Background:
Acute kidney injury (AKI) occurs commonly after major surgery and is correlated with increased in-hospital morbidity and mortality. There is no consensus on whether intraoperative oliguria affects postoperative AKI. We conducted a meta-analysis to systematically assess the correlation of intraoperative oliguria with postoperative AKI.
Methods:
PubMed, Embase, Web of Science, and Cochrane Library databases were searched to identify reports on the relationship between intraoperative oliguria and postoperative AKI. Quality was assessed using the Newcastle–Ottawa Scale. The primary outcomes were the unadjusted and multivariate-adjusted odds ratios (ORs) for intraoperative oliguria to correlate with postoperative AKI. The secondary outcomes included intraoperative urine output in the AKI and non-AKI groups, the demand for postoperative renal replacement therapy (RRT), in-hospital mortality, and length of hospital stay in the oliguria and non-oliguria groups.
Results:
Nine eligible studies with 18 473 patients were included. The meta-analysis revealed that patients with intraoperative oliguria had a considerably greater risk of postoperative AKI (unadjusted OR: 2.03, 95% CI: 1.60–2.58, I 2=63%, P<0.00001; multivariate-adjusted OR: 2.00, 95% CI: 1.64–2.44, I 2=40%, P<0.00001). Further subgroup analysis did not find differences between different oliguria criteria or surgical types. Furthermore, the AKI group’s pooled intraoperative urine output was less (mean differences: −0.16, 95% CI: −0.26 to −0.07, P<0.001). Intraoperative oliguria was associated with increased demand for postoperative RRT (risk ratios: 4.71, 95% CI: 2.83–7.84, P<0.001) and in-hospital mortality (risk ratios: 1.83, 95% CI: 1.24–2.69, P=0.002), but not with prolonged length of hospital stay (mean differences: 0.55, 95% CI: −0.27 to 1.38, P=0.19).
Conclusions:
Intraoperative oliguria was significantly associated with a higher incidence of postoperative AKI, as well as increased in-hospital mortality and demand for postoperative RRT, but not with prolonged hospitalization.
Keywords: acute kidney injury, complications, meta-analysis, oliguria, renal, urine output
Introduction
Highlights
There is no consensus on whether intraoperative oliguria affects postoperative acute kidney injury (AKI).
Intraoperative oliguria was significantly associated with a higher incidence of postoperative AKI.
Intraoperative oliguria has important clinical significance as a risk factor for postoperative AKI that can be recognized early.
Postoperative acute kidney injury (AKI) occurs frequently and carries serious consequences, affecting 13.4% of patients undergoing major surgery and is associated with a sixfold increase in-hospital mortality1–3, as well as an increased incidence of other complications, length of hospital stay (LOS), and hospitalization costs4,5. Unfortunately, although there are numerous beneficial treatments proven to reduce the risk of developing AKI in experimental animals, no widely available therapeutic strategies were found for patients in clinical practice6,7.
The treatment of postoperative AKI remains supportive, making prevention of the AKI critical. Typically, strategies to prevent AKI include early recognition of individuals at risk for AKI after surgery and optimizing their clinical status8. Several baseline factors (e.g. hypertension, elderly patients, male sex, obesity, diabetes) and perioperative factors (e.g. hypotension, systemic inflammation, fluid overload, venous congestion, nephrotoxic drugs) for postoperative AKI have been identified, which may aid in the identification of high-risk individuals with postoperative AKI9–12.
Urine output (UO) is an essential indicator of renal perfusion, intraoperative oliguria is generally caused by reduced intravascular flow or sustained general hypoperfusion13,14. Both conditions are thought to result in a reduction in renal perfusion and lead to decreased filtration load and UO, increasing the risk of postoperative AKI15. Meanwhile, intraoperative UO can be obtained efficiently and instantly at no additional cost to the patient, which can be considered a convenient and valuable indicator in forecasting the development of postoperative AKI16. However, contradictory results have been reported in studies examining the effect of predicting postoperative AKI of intraoperative oliguria. Some studies16–22 indicated that intraoperative oliguria raised the risk of postoperative AKI. In contrast, the remaining studies9,23–27 did not report this association. Each element cannot be clarified by a single study, and diverse research methodologies may lead to selection bias.
Therefore, the primary goal of this meta-analysis was to assess the association of intraoperative oliguria with postoperative AKI. In addition, we explored intraoperative UO in the AKI and non-AKI groups, the relationship between intraoperative oliguria and the demand for postoperative renal replacement therapy (RRT), in-hospital mortality, and LOS. This project hopes to raise consciousness about the role of intraoperative oliguria in developing AKI.
Materials and methods
This systematic review and meta-analysis was registered on the PROSPERO (Prospective Register of Systematic Reviews), protocol number CRD42022326281, and reported in accordance with PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses)28. A PRISMA checklist is available as a supplement (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/JS9/A96).
