Abstract
BACKGROUND.
Recent professional society guidelines for radionuclide imaging of sporadic pheochromocytoma (PHEO) recommend 18F-fluorodihydroxyphenylalanine (18F-FDOPA) as the radiotracer of choice, deeming 68Ga-DOTATATE and FDG to be second- and third-line agents, respectively. An additional agent, 18F-fluorodopamine (18F-FDA), remains experimental for PHEO detection. A paucity of research has performed head-to-head comparison among these agents.
OBJECTIVE.
The purpose of this study was to perform an intraindividual comparison of 68Ga-DOTATATE PET/CT, FDG PET/CT, 18F-FDOPA PET/CT, 18F-FDA PET/CT, CT, and MRI in visualization of sporadic primary PHEO.
METHODS.
This prospective study enrolled patients referred with clinical suspicion for sporadic PHEO. Patients were scheduled for 68Ga-DOTATATE PET/CT, FDG PET/CT, 18F-FDOPA PET/CT, 18F-FDA PET/CT, whole-body staging CT (portal venous phase), and MRI within a 3-month period. PET/CT examinations were reviewed by two nuclear medicine physicians, and CT and MRI were reviewed by two radiologists; differences were resolved by consensus. Readers scored lesions in terms of confidence in diagnosis of PHEO (1–5 scale; 4–5 considered positive for PHEO). Lesion-to-liver SUVmax was computed using both readers’ measurements. Interreader agreement was assessed using intraclass correlation coefficients (ICCs) for SUVmax. Analysis included only patients with histologically confirmed PHEO on resection.
RESULTS.
The analysis included 14 patients (eight women, six men; mean age, 52.4 ± 16.8 [SD] years) with PHEO. Both 68Ga-DOTATATE PET/CT and FDG PET/CT were completed in all 14 patients, 18F-FDOPA PET/CT in 11, 18F-FDA PET/CT in 7, CT in 12, and MRI in 12. Mean conspicuity score for PHEO was 5.0 ± 0.0 for 18F-FDOPA PET/CT, 4.7 ± 0.5 for MRI, 4.6 ± 0.8 for 18F-FDA PET/CT, 4.4 ± 1.0 for 68Ga-DOTATATE PET/CT, 4.3 ± 1.0 for CT, and 4.1 ± 1.5 for FDG PET/CT. The positivity rate for PHEO was 100.0% (11/11) for 18F-FDOPA PET/CT, 100.0% (12/12) for MRI, 85.7% (6/7) for 18F-FDA PET/CT, 78.6% (11/14) for FDG PET/CT, 78.6% (11/14) for 68Ga-DOTATATE PET/CT, and 66.7% (8/12) for CT. Lesion-to-liver SUVmax was 10.5 for 18F-FDOPA versus 3.0–4.2 for the other tracers. Interreader agreement across modalities ranged from 85.7% to 100.0% for lesion positivity with ICCs of 0.55–1.00 for SUVmax measurements.
CONCLUSION.
Findings from this small intraindividual comparative study support 18F-FDOPA PET/CT as a preferred first-line imaging modality in evaluation of sporadic PHEO.
CLINICAL IMPACT.
This study provides data supporting current guidelines for imaging evaluation of suspected PHEO.
TRIAL REGISTRATION.
Keywords: 18F-FDA, 18F-FDG, 18F-FDOPA, 68Ga-DOTATATE, pheochromocytoma
Pheochromocytoma (PHEO) is a rare neuroendocrine tumor with the potential for life-threatening manifestations of catecholamine overproduction. PHEOs arise exclusively from chromaffin cells in the adrenal glands; when arising outside of the adrenal glands (i.e., extraadrenal PHEOs), these tumors are termed paragangliomas (PGLs) [1, 2]. Though 22 known susceptibility genes are associated with PHEO/PGL pathogenesis [1, 3], 90–95% of solitary PHEOs are sporadic [4]. The workup and management of sporadic and hereditary PHEO/PGL differ [1].
