Background:
The increase in elective surgeries and varied postoperative patient outcomes has boosted the use of patient decision support interventions (PDSIs). However, evidence on the effectiveness of PDSIs are not updated. This systematic review aims to summarize the effects of PDSIs for surgical candidates considering elective surgeries and to identify their moderators with an emphasis on the type of targeted surgery.
Design:
Systematic review and meta-analysis.
Methods:
We searched eight electronic databases for randomized controlled trials evaluating PDSIs among elective surgical candidates. We documented the effects on invasive treatment choice, decision-making–related outcomes, patient-reported outcomes, and healthcare resource use. The Cochrane Risk of Bias Tool version 2 and Grading of Recommendations, Assessment, Development, and Evaluations were adopted to rate the risk of bias of individual trials and certainty of evidence, respectively. STATA 16 software was used to conduct the meta-analysis.
Results:
Fifty-eight trials comprising 14 981 adults from 11 countries were included. Overall, PDSIs had no effect on invasive treatment choice (risk ratio=0.97; 95% CI: 0.90, 1.04), consultation time (mean difference=0.04 min; 95% CI: −0.17, 0.24), or patient-reported outcomes, but had a beneficial effect on decisional conflict (Hedges’ g=−0.29; 95% CI: −0.41, −0.16), disease and treatment knowledge (Hedges’ g=0.32; 95% CI: 0.15, 0.49), decision-making preparedness (Hedges’ g=0.22; 95% CI: 0.09, 0.34), and decision quality (risk ratio=1.98; 95% CI: 1.15, 3.39). Treatment choice varied with surgery type and self-guided PDSIs had a greater effect on disease and treatment knowledge enhancement than clinician-delivered PDSIs.
Conclusions:
This review has demonstrated that PDSIs targeting individuals considering elective surgeries had benefited their decision-making by reducing decisional conflict and increasing disease and treatment knowledge, decision-making preparedness, and decision quality. These findings may be used to guide the development and evaluation of new PDSIs for elective surgical care.
Keywords: decisional conflict, elective surgery, meta-analysis, patient decision support interventions, systematic review, treatment choice
Introduction
Highlights
Patient decision support interventions (PDSIs) had no effect on invasive treatment choice, consultation time, or patient-reported outcomes (PROs).
PDSIs had a beneficial effect on decisional conflict, disease and treatment knowledge, decision-making preparedness, and decision quality.
Treatment choice varied with surgery type.
Self-guided PDSIs had a greater effect on disease and treatment knowledge enhancement than clinician-delivered PDSIs.
The global increase in elective surgeries1,2 and varied postoperative patient outcomes are growing public health concerns3. Undesirable patient outcomes such as postoperative complications3 and decreased health-related quality of life (HRQoL)4 are common. Therefore, surgical decisions should be ‘preference-sensitive’5, namely, guided by patient preferences when several options are available or patient outcomes are uncertain.
PDSIs have been used to enhance the preference-sensitive nature of clinical decision-making. PDSIs present evidence-based information to patients about a health condition, treatment options, and the associated benefits and risks, and implicitly or explicitly clarify the value patients place on the treatment benefits and risks5. The primary goal of PDSIs is to enhance decision-making quality and facilitate patient engagement during consultations6. PDSIs may also assist patients by increasing their knowledge of the available options and outcomes, thereby equipping them with more realistic expectations6.
With the increasing availability of validated PDSIs for elective surgical candidates faced with a treatment decision7–9, it is essential to investigate the effects for various types of elective surgery. In general, PDSIs have been found to improve disease and treatment knowledge5,8,9, satisfaction with decision-making9, decision quality8, and reduce decision conflict5,8,9. However, these reviews included different types of PDSIs8,9, study designs8,9, and elective surgery is quite unique.
Knops et al. 7 concluded that PDSIs increased knowledge and decreased decision conflict, but had no effect on anxiety and postoperative HRQoL. However, the review is outdated7. Therefore, we updated this review with the aims of (1) summarizing the effects of PDSIs on invasive treatment choice, decision-making–related outcomes, PROs, and healthcare resource utilization outcomes for patients considering elective surgery, and (2) identifying the moderators of PDSIs effects, with an emphasis on the type of targeted surgery.
Methods
Protocol and registration
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Supplementary Table 1, Supplemental Digital Content 1, http://links.lww.com/JS9/A238)10. The protocol was registered in the International Prospective Register of Systematic Reviews database (PROSPERO Number: CRD42021273767).
Eligibility criteria
The eligibility criteria is illustrated in Supplementary Table 2 (Supplemental Digital Content 1, http://links.lww.com/JS9/A238). We considered all types of randomized controlled trials (RCTs), including both published and unpublished trials. The trials had to enroll surgical candidates who were contemplating elective surgeries, defined as a surgical procedure that is scheduled in advance because it does not involve a medical emergency. The included trials must have assessed one or more PDSIs, defined as tools designed to inform patients and clinicians of their elective treatment options, surgical or both surgical and nonsurgical, among which none is the undisputable choice to all patients. They could take the form of computer software or physical tools. Comparators had to include an active control group, a standard care group, or a waitlist group. Outcomes had to include invasive treatment choices, decision-making–related outcomes, PROs, and outcomes related to healthcare resource use. The trials were limited to studies published in the English language, but not publication period. We excluded trials in which individuals had cognitive impairment or psychiatric disease.
Information sources and search strategy
A three-step comprehensive search strategy was employed from inception to 30 August 2021, under the guidance of an experienced librarian team. First, we searched eight databases (search engines) (index and key terms provided in Supplementary Table 3, Supplemental Digital Content 1, http://links.lww.com/JS9/A238): Cumulative Index to Nursing and Allied Health Literature (EBSCO), Cochrane Central Register of Controlled Trials (Ovid), Excerpta Medica Database (Elsevier), ProQuest Dissertations and Theses (ProQuest), PsycINFO (Ovid), MEDLINE (PubMed), Scopus (Elsevier), and Web of Science (Clarivate). Second, we examined various clinical trial registries (Supplementary Table 4, Supplemental Digital Content 1, http://links.lww.com/JS9/A238) for relevant ongoing and unpublished trials. Third, we conducted a manual search on the reference lists of the related primary studies or systematic reviews. In addition, we searched specialized journals and grey literature databases for potential trials. The EndNote X20 software was used to manage the references and excluded duplicates11.
Study selection and data extraction
Study selection was graphically illustrated using the PRISMA 2020 flow diagram (Fig. 1). Two reviewers (L.J.C. and M.X.L.) independently screened the titles and abstracts to identify their relevance. When multiple reports of the same study were identified, studies were collated. The potential full texts were selected based on the eligibility criteria, and the reasons for inclusion/exclusion were recorded (Supplementary Table 5, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). Any disagreements were resolved by a third reviewer (N.L.).
Figure 1.
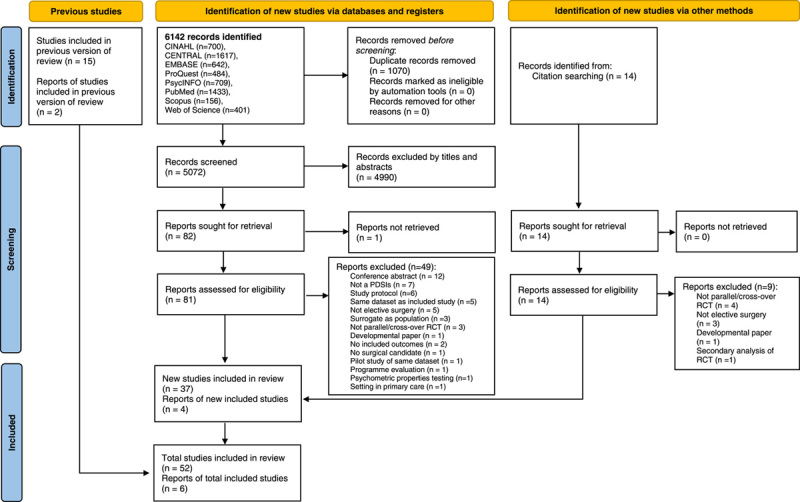
Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 flow diagram for updated systematic reviews. PDSI, patient decision support intervention; RCT, randomized controlled trial.
A standardized data extraction form was developed using the Cochrane Handbook for Systematic Reviews of Interventions12. Three essential components were included: trial characteristics, PDSIs characteristics, and key outcomes related to decision-making, PROs, and healthcare resource use. Based on a reporting guide13,14, the following characteristics of PDSIs were extracted: aim, element, platform, co-intervention, duration, media format, use of PROs data, artificial intelligence embodiment, value consideration, theoretical framework, communication, facilitator, survey administration methods at various time points, and assessment intervals.
Invasive treatment choice included the actual choice of invasive treatment implemented; if not specified, the participant’s preferred option was used as a surrogate measure7. Decision-making–related outcomes (measures) included decisional conflict (different versions of Decisional Conflict Scale15), satisfaction with decision-making (effective decision subscale of Decisional Conflict Scale and self-developed patient satisfaction surveys), disease and treatment knowledge (self-developed questionnaires), decisional regret (Decision Regret Scale16), Preparedness For Decision-Making (Rochester Participatory Decision-Making Scale17 and Preparedness For Decision-Making Scale18), decision quality (Decision Quality Instrument19), Shared Decision-Making (CollaboRATE20 and Nine-Item Shared Decision-Making Questionnaire21), Decision Self-Efficacy (Self-Efficacy For Managing Chronic Diseases Six-Item22 and Decision Self-Efficacy Scale23), and outcome expectations. PROs included HRQoL (general or condition-specific), physical health, mental health, depression, anxiety, and perceived stress. Outcomes related to healthcare resource use included consultation time. Detailed definitions for all extracted outcomes and the measures used are listed in Supplementary Table 6 (Supplemental Digital Content 1, http://links.lww.com/JS9/A238).
The data extraction form was piloted on ten trials to ensure that the items were accurate and appropriate. Two independent reviewers (L.J.C. and M.X.L.) retrieved data from 58 trials following item confirmation. If trials reported data on the median, range, interquartile range, and SE, data conversion was utilized to compute the mean and SD using recommended formulae12,24,25. When data was questionable, insufficient, or missing, trial authors were contacted via email and asked to provide additional unpublished details or results.
Quality of patient decision support interventions design
International Patient Decision Aid Standards instrument short-form (IPDASi-SF) was used to assess the quality of PDSIs26. The IPDASi-SF contains 16 items addressing seven dimensions related to the information about the options, probabilities, values, development, disclosure of funding, decision aid evaluation, and evidence used26. The IPDASi-SF score ranged from 0 to 16, with a higher score indicating better PDSIs quality.
Risk of bias in individual studies
The Cochrane risk of bias (RoB 2.0)27 tool was used to assess the risk of bias in the selected randomised trials on five domains of bias: bias arising from the randomization process, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. Two reviewers (L.J.C. and M.X.L.) independently responded to each of the signaling questions with (1) yes, (2) probably yes, (3) probably no, (4) no, or (5) no information. The RoB 2.0 algorithmic tool rates the risk of bias as (1) low risk of bias, (2) some concerns, or (3) high risk of bias for each domain.
Statistical analysis
The Meta command procedures in Stata 16 software were used to perform a meta-analysis and subgroup analysis28,29. Z-statistics with a significance level of P-value less than 0.05 was used to assess the overall effect30. A minimum of two studies was needed to perform a meta-analysis31. A weighted risk ratio (RR) (dichotomous data), standardized effect size, or mean difference (continuous data) was calculated for each outcome measure32. As most of the trials used a small sample size, the pooled effect sizes of outcomes (continuous data) were assessed using Hedges’ g 33. The effect magnitude was classified as small (≥0.2), medium (≥0.5), large (≥0.8), or very large (≥1.2)34. We used the DerSimonian and Laird procedure to estimate the variability between studies for random-effects meta-analysis35.