Data collection and retrieval strategies
The databases listed below were used to look for relevant research: PubMed, Embase, Web of Science, and Cochrane library, with a search time frame of database creation to June 2022. Medical Subject Headings (MeSH) or keywords ‘acute kidney injury,’ ‘oliguria,’ and ‘intraoperative period’ were used to search the literature without language restrictions. The specific search strategy is shown in the supplementary material (Supplementary Table 2, Supplemental Digital Content 1, http://links.lww.com/JS9/A96). In addition, we meticulously combed through the references of all included studies and pertinent review articles for possibly qualifying research.
Selection criteria
Studies that met the following criteria were included: observational studies; participants were adults who received noncardiac surgery; studies that used a clear definition of oliguria and AKI; studies that provided data on the correlation of intraoperative oliguria with postoperative AKI.
Studies with any of the following conditions were excluded: the type of study design was not described; participants underwent urological or transplantation procedures; literature that is duplicated or from which data cannot be extracted; the full text was not available.
Literature screening and data extraction
Two reviewers (Pang and Liang) screened all retrieved literature independently in strict adherence to predefined inclusion and exclusion criteria. First of all, an initial screening was conducted by reviewing the titles and abstracts of all retrieved studies. After excluding studies that were duplicates or did not match the inclusion criteria, the remaining studies were analyzed, and all potentially eligible studies were identified by reading the full text.
Additionally, the following details were retrieved from each eligible study: first author, country, publication year, study design, sample size, oliguria criteria, diagnostic criteria of AKI, the incidence of AKI, and outcome assessment. The primary outcomes were the unadjusted and multivariate-adjusted odds ratios (ORs) of intraoperative oliguria predicting the incidence of postoperative AKI. Besides, the secondary outcomes included intraoperative UO in the AKI and non-AKI groups, the demand for postoperative RRT, in-hospital mortality, and LOS in oliguria and non-oliguria groups. The extracted information was entered into a predesigned literature data extraction form for data generalization and analysis. All discrepancies were resolved through discussions with a third investigator.
Methodology and literature quality evaluation
To measure the risk of bias, we used the Newcastle–Ottawa Scale29, which consists of three parts: study population selection, comparability between groups, and outcomes. Out of a total score of 9, we awarded scores of 0–3, 4–6, and 7–9 for studies of the low, medium, and high quality. If the score was less than 7, the literature was excluded. Two reviewers (Pang and Liang) performed the quality assessment independently. In addition, the above two reviewers followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach and independently used online software (https://gdt.gradepro.org/app) to evaluate the certainty of the evidence. Each outcome was assessed for the risk of bias, inconsistency, indirectness, imprecision, and other bias and the evidence was classified as very low, low, moderate, and high.
Statistical analysis
We used RevMan 5.4.1 software and STATA 14 software to process the data for the meta-analysis. The heterogeneity of the included studies was analyzed using the Q statistic for χ 2 test (test level: α=0.1), while the I 2 value was applied to identify the heterogeneity level. Suppose there was statistical heterogeneity (P<0.1 or I 2≥40%), the random-effects model was adopted for pooled effect size analysis; otherwise (P≥0.1 and I 2<40%), the fixed-effects model was adopted for pooled effect size analysis. For the primary outcome, the strength of the correlation between intraoperative oliguria and postoperative AKI was estimated primarily by combining unadjusted and adjusted OR values, respectively. It was reported as an OR value of 95% CI. In addition, for secondary outcomes (intraoperative urine volume, postoperative RRT demand rate, in-hospital mortality, and LOS), we calculated mean differences (MDs) with 95% CI for continuous variables and risk ratios (RRs) with 95% CI for dichotomous variables. Moreover, we converted the median and interquartile range (IQR) of continuous data to mean and SD using the formula provided by Luo et al.30 and Wan et al.31. Publication bias was tested using a visual funnel plot and assessed by the Egger test. A significant statistical difference was considered as P less than 0.05.
Furthermore, the correlation of intraoperative oliguria with postoperative AKI was analyzed by subgroup analysis: different oliguria criteria (UO<0.5 or 0.3 ml/kg/h); different types of surgery (abdominal or thoracic surgery).
Trial sequential analysis (TSA)
To assess the credibility of our primary outcome, we used TSA viewer (version 0.9.5.10 beta) to perform TSA. TSA based on the O’Brien–Fleming method to calculate α-spending boundaries. The required information size that estimated sample size was calculated based on a two-sided random-effects model, with type I and type II error set as 5 and 20%, respectively.
Results
Literature retrieval and screening
The study screening process is shown in Figure 1. Based on the above retrieval strategy for electronic database search, 205 studies were initially identified. In the initial screening stage, 70 studies remained after eliminating duplicates and studies that did not match the inclusion criteria by reviewing the titles and abstracts of all retrieved studies. Then full-text articles were read for re-screening to confirm all potentially eligible studies. Finally, our meta-analysis comprised nine studies16–22,26,27.
Figure 1.