Multiple imaging modalities may localize PHEO/PGL tumors, but these tests differ in performance depending on the subpopulation of PHEO/PGL being studied. The radiotracer 68Ga-DOTATATE has shown excellent results in localizing PHEO/PGL tumors [5]. However, the 2019 European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging (EANM/SNMMI) guidelines for radionuclide imaging of sporadic PHEO/PGL recommend use of 18F-fluorodihydroxyphenylalanine (18F-FDOPA) or 123I-MIBG as the radiotracers of choice, followed by 68Ga-DO-TATATE and FDG as second- and third-line agents, respectively [4]. Use of 18F-FDOPA for evaluation of PHEO/PGL is currently investigational in the United States. Additional agents for localization of PHEO/PGL remain in the experimental phase, including 18F-fluorodopamine (18F-FDA) [4]. To our knowledge, no head-to-head study has compared these various radiopharmaceuticals in patients with sporadic PHEO. Therefore, the objective of this study was to perform an intraindividual comparison of 68Ga-DO-TATATE PET/CT, FDG PET/CT, 18F-FDOPA PET/CT, 18F-FDA PET/CT, CT, and MRI in visualization of sporadic primary PHEO.
Methods
Study Participants
This prospective open-label single-center HIPAA-compliant study was approved by the institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Development. Informed consent was obtained from all participants for all clinical, genetic, biochemical, and imaging studies performed as part of the investigation. Patients were referred to the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) for participation in an institutional PHEO/PGL protocol. Patient enrollment for this study began in January 2014, when an investigational 68Ga-DOTATATE PET/CT examination was incorporated into the institutional protocol, and ended in May 2019. Patients were referred because of a clinical suspicion or known diagnosis by the referring physician for PHEO (e.g., symptoms and signs of catecholamine excess, biochemical elevation of catecholamines or metanephrines, prior imaging studies not performed as part of this study, histopathologic proof of PHEO on biopsy). Patients were ineligible if pregnant or breastfeeding, younger than 18 years old, or if they had known extraadrenal PGL or metastatic or multiple PHEO/PGL. Enrolled patients underwent FDG PET/CT, whole-body contrast-enhanced CT, and whole-body contrast-enhanced MRI as standard-of-care examinations for whole-body staging in the workup of patients referred to our institution with suspected or confirmed PHEO/PGL. Enrolled patients also underwent 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT, and 18F-FDA PET/CT for research purposes. Performance of the latter two examinations depended on scheduling availability at the time of the patient’s evaluation; patients were not excluded from the analysis if either of these two examinations were not performed. All examinations in a given patient were performed within a 3-month window of one another. Enrolled patients also underwent genetic testing for PHEO susceptibility genes. The final analysis excluded patients who had a family history of PHEO, PGL, who did not complete the genetic testing or in whom genetic testing identified a germline mutation in one of 20 PHEO susceptibility genes (SDHA, SDHAF2, SDHB, SDHC, SDHD, FH, MAX, MEN1, NF1, RET, TMEM127, VHL, HIF2A, KIF1β, EGLN2, EGLN1, H-Ras, IDH2, IDH1, MDH2, or PGL), and those in whom extraadrenal PGL or metastatic or multiple PHEO/PGL was diagnosed over the course of the study. This process resulted in a final study sample of adult patients with histologically confirmed sporadic primary adrenal PHEO who underwent FDG PET/CT, 68Ga-DOTATATE PET/CT, 18F-FDOPA PET/CT, and 18F-FDA PET/CT, CT, and MRI.
Histologic and Laboratory Information
For included patients, PHEO size was recorded using the histopathology report. In addition, all included patients underwent a plasma biochemical profile to assess for biochemical elevation. Abnormal results of this profile were categorized as an adrenergic phenotype (i.e., elevation of epinephrine or its metabolite metanephrine), a noradrenergic phenotype (i.e., elevation of norepinephrine or its metabolite normetanephrine), or a dopaminergic phenotype (i.e., an elevation of dopamine).
