Background:
Postoperative mortality is an important indicator for evaluating surgical safety. Postoperative mortality is influenced by hospital volume; however, this association is not fully understood. This study aimed to investigate the volume–outcome association between the hospital surgical case volume for gastrectomies per year (hospital volume) and the risk of postoperative mortality in patients undergoing a gastrectomy for gastric cancer.
Methods:
Studies assessing the association between hospital volume and the postoperative mortality in patients who underwent gastrectomy for gastric cancer were searched for eligibility. Odds ratios were pooled for the highest versus lowest categories of hospital volume using a random-effects model. The volume–outcome association between hospital volume and the risk of postoperative mortality was analyzed. The study protocol was registered with Prospective Register of Systematic Reviews (PROSPERO).
Results:
Thirty studies including 586 993 participants were included. The risk of postgastrectomy mortality in patients with gastric cancer was 35% lower in hospitals with higher surgical case volumes than in their lower-volume counterparts (odds ratio: 0.65; 95% CI: 0.56–0.76; P<0.001). This relationship was consistent and robust in most subgroup analyses. Volume–outcome analysis found that the postgastrectomy mortality rate remained stable or was reduced after the hospital volume reached a plateau of 100 gastrectomy cases per year.
Conclusions:
The current findings suggest that a higher-volume hospital can reduce the risk of postgastrectomy mortality in patients with gastric cancer, and that greater than or equal to 100 gastrectomies for gastric cancer per year may be defined as a high hospital surgical case volume.
Keywords: gastric cancer, hospital surgical case volume, postoperative mortality, volume–outcome
Introduction
Highlights
Higher-volume hospitals reduced the risk of postgastrectomy mortality by 35%.
Postgastrectomy mortality was stable or reduced after a plateau of 100 cases per year.
At least 100 gastrectomies per year may be defined as a high hospital volume.
Gastric cancer is a global health burden in terms of cancer mortality, especially in Eastern countries, and radical gastrectomy still plays a decisive role in the management of resectable cases1. Postoperative mortality is one of the most important indicators for evaluating the safety of surgery2–4. This varies greatly among different geographical locations, accounting for 0.1–13.0%, and even up to 17.7%, of patients undergoing radical gastrectomy for gastric cancer5–10. Cancer-related cachexia, emergency surgery, and older age may be associated with increased risks of postoperative mortality11–14. Recent advances in surgical procedures and equipment, together with revised guidelines, have contributed to improvements in the safety of gastrectomy for gastric cancer1,15–18. In addition, the development of prehabilitation, anesthesia, and ICUs have also improved surgical safety, and the risk of postoperative mortality has accordingly decreased worldwide2,19–23. Furthermore, the improvements in perioperative management and rescue treatments have contributed to this decline2,24,25. Hospital surgical case volume has been associated with postoperative mortality26–31; however, the association between hospital surgical case volume and the risk of postoperative mortality in gastric cancer patients undergoing gastrectomy remains unclear.
Recent studies investigating the association between hospital surgical case volume for gastrectomy and the postoperative mortality in gastric cancer patients undergoing gastrectomy have shown inconsistent results5–7,32–42, and information on the volume–outcome association between hospital surgical volume and the risk of postoperative mortality is still lacking. Thus, the current study aimed to investigate the association between hospital surgical case volume and the risk of postoperative mortality in patients undergoing gastrectomy for gastric cancer, with the hypothesis that a higher hospital surgical case volume would lead to a lower risk of postoperative mortality. We also examined the volume–outcome effect of hospital surgical case volume on the risk of postoperative mortality in these patients with the aim of identifying a threshold volume above which there might be a lower risk of postoperative mortality.
Methods
This systematic review was conducted following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA, Supplemental Digital Content 1, http://links.lww.com/JS9/A124, Supplemental Digital Content 2, http://links.lww.com/JS9/A125) guidelines43–45. Each quality assessment was based on AMSTAR 2, Supplemental Digital Content 3, http://links.lww.com/JS9/A126 46, which is highly descriptive and consistent. The protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) prior to conducting this systematic review.
Eligibility criteria
The inclusion criteria were relevant cohort studies assessing the association between hospital surgical case volume for gastrectomy and the postoperative mortality in patients undergoing gastric cancer surgery. In this study, postoperative mortality was defined as death during hospitalization, regardless of the length of hospital stay, and death after hospital discharge within 30 days from the operation date. The exclusion criteria were: case reports, reviews, and studies with insufficient data; studies with no short-term mortality data; studies with 60-day or 90-day mortality data only; studies in which data for benign disease and gastric cancer could not be separated; studies with a mixture of gastric and other cancer types; studies including nonsurgical patients; studies including endoscopic treatments; studies with surgeon-volume data only or hospital-type data only; studies with no or unclear reference groups; and studies with continuous data only.
Data sources and search strategy
Two authors systematically searched PubMed and Embase from their inception to 22 October 2022, without any restrictions. The search terms included those related to gastric cancer, gastrectomy, hospital volume, and their variants. The search strategies are presented in Supplemental Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A127. The reference lists of relevant articles and reviews were also screened to identify eligible studies for inclusion. We also reviewed conference abstracts for potential unpublished studies.
Study selection
Duplicate studies were removed after a systematic search. Two authors assessed the remaining studies, and a third reviewer was consulted to resolve any discrepancies. Relevant studies were initially selected based on titles and abstracts, and the full texts were then read to confirm their relevance. If necessary, potentially relevant studies in languages other than English were translated using translation software or translators.
Data extraction
Two authors independently extracted data from the included studies according to a standardized procedure. The following information was extracted from each study in the adjusted model: first author, year of publication, study design, country, study period, number of gastric cancer patients who underwent gastrectomy, number of hospitals, hospital volume category (annual surgical cases per year), postoperative mortality, definition of postoperative mortality, and covariates. Two other reviewers reviewed the data. Any discrepancies were resolved through discussion and consensus.