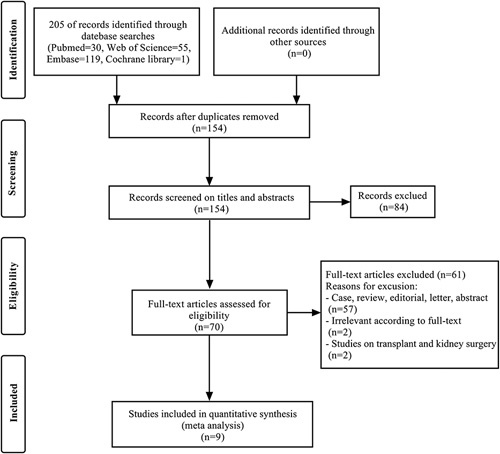
Flow chart of study screening.
Study characteristics and quality assessment
Table 1 summarizes the main characteristics of the included eligible studies. All nine studies were observational studies published between 2013 and 2021, with sample sizes ranging from 153 to 5894. In addition, two of the included study used more than one criterion to define intraoperative oliguria. Meng and Mu20 defined oliguria as UO less than 0.8 or 0.5 ml/kg/h, respectively, while Zhao et al.22 described oliguria as UO less than 0.5 or 0.3 ml/kg/h, respectively, each of these two studies yielded corresponding ORs for the different intraoperative oliguria definitions to predict the incidence of postoperative AKI. Therefore, 11 outcome effect quantities were extracted and combined for the nine included studies.
Table 1.
Main characteristics of the included studies.
| References, year | Country | Study design | Sample size | Surgical type | Oliguria criteria | AKI criteria | Incidence of AKI, N (%) | ORs (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Slankamenac et al.17, 2013 | Switzerland | Single-center, retrospective cohort study | 549 | Liver surgery | UO<400 ml/24 h | RIFLE criteria | 82 (14.9) | 2.14 (1.29–3.55) |
| Goren et al.26, 2017 | Israel | Single-center, retrospective study of prospectively collected data | 153 | Open pancreatic surgery | UO<0.5 ml/kg/h | AKIN criteria | 15 (9.8) | 1.90 (0.21–17.4) |
| Mizota et al.18, 2017 | Japan | Single-center, retrospective cohort study | 3560 | Major abdominal surgery | UO<0.3 ml/kg/h | KDIGO criteria | 224 (6.3) | 1.82 (1.28–2.60) |
| Shiba et al.16, 2018 | Japan | Single-center, retrospective cohort study | 5894 | Noncardiac major surgery | UO<0.5 ml/kg/h, lasting ≥120 min | RIFLE criteria | 430 (7.3) | 2.96 (2.38–3.69) |
| Myles et al.19, 2019 | International | Multicenter, post hoc analysis of prospectively collected data | 2444 | Major abdominal surgery | UO<0.5 ml/kg/h, lasting ≥60 min | KDIGO criteria | 513 (21.0) | 1.52 (1.24–1.85) |
| Inacio et al.27, 2021 | Portugal | Single-center, retrospective cohort study | 165 | Major abdominal surgery | UO<0.5 ml/kg/h | KDIGO criteria | 32 (19.4) | 0.87 (0.33–2.31) |
| Meng and Mu20, 2020 | China | Single-center, retrospective cohort study | 1393 | Lung surgery | UO<0.8 ml/kg/h | KDIGO criteria | 31 (2.2) | 2.77 (1.36–5.67) |
| Meng and Mu20, 2020 | China | Single-center, retrospective cohort study | 1393 | Lung surgery | UO<0.5 ml/kg/h | KDIGO criteria | 31 (2.2) | 1.51 (0.52–4.37) |
| Shim et al.21, 2020 | Korea | Single-center, retrospective matched cohort study | 453 | Laparoscopic colorectal resection | UO<0.5 ml/kg/h | KDIGO criteria | 79 (17.4) | 2.84 (1.44–5.63) |
| Zhao et al.22, 2021 | China | Single-center, retrospective cohort study | 3862 | Pulmonary resection or esophagectomy | UO<0.5 ml/kg/h | KDIGO criteria | 205 (5.3) | 1.60 (1.03–2.49) |
| Zhao et al.22, 2021 | China | Single-center, retrospective cohort study | 3862 | Pulmonary resection or esophagectomy | UO<0.3 ml/kg/h | KDIGO criteria | 205 (5.3) | 2.85 (1.53–5.33) |
AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease Improving Global Guidelines; ORs, odds ratios; RIFLE, Risk, Injury, Failure, Loss and End-stage kidney disease; UO, urine output.
Supplementary Table 3, Supplemental Digital Content 1, http://links.lww.com/JS9/A96 summarizes the details of the quality assessment of each included eligible study using the Newcastle–Ottawa Scale, with an average score of 7.6. Each study scored at least 7, and one of the studies had a maximum score of 9, indicating that the quality of all included studies was high.