Statistical heterogeneity was assessed using I 2 statistics and Cochran’s Q test, with a P-value less than 0.10 indicating evidence of heterogeneity36. The degree of heterogeneity using overlapping intervals for I 2 was set as 0–40% (might not be important), 30–60% (moderate), 50–90% (substantial), and 75–100% (considerable)37. The source of heterogeneity was investigated using subgroup analysis37. For each outcome measure, whenever 10 or more trials were available, separate subgroup analysis was performed to evaluate five moderators coded into categorical variables including: types of elective surgery, patient-reported outcome measures–based PDSIs (yes/no), mode of delivery (self-guided/clinician-administered), value consideration (yes/no), and use of theoretical framework (yes/no).
Reporting bias assessment
We used Begg’s test38, Egger’s regression test39, the asymmetry of the funnel plot40, and the trim-and-fill approach40 to examine publication bias in meta-analyses with 10 or more trials41. Egger regression and Begg’s test were performed using Stata 16 software28, with a P-value more than 0.05 indicating no small-study effects existed. We identified the possibility of heterogeneity in effect sizes across studies, limiting the conclusions drawn from Egger’s tests and the funnel plot42,43. We also applied the Copas selection model, using the metafor package in R software44, to account for selection bias according to funnel plot asymmetry45,46.
Certainty assessment
GRADEpro 3.6 software47 was used to assess the certainty of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach48. The GRADE assessment focuses on five factors: methodological limitations, imprecision, inconsistency, indirectness, and publication bias49. When issues were detected in the five factors, the evidence was downgraded. The certainty of the evidence was graded as high, moderate, low, and very low.
Results
Study selection
Figure 1 illustrates the study selection results. We identified 6142 records and eliminated 1070 duplicates. In addition, citation searching yielded 14 records and 15 studies, as well as two reports, from a previous review7. After two reviewers screened independently, 4990 records were excluded based on their titles and abstracts. Eighty-two full-text articles were selected, and 50 were excluded for several reasons (Supplementary Table 5, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). Finally, 58 trials50–107 were included in the review, with 52 unique trials and six reports linked to the unique trials (Supplementary Table 7, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). The inter-rater agreement of the two reviewers was consistent (κ=0.87, P<0.001).
Trial characteristics
The characteristics of trials, published between 1995 and 2021, are summarized in Table 1. The sample size ranged from 16 to 1485 participants, with a mean age between 23.6 and 72.0 years. The attrition rate varied between 0% and 44.8%. Of these, 52 trials (89.7%) compared PDSIs with usual care, with the remaining six trials comparing different PDSIs. Thirty-two trials (55.2%) were conducted in the USA, with seven in Canada (12.1%), six in The Netherlands (10.3%), three in Australia (5.2%), three in the UK (5.2%), two in Finland (3.4%), and only one in China, Germany, Hong Kong, Spain, and Turkey. Fifty-two trials (89.7%) utilized a two-arm RCT design, two trials used a three-arm RCT and a cluster RCT design, and one trial used a stepped wedge trial design. Most of the trials studied PDSIs developed for patients with neoplasms (number of trials, k=25, 43.1%), diseases of the musculoskeletal system (k=18, 31.0%), or diseases of the genitourinary system (k=8, 13.8%). Most of the PDSIs evaluated involved decisions about elective gynecology and obstetrics surgery (k=21, 36.2%) or orthopedic surgery (k=14, 24.1%).
Table 1.
Characteristics of selected randomized controlled trials
| References/country | Design | Nature of population | Types of elective surgery | Age (mean±SD) | Sample size | Sex (female/male) | Interventions/mode of delivery | Comparisons (comparator) | Outcomes (measures) | Attrition rates (%) | ITT/MDM | Protocol/registration/grant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allen et al. 50/USA | 2-arm RCT | Hip and knee osteoarthritis | Total hip or knee joint replacement | 61.8±11.7 | T: 155 I: 80 C: 75 |
94/61 | Video-based/self-guided | Active control (computer-based) | Decision conflict (DCS); Knowledge (K-DQI); Preparedness for decision-making; Outcome expectations | 5.8 | Y/N | N/N/N |
| Allen et al. 51/USA | 2-arm stepped-wedge RCT | Heart disease | Destination therapy LVAD placement | 63.4±9.9 | T: 248 I: 113 C: 135 |
39/209 | Video-based/clinician | Usual care | Invasive treatment choice; Decisional conflict (DCS); Decisional regret (DRS); General HRQoL (EQ VAS); Depression (PHQ-2); Perceived stress (PSS) | 33.5 | Y/Y | Y/Y/Y |
| Arterburn et al. 52/USA | 2-arm RCT | Obesity-related chronic health condition | Bariatric surgery | 50.5±9.9 | T: 152 I: 75 C: 77 |
111/41 | Video-based/self-guided | Usual care (booklet control) | Invasive treatment choice; Decisional conflict (DCS); Knowledge; Decision self-efficacy (DSES) | 4.6 | Y/N | N/N/Y |
| Auvinen et al. 53/Finland | 2-arm RCT | Prostate cancer | Radical prostatectomy/orchidectomy | 59.9±26.6 | T: 210 I: 104 C: 106 |
0/210 | Paper-based/clinician | Usual care (standardized treatment protocol) | Invasive treatment choice | 3.3 | Y/Y | N N/Y |
| Berry et al. 54/USA | 2-arm RCT | Prostate cancer | Prostatectomy | 57.4±13.5 | T: 392 I: 198 C: 194 |
0/392 | Computer-based/self-guided | Usual care (links+materials provided in clinic) | Decisional conflict (DCS) | 29.6 | N/Y | N/Y/Y |
| Bozic et al. 55/USA | 2-arm RCT | Osteoarthritis | Hip or knee replacement | 60±NR | T: 198 I: 95 C: 103 |
NR/NR | Video-based/self-guided | Usual care | Invasive treatment choice | 37.9 | N/N | N/Y/Y |
| Coylewright et al. 56/USA | 2-arm RCT | Heart disease | Percutaneous coronary intervention | 68.2±10.5 | T: 124 I: 65 C: 59 |
33/91 | Paper-based/clinician | Usual care | Decisional conflict (DCS) | 7.3 | Y/Y | N/Y/Y |
| De Achaval et al. 57/USA | 3-arm RCT | Knee osteoarthritis | Total knee arthroplasty | 62.8±9.0 | T: 208 I1: 70 I2: 69 C: 69 |
141/67 | Video-based/self-guided | Usual care (booklet control) | Decisional conflict (DCS); Satisfaction with decision-making (DCS) | 1.4 | N/N | N/N/Y |
| Deyo et al. 58/USA | 2-arm RCT | Lumbar spinal stenosis and herniated disk, or nonspecific back pain | Lumbar spine surgery | 52.4±16.5 | T: 393 I: 190 C: 203 |
188/205 | Video-based/self-guided | Usual care (booklet control) | Invasive treatment choice | 12.5 | Y/N | N/N/Y |
| Eden et al. 59/USA | 2-arm RCT | Pregnant women |
Cesarean section | 31.1±NR | T: 131 I: 66 C: 65 |
131/0 | Computer-based/self-guided | Usual care (brochure) | Invasive treatment choice; Decisional conflict (DCS) | 0 | Y/Y | N/Y/Y |
| Goel et al. 60/Canada | 2-arm cluster RCT | Breast cancer | Lumpectomy and mastectomy | 57.5±12.2 | T: 136 I: 86 C: 50 |
136/0 | Audio-based/self-guided | Usual care (pamphlet) | Decisional conflict (DCS); Satisfaction with decision-making (DCS); Knowledge (BCIT-R) | 21.3 | N/N | N/N/N |
| Gökce et al. 61/Turkey | 2-arm RCT | Renal stone | SWL and RIRS | 46.3±5.8 | T: 119 I: 60 C: 59 |
52/67 | Paper-based/self-guided | Usual care (standard information) | Invasive treatment choice; Decisional conflict (DCS) | 3.4 | N/N | N/N/N |
| Hawley et al. 62/USA | 2-arm RCT | Breast cancer | Mastectomy | 56.8±10.8 | T: 537 I: 267 C: 270 |
537/0 | Computer-based/self-guided | Active control (static version of icandecide) | Preparedness for decision-making; Decision quality (SDQ) | 7.6 | Y/Y | Y/Y/Y |
| Heller et al. 63/USA | 2-arm RCT | Breast cancer | Breast reconstruction | 47.0±9.4 | T: 133 I: 66 C: 67 |
133/0 | Computer-based/self-guided | Usual care (standard patient education) | Satisfaction with decision-making | 0 | N/N | N/N/N |
| Hutyra et al. 64/USA | 2-arm RCT | Anterior shoulder dislocations | Operative treatment | 23.6±5.3 | T: 199 I: 100 C: 99 |
45/154 | Computer-based/self-guided | Active control (text-based) | Invasive treatment choice; Decisional conflict (DCS) | 0.5 | N/N | N/N/Y |
| Ibrahim et al. 65/USA | 2-arm RCT | Knee osteoarthritis | Total knee replacement | 59.1±7.2 | T: 336 I: 168 C: 168 |
235/101 | Video-based/self-guided | Usual care (educational booklet) | Invasive treatment choice | 9.5 | Y/N | Y/Y/Y |
| Jayakumar et al. 66/USA | 2-arm RCT | Knee osteoarthritis | Total knee arthroplasty | 62.6±8.4 | T: 145 I: 76 C: 69 |
83/62 | Computer-based/clinician | Usual care (educational material) | Invasive treatment choice; Satisfaction with decision-making; Decision quality (K-DQI); Shared decision-making (CollaboRATE); Condition-specific HRQoL (KOOS JR) | 11 | N/N | Y/Y/Y |
| Jibaja-Weiss et al. 67/USA | 2-arm RCT | Breast cancer | Mastectomy | 51.0±10.9 | T: 100 I: 51 C: 49 |
100/0 | Computer-based/clinician | Usual care | Invasive treatment choice; Satisfaction with decision-making (SWDMP) | 24 | N/N | N/N/Y |
| Kearing et al. 68/USA | 2-arm RCT | Lumbar spinal stenosis | Operative treatment | 66.6±9.7 | T: 199 I: 98 C: 101 |
81/118 | Video-based/self-guided | Active control (video-based) | Invasive treatment choice | 15.6 | N/N | N/N/Y |
| Kennedy et al. 69/UK | 3-arm RCT | Menorrhagia | Hysterectomy | 40.3±7.0 | T: 894 I1: 296 I 2: 300 C: 298 |
894/0 | Video-based/self-guided | Usual care | Invasive treatment choice | 30.1 | N/Y | N/N/Y |
| Kleiss et al. 70/USA | 2-arm RCT | Upper-extremity conditions | Operative treatment | 55±14 | T: 147 I: 76 C: 71 |
98/49 | Computer-based/clinician | Usual care (no intervention) | Satisfaction with decision-making; Decisional regret (DRS); Physical Health (PROMIS PF) | 31.3 | N/N | N/Y/N |
| Korteland et al. 71/The Netherlands | 2-arm RCT | Heart disease | Prosthetic heart valve selection | 61.0±16.3 | T: 138 I: 67 C: 71 |
34/104 | Computer-based/self-guided | Usual care (standard preoperative care) | Decisional conflict (DCS); General Health (SF-36); Physical Health (SF-36); Mental Health (SF-36) | 11 | Y/Y | N/Y/Y |
| Kostick et al. 72/USA | 2-arm RCT | Heart disease | LVAD support | 59.8±12.1 | T: 98 I: 47 C: 51 |
23/75 | Paper-based/clinician | Usual care (standard care) | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making; Decisional regret (ORS); Preparedness for decision-making; Shared decision-making (CollaboRATE); General Health | 44.8 | Y/Y | N/Y/Y |
| Kuppermann et al. 73/USA | 2-arm RCT | Pregnant women |
Repeat cesarean section | 34.1±4.5 | T: 1485 I: 742 C: 743 |
1485/0 | Computer-based/clinician | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making (SWD); Knowledge; Shared decision-making (SDM-Q-9); Decision self-efficacy (DSES) | 1.0 | N/Y | Y/N/Y |
| Lam et al. 74/Hong Kong | 2-arm RCT | Breast cancer | Mastectomy | 55.7±10.5 | T: 276 I: 138 C: 138 |