Image Acquisition
The agents 68Ga-DOTATATE, 18F-FDOPA, and 18F-FDA were manufactured in our PET department under an investigational new drug application. PET/CT examinations from the upper thighs to the skull were performed 60.2 ± 0.8 (SD) minutes after IV injection of a mean administered activity of 5.2 ± 0.1 mCi (92.4 ± 3.7 MBq) of 68Ga-DOTATATE, 58.9 ± 3.7 minutes after 7.7 ± 2.2 mCi (284.9 ± 81.4 MBq) of FDG, 30.0 minutes after 12.5 ± 0.2 mCi (462.5 ± 7.4 MBq) of 18F-FDOPA, and 8.2 ± 1.7 minutes after 1.0 mCi (37.0 MBq) of 18F-FDA. Patients fasted for at least 4 hours before FDG injection, and the mean serum glucose level before FDG PET/CT was 103.1 ± 11.6 mg/dL. Sixty minutes before the 18F-FDOPA injection, 200 mg carbidopa was administered orally. PET/CT examinations were performed on a Biograph mCT 64 or Biograph mCT 128 (Siemens Healthcare) PET/CT scanner. PET was performed in 3D mode with time of flight and with an iterative reconstruction algorithm provided by the manufacturer. The 68Ga-DOTATATE, 18F-FDOPA, and 18F-FDA PET/CT images were reconstructed using a 400 × 400 image matrix with 1.5-mm slice thickness. The FDG PET/CT images were reconstructed using a 256 × 256 matrix with 3-mm thickness. All PET/CT examinations included low-dose CT without oral or IV contrast material for attenuation correction and anatomic coregistration (called the “attenuation CT”).
The whole-body CT examinations were performed using a Somatom Force or Definition Flash (Siemens Healthcare) or Toshiba Aquilion One (Canon Medical Systems) scanner. The IV contrast agent dose (range, 90–130 mL; median, 119 mL) varied according to the patient’s body mass index. A nonionic low-osmolality agent (Isovue 300, Bracco Diagnostics) was used in all patients except for one patient who received Isovue 370 (Bracco Diagnostics) because they were undergoing a concomitant pulmonary CTA to exclude pulmonary embolism. An injection rate of 2 mL/s was used in all patients except the patient undergoing pulmonary CTA (injection rate of 4 mL/s) and two patients with limited venous access (injection rates of 1.4 mL/s and 1.6 mL/s). A single acquisition was acquired in the portal venous phase (median, 73 seconds; range, 60–80 seconds after contrast administration). The slice thickness was 2 mm for all CT examinations. A dedicated adrenal washout protocol was not used to reduce radiation exposure for study participants.
The whole-body MRI examinations were performed using Achieva 1.5- or 3-T (Philips Healthcare) or Aera 1.5-T or Verio 3-T (Siemens Healthcare) scanners. MRI examinations of the abdomen and pelvis included DWI, T2-weighted images with and without fat suppression, and multiphase multiplanar T1-weighted images before and after IV administration of 0.2 mL/kg of a gadolinium-based contrast agent. The slice thickness varied slightly across MRI examinations but was typically 3 mm for contrast-enhanced sequences and no greater than 6 mm for unenhanced sequences.
Image Analysis
Two board-certified nuclear medicine physicians (J.A.C. and C.C.C., with 35 and 32 years of experience, respectively, including 21 years each in interpreting imaging of PHEOs) independently interpreted all PET/CT examinations. One board-certified diagnostic radiologist (A.L., with 35 years of experience, including 14 years in interpreting imaging of PHEOs) and one physician with dual-board certification in diagnostic radiology and nuclear medicine (B.S., with 5 years of experience, including 2 years in interpreting PHEOs) independently interpreted all CT and MRI examinations. Readers were aware of patients’ age and sex and that all patients had clinical suspicion for PHEO but were not informed that PHEO had been histologically confirmed in all patients. Readers were blinded to all other clinical data and to the other imaging examinations for the patient. The various imaging examinations reviewed by the pairs of readers were reviewed in separate sessions for each modality (i.e., four separate sessions for the two readers who reviewed PET/CT examinations using the four different agents in random order; two separate sessions for the two readers who reviewed CT and MRI).