Association of intraoperative oliguria with the incidence of postoperative AKI
All 11 outcome effect quantities in the nine studies involved postoperative AKI, and the total number of included study subjects was 18 473. The pooled incidence of AKI in the oliguria and non-oliguria groups was 13% (95% CI: 8–19%) and 8% (95% CI: 5–10%), respectively (Fig. 2A). Meta-analysis results are shown in Figure 3. Because of the statistical heterogeneity among studies (I 2=63%), a random-effects model was applied for analysis, which showed a combined unadjusted OR value was 2.03 (95% CI: 1.60–2.58, P<0.00001), indicating that intraoperative oliguria significantly related to a higher risk of postoperative AKI. The funnel plot (Supplementary Figure 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A97) and Egger test (P=0.925) showed no significant publication bias. Besides, the association remained significant in the sensitivity analysis of the combined adjusted nine outcome effect quantities, with a combined adjusted OR value of 2.00 (95% CI: 1.64–2.44, P<0.00001) (Fig. 4). TSA showed that the cumulative Z-curve across the conventional boundary (Z=1.96) and monitoring boundary, and reached the required information size. Therefore, current result was reliable and conclusive (Supplementary Figure 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A97).
Figure 2.
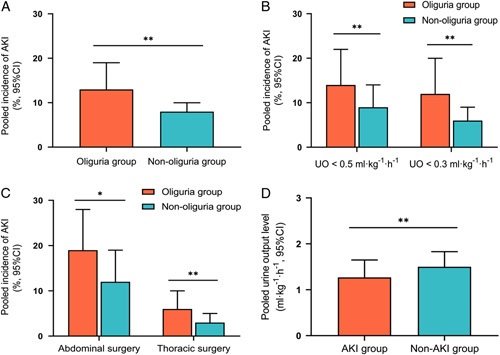
Intraoperative oliguria and postoperative acute kidney injury (AKI). (A) The pooled incidence of postoperative AKI in the oliguric and non-oliguric group; (B) subgroup analysis of different oliguria criteria; (C) subgroup analysis of different surgical types; (D) the pooled intraoperative urine output level in the AKI and non-AKI group; *P<0.01 and **P<0.001.
Figure 3.
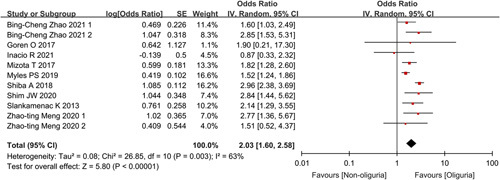
Forest plot showing combined unadjusted odds ratios for the association of intraoperative oliguria with postoperative acute kidney injury.
Figure 4.
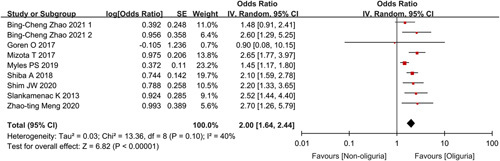
Forest plot showing combined multivariate-adjusted odds ratios for the association of intraoperative oliguria with postoperative acute kidney injury.
Subgroup analysis
In the subgroup analysis based on different oliguria criteria, there was significant heterogeneity between the two subgroups (UO<0.5 or 0.3 ml/kg/h) (test for subgroup difference: P=0.04 and I 2=75.8%) (Fig. 5), implying that the choice of oliguria criteria significantly influenced the results of the meta-analysis. Taking UO less than 0.5 ml/kg/h as the oliguria standard, the pooled incidence of AKI in the oliguria group and non-oliguria group was 14% (95% CI: 8–22%) and 9% (95% CI: 5–14%), respectively (OR: 1.72, 95% CI: 1.39–2.14, P<0.00001). Secondly, with UO less than 0.3 ml/kg/h as the oliguria standard, the pooled incidence of AKI was 11% (95% CI: 8–14%) and 5% (95% CI: 5–6%), respectively (OR: 2.64, 95% CI: 1.86–3.74, P<0.00001) (Figs. 2B, 5).
Figure 5.
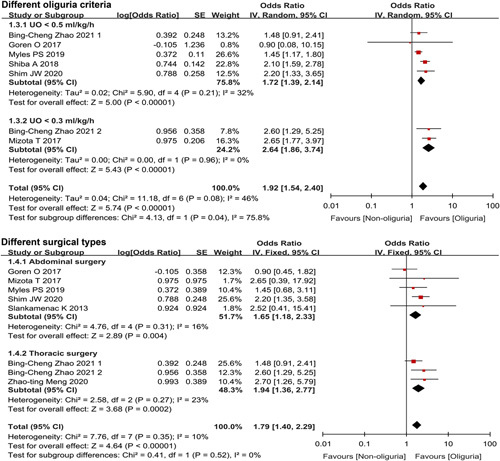
Forest plot of the effect of intraoperative oliguria on the incidence of postoperative acute kidney injury in the subgroup analysis of different oliguria criteria and surgical types.
The results of subgroup analysis based on different types of surgery showed, after abdominal surgery, the pooled incidence of AKI in the oliguria and non-oliguria groups was 19% (95% CI: 12–28%) and 12% (95% CI: 7–19%), respectively (OR: 1.67, 95% CI: 1.18–2.33, P=0.004). After thoracic surgery, the pooled incidence of AKI was 6% (95% CI: 3–10%) and 3% (95% CI: 2–5%), respectively (OR: 1.94, 95% CI: 1.36–2.77, P<0.001) (Figs. 2C, 5).