276/0 | Paper-based/self-guided | Usual care (standard information booklet) | Invasive treatment choice; Decisional conflict (DCS); Knowledge; Decisional regret (DRS); Outcome expectations; Mental health (CHQ); Anxiety (HADS); Depression (HADS) | 18.5 | Y/N | N/N/Y |
| Lamers et al. 75/The Netherlands | 2-arm RCT | Prostate cancer | Radical prostatectomy | 65.3±5.9 | T: 382 I: 273 C: 109 |
0/382 | Computer-based/self-guided | Usual care | Invasive treatment choice | 12.0 | N/N | Y/Y/Y |
| Luan et al. 76/USA | 2-arm RCT | Breast cancer | Breast reconstruction for mastectomy | 49.2±3.4 | T: 16 I: 8 C: 8 |
16/0 | Paper-based/self-guided | Usual care (standard preconsultation material) | Invasive treatment choice; Decisional conflict (DCS); Decisional regret (DRS); Condition-specific HRQoL (BREAST-Q) | 0 | N/N | N/N/N |
| Manne et al. 78/USA | 2-arm RCT | Breast cancer | Breast reconstruction for mastectomy | 50.2±10.6 | T: 55 I: 31 C: 24 |
55/0 | Computer-based/self-guided | Usual care (pamphlet) | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making; Knowledge; Anxiety (STAI) | 21.8 | Y/Y | N/N/Y |
| Manne et al. 77/USA | 2-arm RCT | Breast cancer | Contralateral prophylactic mastectomy | 46.5±8.4 | T: 93 I: 46 C: 47 |
93/0 | Computer-based/self-guided | Usual care | Invasive treatment choice; Satisfaction with decision-making; Knowledge; Preparedness for decision-making (OPDMS) | 10.8 | Y/Y | N/N/Y |
| Metcalfe et al. 79/Canada | 2-arm RCT | BRCA1/2 mutation | Prophylactic mastectomy/oophorectomy | 39.1±8.8 | T: 150 I: 76 C: 74 |
150/0 | Paper-based/self-guided | Usual care (standard genetic counseling) | Decisional conflict (DCS); Knowledge; Perceived stress (IES) | 7.0 | Y/Y | N/N/N |
| Montgomery et al. 80/UK | 3-arm RCT | Pregnant women | Cesarean section | 32.4±4.7 | T: 742 I1: 245 I 2: 250 C: 247 |
742/0 | Computer-based/self-guided | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making (SDS); Knowledge; Anxiety (STAI) | 3.6 | Y/N | Y/Y/Y |
| Parkinson et al. 81/Australia | 2-arm RCT | Women with breast cancer or ductal carcinoma | Breast reconstruction following mastectomy | 51.9±9.5 | T: 222 I: 116 C: 106 |
222/0 | Computer-based/self-guided | Usual care (standard online information) | General HRQoL (QALYs) | 26.9 | Y/Y | N/Y/Y |
| Phelan et al. 82/USA | 2-arm RCT | Lumbar spinal stenosis | Lumbar spine surgery | 49.5±18.8 | T: 100 I: 47 C: 53 |
44/56 | Computer-based/self-guided | Usual care (booklet control) | Invasive treatment choice | 10 | Y/N | N/N/Y |
| Politi et al. 83/USA | 2-arm RCT | Breast cancer | Post-mastectomy breast reconstruction | 50.7±10.8 | T: 120 I: 60 C: 60 |
120/0 | Computer-based/self-guided | Enhanced usual care | Invasive treatment choice; Decisional conflict (SURE); Decision quality (DQI); Condition-specific HRQoL (BREAST-Q) | 0 | Y/Y | N/Y/Y |
| Rivero-Santana et al. 84/Spain | 2-arm RCT | Knee osteoarthritis | Total knee replacement | 66.8±8.4 | T: 193 I: 97 C: 96 |
139/54 | Computer-based/self-guided | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making; Knowledge (K-DQI); Decisional regret (DRS) | 3.6 | N/N | N/Y/Y |
| Schwartz et al. 85/USA | 2-arm RCT | BRCA1/BRCA2 mutation carriers | Mastectomy | 43.9±10.9 | T: 214 I: 100 C: 114 |
214/0 | Computer-based/self-guided | Usual care (booklet control) | Invasive treatment choice | 12.1 | Y/N | N/N/Y |
| Sherman et al. 86/Australia | 2-arm RCT | Women with breast cancer or ductal carcinoma | Breast reconstruction following mastectomy | 51.9±9.5 | T: 222 I: 116 C: 106 |
222/0 | Computer-based/SELF-guided | Usual care (standard online information) | Decisional conflict (DCS); Satisfaction with decision-making; Decisional regret (DRS) | 26.9 | Y/N | N/Y/Y |
| Shorten et al. 87/Australia | 2-arm RCT | Pregnant Women |
Repeat cesarean section | 31.8±NR | T: 227 I: 115 C: 112 |
227/0 | Paper-based/self-guided | Usual care (routine pregnancy care) | Invasive treatment choice | 25.6 | N/N | N/N/Y |
| Shue et al. 88/USA | 2-arm RCT | Hip and knee osteoarthritis | Total hip or knee joint replacement | 61±11 | T: 147 I: 73 C: 74 |
78/69 | Video-based/self-guided | Active control (booklet-based) | Invasive treatment choice; Knowledge | 10.2 | N/N | N/N/Y |
| Stacey et al. 89/Canada | 2-arm RCT | Osteoarthritis | Total joint arthroplasty | 66.5±9.8 | T: 334 I: 167 C: 167 |
192/142 | Video-based/self-guided | Usual care (standard patient education) | Invasive treatment choice; Knowledge (K-DQI); Decision quality (K-DQI) | 37 | N/N | N/Y/Y |
| Stiggelbout et al. 90/The Netherlands | 2-arm RCT | Asymptomatic abdominal aneurysm | Elective aneurysm repair | 72.0±8.0 | T: 100 I: 49 C: 51 |
7/93 | Paper-based/clinician | Usual care (general brochure) | Invasive treatment choice | 11.5 | N/N | N/N/Y |
| Street et al. 91/USA | 2-arm RCT | Breast cancer | Mastectomy | 58.1±12.7 | T: 60 I: 30 C: 30 |
60/0 | Computer-based/self-guided | Usual care (Brochure) | Invasive treatment choice | 0 | N/N | N/N/Y |
| Trenaman et al. 93/Canada | 2-arm RCT | Osteoarthritis | Total joint replacement | 66.5±9.5 | T: 334 I: 167 C: 167 |
192/142 | Video-based/self-guided | Usual care (standard patient education) | General HRQoL (QALYs) | 37 | N/Y | N/Y/Y |
| Trenaman et al. 92/Canada | 2-arm RCT | Osteoarthritis | Total joint replacement | 66.6±9.8 | T: 324 I: 161 C: 163 |
185/139 | Video-based/self-guided | Usual care (standard patient education) | Invasive treatment choice | 37 | N/N | N/Y/Y |
| Tucholka et al. 94/USA | 2-arm RCT | Breast cancer | Mastectomy | 56.3±14.7 | T: 227 I: 116 C: 111 |
227/0 | Computer-based/self-guided | Usual care (standard website) | Knowledge (BCSDQI) | 7.0 | Y/N | N/Y/Y |
| van Roosmalen et al. 95/The Netherlands | 2-arm RCT | Deleterious BRCA1/2 mutation | Prophylactic mastectomy/oophorectomy | 43.6±10.8 | T: 368 I: 184 C: 184 |
368/0 | Video-based/self-guided | Usual care | Invasive treatment choice; Knowledge; General HRQoL; Anxiety (STAI); Depression (CES-D) | 3.3 (only those withdrew) | Y/Y | N/N/Y |
| van Roosmalen et al. 96/The Netherlands | 2-arm RCT | Deleterious BRCA1/2 mutation | Prophylactic mastectomy/oophorectomy | 39.5±10.0 | T: 88 I: 44 C: 44 |
88/0 | Computer-based/clinician | Usual care | Invasive treatment choice; General HRQoL; Anxiety (STAI); Depression (CES-D); Perceived stress (IES) | 1.1 | Y/Y | N/N/Y |
| van Tol-Geerdink et al. 97/The Netherlands | 2-arm RCT | Localized prostate cancer | Prostatectomy | 64±5.0 | T: 240 I: 163 C: 77 |
0/240 | Paper-based/clinician | Usual care | Invasive treatment choice | 0 | Y/N | N/Y/Y |
| Vandemheen et al. 98/Canada, Australia | 2-arm RCT | Cystic fibrosis | Lung transplantation | 30.4±8.9 | T: 149 I: 70 C: 79 |
68/81 | Computer-based/self-guided | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making; Knowledge; Preparedness for decision-making; Outcome expectations | 16.1 | Y/N | N/Y/Y |
| Varelas et al. 99/USA | 2-arm RCT | Breast cancer | Breast reconstruction following mastectomy | 49.6±11.2 | T: 47 I: 25 C: 22 |
26/0 | Computer-based/self-guided | Usual care (consultation) | Decisional conflict (DCS); Condition-specific HRQoL (BREAST-Q); Anxiety (STAI) | 44.7 | N/N | N/N/N |
| Vina et al. 100/USA | 2-arm RCT | Osteoarthritis | Knee replacement | 61.6±8.0 | T: 493 I: 240 C: 253 |
251/242 | Video-based/self-guided | Usual care (booklet control) | Invasive treatment choice | 0.6 | N/Y | N/Y/Y |
| Vodermaier et al. 101/Germany | 2-arm RCT | Breast cancer | Mastectomy | 55.2±11.0 | T: 111 I: 55 C: 56 |
111/0 | Paper-based/clinician | Usual care (standard care) | Invasive treatment choice; Decisional conflict (DCS) | 7.9 | N/N | N/N/Y |
| Vuorma et al. 102/Finland | 2-arm RCT | Menorrhagia | Hysterectomy | 44.4±4.18 | T: 363 I: 184 C: 179 |
363/0 | Paper-based/self-guided | Usual care | Invasive treatment choice | 13.2 | Y/Y | N/N/Y |
| Whelan et al. 103/Canada | 2-arm cluster RCT | Breast cancer | Mastecty/lumpectomy plus radiation | Median: 58.35 | T: 201 I: 94 C: 107 |
201/0 | Paper-based/clinician | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making (DCS); Anxiety (STAI); Depression (CES-D) | 17.9 | Y/N | N/N/Y |
| Wilkens et al. 104/USA | 2-arm RCT | Osteoarthritis | Operative treatment | 63.5±1.4 | T: 90 I: 45 C: 45 |
65/25 | Computer-based/self-guided | Usual care | Invasive treatment choice; Decisional conflict (DCS); Satisfaction with decision-making; Decisional regret (DRS); Condition-specific HRQoL (QuickDASH); Depression (PHQ-2) | 7.8 | Y/Y | N/N/N |
| Wilkins et al. 105/USA | 2-arm RCT | Breast cancer | Mastectomy | 54.9±9.80 | T: 101 I: 52 C: 49 |
101/0 | Video-based/self-guided | Active control (written educational materials) | Invasive treatment choice; Satisfaction with decision-making; Knowledge; Decision self-efficacy (SECPMD); Anxiety (STAI) | NR | N/N | N/N/Y |
| Wong et al. 106/UK | 2-arm RCT | Pregnant women | Surgical termination of pregnancy | 25±NR | T: 328 I: 163 C: 165 |
328/0 | Paper-based/self-guided | Usual care (placebo leaflet) | Invasive treatment choice; Decisional conflict (DCS); Knowledge; Anxiety (STAI) | 14.0 | Y/N | N/N/N |
| Ye et al. 107/China | 2-arm RCT | Cataract | Cataract surgery | 64.3±0.3 | T: 773 I: 386 C: 387 |
556/217 | Paper-based/self-guided | Usual care (booklet control) | Invasive treatment choice; Decisional conflict (DCS); Decision quality | 3.4 | Y/Y | Y/Y/Y |
BCIT-R, Breast Cancer Information Test – Revised; BCSDQI, Breast Cancer Surgery Decision Quality Instrument; CES-D, Center for Epidemiologic Studies Depression Scale; CHQ, Chinese Health Questionnaire; DCS, Decision Conflict Scale; DRS, Decision Regret Scale; DSES, Decision Self-Efficacy Scale; HADS, Hospital Anxiety and Depression Scale; HRQoL, health-related quality of life; IES, Impact of Event Scale; ITT, Intention-to-treat; K-DQI, Knee Osteoarthritis Decision Quality Instrument; KOOS JR, Knee Injury and Osteoarthritis Outcome Score Joint Replacement; LVAD, left ventricular assist device; MDM, missing data management; N, no; NR, not recorded; OPDMS, Ottawa Preparation for Decision-Making Scale; ORS, Ottawa Regret Scale; PDSI, patient decision support intervention; PHQ, Patient Health Questionnaire; PROMIS PF, Patient-Reported Outcomes Measurement Information System Physical Function; PSS, Perceived Stress Scale; QALY, quality-adjusted life-year; QoL, quality of life; QuickDASH, Quick Disabilities of Arm, Shoulder, and Hand; RCT, randomized controlled trial; RIRS, retrograde intrarenal surgery; SDM-Q-9, Shared Decision-Making Questionnaire 9-item; SDQ, Subjective Decision Quality; SDS, Satisfaction with Decision Scale; SECPMD, Self-Efficacy to Communicate with Physician/Manage Disease; SF, Short-Form Health Survey; STAI, State-Trait Anxiety Inventory; SWD, Satisfaction with Decision Scale; SWDMP, Satisfaction with the Process of Making a Treatment Decision Scale; SWL, shock wave lithotripsy; Y, yes.
