Background:
Targeted axillary dissection (TAD) includes biopsy of clipped lymph node and sentinel lymph nodes. However, clinical evidence regarding clinical feasibility and oncological safety of non-radioactive TAD in a real-world cohort remains limited.
Methods:
In this prospective registry study, patients routinely underwent clip insertion into biopsy-confirmed lymph node. Eligible patients received neoadjuvant chemotherapy followed by axillary surgery. Main endpoints included the false-negative rate (FNR) of TAD and nodal recurrence rate.
Results:
Data from 353 eligible patients were analyzed. After completion of neoadjuvant chemotherapy, 85 patients directly proceeded to axillary lymph node dissection (ALND), furthermore, TAD with or without ALND was performed in 152 and 85 patients, respectively. Overall detection rate of clipped node was 94.9% (95% CI, 91.3–97.4%) and FNR of TAD was 12.2% (95% CI, 6.0–21.3%) in our study, with FNR decreasing to 6.0% (95% CI, 1.7–14.6%) in initially cN1 patients. During a median follow-up of 36.6 months, 3 nodal recurrences occurred (3/237 with ALND; 0/85 with TAD alone), with a 3-year freedom-from-nodal-recurrence rate of 100.0% among the TAD-only patients and 98.7% among the ALND patients with axillary pathologic complete response (P=0.29).
Conclusions:
TAD is feasible in initially cN1 breast cancer patients with biopsy-confirmed nodal metastases. ALND can safely be foregone in patients with negativity or a low volume of nodal positivity on TAD, with a low nodal failure rate and no compromise of 3-year recurrence-free survival.
Keywords: Clinical feasibility, neoadjuvant chemotherapy, oncological safety, targeted axillary dissection
Introduction
Highlights
This article is the first prospective study to show that the false-negative rate of non-radioactive targeted axillary dissection (TAD) was acceptable in patients (6.0%) with initially cN1 diseases as compared with those with initially cN2/3 diseases.
This prospective registry study demonstrated the oncological safety of non-radioactive TAD alone even with low volume nodal positivity.
The study revealed that preoperative ultrasound-guided localization might help to decrease the false-negative rate of non-radioactive TAD (instead of localization by mammography).
Neoadjuvant chemotherapy (NACT) was initially administrated in locally advanced breast cancer to render them surgically operable or eligible for breast-conserving surgery1. Nowadays with the advances of chemotherapy and targeted therapy regimens, NACT is increasingly used in early-stage breast cancer to enable in vivo chemosensitivity, thus tailoring adjuvant treatment2,3. Subsequently, pathologic complete response (pCR) in breast and axilla can be achieved in a substantial proportion of patients with indications for the most favourable survival, which further allows for the opportunity to de-escalate surgery and spare the patients morbidities caused by unnecessary operations4,5. Specifically, in initially pathologically-proven node-positive breast cancer, it is estimated that nodal disease can be eradicated in approximately 40% of patients undergoing NACT, and axillary pCR rate can reach to as high as 50-60% in estrogen receptor (ER)-negative, human epidermal growth factor receptor 2 (HER2)-positive and triple-negative breast cancer6. However, it still remains a matter of debate concerning the optimal surgical management in the axilla for this patient population7–9.
The accuracy of sentinel lymph node biopsy (SLNB) after NACT in initially node-positive breast cancer has been extensively investigated in three consecutive large prospective trials including ACOSOG Z1071, SENTInel neoadjuvant (SENTINA), and sentinel node biopsy following neoadjuvant chemotherapy (SN FNAC)10–12. Despite the collectively negative results that overall false-negative rate (FNR) exceeded 10% in these three studies, subgroup analyses established that FNR can be lowered to less than 10% if dual-agent tracers are used or more than three SLNs are retrieved intraoperatively13. Furthermore, several new techniques enabling the removal and evaluation of metastatic lymph node at diagnosis have been developed to improve the accuracy to assess nodal status after NACT14,15. One of those techniques, marking metastatic lymph node with radioactive iodine seeds (MARI), has been proven to accurately determine the status of the axilla after NACT with an acceptable FNR of 7%, but it cannot be globally applied as radioactive iodine seeds are not approved for such use in some countries, limiting its use in clinical practice16. The issue also applies to a novel surgical technique named targeted axillary dissection (TAD) proposed by MD Anderson Cancer Center (MDACC)17. TAD involves a combination biopsy of SLNs and clipped lymph node (CLN) localized by ultrasound with iodine-125 radioactive seeds, which ultimately yields a very low FNR of 2.0%18. Recent data from the SenTa study demonstrated the feasibility of non-radioactive TAD without radioactive iodine seeds placed prior to NACT in a multicenter cohort of early breast cancer patients19. In consequence, clip placement of biopsy-proven metastatic node has been recommended in the national guidelines20. However, whether TAD can apply to initially cN2/3 patients in clinical practice remained unknown. Two retrospective studies from Mayo Clinic and Memorial Sloan Kettering Cancer Center (MSKCC) reported an extremely low nodal failure rate in this patient group with omission of axillary lymph node dissection (ALND) after NACT, with a regional recurrence rate of 0.6% and 0.4%, respectively21,22. However, the availability of outcomes data regarding oncological safety of non-radioactive TAD alone is limited.
The goal of this prospective registry study was to investigate the clinical feasibility and oncological safety of non-radioactive TAD after NACT without the use of radioactive iodine seeds in a single-centre, real-world cohort of breast cancer patients.