Intraoperative urine volume levels in the AKI and non-AKI groups
Five studies18,20,22,26,27 reported differences in intraoperative urine volume between the AKI and non-AKI groups. In the AKI groups, the pooled postoperative UO level of 1.27 ml/kg/h (95% CI: 0.88–1.65) was substantially less than that of 1.50 ml/kg/h (95% CI: 1.18–1.83) in non-AKI groups (MD: −0.16, 95% CI: −0.26 to −0.07, P<0.001), and the difference showed statistical significance (Fig. 2D).
Association of intraoperative oliguria with in-hospital mortality, LOS, and postoperative RRT requirement
Three studies16,19,27 reported postoperative RRT requirements of 8503 patients, with a higher value in the oliguria group compared to the non-oliguria group (RR: 4.71, 95% CI: 2.83–7.84, P<0.001). Two studies16,27 from the evaluation of 6059 patients showed that in-hospital mortality of the oliguria group was substantially greater than the non-oliguria group (RR: 1.83, 95% CI: 1.24–2.69, P=0.002). A total of 8663 patients from four studies reported LOS (in days)16,19,21,27, noticing that the time of the oliguria group was longer than the non-oliguria group. Still, the difference was slight and not statistically significant (MD: 0.55, 95% CI: −0.27 to 1.38, P=0.19).
GRADE certainty of evidence
The GRADE evidence profile was presented in Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/JS9/A96. The certainty of evidence was low for UO and LOS, moderate for the incidence of postoperative AKI, demand for postoperative RRT, and in-hospital mortality.
Discussion
In this systematic review and meta-analysis, we assessed the correlation of intraoperative oliguria with postoperative AKI, and the combined results suggested that intraoperative oliguria significantly related to a higher risk of postoperative AKI, but the strength of this association was weak. TSA analyses showed that there was enough information to confirm the results. Further subgroup analysis did not find differences between different oliguria criteria or surgical types. Moreover, the combined results showed that AKI individuals had considerably lower intraoperative urine volume. Patients with intraoperative oliguria were accompanied with increased in-hospital mortality and demand for postoperative RRT. Still, without prolonging LOS.
Our meta-analysis revealed that the occurrence of postoperative AKI for individuals who suffered intraoperative oliguria was substantially greater, consistent with previous research16–22. The underlying reasons are analyzed as follows. Firstly, UO is an important index to reflect organ perfusion. Intravascular hypovolemia and prolonged hypotension contribute to reduced perioperative renal perfusion and elevate the risk of postoperative AKI15,32,33. The compensatory response of the kidney at this time is the dilatation of the small afferent arteries and the vasoconstriction of the small efferent arteries to sustain glomerular filtration. Simultaneously, renin–angiotensin system mobilization leads to water and sodium ions storage, excretion of potassium ions, and eventually results in low UO34–37. Secondly, oliguria may indicate that early renal function has been compromised, preceding the rise in creatinine37–39. In such cases, the kidney cannot provide adequate sodium urine, which may be caused by impaired glomerular filtration, primary urine caused by renal tubule leakage back to the interstitium of necrotic units, renal tubular obstruction, or renin–angiotensin system excitement40. Therefore, patients with intraoperative oliguria may have already developed subclinical renal dysfunction, making them more likely to progress to AKI postoperatively.
Additionally, the combined ORs of the meta-analysis showed a weak association (moderate heterogeneity) between intraoperative oliguria and postoperative AKI. The possible causes are analyzed as follows. Firstly, the results of the nine included original studies are inconsistent. Seven of the included studies showed a weak correlation16–22, while the other two studies did not support that intraoperative oliguria was a risk factor for AKI26,27, which further reduced the effective size of the combined meta-analysis and weakened the association. Secondly, the occurrence of oliguria does not always mean a decrease in renal perfusion pressure or glomerular perfusion. UO is also influenced by many nonrenal factors, including hemodynamics, stress, sympathetic tension, aldosterone, cardiac natriuretic hormone, antidiuretic hormone, etc41,42. Finally, intraoperative oliguria is only one of many perioperative risk factors for postoperative AKI, and the multifaceted etiology of AKI may undermine the role of oliguria. Several baseline risk factors (e.g. elderly, hypertension, diabetes) and perioperative factors contributing to postoperative AKI (e.g. systemic inflammation, hemodynamic changes, nephrotoxins) have been recognized, which can help identify patients at risk of postoperative AKI9–12. Overall, this weak association reminds us that intraoperative oliguria should not be used as a single predictor or screening indicator for the development of postoperative AKI, but intraoperative oliguria still has important clinical significance because it is an avoidable or modifiable risk factor for postoperative AKI.
However, two recent studies26,27 suggested that intraoperative oliguria is not a reliable indicator for the development of postoperative AKI. We consider that this discrepancy may be attributable to the sample size. The sample sizes of both retrospective cohort studies (Goren’s sample size is 153 and Inacio’s sample size is 165) were too small to reliably assess the relationship between exposure and outcome with sufficient statistical power, which may have contributed to the false-negative result that oliguria was not identified as a risk factor for AKI. Additionally, we noticed that our findings differ from earlier studies showing no correlation of intraoperative oliguria with postoperative acute renal failure9,23–25. Possible reasons for these findings are that most of the sample sizes in the study are small, and acute renal failure is used to measure postoperative renal function changes, resulting in consideration of only advanced and serious renal insufficiency. Therefore, the relationship between intraoperative oliguria and mild postoperative renal damage may have been overlooked. However, mild renal damage is the leading cause of AKI after noncardiac surgery.