Readers assigned each examination a conspicuity score, reflecting their overall impression for the likelihood of PHEO being present using a 5-point Likert scale: 1, PHEO definitely absent; 2, PHEO unlikely; 3, presence of PHEO is equivocal; 4, PHEO likely; 5, PHEO definitely present. Conspicuity scores of 1–3 were considered negative for PHEO, and scores of 4 or 5 were considered positive for PHEO, consistent with the approach in earlier studies [6, 7]. Confidence in PHEO on the PET/CT examinations was based primarily on a visual assessment of the lesions’ degree of uptake of the given agent. Though the attenuation CT images were used to localize uptake to adrenal lesions, lesions were required to be identifiable on the PET images independent of the attenuation CT images to be confident in the diagnosis of PHEO. This approach was taken to avoid the readers inferring the diagnosis of PHEO for a photopenic lesion because of mass effect on other structures apparent on the attenuation CT images. Visual criteria used to consider a mass positive for PHEO were uptake greater than normal liver or uptake similar to or greater than the contralateral normal adrenal gland. Such uptake could be distributed homogeneously or heterogeneously throughout the lesion or could be located within the periphery of a lesion exhibiting a photopenic center.
In the same sessions for which readers assigned conspicuity scores for the PET/CT examinations, the readers also measured SUVmax (corrected for body weight) of each adrenal lesion and the contralateral normal adrenal gland. The ROIs were placed using either MIM (version 7.0.7, MIM Software) (reader J.A.C.) or MedImage (version 12.2.3, MedImage) (reader C.C.C.) software. ROIs were drawn to encompass the entirety of the visually identified adrenal lesion or normal adrenal gland. If the normal adrenal gland could not be definitively identified on FDG PET/CT examinations because of low adrenal uptake, then attenuation CT scans were used to assist in ROI placement. The readers also measured SUVmax of the liver using a spherical ROI placed over a normal-appearing right hepatic lobe. The SUVmax measurements were available when scoring confidence in PHEO but were secondary to qualitative visual assessment in judging confidence.
Confidence in PHEO on CT or MRI was a result of assessment for typical imaging features of PHEO, including hypervascularity, persistent delayed enhancement, heterogeneous enhancement, lack of internal fat, and hyperintensity on T2-weighted sequences [8-10]. Lower conspicuity scores were assigned to lesions exhibiting imaging features associated with other adrenal entities (e.g., adenoma, metastasis, or adrenal cortical carcinoma).
For examinations in which the two readers for the given modality had a concordant dichotomized conspicuity score (negative vs positive), the first reader’s conspicuity scores were used for subsequent statistical analyses. For examinations in which the two readers for the given modality had a discordant dichotomized conspicuity score (negative vs positive), the two readers performed a subsequent joint analysis in which they reached consensus for a single conspicuity score to use for subsequent statistical analyses. For PET/CT examinations, the analysis used the SUVmax measurements of adrenal lesions by one reader (J.A.C.) and the SUVmax measurements of contralateral adrenal gland and normal liver by the other reader (C.C.C.). After completion of the independent readings, the readers performed a post hoc image review in consensus of negative studies to assess for possible causes of the false-negative interpretations on each modality.
Statistics
Standard summary statistics were computed, along with calculation of 95% CIs. Ratios were calculated between SUVmax of lesions and of the contralateral normal adrenal gland and the liver. The Friedman test was used to perform a global comparison of the conspicuity score and SUVmax ratios across the tests, and the Wilcoxon signed rank test was used for subsequent pairwise comparisons among the modalities. The Cochrane Q test was used to perform a global comparison of the positivity rate across the tests, and the McNemar test was used for subsequent pairwise comparisons among the modalities. As a sensitivity analysis, these comparisons were also performed excluding 18F-FDA PET/CT because of the number of patients in whom this examination was not performed. SUVmax values were summarized among all lesions and among true-positive and false-negative lesions. Interreader agreement for the 1–5 conspicuity scores was computed using weighted kappa coefficients and summarized using a classification provided by Landis and Koch [11]: < 0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1.00, almost-perfect agreement. The percentage agreement between readers was also calculated for the dichotomized confidence scores. Interreader agreement for the SUVmax measurements was computed using intraclass correlation coefficients (ICCs) and summarized using a classification provided by Cicchetti [12]: 0.00–0.39, poor; 0.40–0.59, fair; 0.60–0.74, good; 0.75–1.00, excellent agreement. Two-sided p values were calculated and deemed different at p < .05. Analysis was performed using the SAS version 9.4 software (SAS Institute).
