can help improve postoperative pain management in lumbar spinal surgery. The TLIP block showed outstanding analgesic effects and a significant reduction in opioid consumption even when compared with ESPB and WI. However, given that a significant reduction in opioid consumption was seen with the three regional analgesic techniques evaluated, using any of these regional blocks after lumbar spinal surgery seems reasonable. Thus, more refined studies are needed to determine the optimal regional analgesia technique that can improve postoperative pain management after lumbar spinal surgery.
Ethical approval
Not required.
Sources of funding
This work was supported by research fund of National Research Foundation of Korea (NRF- 2022R1C1C1007982) and Chungnam National University.
Author contribution
B.H. and S.P.: conception and design. B.H. and S.P.: acquisition and data. B.H. and S.P.: analysis and interpretation of data. B.H. and S.B.: drafting of manuscript. S.B., H.K., C.O., Y.J., S.L., and S.P.: critical revision of the manuscript for important intellectual content. B.H., S.B., and S.P.: statistical analysis. B.H. and S.P.: obtaining funding. S.P.: administrative, technical, or material support. B.H. and S.P.: supervision.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: Prospero.
Unique identifying number or registration ID: CRD42022309271.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022309271
Guarantor
Seyeon Park, RN, PhD, Department of Nursing, College of Nursing, Chungnam National University, Jung-gu, Munhwa-ro 266, Daejeon 35015, South Korea. Tel: +82 425 808 329, Fax: +86 425 808 309. E-mail: park_sy@cnu.ac.kr
Data availability statement
The original data reported in this article are accessible in the manuscript/supplementary materials; further queries may be sent to the corresponding author.
Supplementary Material
Footnotes
B.H. and S.B. contributed equally to this work as co-first author.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 13 March 2023
Contributor Information
Boohwi Hong, Email: koho0127@gmail.com.
Sujin Baek, Email: iamsuza@cnuh.co.kr.
Hyemin Kang, Email: man4ok2017@naver.com.
Chahyun Oh, Email: 5chahyun@naver.com.
Yumin Jo, Email: lemonny87@naver.com.
Soomin Lee, Email: bimily0526@naver.com.
Seyeon Park, Email: park_sy@cnu.ac.kr.
References
- 1. Yang MMH, Riva-Cambrin J, Cunningham J, et al. Development and validation of a clinical prediction score for poor postoperative pain control following elective spine surgery. J Neurosurg Spine 2021;34:3–12. [DOI] [PubMed] [Google Scholar]
- 2. Grotle M, Smastuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 2019;9:e028743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang S, Brennan TJ. Mechanisms of postoperative pain. Anesth Pain Med 2016;11:236–248. [Google Scholar]
- 4. Kjaergaard M, Moiniche S, Olsen KS. Wound infiltration with local anesthetics for post-operative pain relief in lumbar spine surgery: a systematic review. Acta Anaesthesiol Scand 2012;56:282–290. [DOI] [PubMed] [Google Scholar]
- 5. Brandao J, Graca R, Sa M, et al. Lumbar erector spinae plane block: successful control of acute pain after lumbar spine surgery – a clinical report. Rev Esp Anestesiol Reanim 2019;66:167–171. [DOI] [PubMed] [Google Scholar]
- 6. Melvin JP, Schrot RJ, Chu GM, et al. Low thoracic erector spinae plane block for perioperative analgesia in lumbosacral spine surgery: a case series. Can J Anaesth 2018;65:1057–1065. [DOI] [PubMed] [Google Scholar]
- 7. Liang X, Zhou W, Fan Y. Erector spinae plane block for spinal surgery: a systematic review and meta-analysis. Korean J Pain 2021;34:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farag E, Seif J. Thoracolumbar interfascial block (TLIP): a new technique of interfascial plane blocks. J Clin Anesth 2020;61:109640. [DOI] [PubMed] [Google Scholar]
- 9. Hand WR, Taylor JM, Harvey NR, et al. Thoracolumbar interfascial plane (TLIP) block: a pilot study in volunteers. Can J Anaesth 2015;62:1196–1200. [DOI] [PubMed] [Google Scholar]