Methods
Patient enrolment
Early breast cancer patients aged above 18 and below 70 years with biopsy-confirmed, clinically T1–4, N1–3, and M0 disease were eligible for this study. Enroled patients were initially present with histologically-confirmed lymph node diagnosed with core-needle biopsy (CNB) or fine-needle aspiration (FNA). Patients with distant metastases, inflammatory or extramammary breast cancer, pregnancy, contraindications to surgery were excluded. Patients were staged according to the AJCC staging system as cN1 (disease in movable axillary lymph nodes), cN2 (disease in fixed or matted axillary lymph nodes), or cN3 (pathologically confirmed or initially highly suspected ipsilateral supraclavicular node metastases on image examination). As NACT could not be fully determined prior to enrolment, the current analysis only included patients who underwent completion of NACT followed by axillary surgery. All participants provided written informed consent and were enroled after study approval by all relevant ethics committees, and this study was registered with concerning authorities.
Study procedures
Details had been described in a previous study23. Briefly, if nodal metastasis was confirmed pathologically, a clip was placed in the biopsied node under ultrasound prior to NACT. After NACT, the CLN was visualized by mammography or ultrasound. If the CLN was visible, mammography- or ultrasound-guided wire localization was conducted before surgery. We had changed the titanium clip (before 2017, visible by mammography) to another type of clip with improved visualization under ultrasound (Breast Tissue marker ultrasound enhanced coil, BARD, #864017D) in our centre since 2017, instead of mammography, an experienced ultrasound radiologist who was in charge of clip placement localized the CLN with hook wire under ultrasound (Supplementary Figure 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A420). Of note, for the patients with multiple abnormal nodes or N3 disease under ultrasound at diagnosis, only one clip is placed into the pathologically-proven lymph node.
After the completion of NACT, the surgical approach was determined by the treating breast surgical oncologist. Commonly, TAD, which included the excision of both SLNs and CLN, was suggested but not mandatory. Instead of attempting TAD, breast surgeons had the option to directly perform ALND, mainly based on imaging- and biopsy-related results indicating a low possibility to achieve pCR, which included serial 18F-FDG PET/computed tomography, second CNB in breast, or preoperative targeted FNA of CLN24–26. Notably, clinically positive nodal status after NACT (ypN+) was not deemed as a contraindication of TAD in our centre. Axillary surgery was performed according to the discretion of the surgeons: with or without TAD, and/or ALND. For SLNB, lymphatic mapping was conducted by single tracer (radiolabeled colloid or blue dye) or both. During the surgery, CLN and SLNs together were detected and defined as targeted axillary nodes (TANs). If SLNB was not successful, any lymph nodes surrounding CLN detected by intraoperative palpation could also be submitted as TANs. The specimens were imaged by intraoperative X-ray for confirming the removal of the CLN. All the detected TANs were submitted as TAD specimens and then sent to the pathology department for final histopathologic evaluation.
Of note, between March 2014 and January 2017, we firstly evaluated the accuracy and feasibility of TAD in our centre. Hence all the patients ultimately received ALND to determine the pathological status of axillary lymph nodes during this period regardless of TAD results. After verification, we began to incorporate TAD surgical operation into clinical practice afterwards.
Response and pathology evaluation
ER and progesterone receptor status were evaluated as positive or negative, with positive defined as 1% or higher, and HER2 positivity defined as 3+ staining by immunohistochemistry (IHC) for the HER2 protein or gene amplification by fluorescence in situ hybridization. ycN0 was defined by the absence of suspicious nodes on ultrasound imaging and physical examination after completion of NACT, whereas ycN+ was defined by the presence of suspicious nodes on ultrasound imaging or physical examination after NACT.
During the TAD, the majority of surgically harvested TANs were subjected to intraoperative touch imprint cytology (ITPC), which made non-radioactive TAD more clinically feasible in our centre. As mentioned before, breast surgeons perform ALND if ITPC turned to be positive and TAD alone was conducted if it is negative since 2017. In terms of negative-false cases present in ITPC, whether to further perform ALND was left to multi-disciplinary discussion. Commonly, ALND could be omitted in terms of no more than two micrometastases and isolated tumour cells (ITCs) in the TANs. TANs were ultimately sent for histopathologic assessment and subjected to conventional hematoxylin and eosin staining and serial sectioning according to national guidelines. IHC was not mandatory in our study. In terms of unclear findings or lobular carcinoma, IHC was performed. Any other lymph nodes excised during ALND were subjected to conventional histology only. ypN0 was defined by the absence of viable tumour cells. In addition to macrometastases, residual tumour deposits of any size including micrometastases and ITCs were considered positive. The pathological response of the breast was evaluated using the Miller and Payne grading score devised by Ogston et al. 27.
Treatments and follow-up
Most of enroled patients preoperatively received standard chemotherapy regimens according to the current national and international guidelines, which included anthracycline/taxane and carboplatin/paclitaxel-based regimens. All the patients with HER2-positive tumours received HER2-targeted therapy. Administration of adjuvant chemotherapy and radiotherapy was left primarily to the discretion of the treating physicians.
The patients were regularly followed up postoperatively every 3–6 months for the first 5 years and annually afterwards. Nodal recurrence was defined as a recurrence in the ipsilateral axillary, supraclavicular, or internalmammary nodal basins. Local recurrence was defined as an ipsilateral breast tumour recurrence, and distant recurrence included any distant metastases. Time to recurrence was calculated from date of surgery. Patients were censored at time of recurrence or death, or at last known follow-up.