Results
Patient and Tumor Characteristics
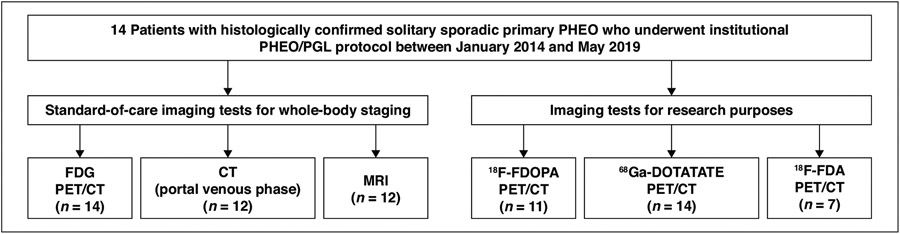
The study sample included 14 patients (eight women, six men; mean age, 52.4 ± 16.8 years; age range, 20–76 years) with a histologically confirmed solitary sporadic primary PHEO (Fig. 1 and Table S1; Table S1 can be viewed in the AJR electronic supplement to this article available at doi.org/10.2214/AJR.21.26071). Six PHEOs were in the left adrenal gland, and eight PHEOs were in the right adrenal gland. All 14 patients underwent surgical resection of the PHEO after completion of the imaging examinations. The mean PHEO size on histopathology was 5.2 ± 2.6 cm (range, 2.0–9.5 cm).The biochemical profile showed biochemical elevation in all 14 patients. All 14 patients showed a noradrenergic phenotype, 10 showed an adrenergic phenotype, and 10 showed a dopaminergic phenotype. Twelve patients also had elevated chromogranin A levels.
Fig. 1—
Diagram of flow of patient inclusions and exclusions. PHEO = pheochromocytoma, PGL = paraganglioma, 18F-FDOPA = 18F-fluorodihydroxyphenylalanine, 18F-FDA = 18F-fluorodopamine..
All 14 patients underwent 68Ga-DOTATATE PET/CT and FDG PET/CT. Ten patients completed both the whole-body CT and whole-body MRI; two patients completed only the whole-body CT, and two patients completed only the whole-body MRI. Eleven patients underwent 18F-FDOPA PET/CT and seven patients underwent 18F-FDA PET/CT. The mean duration was 11 ± 16 days between 68Ga-DOTATATE PET/CT and FDG PET/CT, 5 ± 8 days between 68Ga-DOTATATE PET/CT and 18F-FDOPA PET/CT, 17 ± 19 days between 68Ga-DOTATATE PET/CT and 18F-FDA PET/CT, 10 ± 16 days between 68Ga-DOTATATE PET/CT and CT, and 12 ± 17 days between 68Ga-DOTATATE PET/CT and MRI.
Comparison of Imaging Modalities
Table 1 summarizes the conspicuity scores and positivity rates for the various imaging modalities. The mean conspicuity score for PHEO for 18F-FDOPA PET/CT was 5.0 ± 0.0, for MRI was 4.7 ± 0.5, for 18F-FDA PET/CT was 4.6 ± 0.8, for 68Ga-DOTATATE PET/CT was 4.4 ± 1.0, for single-phase CT was 4.3 ± 1.0, and for FDG PET/CT was 4.1 ± 1.5. These values were not significantly different (p = .17; p = .16 without 18F-FDA PET/CT). The median conspicuity score was 5 for all six modalities.