- 10. Ahiskalioglu A, Yayik AM, Alici HA. Ultrasound-guided lateral thoracolumbar interfascial plane (TLIP) block: description of new modified technique. J Clin Anesth 2017;40:62. [DOI] [PubMed] [Google Scholar]
- 11. Ahn E, Kang H. Concepts and emerging issues of network meta-analysis. Korean J Anesthesiol 2021;74:371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grape S, El-Boghdadly K, Albrecht E. Analgesic efficacy of PECS vs paravertebral blocks after radical mastectomy: a systematic review, meta-analysis and trial sequential analysis. J Clin Anesth 2020;63:109745. [DOI] [PubMed] [Google Scholar]
- 15. Grape S, Jaunin E, El-Boghdadly K, et al. Analgesic efficacy of PECS and serratus plane blocks after breast surgery: a systematic review, meta-analysis and trial sequential analysis. J Clin Anesth 2020;63:109744. [DOI] [PubMed] [Google Scholar]
- 16. Shim SR, Kim SJ, Lee J, et al. Network meta-analysis: application and practice using R software. Epidemiol Health 2019;41:e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harrer M, Cuijpers P, Furukawa TA, et al. Doing Meta-Analysis With R: A Hands-On Guide, 1st ed. Chapman & Hall/CRC Press; 2021. [Google Scholar]
- 18. Jackson D, Barrett JK, Rice S, et al. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med 2014;33:3639–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rucker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JP, Altman DG, Gotzsche PC, et al. G Cochrane Bias Methods, G. Cochrane Statistical Methods, The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Milligan KR, Macafee AL, Fogarty DJ, et al. Intraoperative bupivacaine diminishes pain after lumbar discectomy. A randomised double-blind study. J Bone Joint Surg Br 1993;75:769–771. [DOI] [PubMed] [Google Scholar]
- 22. Mack PF, Hass D, Lavyne MH, et al. Postoperative narcotic requirement after microscopic lumbar discectomy is not affected by intraoperative ketorolac or bupivacaine. Spine (Phila Pa 1976) 2001;26:658–661. [DOI] [PubMed] [Google Scholar]
- 23. Mirzai H, Tekin I, Alincak H. Perioperative use of corticosteroid and bupivacaine combination in lumbar disc surgery: a randomized controlled trial. Spine 2002;27:343–346. [DOI] [PubMed] [Google Scholar]
- 24. Yorukoglu D, Ates Y, Temiz H, et al. Comparison of low-dose intrathecal and epidural morphine and bupivacaine infiltration for postoperative pain control after surgery for lumbar disc disease. J Neurosurg Anesthesiol 2005;17:129–133. [DOI] [PubMed] [Google Scholar]
- 25. Ersayli DT, Gurbet A, Bekar A, et al. Effects of perioperatively administered bupivacaine and bupivacaine-methylprednisolone on pain after lumbar discectomy. Spine (Phila Pa 1976) 2006;31:2221–2226. [DOI] [PubMed] [Google Scholar]
- 26. Esmail F, Mohammad-Reza F, Homayoon T. Preemptive analgesia with local lidocaine infiltration for single-level open disc operation. Pak J Biol Sci 2008;11:1868–1871. [DOI] [PubMed] [Google Scholar]
- 27. Gurbet A, Bekar A, Bilgin H, et al. Pre-emptive infiltration of levobupivacaine is superior to at-closure administration in lumbar laminectomy patients. Eur Spine J 2008;17:1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozyilmaz K, Ayoglu H, Okyay RD, et al. Postoperative analgesic effects of wound infiltration with tramadol and levobupivacaine in lumbar disk surgeries. J Neurosurg Anesthesiol 2012;24:331–335. [DOI] [PubMed] [Google Scholar]
- 29. Ahiskalioglu A, Yayik AM, Doymus O, et al. Efficacy of ultrasound-guided modified thoracolumbar interfascial plane block for postoperative analgesia after spinal surgery: a randomized-controlled trial. Can J Anaesth 2018;65:603–604. [DOI] [PubMed] [Google Scholar]
- 30. Ammar MA, Taeimah M. Evaluation of thoracolumbar interfascial plane block for postoperative analgesia after herniated lumbar disc surgery: a randomized clinical trial. Saudi J Anaesth 2018;12:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen K, Wang L, Ning M, et al. Evaluation of ultrasound-guided lateral thoracolumbar interfascial plane block for postoperative analgesia in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. PeerJ 2019;7:e7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El Ghamry MR, Elgebaly AS, Anwar AG, et al. Ultrasound-guided erector spinae plane block for acute pain management in patients undergoing posterior lumbar interbody fusion under general anaesthesia. South Afr J Anaesth Analg 2019;25:26–31. [Google Scholar]