Statistical analysis
The accuracy of TAD was calculated per patient, and corresponding statistical analyses were carried out using exact (Clopper–Pearson) confidence limits for the binomial proportion. Cox regression analyses were done using to determine the independent prognostic factors of recurrence-free survival (RFS), initially by univariate analysis, followed by multivariate analysis for factors with P less than 0.05. Statistical analyses were performed using STATA statistical software (version 12.0; StataCorp LLC). A two-sided P value of 0.05 was defined as significant. The work has been reported in line with the STARD (Standards for the Reporting of Diagnostic accuracy studies) criteria, Supplemental Digital Content 1, http://links.lww.com/JS9/A419.
Results
Baseline characteristics of patients
Between March 2014 and April 2021, 353 consecutive patients with node-positive breast cancer underwent clip placement into biopsy-confirmed lymph node (Fig. 1). Of these patients, a total of 322 patients met eligibility criteria and were prospectively enroled in our study (Fig. 1).
Figure 1.
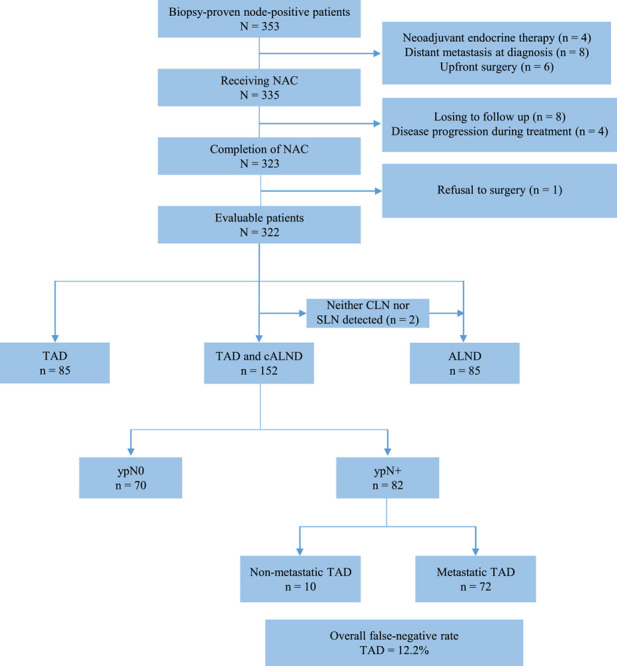
Study flowchart of our study. ALND, axillary lymph node dissection; CLN, clipped lymph node; NACT, neoadjuvant chemotherapy; SLN, sentinel lymph node; TAD, targeted axillary dissection.
The median age was 47 years (range, 20–70 years). Of these patients, 269 (83.5%) had cN1, 44 (13.7%) had cN2, and 9 (2.8%) had cN3 disease. A chemotherapy regimen combining anthracyclines and taxanes was administered to 112 of 322 patients (34.8%). Carboplatin and paclitaxel were given to 169 patients (52.5%). Axillary pCR was attained in 168 patients (pCR rate: 53.8%), varying by biologic subtype with 68.2% in ER−/HER2+, 62.1% in ER−/HER2-, 60.4% in ER+/HER2+, and 20.7% in ER+/HER2− cases. Furthermore, no residual disease (ductal carcinoma in situ is allowed) in breast was observed in 141 patients (40.1%).
According to different axillary surgeries, patients consisted of three groups: TAD (n=85), TAD with ALND (n=152) and ALND (n=85). Among 237 patients successfully undergoing TAD, single mapping agent for SLNB was adopted in 170 patients, including 47.6% with blue dye radiolabeled colloid alone and 52.4% with blue dye alone (Table 1). Baseline clinicopathological and treatment characteristics of 322 patients by axillary surgery were displayed in Table 1.
TABLE 1.
Clinicopathological and treatment characteristics of study cohort.
| Characteristics | Overall (n=322), n (%) | TAD (n=85), n (%) | TAD + ALND (n=152), n (%) | ALND (n=85), n (%) |
|---|---|---|---|---|
| Age (year) | ||||
| Median (range) | 47 (20–70) | 47 (28–70) | 46.5 (20–69) | 48 (29–69) |
| Menopausal status | ||||
| Premenopausal | 173 (53.7) | 40 (47.1) | 94 (61.8) | 39 (46.3) |
| Menopausal | 149 (46.3) | 45 (52.9) | 58 (38.2) | 46 (53.7) |
| Time period at surgery | ||||
| 2014–2016 | 128 (39.8) | 17 (20.0) | 94 (61.8) | 17 (21.3) |
| 2017–2018 | 99 (30.7) | 21 (24.7) | 33 (21.7) | 35 (37.5) |
| 2019–2021 | 95 (29.5) | 47 (55.3) | 25 (16.5) | 33 (41.2) |
| Clinical T stage | ||||
| 1a | 44 (13.6) | 18 (21.2) | 21 (13.8) | 5 (5) |
| 2 | 209 (64.0) | 52 (61.2) | 100 (75.8) | 57 (67.5) |
| 3 | 55 (17.1) | 13 (15.3) | 26 (17.1) | 16 (20.0) |
| 4 | 14 (4.3) | 2 (2.3) | 5 (3.3) | 7 (7.5) |
| Clinical N stage | ||||
| 1 | 269 (83.5) | 76 (89.4) | 128 (84.2) | 65 (76.5) |
| 2–3 | 53 (16.5) | 9 (10.6) | 24 (15.8) | 20 (23.5) |
| Biologic subtype | ||||
| ER+/HER2− | 87 (27.0) | 11 (12.9) | 39 (25.7) | 37 (43.5) |
| ER+/HER2+ | 81 (25.2) | 29 (34.1) | 37 (24.3) | 15 (17.6) |
| ER−/HER2+ | 88 (27.3) | 25 (29.4) | 44 (28.9) | 19 (22.4) |
| ER−/HER2− | 66 (20.5) | 20 (23.5) | 32 (21.1) | 14 (16.5) |
| Ki67 | ||||
| <20% | 34 (10.6) | 4 (4.7) | 18 (11.8) | 12 (13.8) |
| ≥20% | 288 (89.4) | 81 (95.3) | 134 (88.2) | 73 (86.2) |
| NACT regimen | ||||
| Anthracycline/taxanes | 112 (34.8) | 31 (36.5) | 43 (28.3) | 38 (42.5) |