Patient decision support interventions characteristics and quality
A detailed summary of the PDSIs interventions is provided in Supplementary Table 8 (Supplemental Digital Content 1, http://links.lww.com/JS9/A238). The PDSIs were primarily computer-based (k=26, 44.8%) or video-based (k=16, 27.6%), and used a digital platform (k=43, 74.1%). Some employed artificial intelligence (k=12, 20.3%) to predict postoperative PROs (k=5, 8.6%). The majority of the PDSIs utilized asynchronous communication mechanisms (k=50, 86.2%), were designed for self-administration by patients (k=45, 77.6%), incorporated patient’s values (k=39, 67.2%), and lacked a theoretical basis (k=40, 69.0%).
The evidence on the quality of PDSIs was available for all 58 trials (Supplementary Table 9, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). Eleven PDSIs met all IPDASi-SF criteria, and their total score ranged from 5 to 16 (median=12). Full information on the available options, their positive and negative features, and fair comparisons, was provided for all PDSIs. However, incomplete information was provided regarding the impartial reviews (33 of 58 PDSIs; 56.9%), citations to referenced studies (32 of 58 PDSIs; 55.2%), testing details with patients (29 of 58 PDSIs; 50%), and production date (24 of 58 PDSIs; 41.4%). Ten trials incorporated the Ottawa Decision Support Framework (ODSF) to inform the design of the decision assistance, out of 18 trials that incorporated the theoretical framework.
Risk of bias in included studies
Forty-eight trials (82.8%) were rated as having some concerns, six (10.3%) were rated to have low risk, and four (6.9%) were at high risk of overall bias (Supplementary Fig. 1, Supplemental Digital Content 1, http://links.lww.com/JS9/A238 and Supplementary Fig. 2, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). The risks and concerns were mostly attributed to the absence of a published protocol to evaluate the selection of reported results, lack of information about the randomization process, deviations from the intended interventions, and measurement of the outcome with inadequate masking of the outcome assessors. All RCTs adequately addressed the issue of missing outcome data. Supplementary Figure 2 (Supplemental Digital Content 1, http://links.lww.com/JS9/A238) illustrates the risk of bias graph stratified by intention-to-treat and per-protocol analyses. The inter-rater agreement was almost perfect (κ=0.96, P<0.001).
Effects of patient decision support interventions on invasive treatment choice and decision-making–related outcomes
Forty-two trials (72.4%) reported on the invasive treatment choice51–53,55,58,59,61,64–69,72–78,80,82–85,87,89–91,95–98,100–107. Of the 5136 patients who used PDSIs, 2396 (46.7%) chose the invasive treatment option, compared with 2302 of 4802 patients (47.9%) in the control groups. The absolute difference was 1.2% and RR was 0.97 (95% CI: 0.90, 1.04; I 2 =64.5%). A similar effect was observed in a follow-up assessment (median: 9 months) of treatment choice (Table 2, Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/JS9/A238).
Table 2.
Effectiveness of PDSIs on invasive treatment choice, decision-making–related outcomes, PROs, and outcomes related to healthcare resource use
| Overall effect | Test of heterogeneity | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Number of trials (references) | Sample size | Effect estimate (95% CI) | Z | P | I 2 (%) | χ2 | P | Certainty of evidence |
| Invasive treatment choice | |||||||||
| Postintervention | 4251–53,55,58,59,61,64–69,72–78,80,82–85,87,89–91,95–98,100–107 | 9938 | RR=0.97 (0.90, 1.04) | −0.83 | 0.41 | 64.5 | 115.35 | <0.001 | Very low |
| Follow-up (1–85 months) | 651,72,84,92,100,102 | 1429 | RR=1.05 (0.85, 1.30) | 0.46 | 0.65 | 82.2 | 28.15 | <0.001 | Very low |
| Decision-making–related | |||||||||
| Decisional conflict | |||||||||
| Postintervention | 2750–52,54,56,57,60,61,64,71–74,76,78–80,83,84,86,98,99,101,103,104,107 | 5726 | g=−0.29 (−0.41, −0.16) | −4.41 | <0.001** | 79.7 | 128.27 | <0.001 | Very low |
| Follow-up (1–6 months) | 650,51,79,84,86,103 | 1100 | g=−0.11 (−0.23, 0.01) | −1.84 | 0.07 | 0.0 | 2.70 | 0.75 | Low |
| Subscale of Decisional Conflict Scale | |||||||||
| Informed subscale | 1356,57,60,64,71–73,78,80,84,101,106 | 3215 | g=−0.38 (−0.61, −0.14) | −3.11 | <0.001** | 88.9 | 107.84 | <0.001 | Very low |
| Values clarity subscale | 1256,57,60,64,71–73,78,80,84,101 | 2973 | g=−0.25 (−0.41, −0.08) | −2.89 | <0.001** | 74.8 | 43.69 | <0.001 | Very low |
| Support subscale | 1256,57,60,64,71–73,78,80,84,101 | 2903 | g=−0.17 (−0.29, −0.04) | −2.65 | 0.01* | 50.7 | 22.30 | 0.02 | Very low |
| Uncertainty subscale | 1356,57,60,64,71–73,78,80,84,101,106 | 3218 | g=−0.10 (−0.17, −0.02) | −2.52 | 0.01* | 5.6 | 12.71 | 0.39 | Low |
| Effective decision subscale | 1356,57,60,64,71–73,78,80,84,101,106 | 3212 | g=−0.14 (−0.23, −0.04) | −2.80 | 0.01* | 28.6 | 16.80 | 0.16 | Very low |
| Satisfaction with decision-making | |||||||||
| Postintervention | 1857,60,63,66,67,70,72,73,77,78,80,84,86,98,103–105 | 3744 | g=0.09 (−0.05, 0.22) | 1.26 | 0.21 | 71.7 | 60.0 | <0.001 | Very low |
| Follow-up (6 months) | 484,86,103,104 | 649 | g=0.16 (0.01, 0.32) | 2.09 | 0.04* | 0.0 | 1.55 | 0.67 | Moderate |
| Disease and treatment knowledge | |||||||||
| Postintervention | 1550,52,60,73,74,77–80,84,89,95,98,104,106 | 4118 | g=0.32 (0.15, 0.49) | 3.65 | <0.001** | 84.6 | 90.98 | <0.001 | Very low |
| Follow-up (1–6 months) | 450,52,79,84 | 586 | g=0.10 (−0.06, 0.27) | 1.27 | 0.20 | 0.0 | 0.32 | 0.96 | Moderate |
| Decisional regret | |||||||||
| Postsurgery (immediately–6 months) | 851,70,72,74,76,84,86,104 | 1043 | g=−0.20 (−0.53, 0.13) | −1.21 | 0.23 | 83.6 | 42.68 | <0.001 | Very low |
| Follow-up (4–6 months) | 351,74,104 | 551 | g=0.03 (−0.36, 0.42) | 0.14 | 0.89 | 80.2 | 10.10 | 0.01 | Low |
| Decision quality | |||||||||
| Postintervention | 362,66,83 | 745 | g=0.53 (−0.02, 1.09) | 1.88 | 0.06 | 90.5 | 20.95 | <0.001 | Very low |
| Values-concordance | 462,66,89,107 | 1712 | RR=1.98 (1.15, 3.39) | 2.48 | 0.01* | 90.2 | 30.68 | <0.001 | Very low |
| Preparedness for decision-making | 550,62,72,77,98 | 953 | g=0.22 (0.09, 0.34) | 3.36 | <0.001** | 0.0 | 3.27 | 0.51 | Low |
| Shared decision-making | 366,72,73 | 1527 | g=0.22 (−0.31, 0.75) | 0.80 | 0.42 | 88.7 | 17.76 | <0.001 | Very low |
| Decision self-efficacy | 352,73,105 | 1595 | g=0.02 (−0.07, 0.12) | 0.48 | 0.63 | 0.0 | 0.32 | 0.85 | Moderate |
| Outcome expectations | 352,74,98 | 526 | g=0.11 (−0.57, 0.80) | 0.32 | 0.75 | 93.5 | 30.55 | <0.001 | Very low |
| PROs | |||||||||
| General HRQoL | |||||||||
| Postintervention | 551,71,72,95,96 | 862 | g=0.02 (−0.20, 0.25) | 0.21 | 0.84 | 58.3 | 9.59 | 0.05 | Very low |
| Follow-up (3–9 months) | 351,71,96 | 461 | g=0.13 (−0.18, 0.44) | 0.84 | 0.40 | 61.3 | 5.17 | 0.08 | Low |
| Physical health | 270,71 | 285 | g=0.15 (−0.08, 0.38) | 1.30 | 0.20 | 0.0 | 0.05 | 0.82 | Moderate |
| Mental health | |||||||||
| Postintervention | 271,74 | 363 | g=0.14 (−0.34, 0.62) | 0.59 | 0.57 | 80.3 | 5.08 | 0.02 | Low |
| Follow-up (postoperative–4 months) | 271,74 | 363 | g=0.07 (−0.13. 0.28) | 0.69 | 0.49 | 0.0 | 0.04 | 0.84 | High |
| Condition-specific HRQoL | 566,76,83,99,104 | 379 | g=0.28 (−0.11, 0.67) | 1.39 | 0.17 | 67.8 | 12.4 | 0.01 | Very low |
| Anxiety | |||||||||
| Postintervention | 974,78,80,95,96,99,103,105,106 | 1640 | g=−0.03 (−0.20, 0.13) | −0.40 | 0.69 | 57.9 | 19.0 | 0.01 | Very low |
| Follow-up (4–9 months) | 374,96,103 | 513 | g=−0.05 (−0.25, 0.16) | −0.44 | 0.66 | 27.5 | 2.76 | 0.25 | Moderate |
| Depression | |||||||||
| Postintervention | 651,74,95,96,103,104 | 1191 | g=0.04 (−0.10, 0.17) | 0.51 | 0.61 | 24.7 | 6.64 | 0.25 | Moderate |
| Follow-up (4–9 months) | 451,74,96,103 | 749 | g=−0.02 (−0.19, 0.14) | −0.27 | 0.78 | 24.0 | 3.95 | 0.27 | High |
| Perceived stress | 351,79,95 | 729 | g=−0.00 (−0.19, 0.19) | −0.00 | 1.00 | 40.6 | 3.37 | 0.19 | High |
| Healthcare resources use | |||||||||
| Consultation time (min) | 466,83,99,104 | 362 | MD=0.04 (−0.17, 0.24) | 0.37 | 0.71 | 0.0 | 1.42 | 0.70 | Moderate |
g, Hedges’s g; HRQoL, health-related quality of life; I 2, percentage of variation across studies that is due to heterogeneity rather than chance; MD, mean difference; PDSI, patient decision support intervention; PRO, patient-reported outcomes; RR, risk ratio; Z, overall effect size (Z-statistics); χ2, Cochran’s Q test.