Quality assessment
Two authors independently assessed the quality of the included studies using the Newcastle–Ottawa scale for cohort design47. All studies were evaluated regarding participant selection and measurement of exposure, comparability, assessment of outcomes, and adequacy of follow-up. The studies were then classified as high (7–9), moderate (4–6), or low quality (0–3).
Statistical analysis
Odds ratios (ORs) and the corresponding 95% CIs were pooled to compare the association between hospital surgical case volume (highest vs. lowest category) and the risk of postoperative mortality in gastric cancer patients undergoing gastrectomy. The lowest hospital volume was used as the reference group. We applied a random-effects model, considering the high possibility of clinical heterogeneity among the included studies. Heterogeneity across studies was assessed using the Q statistic (I 2 and P value), with I 2 values less than 25, 25–50, and greater than 50% indicating low, moderate, and high heterogeneity, respectively48. Publication bias was assessed using funnel plots with Begg’s and Egger’s tests48,49. P<0.05 was considered statistically significant. All statistical analyses were performed using Stata software, Version 13.1 (StataCorp).
Subgroup analyses
We further confirmed the robustness of the findings by conducting subgroup analyses according to the study period (1982–1999 and 2000–2018), country (Eastern and Western), sample size (<5000 and ≥5000), hospital number (<100 and ≥100), study design (retrospective and prospective), study quality (high and moderate), volume grouping (dichotomies, tertiles, quartiles, and quintiles), and adjusted ORs (yes and no).
Volume–outcome analysis
We also conducted a volume–outcome analysis of the relationship between hospital surgical case volume and the risk of postoperative mortality in patients with gastric cancer undergoing gastrectomy50,51. This method required the following information: at least three quantitative categories of hospital volume, number of postoperative deaths, total number of patients, and ORs with 95% CIs. If no median (or mean) value was indicated, it was estimated from the midpoints between the upper and lower bounds: the lower boundary was assumed to be 0 when the lower boundary was open-ended; otherwise, the median value was assumed to be 1.5 times the lower boundary if the upper boundary was open-ended, as described previously52. The volume–outcome relationship was compared with a linear trend relationship, with P greater than or equal to 0.05 indicating a linear relationship and P less than 0.05 indicating a nonlinear trend. We produced a scatter plot to illustrate the distribution between hospital surgical case volume (x-axis) and the postoperative mortality (y-axis) in patients with gastric cancer undergoing gastrectomy.
Results
The initial search identified 4163 studies. After removing 1061 duplicates, 3102 studies remained, of which 112 were discarded after reviewing the titles and abstracts. Thirty cohort studies were finally included after reviewing the full texts5–10,32–42,53–65. Details of the literature search and study selection are shown in Figure 1.
Figure 1.

Flowchart of literature search and study selection.
Characteristics of included studies
The characteristics of the 30 included studies are summarized in Table 1. The number of patients in the included studies ranged from 188 to 145 523, with a total of 586 993 patients. Four studies were prospective36,41,58,60, and 26 were retrospective cohort studies5–10,32–35,37–40,42,53–57,59,61–65. Nine studies were from Eastern countries5,6,32,34,36,42,55,57,62, and 21 were from Western countries7–10,33,35,37–41,53,54,56,58–61,63–65. The postoperative mortality in the included studies ranged from 0.07 to 17.7% in different categories. The hospital volume categories, ORs, and adjusted factors are listed in Supplemental Table 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A127. The Newcastle–Ottawa scale for the quality assessment of the included studies is presented in Supplemental Table 3, Supplemental Digital Content 4, http://links.lww.com/JS9/A127. The average total score was 7.6 (range: 5–9), indicating a high or moderate quality of all included studies.
Table 1.
Clinical characteristics of gastric cancer patients undergoing gastrectomy from 30 included studies.
| References | Study design | Country | Period | Number of patients | Number of hospitals | Hospital volume (cases/year) | POM (death/total) (%) | Definition of POM |
|---|---|---|---|---|---|---|---|---|
| Wirth et al.53 | Retrospective | Switzerland | 2014–2018 | 188 | 62 | ≤10 | 1.9 | In-hospital |
| >10 | 1.3 | |||||||
| Narendra et al.54 | Retrospective | Australia | 2001–2015 | 796 | 49 | <5 | 5.0 | 30-day |
| ≥5 | 3.0 | |||||||