TABLE 1:
Comparison Among Imaging Modalities of Conspicuity Score and Positivity Rate for Sporadic Adrenal Pheochromocytoma
| Conspicuity Score | Positivity Rate | ||||
|---|---|---|---|---|---|
| Modality | No. of Patients | Mean ± SD | Median (Range) | Raw Data | Percentage (95% CI) |
| 68Ga-DOTATATE PET/CT | 14 | 4.4 ± 1.0 | 5.0 (2.0–5.0) | 11/14 | 78.6 (49.2–95.3) |
| FDG PET/CT | 14 | 4.1 ± 1.5 | 5.0 (1.0–5.0) | 11/14 | 78.6 (49.2–95.3) |
| 18F-FDOPA PET/CT | 11 | 5.0 ± 0.0 | 5.0 (5.0–5.0) | 11/11 | 100 (71.5–100) |
| 18F-FDA PET/CT | 7 | 4.6 ± 0.8 | 5.0 (3.0–5.0) | 6/7 | 85.7 (42.1–99.6) |
| Portal venous phase CT | 12 | 4.3 ± 1.0 | 5.0 (3.0–5.0) | 8/12 | 66.7 (34.9–90.1) |
| MRI | 12 | 4.7 ± 0.5 | 5.0 (4.0–5.0) | 12/12 | 100 (73.5–100) |
The positivity rate for PHEO for 18F-FDOPA PET/CT was 100.0% (11/11), for MRI was 100.0% (12/12), for 18F-FDA PET/CT was 85.7% (6/7), for FDG PET/CT was 78.6% (11/14), for 68Ga-DOTATATE PET/CT was 78.6% (11/14), and for single-phase CT was 66.7% (8/12). These values were significantly different at the global level (p = .02; p < .001 without 18F-FDA PET/CT), though no pairwise difference among modalities was identified (all p > .25).
Table 2 summarizes data regarding SUVmax for the four PET agents. The mean ratio of SUVmax between the adrenal lesion and the contralateral normal adrenal gland for 68Ga-DOTATATE PET/CT was 1.6 ± 0.8, for FDG PET/CT was 2.3 ± 1.5, for 18F-FDOPA PET/CT was 5.7 ± 4.7, and for 18F-FDA PET/CT was 2.5 ± 1.3. These ratios were significantly different globally (p = .004; p = .005 without 18F-FDA PET/CT). Using pairwise testing, the ratios were different between 18F-FDOPA PET/CT and 68Ga-DOTATATE PET/CT (p = .001), between 18F-FDOPA PET/CT and FDG PET/CT (p = .04), and between 18F-FDO-PA PET/CT and 18F-FDA PET/CT (p = .03). The mean ratio of SUVmax between the adrenal lesion and normal liver for 68Ga-DOTATATE PET/CT was 4.2 ± 2.7, for FDG PET/CT was 3.0 ± 2.0, for 18F-FDOPA PET/CT was 10.5 ± 7.3, and for 18F-FDA PET/CT was 3.5 ± 1.0. These ratios were significantly different (p = .002; p < .001 without 18F-FDA). According to pairwise testing, the ratios were significantly different between 18F-FDOPA PET/CT and 68Ga-DOTATATE PET/CT (p = .01), and between 18F-FDOPA PET/CT and FDG PET/CT (p = .001).