| Carboplatin/paclitaxel | 169 (52.5) | 48 (56.5) | 89 (58.6) | 32 (38.8) |
| Other | 41 (12.7) | 6 (7.0) | 20 (13.1) | 15 (18.7) |
| Neoadjuvant anti-HER2 therapy | ||||
| Trastuzumab | 124 (38.5) | 35 (41.2) | 65 (42.8) | 24 (28.2) |
| Trastuzumab + Pertuzumab/Pyrotinib | 45 (14.0) | 19 (22.4) | 16 (10.5) | 10 (11.8) |
| Clinical N stage after NACT | ||||
| ycN0 | 242 (75.2) | 76 (89.4) | 121 (79.6) | 45 (52.9) |
| ycN+ | 80 (24.8) | 9 (10.6) | 31 (20.4) | 40 (47.1) |
| SLN mapping agent | ||||
| Single | 170 (52.8) | 60 (70.6) | 110 (72.3) | 0 |
| Dual | 67 (20.8) | 25 (29.4) | 42 (27.7) | 0 |
| Not used | 85 (26.4) | 0 | 0 | 85 (100.0) |
| Breast surgery | ||||
| Lumpectomy | 112 (34.8) | 47 (55.3) | 48 (31.6) | 17 (20.0) |
| Mastectomy | 210 (65.2) | 38 (44.7) | 104 (68.4) | 68 (80.0) |
| Breast pCR | ||||
| Yes | 141 (40.1) | 58 (68.2) | 67 (44.1) | 16 (18.8) |
| No | 181 (59.9) | 27 (31.8) | 85 (55.9) | 69 (81.2) |
| Axillary pCR | ||||
| Yes | 168 (52.2) | 75 (88.2) | 70 (46.1) | 23 (28.8) |
| No | 154 (47.8) | 10 (11.8) | 82 (53.9) | 62 (71.2) |
| ITCs only | 3 (0.9) | 2 (2.4) | 1 (0.7) | 0 |
| Micrometastases only | 16 (5.0) | 5 (5.9) | 11 (7.2) | 0 |
| Macrometastases | 135 (41.9) | 3 (3.5) | 70 (46.1) | 62 (71.2) |
ALND, axillary lymph node dissection; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; ITC, isolated tumour cell; NACT, neoadjuvant chemotherapy; pCR, pathologic complete response; SLN, sentinel lymph node.
Including four cases of occult breast cancer.
FNR and detection rate of TAD
As shown in Fig. 1, TAD was initially attempted in 239 patients (including TAD with or without ALND). Neither CLN nor SLNs were detected in two patients, and these two patients subsequently received ALND. The clip was discovered in the ALND specimen. Of remaining 237 patients, CLNs were successfully detected in 225 patients, with 6 failure cases by mammography and other 6 cases by ultrasound. Instead of going back to locate the previously clipped node, the CLN was affirmed to be removed in the ALND specimen postoperatively of these 12 patients via X-ray. As a result, nodal staging was based on SLNB in 12 patients with failure in the detection of CLN. SLN mapping was routinely performed in these 237 patients, 81 (34.2%) had mapping performed with blue dye only, 89 (37.6%) had mapping with radiolabeled colloid only, and 67 (28.3%) had mapping with both blue dye and radiolabeled colloid. No SLNs were found intraoperatively in 10 patients, of whom 7 cases only one CLN and 3 cases the CLN in addition to palpable lymph nodes surrounding CLN were submitted to pathological evaluation, respectively.
ALND was performed in 152 of 239 patients with TAD (Fig. 1), rendering these patients evaluable for assessment of FNR in TAD. Of 152 patients, 70 (45.3%) were ypN0 and 82 (54.7%) were ypN+ (Fig. 1). Specifically, residual nodal disease was confined to the TANs in 27 patients (32.9%), confined to the nodes removed on ALND in 10 patients (12.2%), and present in nodes from both procedures in 45 patients (54.9%). Thus, the FNR of TAD was 12.2% (10 of 82, 95% CI: 6.0–21.3) and the negative-predictive value was 87.5% (70 of 80, 95% CI: 78.2–93.8). Hence, the TANs accurately predicted axillary nodal status in 142 of 152 patients (93.4%, 95% CI: 88.2–96.8).
Interestingly enough, the FNR of TAD was lowered to 6.0% in initially cN1 patients (4 of 67, 95% CI: 1.7–14.6); however, calculations were based on small sample sizes and did not gain enough statistical power. Based on several previously reported factors influencing the accuracy of SLNB after NACT separating initially cN1 and cN2/3 patients, subgroup analyses revealed that localizing method via ultrasound and time period (2017–2021) might be associated with enhanced accuracy of TAD. Surprisingly, limited effectiveness in reducing FNR was observed with adoption of dual SLN mapping agents as compared with a single agent in light of non-radioactive TAD (Table 2).