| Iwatsuki et al.34 | Retrospective | Japan | 2011–2015 | 71 307 | 2051 | 0–11 | 3.1 | In-hospital |
| 12–26 | 1.7 | |||||||
| 27–146 | 1.2 | |||||||
| Ji et al.5 | Retrospective | China | 2013–2018 | 125 683 | 515 | 1–83 | 0.44 | In-hospital |
| 84–238 | 0.29 | |||||||
| 239–579 | 0.24 | |||||||
| 580–1193 | 0.15 | |||||||
| Diers et al.35 | Retrospective | Germany | 2009–2017 | 46 187 | 1084 | ≤10 | 6.9 | In-hospital |
| 11–29 | 5.8 | |||||||
| ≥30 | 3.9 | |||||||
| Tian et al.8 | Retrospective | Australia | 2001–2015 | 1253 | 49 | <5 | 6.4 | In-hospital |
| ≥5 | 4.3 | |||||||
| Levy et al.56 | Retrospective | Canada | 2004–2015 | 1660 | 69 | 0–2 | 6.6 | 30 days |
| 2.5–5 | NA | |||||||
| 5.5–7.5 | NA | |||||||
| 8–11.5 | NA | |||||||
| 12–22 | 3.1 | |||||||
| Shibao et al.55 | Retrospective | Japan | 2012–2013 | 37 752 | 1074 | 1–35 | NA | In-hospital |
| 36–61 | NA | |||||||
| 62–97 | NA | |||||||
| 98–458 | NA | |||||||
| Iwatsuki et al.6 | Retrospective | Japan | 2011–2015 | 145 523 | 2182 | 1–22 | 1.9 | In-hospital |
| 23–51 | 1.0 | |||||||
| 52–404 | 0.5 | |||||||
| Wu et al.57 | Retrospective | China | 2000–2010 | 7905 | 185 | <10 | 4.1 | 30 days |
| ≥10 | 1.9 | |||||||
| Claassen et al.7 | Retrospective | Netherlands | 2007–2015 | 494 | NA | 1–10 | 1.6 | In-hospital/30 days |
| 11–20 | 4.3 | |||||||
| 21–30 | 2.0 | |||||||
| ≥31 | 0.63 | |||||||
| Haga et al.36 | Retrospective | Japan | 2000–2003 | 2045 | NA | ≤90 | 0.84 | 30 days |
| >90 | 0.07 | |||||||
| Ptok et al.58 | Prospective | Germany | 2007–2009 | 2897 | 140 | <5 | 7.8 | In-hospital |
| 5–10 | 5.5 | |||||||
| 11–20 | 6.6 | |||||||
| >20 | 5.8 | |||||||
| Busweiler et al.61 | Retrospective | Netherlands | 2007–2015 | 4837 | NA | <15 | 7.4 | 30 days |
| 15–39 | 6.1 | |||||||
| ≥40 | 4.8 | |||||||
| Güller et al.60 | Prospective | Switzerland | 1999–2012 | 4404 | NA | 1–10 | 4.9 | In-hospital |
| >10 | 3.3 | |||||||
| Liu et al.59 | Retrospective | United States | 2010–2013 | 17 923 | 1297 | ≤4 | NA | 30 days |
| >4 | NA | |||||||
| Murata et al.62 | Retrospective | Japan | 2009–2011 | 5941 | 741 | <40 | 0.5 | In-hospital |
| ≥40 | 0.3 | |||||||
| Altini et al.37 | Retrospective | Italy | 2004–2008 | 1314 | 16 | <7 | 11.9 | In-hospital/30 days |
| 7–21 | NA | |||||||
| ≥21 | 4.2 | |||||||
| Smith et al.63 | Retrospective | Australia | 2000–2009 | 1621 | 84 | ≤6 | 5.1 | 30 days |
| >6 | 3.8 | |||||||
| Dikken et al.39 | Retrospective | Netherlands, | 2004–2009 | 9010 | NA | 1–10 | 4.4 | 30 days |
| England, | 11–20 | NA | ||||||
| Sweden, | ≥21 | 6.7 | ||||||
| Denmark, | ||||||||
| Ho et al.38 | Retrospective | Netherlands | 2008–2010 | 1024 | 88 | 1–6 | 6.1 | 30 days |
| 7–9 | 6.4 | |||||||
| ≥10 | 4.6 | |||||||
| Ghaferi et al.9 | Retrospective | United States | 2005–2007 | 8838 | NA | 1–4 | 17.7 | In-hospital/30 days |
| NA | NA | |||||||
| NA | NA | |||||||
| 11–110 | 7.5 | |||||||
| Kim et al.32 | Retrospective | South Korea | 2002–2005 | 11 071 | NA | <39 | 0.71 | 30 days |
| 39–126 | 0.86 | |||||||
| >126 | 0.36 | |||||||
| Skipworth et al.40 | Retrospective | Scotland | 1982–2003 | 4589 | 61 | 1–3 | 8.9 | In-hospital |
| 4–5 | 12.0 | |||||||
| 6–9 | 9.4 | |||||||
| ≥10 | 8.6 | |||||||
| Baré et al.33 | Retrospective | Spain | 2001–2002 | 3241 | 144 | <18 | 7.9 | In-hospital |
| 18–35 | 11.7 | |||||||
| >35 | 11.6 | |||||||
| Thompson et al.41 | Prospective | Scotland | 1997–1999 | 639 | 39 | <13 | 11.9 | In-hospital/30 days |
| 13–19 | 9.1 | |||||||
| 20–34 | 10.3 | |||||||
| ≥35 | 10.0 | |||||||
| Lin et al.42 | Retrospective | China | 2000–2003 | 11 348 | 174 | Q1 | 5.4 | In-hospital |
| Q2 | 3.4 | |||||||
| Q3 | 3.1 | |||||||
| Q4 | 1.6 | |||||||
| Q5 | 1.4 | |||||||
| Wainess et al.64 | Retrospective | United States | 1988–2000 | 23,690 | NA | 1–4 | 8.3 | In-hospital |
| 5–8 | 7.1 | |||||||
| ≥9 | 6.5 | |||||||
| Damhuis et al.65 | Retrospective | Netherlands | 1987–1997 | 1978 | 22 | <7 | 8.0 | 30 days |
| 7–10 | 9.8 | |||||||
| >10 | 6.8 | |||||||
| Birkmeyer et al.10 | Retrospective | United States | 1994–1999 | 31,944 | 3423 | <5 | 13.0 | In-hospital/30 days |
| 5–8 | 12.7 | |||||||
| 9–13 | 11.1 | |||||||
| 14–21 | 11.3 | |||||||
| >21 | 8.7 |
NA, not available; OR, odds ratio; POM, postoperative mortality; Q, quintiles.
Hospital volume and risk of postoperative mortality
Thirty studies with available data were included in the quantitative analysis5–10,32–42,53–65. A higher-volume hospital could reduce the risk of postoperative mortality among patients undergoing gastric cancer surgery by 35% compared with lower-volume hospitals (OR: 0.65; 95% CI: 0.56–0.76; P<0.001) (Fig. 2).
Figure 2.

Forest plot of association between hospital surgical case volume per year and the risk of postoperative mortality among gastric cancer patients undergoing gastrectomy according to volume grouping. OR, odds ratio.