P<0.05.
P<0.001.
Thirty-nine trials reported on decision-making–related outcomes (Supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/JS9/A238), including 27 trials (46.6%) reporting on decisional conflict50–52,54,56,57,60,61,64,71–74,76,78–80,83,84,86,98,99,101,103,104,107, 18 (31.0%) on decisional satisfaction57,60,63,66,67,70,72,73,77,78,80,84,86,98,103–105, 15 (25.9%) on disease and treatment knowledge50,52,60,73,74,77–80,84,89,95,98,104–106, eight (13.8%) on decisional regret51,70,72,74,76,84,86,104, five (8.6%) on preparedness for decision-making50,62,72,77,98, four (6.9%) on decision quality measured through value concordance62,66,89,107, and three (5.2%) on shared decision-making66,72,73, decisional self-efficacy52,73,105, and outcome expectations52,74,98.
A small effect size (g) of PDSIs on decisional conflict (−0.29; 95% CI, −0.41, −0.16; I 2 =79.7%) was observed. The effect size at follow-up assessment (median: 6 months) was −0.11 (95% CI: −0.23, 0.01; I 2 =0.0%)50,51,79,84,86,103. The effect size ranged from −0.38 to −0.10 for subdomains of decisional conflict (Table 2).
A negligible effect size of 0.09 (95% CI: −0.05, 0.22; I 2 =71.7%) was observed for PDSIs on satisfaction with decision-making. At a follow-up assessment (median: 6 months), the effect size was 0.16 (95% CI: 0.01, 0.32; I 2 =0.0%)84,86,103,104.
The effect size of PDSIs on disease and treatment knowledge was 0.32 (95% CI: 0.15, 0.49; I 2=84.6%). The effect size of PDSIs on knowledge was 0.10 (95% CI: −0.06, 0.27; I 2 =0.0%) at a follow-up assessment (median: 4.5 months) based on four trials50,52,79,84.
The effect size on decision-making preparedness was 0.22 (95% CI: 0.09, 0.34; I 2 =0.0%). The pooled estimate revealed that the patients who used PDSIs were more likely to have experienced better decision quality (RR=1.98; 95% CI: 1.15, 2.36; I 2 =90.2%) as compared with the control groups.
There were no significant changes in the following decision-making–related outcomes: decisional regret, shared decision-making, decision self-efficacy, and outcome expectations (Table 2).
Effects of patient decision support interventions on patient-related outcomes and healthcare resource utilization
There were 18 trials that reported on PROs (Supplementary Fig. 5, Supplemental Digital Content 1, http://links.lww.com/JS9/A238), including nine trials that reported on anxiety74,78,80,95,96,99,103,105,106, six on depression51,74,95,96,103,104, five on general HRQoL51,71,72,95,96, five on condition-specific HRQoL66,76,83,99,104, and three on perceived stress51,79,95. The use of PDSIs had no effect on HRQoL (general or condition-specific), physical health, mental health, depression, anxiety, and perceived stress (Table 2).
Four trials66,83,99,104 assessed healthcare resource utilization in terms of consultation time but the overall effect was negligible (mean difference=0.04 min; 95% CI: −0.17, 0.24; P=0.71) (Supplementary Fig. 6, Supplemental Digital Content 1, http://links.lww.com/JS9/A238).
Subgroup analysis
Subgroup analysis showed a statistically significant subgroup difference in decision conflict (χ2=12.80; P=0.03), with a very large effect size of −1.07 on reducing decisional conflict in patients considering breast reconstruction76,78,83,86,99 (g=−1.07; 95% CI: −1.91, −0.23), but no effect in patients considering cesarean section73,80 (g=−0.13; 95% CI: −0.38, 0.12) and those contemplating destination therapy left ventricular assist device (LVAD) placement51,72 (g=0.06; 95% CI: −0.17, 0.29). In addition, self-guided PDSIs (g=0.35; 95% CI: 0.19, 0.51) had a much greater effect on disease and treatment knowledge50,52,60,74,77–80,84,89,95,98,104,106 as compared with clinician-delivered PDSIs (g=0.00; 95% CI: −0.11, 0.11) with a subgroup difference (χ2=12.82; P<0.001). No other differences were observed in subgroup comparisons (Table 3 (Supplementary Figs. 7–10, Supplemental Digital Content 1, http://links.lww.com/JS9/A238).
Table 3.
Subgroup analysis of the PDSIs on invasive treatment choice and decision-making–related outcomes
| Test of heterogeneity | ||||||
|---|---|---|---|---|---|---|
| Variables | Number of trials (references) | Pooled estimate (95% CI) | I 2 (%) | χ2 | P | χ2, subgroup differences |
| Invasive treatment choice | ||||||
| Types of elective surgery | ||||||
| Breast reconstruction | 376,78,83 | RR=0.99 (0.83, 1.18) | 0.0 | 1.26 | 0.53 | 14.06, P=0.08 |
| Destination therapy LVAD placement | 251,72 | RR=0.77 (0.51, 1.15) | 80.0 | 4.99 | 0.03 | |
| First/repeat cesarean section | 459,73,80,87 | RR=0.99 (0.87, 1.14) | 41.8 | 5.15 | 0.16 | |
| Lumbar spine surgery | 258,82 | RR=0.70 (0.49, 1.01) | 19.9 | 1.25 | 0.26 | |
| Lumpectomy and/or mastectomy | 867,74,77,85,91,101,103,105 | RR=1.13 (0.88, 1.46) | 59.2 | 17.14 | 0.02 | |
| Prophylactic mastectomy/oophorectomy/hysterectomy | 5 69,95,96,102 | RR=1.12 (1.01, 1.24) | 0.0 | 1.53 | 0.82 | |
| Prostatectomy/radical prostatectomy or orchidectomy | 353,75,97 | RR=0.91 (0.65, 1.26) | 86.7 | 15.03 | <0.001 | |
| Total knee/hip/joint replacement/arthroplasty | 655,65,66,84,89,100 | RR=1.01 (0.89, 1.15) | 52.7 | 10.57 | 0.06 | |
| Othersa | 952,61,64,68,90,98,104,106,107 | RR=0.87 (0.73, 1.03) | 53.5 | 17.19 | 0.03 | |
| Mode of delivery | ||||||
| Clinician-delivered | 1151,53,66,67,72,73,90,96,97,101,103 | RR=0.88 (0.73, 1.07) | 79.2 | 48.16 | <0.001 | 1.33, P=0.25 |
| Self-guided | 3152,55,58,59,61,64,65,68,69,74–78,80,82–85,87,89,91,95,98,100,102,104–107 | RR=1.00 (0.93, 1.07) | 52.9 | 63.74 | <0.001 | |
| Consider value | ||||||
| Yes | 2651,59,64–69,73–78,80,83–85,87,89,90,96,97,104,106,107 | RR=0.98 (0.90, 1.07) | 60.6 | 63.39 | <0.001 | 0.27, P=0.61 |
| No | 1652,53,55,58,61,72,82,91,95,98,100–103,105 | RR=0.94 (0.83, 1.07) | 71.1 | 51.95 | <0.001 | |
| Use of theoretical framework | ||||||
| Yes | 1251,59,61,64,67,72,77,78,85,87,98,104 | RR=0.98 (0.83, 1.17) | 61.0 | 28.23 | <0.001 | 0.03, P=0.87 |
| No | 3052,53,55,58,65,66,68,69,73–76,80,82–84,89–91,95–97,100–103,105–107 | RR=0.97 (0.89, 1.05) | 66.6 | 86.73 | <0.001 | |
| PROM-based PDSIs | ||||||
| Yes | 366,90,96 | RR=1.24 (0.85, 1.81) | 0.0 | 1.64 | 0.44 | 1.64, P=0.20 |
| No | 3951–53,55,58,59,61,64–69,72–78,80,82–85,87,89,91,95,97,98,100–107 | RR=0.96 (0.90, 1.04) | 64.5 | 112.09 | <0.001 | |
| Decisional conflict | ||||||
| Types of elective surgery | ||||||
| Breast reconstruction | 576,78,83,86,99 | g=−1.07 (−1.91, −0.23) | 92.1 | 50.63 | <0.001 | 12.80, P=0.03* |
| Destination therapy LVAD placement | 251,72 | g=0.06 (−0.17, 0.29) | 0.0 | 0.69 | 0.41 | |
| First/repeat cesarean section | 273,80 | g=−0.13 (−0.38, 0.12) | 73.3 | 3.75 | 0.05 | |
| Lumpectomy and/or mastectomy | 460,74,101,103 | g=−0.31 (−0.46, −0.15) | 0.0 | 1.33 | 0.72 | |
| Total knee/hip/joint replacement/arthroplasty | 450,57,84 | g=−0.48 (−0.96, 0.01) | 86.6 | 22.31 | <0.001 | |
| Othersb | 1052,54,56,61,64,71,79,98,104,107 | g=−0.16 (−0.29, −0.02) | 53.5 | 19.37 | 0.02 | |
| Mode of delivery | ||||||
| Clinician-delivered | 651,56,72,73,101,103 | g=−0.13 (−0.30, 0.03) | 54.5 | 10.99 | 0.05 | 3.21, P=0.07 |
| Self-guided | 2150,52,54,57,60,61,64,71,74,76,78–80,83,84,86,98,99,104,107 | g=−0.35 (−0.51, −0.18) | 81.8 | 109.78 | <0.001 | |
| Consider value | ||||||
| Yes | 1951,54,56,57,60,64,71,73,74,76,78,80,83,84,86,99,104,107 | g=−0.31 (−0.48, −0.15) | 84.4 | 115.70 | <0.001 | 0.26, P=0.61 |
| No | 850,52,61,72,79,98,101,103 | g=−0.26 (−0.40, −0.12) | 28.1 | 9.73 | 0.20 | |
| Use of theoretical framework | ||||||
| Yes | 1051,54,61,64,72,78,79,86,98,104 | g=−0.29 (−0.54, −0.05) | 81.7 | 49.14 | <0.001 | 0.00, P=1.00 |
| No | 1750,52,56,57,60,71,73,74,76,80,83,84,99,101,103,107 | g=−0.29 (−0.45, −0.14) | 79.8 | 79.13 | <0.001 | |
| PROM-based PDSIs | ||||||
| Yes | 254,57 | g=−0.12 (−0.54, 0.29) | 68.4 | 3.16 | 0.08 | 0.65, P=0.42 |
| No | 2650–52,56,57,60,61,64,71–74,76,78–80,83,84,86,98,99,101,103,104,107 | g=−0.30 (−0.44, −0.17) | 80.6 | 123.68 | <0.001 | |
| Satisfaction with decision-making | ||||||
| Types of elective surgery | ||||||
| Breast reconstruction | 363,78,86 | g=0.09 (−0.16, 0.35) | 31.6 | 2.92 | 0.23 | 0.20, P=1.00 |
| First/repeat cesarean section | 273,80 | g=0.13 (−0.25, 0.51) | 91.6 | 11.86 | <0.001 | |
| Lumpectomy and/or mastectomy | 560,67,77,103,105 | g=0.05 (−0.19, 0.30) | 54.2 | 8.74 | 0.07 | |
| Total knee/hip/joint replacement/arthroplasty | 457,66,84 | g=0.14 (−0.34, 0.61) | 85.8 | 21.12 | <0.001 | |
| Othersc | 470,72,98,104 | g=0.05 (−0.33, 0.43) | 74.1 | 11.56 | 0.01 | |
| Mode of delivery | ||||||
| Clinician-delivered | 666,67,70,72,73,103 | g=0.20 (−0.05, 0.44) | 76.1 | 20.89 | <0.001 | 1.23, P=0.27 |
| Self-guided | 1257,60,63,77,78,80,84,86,98,104,105 | g=0.03 (−0.16, 0.21) | 71.6 | 38.69 | <0.001 | |
| Consider value | ||||||
| Yes | 1357,60,66,67,70,73,77,78,80,84,86,104 | g=0.11 (−0.06, 0.27) | 74.3 | 46.75 | <0.001 | 0.16, P=0.69 |
| No | 563,72,98,103,105 | g=0.04 (−0.25, 0.33) | 69.5 | 13.10 | 0.01 | |
| Use of theoretical framework | ||||||
| Yes | 867,70,72,77,78,86,98,104 | g=0.02 (−0.19, 0.23) | 58.2 | 16.76 | 0.02 | 0.57, P=0.45 |
| No | 1057,60,63,66,73,80,84,103,105 | g=0.13 (−0.06, 0.32) | 79.0 | 42.89 | <0.001 | |
| PROM-based PDSIs | ||||||
| Yes | 257,66 | g=0.23 (−0.61, 1.06) | 89.6 | 9.57 | <0.001 | 0.13, P=0.72 |
| No | 1757,60,63,67,70,72,73,77,78,80,84,86,98,103–105 | g=0.07 (−0.06, 0.21) | 68.8 | 48.03 | <0.001 | |
| Disease and treatment knowledge | ||||||
| Types of elective surgery | ||||||
| First/repeat cesarean section | 273,80 | g=0.28 (−0.28, 0.83) | 95.9 | 21.11 | <0.001 | 3.90, P=0.42 |
| Lumpectomy and/or mastectomy | 460,74,77,105 | g=0.15 (−0.04, 0.34) | 17.2 | 3.62 | 0.31 | |
| Prophylactic mastectomy/oophorectomy/hysterectomy | 279,95 | g=0.20 (−0.16, 0.56) | 72.2 | 3.59 | 0.06 | |
| Total knee/hip/joint replacement/arthroplasty | 350,84,89 | g=0.39 (0.18, 0.61) | 46.8 | 3.76 | 0.15 | |
| Othersd | 452,78,98,106 | g=0.50 (0.06, 0.94) | 85.4 | 20.48 | <0.001 | |
| Mode of delivery | ||||||
| Clinician-delivered | 173 | g=0.00 (−0.11, 0.11) | – | – | – | 12.82, P<0.001** |
| Self-guided | 1450,52,60,74,77–80,84,89,95,98,104,106 | g=0.35 (0.19, 0.51) | 76.0 | 54.13 | <0.001 | |
| Consider value | ||||||