TABLE 2.
False-negative rate of TAD for patients receiving TAD and ALND differentiating with initially cN1 and cN2/3 patients.
| Variables | Including cN2 or cN3, n/N (%) | 95% CI | Excluding cN2 or cN3, n/N (%) | 95% CI |
|---|---|---|---|---|
| Adoption of IHC | ||||
| Yes | 10/82 (12.2) | 6.0–21.3 | 4/67 (6.0) | 1.7–14.6 |
| No | 11/82 (13.4) | 6.9–22.7 | 4/67 (6.0) | 1.7–14.6 |
| No. TANa identified | ||||
| 1 | 2/10 (20.0) | 2.5–55.6 | 1/9 (11.1) | 0.3–48.2 |
| 2 | 1/18 (5.6) | 0.1–27.3 | 0/13 (0) | 0.0–24.7 |
| >2 | 7/54 (13.0) | 5.4–24.9 | 3/45 (6.7) | 1.4–18.3 |
| Time period | ||||
| 2014–2016 | 7/45 (15.6) | 6.5–29.5 | 4/35 (11.4) | 3.2–26.7 |
| 2017–2021 | 3/37 (8.1) | 1.7–21.9 | 0/32 (0) | 0.0–10.9 |
| Clinical T stage | ||||
| T1–T2 | 7/60 (11.7) | 4.8–22.6 | 4/53 (7.5) | 2.1–18.2 |
| T3–T4 | 3/22 (13.6) | 2.9–34.9 | 0/14 (0) | 0.0–23.2 |
| SLN mapping agent used | ||||
| Single | 7/57 (12.3) | 5.1–23.7 | 3/48 (6.3) | 1.3–17.2 |
| Dual | 3/25 (12.0) | 2.5–31.2 | 1/19 (5.3) | 0.1–26.0 |
| Detection of clipped node | ||||
| Mammography | 5/42 (11.9) | 4.0–25.6 | 3/33 (9.1) | 1.9–24.3 |
| Ultrasound | 2/34 (5.9) | 0.7–19.7 | 0/30 (0) | 0.0–11.6 |
| Not detected | 3/6 (50.0) | 11.8–88.2 | 1/4 (25.0) | 0.6–80.6 |
including clipped lymph nodes or sentinel lymph nodes.
ALND, axillary lymph node dissection; IHC, immunohistochemistry; SLN, sentinel lymph node; TAD, targeted axillary dissection; TAN, targeted axillary node.
Nodal recurrence and survival by axillary surgery and pathological nodal status
During a median follow-up period of 36.6 months, a total of 28 patients developed recurrence, including 3 cases with regional lymph node recurrence, 2 cases with local recurrence and remaining 23 cases with distant recurrence. Only two patients developed distant recurrence in TAD-only group, including one present with ITC only and another with ypN0 in TAD results. Notably, they both received adjuvant nodal irradiation.
As shown in Fig. 2, the Kaplan–Meier curves by axillary surgery (ALND ± TAD or TAD alone) and pathologic nodal response (ypN0/N+) showed that the patients with ypN0 who had TAD only experienced the best outcomes, similar to those with ypN0 who had ALND ± TAD surgery [P=1.000 for nodal recurrence-free survival and P=0.297 for RFS], and those patients who had ypN+ with ALND experienced the worst outcomes (P=0.195 for NFRS and P=0.002 for RFS).
Figure 2.

Kaplan–Meier estimates of freedom from nodal recurrence and recurrence-free survival by axillary surgery and pathologic nodal response to neoadjuvant chemotherapy. ALND, axillary lymph node dissection; TAD, targeted axillary dissection.
The rate for 3-year nodal recurrence-free survival was 0% for the TAD patients and 98.7% for the ALND patients (P=0.287; Supplementary Figure 2, Supplemental Digital Content 3, http://links.lww.com/JS9/A421). And RFS was significantly better for the TAD-only patients than for the ALND patients (98.8% vs. 89.9% at 3 years; P=0.022; Supplementary Figure 2, Supplemental Digital Content 3, http://links.lww.com/JS9/A421). Multivariate Cox regression analyses also showed that TAD alone had no significant prognostic impact in recurrence-free survival after adjusting for the related confounding factors (P=0.60, Supplementary Table 1, Supplemental Digital Content 4, http://links.lww.com/JS9/A422).
Discussion
TAD has shown promise and accuracy in nodal staging after NACT for initially node-positive breast cancer patients; however, widespread use of this technique was somewhat limited for the radioactive seed. As far as we concerned, it was the first real-world study enroling a substantial number of patients to evaluate the feasibility and safety of non-radioactive TAD in Asian population. Importantly, our study firstly demonstrated that TAD was clinically feasible in initially cN1 patients, and our findings revealed a low nodal recurrence rate achieved by TAD alone in patients with negativity even a low nodal positivity on TAD results.