Subgroup analyses
Variations in hospital volume grouping strategies among the studies may be a potential source of heterogeneity. Compared with their lower-volume counterparts, higher-volume hospitals significantly reduced the risk of postoperative mortality in gastric cancer patients undergoing gastrectomy for dichotomies (OR: 0.65; 95% CI: 0.58–0.74; P<0.001), for tertiles (OR: 0.75; 95% CI: 0.57–0.98; P=0.036), for quartiles (OR: 0.54; 95% CI: 0.35–0.84; P=0.006), and for quintiles (OR: 0.57; 95% CI: 0.36–0.91; P=0.019), although heterogeneity could still be observed (Fig. 2, Table 2). The association between hospital surgical case volume and the postoperative mortality after gastric cancer surgery remained consistent in other subgroup analyses, except for prospective study design, moderate study quality, and unadjusted OR (Supplemental Figs 1–7, Supplemental Digital Content 4, http://links.lww.com/JS9/A127; Table 2).
Table 2.
Subgroup analyses of volume effect on postoperative mortality in gastric cancer patients undergoing gastrectomy.
| Subgroup | Number of patients | Number of studies | Odds ratio (95% CI) | P | Test of heterogeneity | |
|---|---|---|---|---|---|---|
| I 2 value (%) | P | |||||
| Total | 586 993 | 30 | 0.65 (0.56–0.76) | <0.001 | 81.9 | <0.001 |
| Study period | ||||||
| 1982–1999 | 34 561 | 3 | 0.73 (0.64–0.83) | <0.001 | 0 | 0.774 |
| 2000–2018 | 519 749 | 24 | 0.61 (0.50–0.73) | <0.001 | 79.5 | <0.001 |
| Country | ||||||
| Eastern | 418 575 | 9 | 0.50 (0.38–0.66) | <0.001 | 72.3 | <0.001 |
| Western | 168 418 | 21 | 0.72 (0.61–0.85) | <0.001 | 79.3 | <0.001 |
| Sample size | ||||||
| <5000 | 32 871 | 16 | 0.80 (0.66–0.96) | 0.016 | 45.4 | 0.025 |
| ≥5000 | 554 122 | 14 | 0.57 (0.46–0.70) | <0.001 | 88.3 | <0.001 |
| Hospital number | ||||||
| <100 | 15 062 | 10 | 0.77 (0.65–0.92) | 0.003 | 0 | 0.968 |
| ≥100 | 507 542 | 12 | 0.58 (0.46–0.73) | <0.001 | 85.7 | <0.001 |
| Study design | ||||||
| Retrospective | 577 117 | 26 | 0.65 (0.56–0.77) | <0.001 | 84.0 | <0.001 |
| Prospective | 9876 | 4 | 0.66 (0.42–1.03) | 0.066 | 27.7 | 0.246 |
| Study quality | ||||||
| High | 559 670 | 23 | 0.66 (0.58–0.76) | <0.001 | 72.0 | <0.001 |
| Moderate | 27 323 | 7 | 0.54 (0.27–1.11) | 0.092 | 92.1 | <0.001 |
| Volume grouping | ||||||
| Dichotomies | 42 076 | 9 | 0.65 (0.58–0.74) | <0.001 | 0 | 0.852 |
| Tertiles | 319 182 | 11 | 0.75 (0.57–0.98) | 0.036 | 89.7 | <0.001 |
| Quartiles | 180 783 | 7 | 0.54 (0.35–0.84) | 0.006 | 76.3 | <0.001 |
| Quintiles | 44 952 | 3 | 0.57 (0.36–0.91) | 0.019 | 75.8 | <0.001 |
| Adjusted OR | ||||||
| Yes | 561 908 | 19 | 0.63 (0.54–0.73) | <0.001 | 77.7 | <0.001 |
| Unknown | 25 085 | 11 | 0.69 (0.44–1.08) | 0.102 | 87.4 | 0.001 |
OR, odds ratio.
Volume–outcome analysis
Fourteen studies were included in the volume–outcome analysis of hospital surgical case volume and the risk of postoperative mortality in gastric cancer patients undergoing gastrectomy5–7,10,32–35,38,40,41,58,61,65. The volume–outcome association was not a linear association (Fig. 3), and the risk of postgastrectomy mortality remained stable or decreased after the hospital volume reached a plateau of 100 gastrectomy cases per year. Twenty-seven studies were included in the scatter plot to assess the relationship between hospital surgical case volume and the postoperative mortality rates5–10,32–41,53,54,56–58,60–65. Similarly, the postoperative mortality rates showed a rapid decline from 17.7 to 0.3% until the hospital volume reached a plateau of 100 gastrectomies per year, and then remained at 0.07–0.51% (Fig. 4).
Figure 3.

Volume–outcome analysis of association between hospital surgical case volume per year and the risk of postoperative mortality among gastric cancer patients undergoing gastrectomy. Middle green line is the fit curve of odds ratio; upper green line is the upper 95% CI; lower green line is the lower 95% CI.
Figure 4.