| Yes | 960,73,74,77,78,80,84,89,106 | g=0.39 (0.13, 0.65) | 90.3 | 82.82 | <0.001 | 1.15, P=0.28 |
| No | 650,52,79,95,98,104 | g=0.22 (0.07, 0.38) | 36.7 | 7.89 | 0.16 | |
| Use of theoretical framework | ||||||
| Yes | 477–79,98 | g=0.20 (−0.01, 0.41) | 16.4 | 3.59 | 0.31 | 0.98, P=0.32 |
| No | 1150,52,60,73,74,80,84,89,95,104,106 | g=0.35 (0.14, 0.56) | 88.5 | 86.77 | <0.001 | |
g, Hedges’s g; I 2, percentage of variation across studies that is due to heterogeneity rather than chance; LVAD, left ventricular assist devices; PDSI, patient decision support intervention; PROM, patient-reported outcome measure; RCT, randomized controlled trial; RR, risk ratio.
aBariatric surgery, cataract surgery, aneurysm repair, lung transplantation, surgical termination of pregnancy, shock wave lithotripsy, and retrograde intrarenal surgery.
bBariatric, cataract, general surgery, lung transplantation, percutaneous coronary intervention, prophylactic mastectomy, prostatectomy, shock wave lithotripsy, and retrograde intrarenal surgery.
cLVAD placement, lung transplantation, and general surgery.
dBariatric surgery, lung transplantation, and surgical termination of pregnancy.
*P<0.05.
**P<0.001.
Publication bias
Publication bias was not detected for treatment choice, patient satisfaction, and disease and treatment knowledge; however, there was an asymmetrical distribution on the funnel plots for decisional conflict (Supplementary Figs. 11a, c, d, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). The trim-and-fill method imputed three studies and pooled confounder-adjusted estimate increased from −0.29 (95% CI: −0.41, −0.16) to −0.21 (95% CI: −0.35, −0.07) (Supplementary Fig. 12, Supplemental Digital Content 1, http://links.lww.com/JS9/A238). A sensitivity analysis using the Copas selection model suggested that publication bias was unlikely to be an issue (Supplementary Fig. 13, Supplemental Digital Content 1, http://links.lww.com/JS9/A238).
Certainty of evidence
Supplementary Table 10 (Supplemental Digital Content 1, http://links.lww.com/JS9/A238) shows the GRADE summary of evidence. For all primary and secondary outcomes, the certainty of evidence was rated as very low or low, except for disease and treatment knowledge (follow-up), decision self-efficacy, physical health, anxiety (follow-up), depression (postintervention), and consultation time, which were rated as moderate, while mental health and depression (follow-up), as well as perceived stress, which were rated as high.
Discussion
Summary of evidence
Our review demonstrated PDSIs that were intended to guide decision-making for elective surgeries had a beneficial impact on many decision-related outcomes. These effects were small and varied according to the type of elective surgery and mode of delivery of the PDSIs. They did not influence invasive treatment choice, PROs, or healthcare utilization outcomes.
Effects on outcome measures
Many findings in this analysis corroborated with aggregated findings from previous reviews such as: using PDSIs reduces decisional conflict7–9, enhances disease and treatment knowledge7–9, and improves decision quality8. According to the ODSF108 (Fig. 2), PDSIs can assist in meeting decisional needs by providing information on the possible treatment options and health conditions, as well as the associated benefits and harms5. This enables patients to appreciate the value-sensitive nature of decisions, thus enhancing the preference elicitation process5. ODSF theorizes that when adequate decisional support meets decisional needs, decision quality improves with a greater possibility of value concordance108. Nonetheless, a comprehensive needs assessment is required before the implementation of PDSIs in a specific patient population, as our subgroup analysis indicated that the magnitude of the benefits may vary across patient populations and PDSIs designs.
Figure 2.
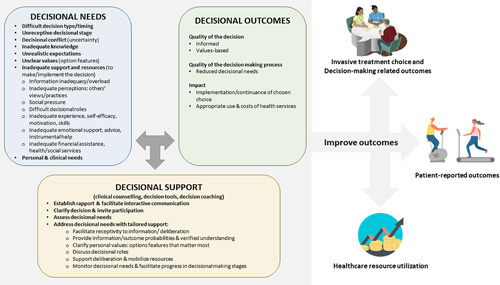
Possible mechanisms of patient decision support interventions in improving outcomes among elective surgical patients. Adapted from Ottawa Decision Support Framework.109
In line with previous systematic reviews, our meta-analyses demonstrated that PDSIs had no effects on PROs7,9. In theory, the use of PDSIs may improve PROs through two mechanisms: (1) encouraging the selection of treatment with greater PROs benefits, and (2) improving an individual’s psychological well-being. The first mechanism contradicts our finding, which has shown that the use of PDSIs had no effects on treatment choice for most elective surgeries. This is because, in addition to the potential improvement in their PROs, the surgical candidate may also assess the risk of surgical adverse events. The second mechanism could work via enhanced shared decision-making5,109 or decisions aligned with the patients’ values and preferences. The insignificant effects on shared decision-making in this review contradicted the former; and the absence of trials that assessed positive psychological constructs such as satisfaction with treatment outcomes rendered the latter uncertain. Hence, future high-quality research is warranted to investigate the downstream effects of PDSIs, such as the positive psychological effects on treatment outcomes.
A recent Cochrane review5 found that the use of PDSIs had no discernible effect on the choice of nonsurgical or invasive surgical intervention, whereas another review7 found a marginal difference in which patients who used PDSIs were less likely to undergo surgical treatment. In line with the former review5, which focused exclusively on RCTs, our meta-analysis demonstrated no effect on the choice of invasive treatment. There are two plausible explanations for the contradictory result in the latter review7. First, the review combined experimental and observational studies, which increased the likelihood of bias. The analysis might also have overestimated the effect size and reported marginal effect due to the relatively smaller sample size (N=2674), as compared with our review (N=9938). Second, our review included trials with more diverse populations. PDSIs typically had a variable effect depending on the target population and the surgery being considered. Indeed, our subgroup analysis found that, while PDSIs had no influence on most elective surgery, they might be able to decrease the likelihood of some invasive procedures, most notably destination therapy LVAD placement and lumbar spine surgery, which is consistent with previous review findings8. Therefore, although the overall effect of PDSIs on treatment choice was largely minimal, it varied with the type of surgery.
Consistent with another meta-analytic review5, our review indicated that the use of PDSIs did not incur increased use of the surgeon’s consultation time. This would imply no increase in resource utilization and the likelihood of acceptance by clinicians if PDSIs were to be implemented in clinical practice. It should be noted that the timing of PDSIs administration might affect consultation time. Two included trials83,99, in which patients received a self-guided PDSIs a few days before the consultation, observed a shorter consultation time (g=−0.06; 95% CI: −0.38, 0.26). In contrast, two other trials66,104, in which patients received a self-guided PDSIs during the waiting time before the routine consultation, observed similar or even a longer consultation time (g=0.10; 95% CI: −0.16, 0.37) compared with the control group.
Effects of investigator moderators
Surprisingly, our review discovered that self-guided PDSIs appeared to be more effective than clinician-administered PDSIs in enhancing disease and treatment knowledge and possibly reducing decisional conflict. This finding could be due to several reasons. First, providing self-guided PDSIs well before the consultation allows patients more time to digest the information and prepare for discussing the decision5. Second, there may be a lack of clinician buy-in for clinician-administered PDSIs, resulting in less effectiveness. A qualitative study among oncological surgeons showed that although two-thirds of them were aware of PDSIs, less than half had used one during routine surgical consultations111. Lastly, it could be due to chance because our subgroup analysis included one trial.
Similarly, theory-guided development, value consideration, or provision of patient-reported outcome measure data showed no effects in our review. Increasingly, PDSIs are developed by taking into account the recommendations of ODSF, which postulates that high-quality decisions are typically those consistent with the patient’s values108. A recent review stressed the need of including longitudinal PROs into the treatment decision-making process112. PROs are particularly relevant for patients considering elective surgeries as the main aim of the treatment is to improve functioning and well-being, or HRQoL. Given that PROs data is increasingly collected in clinical practice, incorporating such data into PDSIs becomes feasible. A possible reason for the insignificant subgroup differences of the design-related factors is that their effects were confounded or moderated by other contextual or implementation-related factors such as suboptimal protocol compliance. It is also possible that the design of PDSIs was inadequate or difficult for the user to comprehend. For example, it appears that the three PDSIs provided PROs data in the form of numerical scale scores without interpretation. Without training in psychometrics, patients are unlikely to be able to fully understand such information.
Limitations
Several limitations should be considered before interpreting these findings. First, while the comprehensive search approach lends credibility to this review, we used a broad search strategy and selected a large amount of data. Although two independent reviewers were involved, reviewer’s fatigue might have led to the misclassification of records for inclusion. Second, the included trials were clinically and statistically heterogeneous, limiting their comparison. To address this issue, our study used subgroup analysis. Last, the English language restriction imposed on the RCTs might have limited the generalizability of the findings.