NACT has been widely accepted as a standard of care in early-stage HER2-positive breast cancer and triple-negative breast cancer, resulting from clinical trials supporting its prognostic role indicating potential benefit for continuing adjuvant systemic treatment in terms of residual disease after NACT3. Correspondingly, there is a continuous effort to explore a less-invasive procedure to surgically stage axilla after NACT. Apart from SLNB, marking metastatic lymph node at diagnosis also serves as an option to evaluate nodal response after NACT, allowing for the development of some novel surgical techniques such as TAD, MARI, clipped lymph node biopsy and so on14. Non-radioactive TAD without the use of radioactive iodine-125 seeds also has showed promising results and gained popularity in some countries for the regulatory issues concerning the use of radioactive iodine-125 seeds28–33. In the SenTa study, FNR was 4.3% for non-radioactive TAD followed by ALND19. As a comparison, 10 false-negative events occurred in our study, yielding a FNR of 12.2%, higher than 10% cut point defined in the trial as an acceptable FNR. We speculated that the differences in SLN mapping agents and levels of experience might result in the discrimination between two studies with a similar design. More importantly, more than 70% of recruiting patients in SenTa study presented with no more than two suspicious lymph nodes at diagnosis while our study enroled more advanced breast cancer with a proportion of initially cN2/3 patients. Accordingly, it was noteworthy that FNR decreased to 6.0% in initially cN1 patients and the FNR was unacceptably high among initially cN2/3 patients. Of note, a study by MDACC revealed that presence of greater than or equal to 4 abnormal nodes on initial ultrasound contributed to the inability to identify the CLN as an SLN and thus inaccuracy of TAD to stage the axilla, which, to some extent, corroborated our findings18. We speculated that differential axillary nodal response to NACT in patients with multiple pathologically positive lymph nodes, and evaluation of more clipped metastatic nodes might render a more accurate evaluation of post-NACT axillary status34.
In routine practice, initial pre-NACT nodal burden of disease (the clinical nodal status), which indicates locoregional recurrence risks, greatly impacts the decision whether to attempt TAD or directly proceed to ALND35. With the progresses in chemotherapy and targeted treatment regimen in recent years, contemporary data have also demonstrated a multimodality therapy approach utilizing neoadjuvant systemic therapy, surgery, and adjuvant radiotherapy could lead to long-term disease-free and overall survival in a subset of N3 patients even without extensive dissection36,37. In addition, a study by Gentile et al. 38 reported that tumour biology (subtypes), but not extent of disease prior to NACT (clinical T or N stage), predicted nodal pCR in patients with locally advanced breast cancer. Therefore, it is of substantial importance to individually select patients with an excellent response to NACT to de-escalate breast or axillary surgery. Rather than clinical trials, in our practice, for patients with clip placement in our centre, TAD is selectively attempted, and the decision to perform TAD is comprehensively determined by preoperative evaluation of treatment response as well as breast cancer subtypes. Based on a series of previous studies by our group, we demonstrated serial18 F-FDG PET/computed tomography, second CNB in breast, or preoperative targeted FNA of CLN could serve as diverse accurate modalities to predict response of NACT and thus distinguish good responders who are unlikely candidates of less-invasive TAD or poor responders suitable to directly proceed to ALND24–26.
Additionally, subgroup analyses were conducted based on several previously reported factors, differentiating with including and excluding cN2/3 patients. Interestingly enough, we found that clip localization by ultrasound might improve the accuracy of TAD as compared with mammography, which was not previously reported by other studies. In our centre, ultrasound localization might be indeed helpful to detect CLN, for clip insertion was also conducted under ultrasound at diagnosis, which theoretically enhanced operating homogeneity. Furthermore, we speculated that it was, to a great extent, attributable to improved visualization of clipped node with another type of clip visible by ultrasound since 2017. However, the existence of learning curve and experience accumulation in TAD technique at early stage should also be considered, coinciding with the localization method transition from mammography to ultrasound following by the advance of time. However, more evidence should be accumulated to support superiority of ultrasound-guided localization in the future.
Following the previous TAD study in MDACC without routine utility of dual agents mapping SLN17,18, our findings further affirmed that non-radioactive TAD might not necessarily require dual agents, for limited benefit in reducing FNR was seen in our study with simultaneous adoption of two mapping agents. Furthermore, failure in SLN detection occurred intraoperatively in 10 patients, only one clipped node and clipped node in addition to palpable surrounding lymph nodes were detected and submitted to pathological evaluation in seven and three cases, respectively. However, it turned out with no presence of false-negative cases with TAD in this setting. Of note, our data was consistent with previous series with regard to TAD or similar procedures, data has shown that an acceptably low FNR can be obtained with clipped node biopsy alone, including 4.2% with TAD procedure in MDACC study and 7% with MARI procedure in Netherlands study16,18.
Despite of several studies to evaluate the feasibility of TAD in clinical studies, evidence remained limited regarding the long-term survival of TAD. Our study firstly demonstrated the oncological safety of TAD alone as compared with patients present with ypN0 and receiving ALND during the same period. It was noteworthy that none of the TAD-only patients in our study experienced axillary failure, and none of the 10 patients with a positive TAN (ypN1) who did not receive ALND had axillary failure, even though only one of these patients developed distant metastases. Similarly, the oncological safety of SLNB alone in clinically node-positive patients has been demonstrated in some studies21,22,39. Besides, we speculated that exceptional lack of locoregional recurrence in the TAD group could be partly attributed to the administration of nodal regional radiotherapy, given that ~65% of patients receiving adjuvant nodal irradiation in our study (data not shown), and it was also reflected by data from MSKCC showing that only one axillary nodal recurrence synchronous with local recurrence occurred in a patient who refused radiotherapy22. Additionally, data from two retrospective studies consistently revealed that adjuvant regional nodal radiotherapy reduced the risk of locoregional recurrence in patients with breast cancer with axillary metastases who underwent NACT40,41. It was noted that these data should be viewed in light of the retrospective nature and the selected use of radiotherapy in the patient population. More importantly, the multivariable Cox regression analyses further supported the idea that TAD is a sufficient axillary treatment, not only when the TAN is pN0 but also in some selected patients with low nodal positivity (pN1). However, these findings deserved further validation in a large population of patients and the results of ongoing Alliance 011202 trials will examine the clinical benefit in selected group of patients with a low volume of residual nodal disease42,43.