This study supported that intraoperative oliguria is the risk factor for postoperative AKI. Thus, we wondered whether rapid reversal of intraoperative oliguria by intravenous fluid volume expansion could help prevent AKI. Recently, two meta-analyses implemented by Egal et al.43,44, examined an identical question in different populations of studied patients and yielded comparable findings. Their findings suggested that target treatment of oliguria reversal had no contribution to preventing AKI development. According to additional studies45,46, extra intravenous fluids or diuretic regimens had no benefit in reducing the incidence of postoperative AKI in patients suffering oliguria. The possible causes are analyzed as follows. UO can be affected by many other factors besides renal perfusion, including hemodynamic changes, sympathetic tension, high intra-abdominal pressure, and hormonal influences such as aldosterone and antidiuretic hormone41,42,47. Oliguria may sometimes not indicate the onset of hypovolemia and inadequate renal perfusion. Thus, reversal of oliguria as a resuscitation goal and blind volume expansion may result in fluid overload, which substantially contributes to a greater risk of AKI48, increased postoperative complications, prolonged LOS49, and elevated mortality50.
Regardless of the source of intraoperative oliguria, intraoperative oliguria is implicated in the development of AKI and therefore requires close attention. However, physicians should not use oliguria alone as a marker to trigger specific interventions (e.g. additional intravenous fluids or diuretic regimens). When intraoperative oliguria occurs, appropriate preventive interventions should be cautiously implemented to reduce the risk of postoperative AKI. The first clinical procedure to be initiated should carefully calculate and estimate the patient’s intraoperative fluid intake and output, whether low blood volume or fluid excess is present, systemic hemodynamic changes, whether hypotension occurs, fluid reactivity, oliguria duration, and other information. Finding and identifying the potential source of oliguria may contribute to the development of appropriate renal protection and management programs, leading to improvements in volume and hemodynamic status (e.g., expansion of intravascular volume and combating hypotension)22,51,52.
Besides, the result of our subgroup analysis suggested that intraoperative oliguria is significantly associated with postoperative AKI regardless of whether UO less than 0.5 or 0.3 ml/kg/h is the oliguria criterion. Thus, we recommend a clinical threshold of 0.5 ml/kg/h for intraoperative oliguria to avoid the risks involved with milder levels of oliguria. Intraoperative oliguria is linked to postoperative AKI in both surgery types (abdominal or thoracic surgery). It is most likely to benefit from the analysis of oliguria and the risk of AKI and requires our attention, as a significant incidence of AKI follows abdominal and thoracic surgery, second only to cardiac surgery41. Moreover, patients undergoing thoracic surgery generally receive restricted intravenous infusions to avoid postoperative pulmonary complications53. However, we should also be aware that overly restrictive fluid therapy may lead to oliguria, which is associated with a higher risk of AKI after surgery.
To the best of our knowledge, this systematic review and meta-analysis is the first to systematically analyze and comprehensively summarize data on the correlation of intraoperative oliguria with postoperative AKI, in-hospital mortality, the requirement for RRT, and LOS, especially after abdominal and thoracic surgery, and completed protocol registration on the PROSPERO. It included data from more than 18 500 patients from nine studies, which we analyzed in detail.
There are several limitations to this study that should be mentioned. Firstly, meta-analysis results based on retrospective and observational studies could not infer whether this association was causal or not. Secondly, the included studies differed in many elements, like surgical types and definitions of intraoperative oliguria or AKI. Therefore, the comparably high clinical heterogeneity may have weakened the authenticity and accuracy of our conclusions. Finally, we excluded studies that did not include data on AKI as the incidence of AKI was our primary outcome and this study was affected by confounding factors. Thus, the applicability of our secondary outcome may be limited.
Conclusions
This meta-analysis found that a significant relationship was seen between intraoperative oliguria and subsequent postoperative acute kidney injury. Furthermore, it also demonstrated that patients with intraoperative oliguria were accompanied with increased in-hospital mortality and demand for postoperative RRT, but not with prolonged hospitalization. We recommend UO less than 0.5 ml/kg/h as the clinical standard of oliguria during operation, which has important clinical significance as a recognizable risk factor of postoperative AKI.
Ethics approval
The study was approved by the Ethics Committee of Xiangya Hospital.
Consent for publication
Not applicable.
Sources of funding
This work was supported by grants from the National Natural Science Foundation of China (82171236 and 81974172 to W.Z.) and the Key Research and Development Program of Hunan Province (2021SK2018 to W.Z.).
Author contribution
W.Z. and Z.P.: study conception and design; Z.P., S.L., and W.Z.: data analysis and interpretation; Z.P. and N.Z.: supervision of data analysis and interpretation; M.X.: data extraction; Z.P.: drafting of the manuscript; Z.P., S.L., Q.G., W.Z.: critical review and revision of the manuscript.