TABLE 2:
Comparison Among Imaging Modalities in SUVmax Measurements
| Modality | Lesions | Contralateral Adrenal Gland |
Ratio of Lesion to Contralateral Adrenal Gland |
Normal Liver |
Ratio of Lesion to Liver |
||
|---|---|---|---|---|---|---|---|
| All | True-Positives | False-Negatives | |||||
| 68Ga-DOTATATE PET/CT (n = 14) | |||||||
| Mean ± SD | 43.6 ± 26.7 | 47.0 ± 29.3 | 31.3 ± 8.4 | 29.3 ± 12.0 | 1.6 ± 0.8 | 11.0 ± 2.4 | 4.2 ± 2.7 |
| Median | 35.3 | 36.0 | 34.6 | 29.8 | 1.3 | 10.6 | 3.4 |
| Range | 15.0–99.4 | 15.0–99.4 | 21.7–37.5 | 10.0–49.8 | 0.7–3.7 | 7.5–14.9 | 1.5–11.0 |
| FDG PET/CT (n = 14) | |||||||
| Mean ± SD | 10.5 ± 5.6 | 12.0 ± 5.5 | 5.2 ± 1.5 | 5.2 ± 2.6 | 2.3 ± 1.5 | 3.8 ± 1.1 | 3.0 ± 2.0 |
| Median | 10.2 | 11.0 | 5.6 | 4.5 | 1.7 | 3.7 | 2.5 |
| Range | 3.6–20.9 | 5.2–20.9 | 3.6–6.5 | 2.9–12.0 | 1.0–6.4 | 1.9–6.8 | 1.0–6.6 |
| 18F-FDOPA PET/CT (n = 11) | |||||||
| Mean ± SD | 34.6 ± 21.7 | 34.6 ± 21.7 | NAa | 7.0 ± 2.1 | 5.7 ± 4.7 | 3.4 ± 0.8 | 10.5 ± 7.3 |
| Median | 31.2 | 31.2 | 6.7 | 4.9 | 3.2 | 8.3 | |
| Range | 11.6–78.4 | 11.6–78.4 | 4.4–11.7 | 1.3–17.7 | 2.6–5.5 | 3.6–27.0 | |
| 18F-FDA PET/CT (n = 7) | |||||||
| Mean ± SD | 39.3 ± 17.7 | 42.4 ± 17.2 | 20.8 ± 0b | 18.2 ± 11.0 | 2.5 ± 1.3 | 10.9 ± 2.3 | 3.5 ± 1.0 |
| Median | 32.4 | 35.5 | 17.2 | 2.0 | 9.8 | 3.3 | |
| Range | 20.8–73.8 | 28.1–73.8 | 7.9–40.9 | 1.1–4.9 | 9.1–15.7 | 2.3–4.7 | |
Note—NA = not applicable.
No false-negatives.
Only one false-negative.
The mean SUVmax for all lesions, true-positive lesions, and false-negative lesions (according to the dichotomized conspicuity score) for 68Ga-DOTATATE PET/CT was 43.6 ± 26.7, 47.0 ± 29.3 (n = 11), and 31.3 ± 8.4 (n = 3), respectively; for FDG PET/CT was 10.5 ± 5.6, 12.0 ± 5.5 (n = 11), and 5.2 ± 1.5 (n = 3), respectively; and for 18F-FDA PET/CT was 39.3 ± 17.7, 42.4 ± 17.2 (n = 6), and 20.8 ± 0 (n = 1), respectively. For 18F-FDOPA PET/CT, all 11 lesions were true-positives, with a mean SUVmax of 34.6 ± 21.7.
Tables S1-S5 provide patient-level results for the six imaging tests (supplemental tables can be viewed in the AJR electronic supplement to this article available at doi.org/10.2214/AJR.21.26071). Figure 2 and Figures S1-S3 provide images from representative patients (supplemental figures are also available at doi.org/10.2214/AJR.21.26071).