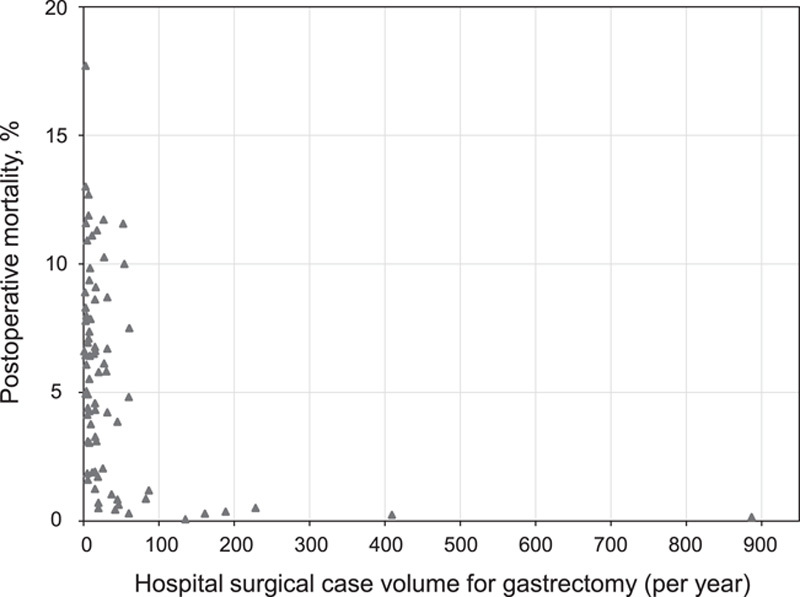
Scatter plot of distribution between median or mean hospital surgical case volume per year in each category from 27 studies and corresponding mortality rates among gastric cancer patients undergoing gastrectomy.
Publication bias
Thirty studies were included in the quantitative meta-analysis5–10,32–42,53–65, and there was no evidence of publication bias based on Begg’s (P=0.412) or Egger’s tests (P=0.795) (Fig. 5).
Figure 5.

Funnel plot for publication bias of association between hospital surgical case volume per year and the risk of postoperative mortality among gastric cancer patients undergoing gastrectomy. OR, odds ratio.
Discussion
This meta-analysis investigated the association between the hospital surgical case volume for gastrectomy and the risk of postoperative mortality in patients with gastric cancer undergoing the procedure. Compared with lower-volume hospitals, the risk of postoperative mortality was reduced by 35% in patients who underwent gastrectomy for gastric cancer in higher-volume hospitals. This volume effect on the risk of postoperative mortality remained robust and consistent in multiple subgroup analyses. Volume–outcome analysis further showed that the risk of postgastrectomy mortality remained stable or decreased after the hospital volume reached a plateau of 100 gastrectomy cases per year. Similarly, postoperative mortality rates decreased rapidly from 17.7 to 0.3% until the hospital surgical case volume reached a plateau of 100 gastrectomies per year and then remained below 0.51%.
A previous pioneering systematic review evaluated the potential relationships between hospital volume and survival outcomes for multiple cancers66, while another recent meta-analysis further investigated the effect of surgical volume on surgical and oncological outcomes for gastric cancer67. However, these meta-analyses included several studies with heterogeneous patients with and without gastrectomy rather than only surgical patients, limiting the analysis to postoperative mortality, which is one of the most important indicators for assessing surgical safety2–4. In contrast, we investigated the association between hospital surgical case volume and the risk of postoperative mortality exclusively in patients who underwent gastrectomy for gastric cancer. In addition, the current analysis included 12 further studies with a total of 213 792 patients, which were not included in previous systematic reviews5,8,9,32,35,40,53,54,58,61–63. We also excluded studies that included patients with benign disease in order to analyze a highly homogeneous patient population with curable gastrectomy. We further confirmed the robustness of our findings using multiple subgroup analyses. For the first time, we showed that greater than or equal to 100 gastrectomies per year for gastric cancer may be defined as a high hospital surgical case volume.
Although an association between hospital surgical volume and postoperative mortality has been proposed for several decades, the mechanism remains unclear68. Surgeons and anesthesia teams in hospitals with higher surgical case volumes may have more experience and the chance of enhanced training, thus reducing postoperative mortality, that is, ‘practice makes perfect’2,69–73. A higher rate of unplanned intensive care admissions in lower-volume hospitals also led to higher postoperative mortality56. High-volume hospitals were also reported to have higher rates of success of rescue treatments compared with low-volume hospitals35,74, due to their structural differences such as the availability of specialized ICUs35.
The current results may provide helpful information to guide patients’ hospital choices, volume-based referrals, and hospital management. High-volume hospitals have been associated with improved patient outcomes, attributed to their subspecialty oncology expertise30,75. The current findings suggest that a higher-volume hospital can reduce the risk of postgastrectomy mortality in patients with gastric cancer, and that greater than or equal to 100 gastrectomies for gastric cancer per year may be defined as a high hospital surgical case volume. It is clinically important to make efforts to reduce postoperative mortality, especially in low-volume hospitals. First, self-training for surgical skills is important. For example, a LapMentor virtual reality laparoscopic simulator (Simbionix Corporation) was shown to help surgeons improve their surgical skills76. Surgeons are also encouraged to enhance their surgical skills through advanced training in high-volume cancer centers. Furthermore, it is critical to identify high-risk patients through an adequate preoperative evaluation. For instance, the surgical risks of most operations can be predicted by a decision-support tool, such as the American College of Surgeons National Surgical Quality Improvement Program surgical risk calculator77. Similarly, a machine-learning project trained on Pythia was built to predict the risks of postoperative complications, and high-risk patients could be identified78. These predictive models can help identify high-risk patients in low-volume hospitals, who can then be selectively transferred to high-volume hospitals79–81 that have more surgical and anesthetic teams with better experience, a higher rate of rescue success, and a higher availability of specialized ICUs2,35,69–74. In addition, perioperative management plays a crucial role in surgical safety2, which has been improved over time25. For example, a uniform perioperative management procedure can be applied to improve surgical safety, such as the clinical application of a surgical safety checklist82 and the CLASSification of Intraoperative Complications (ClassIntra version 1.0, formerly known as CLASSIC)2, which can effectively reduce postoperative mortality. Improved nurse staffing levels can also reduce postoperative mortality, and an increase in a nurse’s workload by one patient was reported to increase the likelihood of 30-day mortality from admission by 7%, while every 10% increase in bachelor-degree nurses decreased this likelihood by 7%83.
This study had several limitations. Most of the included studies were retrospective cohort studies with different populations. In addition, uniform adjustment factors for assessing the association between hospital volume and the risk of postoperative mortality were lacking; however, multiple subgroup analyses confirmed the robustness of the main findings. Furthermore, there was heterogeneity among the cutoff values for categories of annual hospital surgical case volumes among the studies, and further investigations are needed to validate the main findings of the current study.