Conflicts of interest disclosure
The authors have no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO (Prospective Register of Systematic Reviews).
Unique identifying number or registration ID: CRD42022326281.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=326281
Guarantor
Wangyuan Zou.
Data availability
This is a meta-analysis article; data availability is not applicable; please contact the corresponding author if some data are needed.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgments
All authors approved the final version of the manuscript.
Footnotes
Zhaohua Pang and Shuang Liang contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 24 March 2023
Contributor Information
Zhaohua Pang, Email: 208112394@csu.edu.cn.
Shuang Liang, Email: liangshuang@csu.edu.cn.
Manyu Xing, Email: xmy0371@csu.edu.cn.
Nannan Zhou, Email: zhounannan@csu.edu.cn.
Qulian Guo, Email: qulianguo@hotmail.com.
Wangyuan Zou, Email: wangyuanzou@csu.edu.cn.
References
- 1. Garg AX, Kurz A, Sessler DI, et al. Perioperative aspirin and clonidine and risk of acute kidney injury: a randomized clinical trial. JAMA 2014;312:2254–2264. [DOI] [PubMed] [Google Scholar]
- 2. Biteker M, Dayan A, Tekkesin AI, et al. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am J Surg 2014;207:53–59. [DOI] [PubMed] [Google Scholar]
- 3. O’Connor ME, Kirwan CJ, Pearse RM, et al. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med 2016;42:521–530. [DOI] [PubMed] [Google Scholar]
- 4. Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 5. Vaara ST, Bellomo R. Postoperative renal dysfunction after noncardiac surgery. Curr Opin Crit Care 2017;23:440–446. [DOI] [PubMed] [Google Scholar]
- 6. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 7. Zacharias M, Mugawar M, Herbison GP, et al. Interventions for protecting renal function in the perioperative period. Cochrane Database Syst Rev 2013;9:CD003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goren O, Matot I. Perioperative acute kidney injury. Br J Anaesth 2015;115(Suppl 2):ii3–ii14. [DOI] [PubMed] [Google Scholar]
- 9. Kheterpal S, Tremper KK, Englesbe MJ, et al. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology 2007;107:892–902. [DOI] [PubMed] [Google Scholar]
- 10. Romagnoli S, Zagli G, Tuccinardi G, et al. Postoperative acute kidney injury in high-risk patients undergoing major abdominal surgery. J Crit Care 2016;35:120–125. [DOI] [PubMed] [Google Scholar]
- 11. Kheterpal S, Tremper KK, Heung M, et al. Development and validation of an acute kidney injury risk index for patients undergoing general surgery: results from a national data set. Anesthesiology 2009;110:505–515. [DOI] [PubMed] [Google Scholar]
- 12. Long TE, Helgason D, Helgadottir S, et al. Acute kidney injury after abdominal surgery: incidence, risk factors, and outcome. Anesth Analg 2016;122:1912–1920. [DOI] [PubMed] [Google Scholar]
- 13. Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS(R)) Society Recommendations: 2018. World J Surg 2019;43:659–695. [DOI] [PubMed] [Google Scholar]
- 14. Carmichael JC, Keller DS, Baldini G, et al. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and Society of American Gastrointestinal and Endoscopic Surgeons. Dis Colon Rectum 2017;60:761–784. [DOI] [PubMed] [Google Scholar]
- 15. Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014;10:37–47. [DOI] [PubMed] [Google Scholar]
- 16. Shiba A, Uchino S, Fujii T, et al. Association between intraoperative oliguria and acute kidney injury after major noncardiac surgery. Anesth Analg 2018;127:1229–1235. [DOI] [PubMed] [Google Scholar]
- 17. Slankamenac K, Beck-Schimmer B, Breitenstein S, et al. Novel prediction score including pre- and intraoperative parameters best predicts acute kidney injury after liver surgery. World J Surg 2013;37:2618–2628. [DOI] [PubMed] [Google Scholar]
- 18. Mizota T, Yamamoto Y, Hamada M, et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery. Br J Anaesth 2017;119:1127–1134. [DOI] [PubMed] [Google Scholar]
- 19. Myles PS, Mcilroy DR, Bellomo R, et al. Importance of intraoperative oliguria during major abdominal surgery: findings of the Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery trial. Br J Anaesth 2019;122:726–733. [DOI] [PubMed] [Google Scholar]
- 20. Meng ZT, Mu DL. Impact of oliguria during lung surgery on postoperative acute kidney injury. Beijing Da Xue Xue Bao Yi Xue Ban 2020;53:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shim JW, Kim KR, Jung Y, et al. Role of intraoperative oliguria in risk stratification for postoperative acute kidney injury in patients undergoing colorectal surgery with an enhanced recovery protocol: a propensity score matching analysis. PLoS One 2020;15:e0231447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao BC, Lei SH, Yang X, et al. Assessment of prognostic value of intraoperative oliguria for postoperative acute kidney injury: a retrospective cohort study. Br J Anaesth 2021;126:799–807. [DOI] [PubMed] [Google Scholar]