The limitations of this study included by its performance at a single institution and relatively short median follow-up. Furthermore, our findings that preoperative ultrasound-guided localization might be helpful to detect CLN and decrease the FNR of TAD deserved further investigation and validation in the setting of clinical trials that incorporate a strict design and a homogeneous patient population. And small sample size of patients in subgroup limited subsequent statistical power.
In conclusion, our results demonstrate the clinical feasibility and oncological safety of TAD as a less-invasive method for axillary staging after NACT in initially cN1 breast cancer patients with biopsy-proven involvement of axillary lymph nodes.
Ethical approval
Our study was approved by all relevant ethics committees in Fudan University Shanghai Cancer Center (FUSCC), and relevant Judgement’s reference number was 1706173-6.
Source of funding
This study was supported by the Shanghai Sailing Program (22YF1408600), and the Shanghai Science and Technology Commission (19411966700), including patient recruitment and study management.
Author contribution
Conceptualization: S.-Y.W. and G.-Y.L. Methodology: S.-Y.W., J.-W.L., Y.-J.W., K.-R.J., X.-L.Y., M.M., N.H. and G.-Y.L. Formal analysis: S.-Y.W., J.-W.L. and Y.-J.W. Investigation: J.-W.L., Y.-J.W., B.-L.Y., J.-J.L. and N.H. Resources: N.H., Z.-M.S. and G.-Y.L. Data Curation: S.-Y.W., Y.-J.W., K.-R.J. and N.H. Writing—original draft: S.-Y.W. and J.-W.L. Writing—review and editing: N.H. and G.-Y.L. Visualization: S.-Y.W. and N.H. Supervision: Z.-M.S. and G.-Y.L. Project administration: S.-Y.W., J.-W.L. and G.-Y.L. Funding acquisition: S.-Y.W. and G.-Y.L.
Conflicts of interest disclosure
The authors declare that they have no competing interests.
Research registration unique identifying number (UIN)
1. Name of the registry: https://www.clinicaltrials.gov/.
2. Unique Identifying number or registration ID: ClinicalTrials.gov Identifier: NCT05141630.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/show/NCT05141630.
Guarantor
Si-Yu Wu and Guang-Yu Liu.
Data sharing statement
The datasets used and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Supplementary Material
Acknowledgements
The authors thank all patients and their families who participated in the studies that form the basis of this work.
Footnotes
S.-Y. W. and J.-W. L. contributed equally to this work.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 3 May 2023
Contributor Information
Si-Yu Wu, Email: drwu1187343296@163.com.
Jian-Wei Li, Email: ljwdoctor@163.com.
Yu-Jie Wang, Email: yujie.happy.love@163.com.
Kai-Rui Jin, Email: concept111@126.com.
Ben-Long Yang, Email: yblqhdx@163.com.
Jun-Jie Li, Email: lijunjie_ronaldo@hotmail.com.
Xiao-Li Yu, Email: stephanieyxl@hotmail.com.
Miao Mo, Email: woodenbird026@163.com.
Na Hu, Email: carrothuna@sina.com.
Zhi-Ming Shao, Email: zhiminshao_submission@hotmail.com.
Guang-Yu Liu, Email: liugy688@163.com.
References
- 1. Zardavas D, Piccart M. Neoadjuvant therapy for breast cancer. Annu Rev Med 2015;66:31–48. [DOI] [PubMed] [Google Scholar]
- 2. Killelea BK, Yang VQ, Mougalian S, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg 2015;220:1063–1069. [DOI] [PubMed] [Google Scholar]
- 3. Pusztai L, Foldi J, Dhawan A, et al. Changing frameworks in treatment sequencing of triple-negative and HER2-positive, early-stage breast cancers. Lancet Oncol 2019;20:e390–e396. [DOI] [PubMed] [Google Scholar]
- 4. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164–172. [DOI] [PubMed] [Google Scholar]
- 5. Cutress RI, McIntosh SA, Potter S, et al. Opportunities and priorities for breast surgical research. Lancet Oncol 2018;19:e521–e533. [DOI] [PubMed] [Google Scholar]
- 6. Samiei S, Simons JM, Engelen S, et al. Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease: a systematic review and meta-analysis. JAMA Surg 2021;156:e210891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilewskie M, Morrow M. Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 2017;3:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banys-Paluchowski M, Gasparri ML, de Boniface J, et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA Study. Cancers (Basel) 2021;13:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morrow M, Khan AJ. Locoregional management after neoadjuvant chemotherapy. J Clin Oncol 2020;38:2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013;14:609–618. [DOI] [PubMed] [Google Scholar]
- 11. Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013;310:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33:258–264. [DOI] [PubMed] [Google Scholar]
- 13. Boughey JC, Suman VJ, Mittendorf EA, et al. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in ACOSOG Z1071 (Alliance). Ann Surg 2015;261:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swarnkar PK, Tayeh S, Michell MJ, et al. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) 2021;13:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murthy V, Young J, Tokumaru Y, et al. Options to determine pathological response of axillary lymph node metastasis after neoadjuvant chemotherapy in advanced breast cancer. Cancers (Basel) 2021;13:4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 2015;261:378–382. [DOI] [PubMed] [Google Scholar]
- 17. Caudle AS, Yang WT, Mittendorf EA, et al. Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg 2015;150:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 2016;34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuemmel S, Heil J, Rueland A, et al. A prospective, multicenter registry study to evaluate the clinical feasibility of targeted axillary dissection (TAD) in node-positive breast cancer patients. Ann Surg 2022;276:e553–e662. [DOI] [PubMed] [Google Scholar]