- 33. Mohta M, Rani A, Sethi AK, et al. Efficacy of local wound infiltration analgesia with ropivacaine and dexmedetomidine in tubercular spine surgery – a pilot randomised double-blind controlled trial. Indian J Anaesth 2019;63:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ozmen O, Ince I, Aksoy M, et al. The effect of the modified thoracolumbar interfacial nerve plane block on postoperative analgesia and healing quality in patients undergoing lumbar disk surgery: a prospective, randomized study. Medeni Med J 2019;34:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yayik AM, Cesur S, Ozturk F, et al. Postoperative analgesic efficacy of the ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal decompression surgery: a randomized controlled study. World Neurosurg 2019;126:e779–e785. [DOI] [PubMed] [Google Scholar]
- 36. Ciftci B, Ekinci M, Celik EC, et al. Ultrasound-guided erector spinae plane block versus modified-thoracolumbar interfascial plane block for lumbar discectomy surgery: a randomized, controlled study. World Neurosurg 2020;144:e849–e855. [DOI] [PubMed] [Google Scholar]
- 37. Ekinci M, Çiftçi B, Çelik EC, et al. A comparison of the ultrasound-guided modified-thoracolumbar interfascial plane block and wound infiltration for postoperative pain management in lumbar spinal surgery patients. Agri 2020;32:140–146. [DOI] [PubMed] [Google Scholar]
- 38. Eskin MB, Ceylan A, Özhan MÖ, et al. Ultrasound-guided erector spinae block versus mid-transverse process to pleura block for postoperative analgesia in lumbar spinal surgery. Anaesthesist 2020;69:742–750. [DOI] [PubMed] [Google Scholar]
- 39. Kraiwattanapong C, Arnuntasupakul V, Kantawan R, et al. Effect of multimodal drugs infiltration on postoperative pain in split laminectomy of lumbar spine: a randomized controlled trial. Spine (Phila Pa 1976) 2020;45:1687–1695. [DOI] [PubMed] [Google Scholar]
- 40. Singh S, Choudhary NK, Lalin D, et al. Bilateral ultrasound-guided erector spinae plane block for postoperative analgesia in lumbar spine surgery: a randomized control trial. J Neurosurg Anesthesiol 2020;32:330–334. [DOI] [PubMed] [Google Scholar]
- 41. Zhang TJ, Zhang JJ, Qu ZY, et al. Bilateral erector spinae plane blocks for open posterior lumbar surgery. J Pain Res 2020;13:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eltaher E, Nasr N, Abuelnaga ME, et al. Effect of ultrasound-guided thoracolumbar interfascial plane block on the analgesic requirements in patients undergoing lumbar spine surgery under general anesthesia: a randomized controlled trial. J Pain Res 2021;14:3465–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Finnerty D, Ni Eochagain A, Ahmed M, et al. A randomised trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia 2021;76:1499–1503. [DOI] [PubMed] [Google Scholar]
- 44. Goel VK, Chandramohan M, Murugan C, et al. Clinical efficacy of ultrasound guided bilateral erector spinae block for single-level lumbar fusion surgery: a prospective, randomized, case-control study. Spine J 2021;21:1873–1880. [DOI] [PubMed] [Google Scholar]
- 45. Jin Y, Zhao S, Cai J, et al. Erector spinae plane block for perioperative pain control and short-term outcomes in lumbar laminoplasty: a randomized clinical trial. J Pain Res 2021;14:2717–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wahdan AS, Radwan TA, Mohammed MM, et al. Effect of bilateral ultrasound-guided erector spinae blocks on postoperative pain and opioid use after lumbar spine surgery: a prospective randomized controlled trial. Egypt J Anaesth 2021;37:100–106. [Google Scholar]