Conclusions
Treatment in hospitals with higher surgical case volumes could reduce the risk of postoperative mortality among gastric cancer patients undergoing gastrectomy by 35% compared with that in lower-volume hospitals. The current findings suggest that a higher-volume hospital can reduce the risk of postgastrectomy mortality in patients with gastric cancer, and that greater than or equal to 100 gastrectomies for gastric cancer per year may be defined as a high hospital surgical case volume. Patients with gastric cancer may benefit from a lower risk of postoperative mortality if radical gastrectomy procedures are centralized at high-volume hospitals above the threshold of 100 gastrectomies for gastric cancer per year.
Ethical approval
Not applicable.
Sources of funding
This study received no funding.
Author contribution
F.-L.N., W.-J.G., Z.-M.Z., and C.-D.Z: conceived and designed the experiments. F.-L.N., Z.-M.Z., and C.-D.Z.: analyzed the data. F.-L.N., W.-J.G., Z.-M.Z., and C.-D.Z.: contributed reagents/materials/analysis. F.-L.N., W.-J.G., Z.-M.Z., W.-Y.D., M.S., S.-Y.C., Y.-J.Z., M.A., and C.-D.Z.: wrote the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO database.
Unique identifying number or registration ID: CRD42022368649.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022368649
Guarantor
Chun-Dong Zhang.
Data availability statement
All the data generated and analyzed during this study are included in this article. The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Footnotes
F.-L.N., W.-J.G., and Z.-M.Z. contributed equally to this work.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.journal-surgery.net.
Published online 14 March 2023
Contributor Information
Fei-Long Ning, Email: ning_fl@126.com.
Wan-Jie Gu, Email: wanjiegu@hotmail.com.
Zhe-Ming Zhao, Email: 20111253@cmu.edu.cn.
Wan-Ying Du, Email: dwy@g.ecc.u-tokyo.ac.jp.
Min Sun, Email: sunmin-0715@163.com.
Shi-Yi Cao, Email: caoshiyi@hust.edu.cn.
Yong-Ji Zeng, Email: yongji.zeng@bcm.edu.
Masanobu Abe, Email: abem-ora@h.u-tokyo.ac.jp.
Chun-Dong Zhang, Email: zhangchundong2007@126.com;cdzhang@cmu.edu.cn.
References
- 1. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dell-Kuster S, Gomes NV, Gawria L, et al. Prospective validation of classification of intraoperative adverse events (ClassIntra): international, multicentre cohort study. Br Med J 2020;370:m2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Russell EM, Bruce J, Krukowski ZH. Systematic review of the quality of surgical mortality monitoring. Br J Surg 2003;90:527–532. [DOI] [PubMed] [Google Scholar]
- 4. Mise Y, Vauthey JN, Zimmitti G, et al. Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg 2015;262:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ji J, Shi L, Ying X, et al. Associations of centralization with health care quality for gastric cancer patients receiving gastrectomy in China. Chin J Cancer Res 2021;33:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwatsuki M, Yamamoto H, Miyata H, et al. Effect of hospital and surgeon volume on postoperative outcomes after distal gastrectomy for gastric cancer based on data from 145,523 Japanese patients collected from a nationwide web-based data entry system. Gastric Cancer 2019;22:190–201. [DOI] [PubMed] [Google Scholar]
- 7. Claassen YHM, van Sandick JW, Hartgrink HH, et al. Association between hospital volume and quality of gastric cancer surgery in the CRITICS trial. Br J Surg 2018;105:728–735. [DOI] [PubMed] [Google Scholar]
- 8. Tian K, Baade PD, Aitken JF, et al. Procedure-specific outcomes following gastrectomy for cancer compared by hospital volume and service capability. ANZ J Surg 2021;91:2430–2435. [DOI] [PubMed] [Google Scholar]
- 9. Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care 2011;49:1076–1081. [DOI] [PubMed] [Google Scholar]
- 10. Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 11. Surgical site infection after gastrointestinal surgery in high-income, middle-income, and low-income countries: a prospective, international, multicentre cohort study. Lancet Infect Dis 2018;18:516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 13. Solsky I, Friedmann P, Muscarella P, et al. Poor outcomes of gastric cancer surgery after admission through the emergency department. Ann Surg Oncol 2017;24:1180–1187. [DOI] [PubMed] [Google Scholar]
- 14. Nelen SD, Bosscha K, Lemmens V, et al. Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg 2018;105:1163–1170. [DOI] [PubMed] [Google Scholar]
- 15. Kim W, Kim HH, Han SU, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage i gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01. Ann Surg 2016;263:28–35. [DOI] [PubMed] [Google Scholar]
- 16. Katai H, Mizusawa J, Katayama H, et al. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 2017;20:699–708. [DOI] [PubMed] [Google Scholar]
- 17. Lee HJ, Hyung WJ, Yang HK, et al. Short-term outcomes of a multicenter randomized controlled trial comparing laparoscopic distal gastrectomy with d2 lymphadenectomy to open distal gastrectomy for locally advanced gastric cancer (KLASS-02-RCT. Ann Surg 2019;270:983–991. [DOI] [PubMed] [Google Scholar]
- 18. Yu J, Huang C, Sun Y, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: the class-01 randomized clinical trial. JAMA 2019;321:1983–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braz LG, Braz JRC, Modolo MP, et al. Perioperative and anesthesia-related cardiac arrest and mortality rates in Brazil: a systematic review and proportion meta-analysis. PLoS One 2020;15:e0241751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tak Kyu O, Ji E, Ahn S, et al. Admission to surgical intensive care unit in time with intensivist coverage and its association with postoperative 30-day mortality: the role of intensivists in a surgical intensive care unit. Anaesth Crit Care Pain Med 2019;38:259–263. [DOI] [PubMed] [Google Scholar]