- 23. Alpert RA, Roizen MF, Hamilton WK, et al. Intraoperative urinary output does not predict postoperative renal function in patients undergoing abdominal aortic revascularization. Surgery 1984;95:707–711. [PubMed] [Google Scholar]
- 24. Knos GB, Berry AJ, Isaacson IJ, et al. Intraoperative urinary output and postoperative blood urea nitrogen and creatinine levels in patients undergoing aortic reconstructive surgery. J Clin Anesth 1989;1:181–185. [DOI] [PubMed] [Google Scholar]
- 25. Zarbock A, Koyner JL, Hoste E, et al. Update on perioperative acute kidney injury. Anesth Analg 2018;127:1236–1245. [DOI] [PubMed] [Google Scholar]
- 26. Goren O, Levy A, Cattan A, et al. Acute kidney injury in pancreatic surgery; association with urine output and intraoperative fluid administration. Am J Surg 2017;214:246–250. [DOI] [PubMed] [Google Scholar]
- 27. Inacio R, Gameiro J, Amaro S, et al. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis. J Bras Nefrol 2021;43:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 29. Susantitaphong P, Cruz DN, Cerda J, et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018;27:1785–1805. [DOI] [PubMed] [Google Scholar]
- 31. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Zou W. Research progress in influence of perioperative hypotension on postoperative outcome of patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021;46:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang S, Zou W. Effect of the intraoperative use of the Hypotension Prediction Index on postoperative hypotension in the postanaesthesia care unit. Comment on Br J Anaesth 2021; 127: 681–8. Br J Anaesth 2022;128:E340–E341. [DOI] [PubMed] [Google Scholar]
- 34. Sear JW. Kidney dysfunction in the postoperative period. Br J Anaesth 2005;95:20–32. [DOI] [PubMed] [Google Scholar]
- 35. Abelha FJ, Botelho M, Fernandes V, et al. Determinants of postoperative acute kidney injury. Crit Care 2009;13:R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prowle JR, Liu YL, Licari E, et al. Oliguria as predictive biomarker of acute kidney injury in critically ill patients. Crit Care 2011;15:R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Small RG, Witt RE. Major and minor surgery. JAMA 1965;191:180–182. [DOI] [PubMed] [Google Scholar]
- 38. Macedo E, Malhotra R, Bouchard J, et al. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int 2011;80:760–767. [DOI] [PubMed] [Google Scholar]
- 39. Piccinni P, Cruz DN, Gramaticopolo S, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol 2011;77:1072–1083. [PubMed] [Google Scholar]
- 40. Ostermann M, Liu K. Pathophysiology of AKI. Best Pract Res Clin Anaesthesiol 2017;31:305–314. [DOI] [PubMed] [Google Scholar]
- 41. Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis 2016;67:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. du Toit L, Biccard BM. The relationship between intraoperative oliguria and acute kidney injury. Br J Anaesth 2019;122:707–710. [DOI] [PubMed] [Google Scholar]
- 43. Egal M, de Geus HR, van Bommel J, et al. Targeting oliguria reversal in perioperative restrictive fluid management does not influence the occurrence of renal dysfunction: a systematic review and meta-analysis. Eur J Anaesthesiol 2016;33:425–435. [DOI] [PubMed] [Google Scholar]
- 44. Egal M, Erler NS, de Geus HR, et al. Targeting oliguria reversal in goal-directed hemodynamic management does not reduce renal dysfunction in perioperative and critically ill patients: a systematic review and meta-analysis. Anesth Analg 2016;122:173–185. [DOI] [PubMed] [Google Scholar]
- 45. Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. BMJ 2006;333:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matot I, Paskaleva R, Eid L, et al. Effect of the volume of fluids administered on intraoperative oliguria in laparoscopic bariatric surgery: a randomized controlled trial. Arch Surg 2012;147:228–234. [DOI] [PubMed] [Google Scholar]
- 47. Klein SJ, Lehner GF, Forni LG, et al. Oliguria in critically ill patients: a narrative review. J Nephrol 2018;31:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kambhampati G, Ross EA, Alsabbagh MM, et al. Perioperative fluid balance and acute kidney injury. Clin Exp Nephrol 2012;16:730–738. [DOI] [PubMed] [Google Scholar]
- 49. Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005;103:25–32. [DOI] [PubMed] [Google Scholar]
- 50. Teixeira C, Garzotto F, Piccinni P, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care 2013;17:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schortgen F, Schetz M. Does this critically ill patient with oliguria need more fluids, a vasopressor, or neither? Intensive Care Med 2017;43:907–910. [DOI] [PubMed] [Google Scholar]
- 52. Perner A, Prowle J, Joannidis M, et al. Fluid management in acute kidney injury. Intensive Care Med 2017;43:807–815. [DOI] [PubMed] [Google Scholar]
- 53. Wu Y, Yang R, Xu J, et al. Effects of intraoperative fluid management on postoperative outcomes after lobectomy. Ann Thorac Surg 2019;107:1663–1669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a meta-analysis article; data availability is not applicable; please contact the corresponding author if some data are needed.