- 47. Wang L, Wu Y, Dou L, et al. Comparison of two ultrasound-guided plane blocks for pain and postoperative opioid requirement in lumbar spine fusion surgery: a prospective, randomized, and controlled clinical trial. Pain Ther 2021;10:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yeşiltaş S, Abdallah A, Uysal Ö, et al. The efficacy of intraoperative freehand erector spinae plane block in lumbar spondylolisthesis: a randomized controlled study. Spine (Phila Pa 1976) 2021;46:E902–E910. [DOI] [PubMed] [Google Scholar]
- 49. Yorukoglu HU, Icli D, Aksu C, et al. Erector spinae block for postoperative pain management in lumbar disc hernia repair. J Anesth 2021;35:420–425. [DOI] [PubMed] [Google Scholar]
- 50. Yu Y, Wang M, Ying H, et al. The analgesic efficacy of erector spinae plane blocks in patients undergoing posterior lumbar spinal surgery for lumbar fracture. World Neurosurg 2021;147:e1–e7. [DOI] [PubMed] [Google Scholar]
- 51. Zhang JJ, Zhang TJ, Qu ZY, et al. Erector spinae plane block at lower thoracic level for analgesia in lumbar spine surgery: a randomized controlled trial. World J Clin Cases 2021;9:5126–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang Q, Wu Y, Ren F, et al. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth 2021;68:110090. [DOI] [PubMed] [Google Scholar]
- 53. Zhu L, Wang M, Wang X, et al. Changes of opioid consumption after lumbar fusion using ultrasound-guided lumbar erector spinae plane block: a randomized controlled trial. Pain Physician 2021;24:E161–E168. [PubMed] [Google Scholar]
- 54. Asar S, Sari S, Altinpulluk EY, et al. Efficacy of erector spinae plane block on postoperative pain in patients undergoing lumbar spine surgery. Eur Spine J 2022;31:197–204. [DOI] [PubMed] [Google Scholar]
- 55. Yang A, Pechlivanoglou P, Aoyama K. Interpreting and assessing confidence in network meta-analysis results: an introduction for clinicians. J Anesth 2022;36:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Çiftçi B, Ekinci M. A prospective and randomized trial comparing modified and classical techniques of ultrasound-guided thoracolumbar interfascial plane block. Agri 2020;32:186–192. [DOI] [PubMed] [Google Scholar]
- 57. Harbell MW, Seamans DP, Koyyalamudi V, et al. Evaluating the extent of lumbar erector spinae plane block: an anatomical study. Reg Anesth Pain Med 2020;45:640–644. [DOI] [PubMed] [Google Scholar]
- 58. De Lara González SJ, Pomés J, Prats-Galino A, et al. Anatomical description of anaesthetic spread after deep erector spinae block at L-4. Rev Esp Anestesiol Reanim 2019;66:409–416. [DOI] [PubMed] [Google Scholar]
- 59. Creze M, Soubeyrand M, Nyangoh Timoh K, et al. Organization of the fascia and aponeurosis in the lumbar paraspinal compartment. Surg Radiol Anat 2018;40:1231–1242. [DOI] [PubMed] [Google Scholar]
- 60. Hussain N, Brull R, Noble J, et al. Statistically significant but clinically unimportant: a systematic review and meta-analysis of the analgesic benefits of erector spinae plane block following breast cancer surgery. Reg Anesth Pain Med 2021;46:3–12. [DOI] [PubMed] [Google Scholar]
- 61. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth 2017;118:424–429. [DOI] [PubMed] [Google Scholar]
- 62. Peene L, Le Cacheux P, Sauter AR, Joshi GP, Beloeil H. P.W.G. Collaborators. A. European Society of Regional, Pain management after laminectomy: a systematic review and procedure-specific post-operative pain management (prospect) recommendations. Eur Spine J 2021;30:2925–2935. [DOI] [PubMed] [Google Scholar]
- 63. Waelkens P, Alsabbagh E, Sauter A, et al. Pain management after complex spine surgery: a systematic review and procedure-specific postoperative pain management recommendations. Eur J Anaesthesiol 2021;38:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bae S, Alboog A, Esquivel KS, et al. Efficacy of perioperative pharmacological and regional pain interventions in adult spine surgery: a network meta-analysis and systematic review of randomised controlled trials. Br J Anaesth 2022;128:98–117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data reported in this article are accessible in the manuscript/supplementary materials; further queries may be sent to the corresponding author.