- 20. Brackstone M, Baldassarre FG, Perera FE, et al. Management of the Axilla in Early-Stage Breast Cancer: ontario Health (Cancer Care Ontario) and ASCO Guideline. J Clin Oncol 2021;39:3056–3082. [DOI] [PubMed] [Google Scholar]
- 21. Piltin MA, Hoskin TL, Day CN, et al. Oncologic outcomes of sentinel lymph node surgery after neoadjuvant chemotherapy for node-positive breast cancer. Ann Surg Oncol 2020;27:4795–4801. [DOI] [PubMed] [Google Scholar]
- 22. Barrio AV, Montagna G, Mamtani A, et al. Nodal recurrence in patients with node-positive breast cancer treated with sentinel node biopsy alone after neoadjuvant chemotherapy—a rare event. JAMA Oncol 2021;7:1851–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu S, Wang Y, Zhang N, et al. Intraoperative touch imprint cytology in targeted axillary dissection after neoadjuvant chemotherapy for breast cancer patients with initial axillary metastasis. Ann Surg Oncol 2018;25:3150–3157. [DOI] [PubMed] [Google Scholar]
- 24. Wu S, Wang Y, Li J, et al. Subtype-guided (18) F-FDG PET/CT in tailoring axillary surgery among patients with node-positive breast cancer treated with neoadjuvant chemotherapy: a feasibility study. Oncologist 2020;25:e626–e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Shen J, Wu S, et al. The use of a second core needle biopsy to predict response to neoadjuvant chemotherapy in breast cancer patients, especially in the HER2-positive population. Surgery 2020;168:1115–1121. [DOI] [PubMed] [Google Scholar]
- 26. Wu SY, Li JW, Wu HL, et al. Accuracy of ultrasound-guided targeted fine-needle aspiration in assessing nodal response in node-positive breast cancer after neoadjuvant chemotherapy: prospective feasibility study. Br J Surg 2022;109:1194–1197. [DOI] [PubMed] [Google Scholar]
- 27. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003;12:320–327. [DOI] [PubMed] [Google Scholar]
- 28. Choy N, Lipson J, Porter C, et al. Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol 2015;22:377–382. [DOI] [PubMed] [Google Scholar]
- 29. Plecha D, Bai S, Patterson H, et al. Improving the accuracy of axillary lymph node surgery in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol 2015;22:4241–4246. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann S, Reimer T, Gerber B, et al. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol 2018;44:1307–1311. [DOI] [PubMed] [Google Scholar]
- 31. Siso C, de Torres J, Esgueva-Colmenarejo A, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial) : a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol 2018;25:784–791. [DOI] [PubMed] [Google Scholar]
- 32. Laws A, Dillon K, Kelly BN, et al. Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol 2020;27:4819–4827. [DOI] [PubMed] [Google Scholar]
- 33. Hartmann S, Kuhn T, de Boniface J, et al. Carbon tattooing for targeted lymph node biopsy after primary systemic therapy in breast cancer: prospective multicentre TATTOO trial. Br J Surg 2021;108:302–307. [DOI] [PubMed] [Google Scholar]
- 34. Lim GH, Gudi M, Teo SY, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy? Oncologist 2020;25:e1621–e1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol 2012;30:3960–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brito RA, Valero V, Buzdar AU, et al. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: the University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol 2001;19:628–633. [DOI] [PubMed] [Google Scholar]
- 37. Diao K, Andring LM, Barcenas CH, et al. Contemporary outcomes after multimodality therapy in patients with breast cancer presenting with ipsilateral supraclavicular node involvement. Int J Radiat Oncol Biol Phys 2022;112:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gentile LF, Plitas G, Zabor EC, et al. Tumor biology predicts pathologic complete response to neoadjuvant chemotherapy in patients presenting with locally advanced breast cancer. Ann Surg Oncol 2017;24:3896–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martelli G, Barretta F, Miceli R, et al. Sentinel node biopsy alone or with axillary dissection in breast cancer patients after primary chemotherapy: long-term results of a prospective interventional study. Ann Surg 2022;276:e544–e552. [DOI] [PubMed] [Google Scholar]
- 40. Stecklein SR, Park M, Liu DD, et al. Long-term impact of regional nodal irradiation in patients with node-positive breast cancer treated with neoadjuvant systemic therapy. Int J Radiat Oncol Biol Phys 2018;102:568–577. [DOI] [PubMed] [Google Scholar]
- 41. Haffty BG, McCall LM, Ballman KV, et al. Impact of radiation on locoregional control in women with node-positive breast cancer treated with neoadjuvant chemotherapy and axillary lymph node dissection: results from ACOSOG Z1071 clinical trial. Int J Radiat Oncol Biol Phys 2019;105:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krug D, Baumann R, Budach W, et al. Individualization of post-mastectomy radiotherapy and regional nodal irradiation based on treatment response after neoadjuvant chemotherapy for breast cancer : a systematic review. Strahlenther Onkol 2018;194:607–618. [DOI] [PubMed] [Google Scholar]
- 43. Senkus E, Cardoso MJ, Kaidar-Person O, et al. De-escalation of axillary irradiation for early breast cancer—has the time come? Cancer Treat Rev 2021;101:102297. [DOI] [PubMed] [Google Scholar]