- 21. Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018;267:50–56. [DOI] [PubMed] [Google Scholar]
- 22. Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016;160:1189–1201. [DOI] [PubMed] [Google Scholar]
- 23. International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knight SR, Shaw CA, Pius R, et al. GlobalSurg collaborative and national institute for health research global health research unit on global surgery, global variation in postoperative mortality and complications after cancer surgery: a multicentre, prospective cohort study in 82 countries. Lancet 2021;397:387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrianello S, Marchegiani G, Malleo G, et al. Pancreaticojejunostomy with externalized stent vs pancreaticogastrostomy with externalized stent for patients with high-risk pancreatic anastomosis: a single-center, phase 3, randomized clinical trial. JAMA Surg 2020;155:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 27. Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut 2014;63:1393–1400. [DOI] [PubMed] [Google Scholar]
- 29. van de Poll-Franse LV, Lemmens VE, Roukema JA, et al. Impact of concentration of oesophageal and gastric cardia cancer surgery on long-term population-based survival. Br J Surg 2011;98:956–963. [DOI] [PubMed] [Google Scholar]
- 30. El Amrani M, Lenne X, Clement G, et al. Specificity of procedure volume and its association with postoperative mortality in digestive cancer surgery: a nationwide study of 225,752 patients. Ann Surg 2019;270:775–782. [DOI] [PubMed] [Google Scholar]
- 31. Madenci AL, Wanis KN, Cooper Z, et al. Comparison of mortality risk with different surgeon and hospital operative volumes among individuals undergoing pancreatectomy by emulating target trials in us medicare beneficiaries. JAMA Netw Open 2022;5:e221766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim SY, Park JH, Kim SG, et al. Disparities in utilization of high-volume hospitals for cancer surgery: results of a Korean population-based study. Ann Surg Oncol 2010;17:2806–2815. [DOI] [PubMed] [Google Scholar]
- 33. Baré M, Cabrol J, Real J, et al. In-hospital mortality after stomach cancer surgery in Spain and relationship with hospital volume of interventions. BMC Public Health 2009;9:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iwatsuki M, Yamamoto H, Miyata H, et al. Association of surgeon and hospital volume with postoperative mortality after total gastrectomy for gastric cancer: data from 71,307 Japanese patients collected from a nationwide web-based data entry system. Gastric Cancer 2021;24:526–534. [DOI] [PubMed] [Google Scholar]
- 35. Diers J, Baum P, Wagner JC, et al. Hospital volume following major surgery for gastric cancer determines in-hospital mortality rate and failure to rescue: a nation-wide study based on German billing data (2009-2017). Gastric Cancer 2021;24:959–969. [DOI] [PubMed] [Google Scholar]
- 36. Haga Y, Hato S, Ikenaga M, et al. Validation of an assessment tool: estimation of postoperative overall survival for gastric cancer. Eur J Surg Oncol 2018;44:515–523. [DOI] [PubMed] [Google Scholar]
- 37. Altini M, Carretta E, Morgagni P, et al. Is a clear benefit in survival enough to modify patient access to the surgery service? A retrospective analysis in a cohort of gastric cancer patients. Gastric Cancer 2015;18:159–166. [DOI] [PubMed] [Google Scholar]
- 38. Ho VK, Damhuis RA, Hartgrink HH. Adherence to national guidelines for gastric cancer in the Netherlands: a retrospective population-based audit. Int J Cancer 2013;132:1156–1161. [DOI] [PubMed] [Google Scholar]
- 39. Dikken JL, van Sandick JW, Allum WH, et al. Differences in outcomes of oesophageal and gastric cancer surgery across Europe. Br J Surg 2013;100:83–94. [DOI] [PubMed] [Google Scholar]
- 40. Skipworth RJ, Parks RW, Stephens NA, et al. The relationship between hospital volume and post-operative mortality rates for upper gastrointestinal cancer resections: Scotland 1982-2003. Eur J Surg Oncol 2010;36:141–147. [DOI] [PubMed] [Google Scholar]
- 41. Thompson AM, Rapson T, Gilbert FJ, et al. Hospital volume does not influence long-term survival of patients undergoing surgery for oesophageal or gastric cancer. Br J Surg 2007;94:578–584. [DOI] [PubMed] [Google Scholar]
- 42. Lin HC, Xirasagar S, Lee HC, et al. Hospital volume and inpatient mortality after cancer-related gastrointestinal resections: the experience of an Asian country. Ann Surg Oncol 2006;13:1182–1188. [DOI] [PubMed] [Google Scholar]
- 43. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 45. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 46. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Br Med J 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wells GA, Shea B, O’Connell D, et al. , The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp
- 48. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 50. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. The stata journal 2006;6:40–57. [Google Scholar]
- 51. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 52. Gu WJ, Wu XD, Zhou Q, et al. Relationship between annualized case volume and mortality in sepsis: a dose-response meta-analysis. Anesthesiology 2016;125:168–179. [DOI] [PubMed] [Google Scholar]
- 53. Wirth K, Näpflin M, Graber SM, et al. Does hospital volume affect outcomes after abdominal cancer surgery: an analysis of Swiss health insurance claims data. BMC Health Serv Res 2022;22:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Narendra A, Baade PD, Aitken JF, et al. Hospital characteristics associated with better ‘quality of surgery’ and survival following oesophagogastric cancer surgery in Queensland: a population-level study. ANZ J Surg 2021;91:323–328. [DOI] [PubMed] [Google Scholar]