Implications for future research and patient decision support interventions design
In this review, most trials were classified as having some concerns due to the lack of a published protocol for assessing bias in the selection of reported outcomes. In addition, a few trials were rated as having high risk of bias for not blinding those receiving the intervention and not providing the randomization procedure. Hence, investigators assessing the efficacy of PDSIs in future trials should adhere to good trial design as well as reporting standards such as the Consolidated Standards of Reporting Trials (CONSORT) 2010113.
Our review identified a significant gap in the reporting of PDSIs evaluation, including information about impartial review, citations to studies, and patient pilot testing. This made it challenging for reviewers to assess the quality of the PDSIs. Future research should develop and use a standardized International Patient Decision Aid Standards Version26 so that the quality of PDSIs can be properly assessed. In addition, it is difficult to explore the information in the comparator group due to a lack of description. Future studies are recommended to comply with the Standards for UNiversal reporting of patient Decision Aid Evaluation (SUNDAE) checklist to ensure transparent and high-quality reports of PDSIs evaluation studies13.
Conclusions
This review has demonstrated that PDSIs targeting individuals considering elective surgeries had benefited their decision-making by reducing decisional conflict and increasing disease and treatment knowledge, decision-making preparedness, and decision quality. However, the quality of PDSIs varied and the certainty of evidence for many key outcomes was low. Nonetheless, these findings may be used to guide the development and evaluation of new PDSIs for use in elective surgical care. Furthermore, future high-quality research is needed to investigate the downstream treatment outcomes of PDSIs, such as the positive psychological effects of PDSIs.
Ethical approval
Not applicable as this is a review paper.
Sources of funding
None.
Author contribution
L.J.C.: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, and project administration. N.B.: methodology, validation, formal analysis, investigation, resources, writing – review and editing, and visualization. M.L.: methodology, validation, formal analysis, investigation, writing – review and editing, and visualization. V.X.W., W.W., G.K.P.L., and H.W.D.H.: methodology, validation, investigation, writing – review and editing, and supervision. N.L.: conceptualization, methodology, validation, investigation, resources, data curation, writing – review and editing, visualization, and supervision.
Conflicts of interest disclosure
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: PROSPERO
Unique Identifying number or registration ID: CRD42021273767
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=273767
Guarantor
Nan Luo affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
The review team would like to express their gratitude to the authors who provided additional information regarding their studies, which is invaluable for our systematic review. L.J.C. (first author) gratefully acknowledges financial support from the Tan Kah Kee Foundation for the Tan Kah Kee Postgraduate Scholarship.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 10 April 2023
Contributor Information
Ling Jie Cheng, Email: Sphclj@nus.edu.sg.
Nick Bansback, Email: nick.bansback@ubc.ca.
Meixia Liao, Email: meixia.liao@u.nus.edu.
Vivien Xi Wu, Email: nurwux@nus.edu.sg.
Wenru Wang, Email: nurww@nus.edu.sg.
Gabriel Ka Po Liu, Email: dosglkp@nus.edu.sg.
Hwee Weng Dennis Hey, Email: doshhwd@nus.edu.sg.
Nan Luo, Email: ephln@nus.edu.sg.
References
- 1. Weiser TG, Haynes AB, Molina G, et al. Estimate of the global volume of surgery in 2012: an assessment supporting improved health outcomes. Lancet 2015;385(suppl 2):S11. [DOI] [PubMed] [Google Scholar]
- 2. Abbott TEF, Fowler AJ, Dobbs TD, et al. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth 2017;119:249–257. [DOI] [PubMed] [Google Scholar]
- 3. The International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016;117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kauppila JH, Johar A, Lagergren P. Postoperative complications and health-related quality of life 10 years after esophageal cancer surgery. Ann Surg 2020;271:311–316. [DOI] [PubMed] [Google Scholar]
- 5. Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NICE Medicines and Prescribing Centre (UK). Medicines Optimisation: The Safe and Effective Use of Medicines to Enable the Best Possible Outcomes [Patient decision aids used in consultations involving medicines NICE Guideline, No 5]. National Institute for Health and Care Excellence (UK); 2015. [PubMed] [Google Scholar]
- 7. Knops AM, Legemate DA, Goossens A, et al. Decision aids for patients facing a surgical treatment decision: a systematic review and meta-analysis. Ann Surg 2013;257:860–866. [DOI] [PubMed] [Google Scholar]
- 8. Boss EF, Mehta N, Nagarajan N, et al. Shared decision making and choice for elective surgical care: a systematic review. Otolaryngol Head Neck Surg 2016;154:405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leinweber KA, Columbo JA, Kang R, et al. A review of decision aids for patients considering more than one type of invasive treatment. J Surg Res 2019;235:350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The EndNote Team. EndNote 64-bit (EndNote 20). Philadelphia, PA: Clarivate; 2013.
- 12. Li T, Higgins JPT, Deeks JJ, Chapter 5: Collecting data; 2019. Accessed 20 October 2022. https://training.cochrane.org/handbook/current/chapter-05
- 13. Sepucha KR, Abhyankar P, Hoffman AS, et al. Standards for UNiversal reporting of patient Decision Aid Evaluation studies: the development of SUNDAE Checklist. BMJ Qual Saf 2018;27:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 15. Garvelink MM, Boland L, Klein K, et al. Decisional Conflict Scale use over 20 years: the anniversary review. Med Decis Making 2019;39:301–314. [DOI] [PubMed] [Google Scholar]
- 16. O’Connor AM, User manual – Decision Regret Scale [document on the internet]; 2003. Accessed 20 October 2022. https://decisionaid.ohri.ca/docs/develop/User_manuals/UM_Regret_Scale.pdf
- 17. Shields CG, Franks P, Fiscella K, et al. Rochester Participatory Decision-Making Scale (RPAD): reliability and validity. Ann Fam Med 2005;3:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bennett C, Graham ID, Kristjansson E, et al. Validation of a preparation for Decision Making Scale. Patient Educ Couns, 78 2010:130–133. [DOI] [PubMed] [Google Scholar]
- 19. MGH Heath Decision Sciences Center, Decision quality instruments; 2017. Accessed 20 October 2022. https://mghdecisionsciences.org/tools-training/decision-quality-instruments/
- 20. Barr PJ, Thompson R, Walsh T, et al. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res 2014;16:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kriston L, Scholl I, Hölzel L, et al. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). development and psychometric properties in a primary care sample. Patient Educ Couns 2010;80:94–99. [DOI] [PubMed] [Google Scholar]
- 22. Lorig KR, Sobel DS, Ritter PL, et al. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 2001;4:256–262. [PubMed] [Google Scholar]
- 23. O’Connor AM, User manual – Decision Self-efficacy Scale [document on the internet]; 2003. Accessed 20 October 2022. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_decision_selfefficacy.pdf
- 24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elwyn G, O’Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS ONE 2009;4:e4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 28. StataCorp. Stata: Release 16. StataCorp LLC; 2019. [Google Scholar]
- 29. StataCorp. Stata Meta-Analysis Reference Manual. Stata Press; 2019. [Google Scholar]
- 30. McKenzie JE Brennan SE Ryan RE, et al. , Chapter 9: Summarizing study characteristics and preparing for synthesis; 2019. Accessed 20 October 2022. https://training.cochrane.org/handbook/current/chapter-09
- 31. Ryan RE, Cochrane Consumers and Communication Group: meta-analysis; 2016. Accessed 20 October 2022. http://cccrg.cochrane.org/sites/cccrg.cochrane.org/files/public/uploads/meta-analysis_revised_december_1st_1_2016.pdf
- 32. Higgins JPT, Li T, Deeks JJ, Chapter 6: Choosing effect measures and computing estimates of effect; 2019. Accessed 20 October 2022. https://training.cochrane.org/handbook/current/chapter-06
- 33. Rosenthal R, Cooper H, Hedges L. Cooper H, Hedges LV. Parametric measures of effect size. The Handbook of Research Synthesis. Russell Sage Foundation; 1994;621:231–244. [Google Scholar]
- 34. Hedges LV, Olkin I. Statistical Methods for Meta-analysis. Academic Press; 2014. [Google Scholar]
- 35. Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 36. Higgins JPT Thomas J Chandler J, et al. , Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019); 2019. Accessed 20 October 2022. https://training.cochrane.org/handbook/current
- 37. Deeks JJ, Higgins JPT, Altman DG, Chapter 10: Analysing data and undertaking meta-analyses; 2019. Accessed 20 October 2022. https://training.cochrane.org/handbook/current/chapter-10
- 38. Jin Z-C, Zhou X-H, He J. Statistical methods for dealing with publication bias in meta-analysis. Stat Med 2015;34:343–360. [DOI] [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zwetsloot P-P, Van Der Naald M, Sena ES, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. eLife 2017;6:e24260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 42. Terrin N, Schmid CH, Lau J, et al. Adjusting for publication bias in the presence of heterogeneity. Stat Med 2003;22:2113–2126. [DOI] [PubMed] [Google Scholar]
- 43. Pustejovsky JE, Rodgers MA. Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods 2019;10:57–71. [DOI] [PubMed] [Google Scholar]
- 44. Carpenter JR, Rücker G, Schwarzer G. copas: an R package for Fitting the Copas Selection Model. R J 2009;1:31–36. [Google Scholar]
- 45. Schwarzer G, Carpenter J, Rücker G. Empirical evaluation suggests Copas selection model preferable to trim-and-fill method for selection bias in meta-analysis. J Clin Epidemiol 2010;63:282–288. [DOI] [PubMed] [Google Scholar]
- 46. Copas J. What works?: selectivity models and meta-analysis. J R Stat Soc Ser A Stat Soc 1999;162:95–109. [Google Scholar]
- 47. McMaster University and Evidence Prime Inc. GRADEpro GDT: GRADE guideline development tool; 2020. Accessed 20 October 2022. https://www.gradepro.org/ .
- 48. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 50. Allen KD, Sanders LL, Olsen MK, et al. Internet versus DVD decision aids for hip and knee osteoarthritis. Musculoskelet Care 2016;14:87–97. [DOI] [PubMed] [Google Scholar]
- 51. Allen LA, McIlvennan CK, Thompson JS, et al. Effectiveness of an intervention supporting shared decision making for destination therapy left ventricular assist device: the DECIDE-LVAD randomized clinical trial. JAMA Intern Med 2018;178:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arterburn DE, Westbrook EO, Bogart TA, et al. Randomized trial of a video-based patient decision aid for bariatric surgery. Obesity (Silver Spring) 2011;19:1669–1675. [DOI] [PubMed] [Google Scholar]
- 53. Auvinen A, Hakama M, Ala-Opas M, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU Int 2004;93:52–56; discussion 56. [DOI] [PubMed] [Google Scholar]
- 54. Berry DL, Hong F, Blonquist TM, et al. Decision support with the personal patient profile-prostate: a multicenter randomized trial. J Urol 2018;199:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bozic KJ, Belkora J, Chan V, et al. Shared decision making in patients with osteoarthritis of the hip and knee: results of a randomized controlled trial. J Bone Joint Surg Am 2013;95:1633–1639. [DOI] [PubMed] [Google Scholar]
- 56. Coylewright M, Dick S, Zmolek B, et al. PCI choice decision aid for stable coronary artery disease: a randomized trial. Circ Cardiovasc Qual Outcomes 2016;9:767–776. [DOI] [PubMed] [Google Scholar]
- 57. de Achaval S, Fraenkel L, Volk RJ, et al. Impact of educational and patient decision aids on decisional conflict associated with total knee arthroplasty. Arthritis Care Res (Hoboken) 2012;64:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Deyo RA, Cherkin DC, Weinstein J, et al. Involving patients in clinical decisions: impact of an interactive video program on use of back surgery. Med Care 2000;38:959–969. [DOI] [PubMed] [Google Scholar]
- 59. Eden KB, Perrin NA, Vesco KK, et al. A randomized comparative trial of two decision tools for pregnant women with prior cesareans. J Obstet Gynecol Neonatal Nurs 2014;43:568–579. [DOI] [PubMed] [Google Scholar]
- 60. Goel V, Sawka CA, Thiel EC, et al. Randomized trial of a patient decision aid for choice of surgical treatment for breast cancer. Med Decis Making 2001;21:1–6. [DOI] [PubMed] [Google Scholar]
- 61. Gokce M, Akpinar C, Esen B, et al. The role of a novel decision aid to support informed decision making process in patients with a symptomatic non – lower pole renal stone <20 mm in diameter: a prospective randomized study. Int Braz J Urol 2019;45:941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hawley ST, Li Y, An LC, et al. Improving breast cancer surgical treatment decision making: the iCanDecide randomized clinical trial. J Clin Oncol 2018;36:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heller L, Parker PA, Youssef A, et al. Interactive digital education aid in breast reconstruction. Plast Reconstr Surg 2008;122:717–724. [DOI] [PubMed] [Google Scholar]
- 64. Hutyra CA, Smiley S, Taylor DC, et al. Efficacy of a preference-based decision tool on treatment decisions for a first-time anterior shoulder dislocation: a randomized controlled trial of at-risk patients. Med Decis Making 2019;39:253–263. [DOI] [PubMed] [Google Scholar]
- 65. Ibrahim SA, Blum M, Lee GC, et al. Effect of a decision aid on access to total knee replacement for black patients with osteoarthritis of the knee: a randomized clinical trial. JAMA Surg 2017;152:e164225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jayakumar P, Moore MG, Furlough KA, et al. Comparison of an artificial intelligence-enabled patient decision aid vs educational material on decision quality, shared decision-making, patient experience, and functional outcomes in adults with knee osteoarthritis: a randomized clinical trial. JAMA Netw Open 2021;4:e2037107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jibaja-Weiss ML, Volk RJ, Granchi TS, et al. Entertainment education for breast cancer surgery decisions: a randomized trial among patients with low health literacy. Patient Educ Couns 2011;84:41–48. [DOI] [PubMed] [Google Scholar]
- 68. Kearing S, Berg SZ, Lurie JD. Can decision support help patients with spinal stenosis make a treatment choice?: A prospective study assessing the impact of a patient decision aid and health coaching. Spine 2016;41:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kennedy AD, Sculpher MJ, Coulter A, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA 2002;288:2701–2708. [DOI] [PubMed] [Google Scholar]
- 70. Kleiss IIM, Kortlever JTP, Ring D, et al. A randomized controlled trial of decision aids for upper-extremity conditions. J Hand Surg Am 2021;46:338.e1–38.e15. [DOI] [PubMed] [Google Scholar]
- 71. Korteland NM, Ahmed Y, Koolbergen DR, et al. Does the use of a decision aid improve decision making in prosthetic heart valve selection? Circ Cardiovasc Qual Outcomes 2017;10:e003178. [DOI] [PubMed] [Google Scholar]
- 72. Kostick KM, Bruce CR, Minard CG, et al. A multisite randomized controlled trial of a patient-centered Ventricular Assist Device Decision Aid (VADDA Trial). J Card Fail 2018;24:661–671. [DOI] [PubMed] [Google Scholar]
- 73. Kuppermann M, Kaimal AJ, Blat C, et al. Effect of a patient-centered decision support tool on rates of trial of labor after previous cesarean delivery: the PROCEED randomized clinical trial. JAMA 2020;323:2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lam WW, Fielding R, Butow P, et al. Decision aids for breast cancer surgery: a randomised controlled trial. Hong Kong Med J 2014;20(suppl 7):24–27. [PubMed] [Google Scholar]
- 75. Lamers RED, Cuypers M, de Vries M, et al. Differences in treatment choices between prostate cancer patients using a decision aid and patients receiving care as usual: results from a randomized controlled trial. World J Urol 2021;39:4327–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Luan A, Hui KJ, Remington AC, et al. Effects of a novel decision aid for breast reconstruction: a randomized prospective trial. Ann Plast Surg 2016;76(suppl 3):S249–S254. [DOI] [PubMed] [Google Scholar]
- 77. Manne SL, Smith BL, Frederick S, et al. B-Sure: a randomized pilot trial of an interactive web-based decision support aid versus usual care in average-risk breast cancer patients considering contralateral prophylactic mastectomy. Transl Behav Med 2020;10:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Manne SL, Topham N, D’Agostino TA, et al. Acceptability and pilot efficacy trial of a web-based breast reconstruction decision support aid for women considering mastectomy. Psychooncology 2016;25:1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Metcalfe KA, Dennis CL, Poll A, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Genet Med 2017;19:330–336. [DOI] [PubMed] [Google Scholar]
- 80. Montgomery AA, Emmett CL, Fahey T, et al. Two decision aids for mode of delivery among women with previous caesarean section: randomised controlled trial. BMJ 2007;334:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parkinson B, Sherman KA, Brown P, et al. Cost-effectiveness of the BRECONDA decision aid for women with breast cancer: results from a randomized controlled trial. Psychooncology 2018;27:1589–1596. [DOI] [PubMed] [Google Scholar]
- 82. Phelan EA, Deyo RA, Cherkin DC, et al. Helping patients decide about back surgery: a randomized trial of an interactive video program. Spine 2001;26:206–211; discussion 212. [DOI] [PubMed] [Google Scholar]
- 83. Politi MC, Lee CN, Philpott-Streiff SE, et al. A randomized controlled trial evaluating the BREASTChoice Tool for personalized decision support about breast reconstruction after mastectomy. Ann Surg 2020;271:230–237. [DOI] [PubMed] [Google Scholar]
- 84. Rivero-Santana A, Torrente-Jiménez RS, Perestelo-Pérez L, et al. Effectiveness of a decision aid for patients with knee osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage 2021;29:1265–1274. [DOI] [PubMed] [Google Scholar]
- 85. Schwartz MD, Valdimarsdottir HB, DeMarco TA, et al. Randomized trial of a decision aid for BRCA1/BRCA2 mutation carriers: impact on measures of decision making and satisfaction. Health Psychol 2009;28:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sherman KA, Shaw LE, Winch CJ, et al. Reducing decisional conflict and enhancing satisfaction with information among women considering breast reconstruction following mastectomy: results from the BRECONDA randomized controlled trial. Plast Reconstr Surg 2016;138:592e–602e. [DOI] [PubMed] [Google Scholar]
- 87. Shorten A, Shorten B, Keogh J, et al. Making choices for childbirth: a randomized controlled trial of a decision-aid for informed birth after cesarean. Birth 2005;32:252–261. [DOI] [PubMed] [Google Scholar]
- 88. Shue J, Karia RJ, Cardone D, et al. A randomized controlled trial of two distinct shared decision-making aids for hip and knee osteoarthritis in an ethnically diverse patient population. Value Health 2016;19:487–493. [DOI] [PubMed] [Google Scholar]
- 89. Stacey D, Taljaard M, Dervin G, et al. Impact of patient decision aids on appropriate and timely access to hip or knee arthroplasty for osteoarthritis: a randomized controlled trial. Osteoarthritis Cartilage 2016;24:99–107. [DOI] [PubMed] [Google Scholar]
- 90. Stiggelbout AM, Molewijk AC, Otten W, et al. The impact of individualized evidence-based decision support on aneurysm patients’ decision making, ideals of autonomy, and quality of life. Med Decis Making 2008;28:751–762. [DOI] [PubMed] [Google Scholar]
- 91. Street RL, Jr, Voigt B, Geyer C, Jr., et al. , Increasing patient involvement in choosing treatment for early breast cancer. Cancer 1995;76:2275–2285. [DOI] [PubMed] [Google Scholar]
- 92. Trenaman L, Stacey D, Bryan S, et al. Long-term effect of patient decision aids on use of joint replacement and health care costs. Osteoarthritis Cartilage 2020;28:819–823. [DOI] [PubMed] [Google Scholar]
- 93. Trenaman L, Stacey D, Bryan S, et al. Decision aids for patients considering total joint replacement: a cost-effectiveness analysis alongside a randomised controlled trial. Osteoarthritis Cartilage 2017;25:1615–1622. [DOI] [PubMed] [Google Scholar]
- 94. Tucholka JL, Yang D-Y, Bruce JG, et al. A randomized controlled trial evaluating the impact of web-based information on breast cancer patients’ knowledge of surgical treatment options. J Am Coll Surg 2018;226:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. van Roosmalen MS, Stalmeier PF, Verhoef LC, et al. Randomised trial of a decision aid and its timing for women being tested for a BRCA1/2 mutation. Br J Cancer 2004;90:333–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. van Roosmalen MS, Stalmeier PF, Verhoef LC, et al. Randomized trial of a shared decision-making intervention consisting of trade-offs and individualized treatment information for BRCA1/2 mutation carriers. J Clin Oncol 2004;22:3293–3301. [DOI] [PubMed] [Google Scholar]
- 97. van Tol-Geerdink JJ, Willem Leer J, Weijerman PC, et al. Choice between prostatectomy and radiotherapy when men are eligible for both: a randomized controlled trial of usual care vs decision aid. BJU Int 2013;111:564–573. [DOI] [PubMed] [Google Scholar]
- 98. Vandemheen KL, O’Connor A, Bell SC, et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. Am J Respir Crit Care Med 2009;180:761–768. [DOI] [PubMed] [Google Scholar]
- 99. Varelas L, Egro FM, Evankovich N, et al. A randomized controlled trial to assess the use of a virtual decisional aid to improve knowledge and patient satisfaction in women considering breast reconstruction following mastectomy. Cureus 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vina ER, Richardson D, Medvedeva E, et al. Does a patient-centered educational intervention affect African-American access to knee replacement? A randomized trial. Clin Orthop Relat Res 2016;474:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vodermaier A, Caspari C, Koehm J, et al. Contextual factors in shared decision making: a randomised controlled trial in women with a strong suspicion of breast cancer. Br J Cancer 2009;100:590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Vuorma S, Teperi J, Aalto AM, et al. A randomized trial among women with heavy menstruation – impact of a decision aid on treatment outcomes and costs. Health Expect 2004;7:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Whelan T, Levine M, Willan A, et al. Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: a randomized trial. JAMA 2004;292:435–441. [DOI] [PubMed] [Google Scholar]
- 104. Wilkens SC, Ring D, Teunis T, et al. Decision aid for trapeziometacarpal arthritis: a randomized controlled trial. J Hand Surg Am 2019;44:247.e1–47.e9. [DOI] [PubMed] [Google Scholar]
- 105. Wilkins EG, Lowery JC, Copeland LA, et al. Impact of an educational video on patient decision making in early breast cancer treatment. Med Decis Making 2006;26:589–598. [DOI] [PubMed] [Google Scholar]
- 106. Wong SSM, Thornton JG, Gbolade B, et al. A randomised controlled trial of a decision-aid leaflet to facilitate women’s choice between pregnancy termination methods. BJOG 2006;113:688–694. [DOI] [PubMed] [Google Scholar]
- 107. Ye G, Qu B, Tham Y-C, et al. A decision aid to facilitate informed choices among cataract patients: a randomized controlled trial. Patient Educ Couns 2021;104:1295–1303. [DOI] [PubMed] [Google Scholar]
- 108. Stacey D, Légaré F, Boland L, et al. 20th Anniversary Ottawa Decision Support Framework: Part 3. Overview of systematic reviews and updated framework. Med Decis Making 2020;40:379–398. [DOI] [PubMed] [Google Scholar]
- 109. https://decisionaid.ohri.ca/odsf.html Ottawa Hospital Research Institute, Ottawa Decision Support Framework (ODSF) [document on the internet]; 2022. Accessed 15 April 2023. [Google Scholar]
- 110. Wieringa TH, Rodriguez-Gutierrez R, Spencer-Bonilla G, et al. Decision aids that facilitate elements of shared decision making in chronic illnesses: a systematic review. Syst Rev 2019;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. O’Brien MA, Charles C, Lovrics P, et al. Enablers and barriers to using patient decision aids in early stage breast cancer consultations: a qualitative study of surgeons’ views. Implement Sci 2014;9:174 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Briffa N. The employment of patient-reported outcome measures to communicate the likely benefits of surgery. Patient Relat Outcome Meas 2018;9:263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.