- 55. Shibao K, Fujino Y, Joden F, et al. Clinical outcomes of laparoscopic versus laparotomic distal gastrectomy in gastric cancer patients: a multilevel analysis based on a nationwide administrative database in japan. World J Surg 2020;44:3852–3861. [DOI] [PubMed] [Google Scholar]
- 56. Levy J, Gupta V, Amirazodi E, et al. Gastrectomy case volume and textbook outcome: an analysis of the Population Registry of Esophageal and Stomach Tumours of Ontario (PRESTO). Gastric Cancer 2020;23:391–402. [DOI] [PubMed] [Google Scholar]
- 57. Wu JM, Ho TW, Tien YW. Correlation between the increased hospital volume and decreased overall perioperative mortality in one universal health care system. World J Surg 2019;43:2194–2202. [DOI] [PubMed] [Google Scholar]
- 58. Ptok H, Gastinger I, Meyer F, et al. Hospital volume effects in surgical treatment of gastric cancer: Results of a prospective multicenter observational study. Chirurg 2017;88:328–338. [DOI] [PubMed] [Google Scholar]
- 59. Liu JB, Bilimoria KY, Mallin K, et al. Patient characteristics associated with undergoing cancer operations at low-volume hospitals. Surgery 2017;161:433–443. [DOI] [PubMed] [Google Scholar]
- 60. Güller U, Warschkow R, Ackermann CJ, et al. Lower hospital volume is associated with higher mortality after oesophageal, gastric, pancreatic and rectal cancer resection. Swiss Med Wkly 2017;147:w14473. [DOI] [PubMed] [Google Scholar]
- 61. Busweiler LAD, Dikken JL, Henneman D, et al. The influence of a composite hospital volume on outcomes for gastric cancer surgery: a Dutch population-based study. J Surg Oncol 2017;115:738–745. [DOI] [PubMed] [Google Scholar]
- 62. Murata A, Muramatsu K, Ichimiya Y, et al. Influence of hospital volume on outcomes of laparoscopic gastrectomy for gastric cancer in patients with comorbidity in Japan. Asian J Surg 2015;38:33–39. [DOI] [PubMed] [Google Scholar]
- 63. Smith RC, Creighton N, Lord RV, et al. Survival, mortality and morbidity outcomes after oesophagogastric cancer surgery in New South Wales, 2001-2008. Med J Aust 2014;200:408–413. [DOI] [PubMed] [Google Scholar]
- 64. Wainess RM, Dimick JB, Upchurch GR, Jr., et al. Epidemiology of surgically treated gastric cancer in the United States, 1988-2000. J Gastrointest Surg 2003;7:879–883. [DOI] [PubMed] [Google Scholar]
- 65. Damhuis RA, Meurs CJ, Dijkhuis CM, et al. Hospital volume and post-operative mortality after resection for gastric cancer. Eur J Surg Oncol 2002;28:401–405. [DOI] [PubMed] [Google Scholar]
- 66. Gruen RL, Pitt V, Green S, et al. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA Cancer J Clin 2009;59:192–211. [DOI] [PubMed] [Google Scholar]
- 67. Ji J, Shi L, Ying X, et al. Associations of annual hospital and surgeon volume with patient outcomes after gastrectomy: a systematic review and meta-analysis. Ann Surg Oncol 2022;29:8276–8297. [DOI] [PubMed] [Google Scholar]
- 68. Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364–1369. [DOI] [PubMed] [Google Scholar]
- 69. Mamidanna R, Ni Z, Anderson O, et al. Surgeon volume and cancer esophagectomy, gastrectomy, and pancreatectomy: a population-based study in England. Ann Surg 2016;263:727–732. [DOI] [PubMed] [Google Scholar]
- 70. Callahan MA, Christos PJ, Gold HT, et al. Influence of surgical subspecialty training on in-hospital mortality for gastrectomy and colectomy patients. Ann Surg 2003;238:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surg 2002;131:6–15. [DOI] [PubMed] [Google Scholar]
- 72. Schuster KM, Hazelton JP, Rattigan D, et al. Association of acute care surgeon experience with emergency surgery patient outcomes and mortality. JAMA Surg 2021;156:472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Saied NN, Helwani MA, Weavind LM, et al. Effect of anaesthesia type on postoperative mortality and morbidities: a matched analysis of the NSQIP database. Br J Anaesth 2017;118:105–111. [DOI] [PubMed] [Google Scholar]
- 74. Smith DL, Elting LS, Learn PA, et al. Factors influencing the volume-outcome relationship in gastrectomies: a population-based study. Ann Surg Oncol 2007;14:1846–1852. [DOI] [PubMed] [Google Scholar]
- 75. Rocque GB, Williams CP, Miller HD, et al. Impact of travel time on health care costs and resource use by phase of care for older patients with cancer. J Clin Oncol 2019;37:1935–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Crochet P, Aggarwal R, Dubb SS, et al. Deliberate practice on a virtual reality laparoscopic simulator enhances the quality of surgical technical skills. Ann Surg 2011;253:1216–1222. [DOI] [PubMed] [Google Scholar]
- 77. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013;217:833–842.e831-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Corey KM, Kashyap S, Lorenzi E, et al. Development and validation of machine learning models to identify high-risk surgical patients using automatically curated electronic health record data (Pythia): a retrospective, single-site study. PLoS Med 2018;15:e1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hollenbeck BK, Dunn RL, Miller DC, et al. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol 2007;25:91–96. [DOI] [PubMed] [Google Scholar]
- 80. Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA 2000;283:1159–1166. [DOI] [PubMed] [Google Scholar]
- 81. Antunez AG, Kanters AE, Regenbogen SE. Evaluation of access to hospitals most ready to achieve national accreditation for rectal cancer treatment. JAMA Surg 2019;154:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009;360:491–499. [DOI] [PubMed] [Google Scholar]
- 83. Aiken LH, Sloane DM, Bruyneel L, et al. Nurse staffing and education and hospital mortality in nine European countries: a retrospective observational study. Lancet 2014;383:1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated and analyzed during this study are included in this article. The data supporting the findings of this study are available from the corresponding author upon reasonable request.