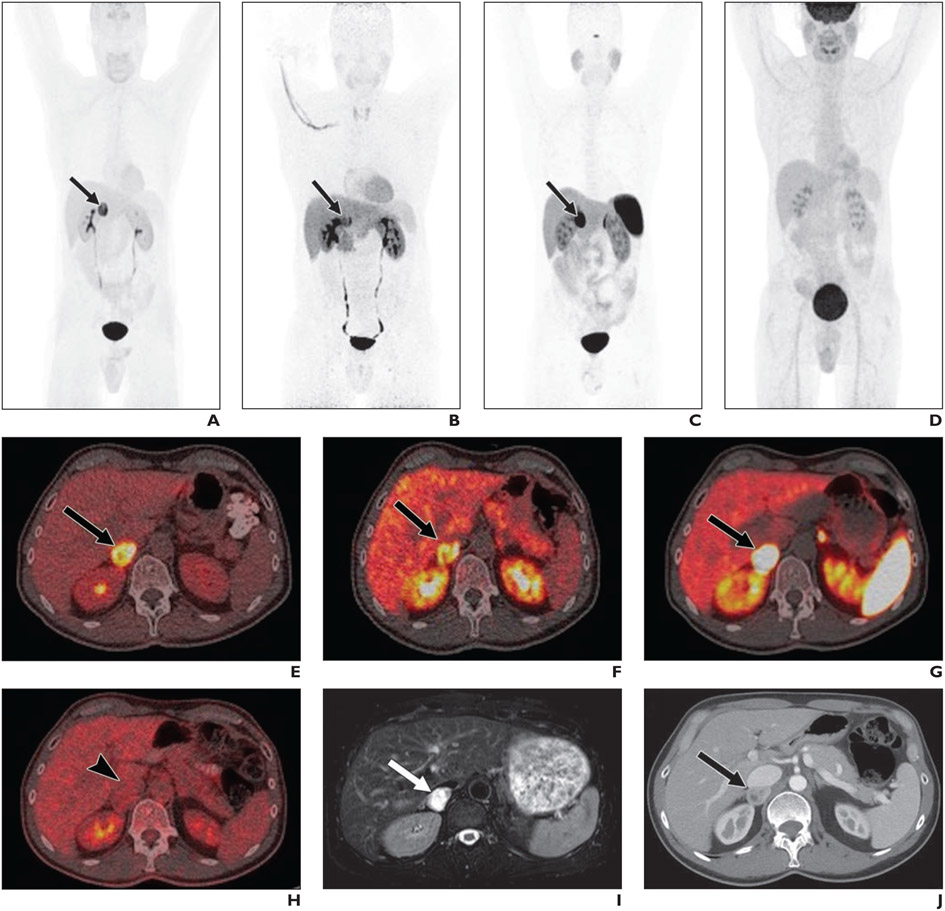
Fig. 2—
Multimodality imaging of 54-year-old man with clinical suspicion for pheochromocytoma (PHEO) based on symptoms of flushing, palpitations, episodes of anxiety, nocturnal sweating, and angina. Patient also had elevated blood pressure, elevated plasma and urinary metanephrines, and positive 123I-MIBG uptake in right suprarenal area on prior imaging. Patient was referred to National Institutes of Health for further evaluation and management and underwent institutional PHEO/PGL protocol.
A–J, Images show maximum-intensity-projection PET using 18F-fluorodihydroxyphenylalanine (18F-FDOPA, A), 18F-fluorodopamine (18F-FDA) (B), 68Ga-DOTATATE (C), and FDG (D); fused axial PET/CT using 18F-FDOPA (E), 18F-FDA (F), 68Ga-DOTATATE (G), and FDG (H); axial T2-weighted MRI (I), and axial portal venous phase contrast-enhanced CT (J). Mass (arrows, A–C, E–G, I, J) was deemed positive for PHEO on all modalities other than on FDG PET/CT, on which mass (arrowhead, H) was deemed negative for PHEO. Conspicuity score (CS) and SUVmax by reader 1 were as follows: 18F-FDOPA: CS, 5 and SUVmax, 30.0; 18F-FDA: CS, 5 and SUVmax, 32.4; 68Ga-DOTATATE: CS, 5 and SUVmax, 99.4; FDG: CS, 1 and SUVmax, 3.6; MRI: CS, 5; and CT: CS, 5. Subsequent surgical resection confirmed PHEO.
Post Hoc Assessment of False-Negative Interpretations for PHEO
In the three false-negative interpretations on 68Ga-DOTATATE PET/CT (patients 6, 9, and 12), the lesions showed photopenia with thin peripheral uptake. In these cases, the readers attributed the lesion’s peripheral uptake to normal adrenal tissue and deemed the lesion to not show increased uptake. Nonetheless, all three lesions were positive on FDG PET/CT and 18F-FDOPA PET/CT (when performed).
For the single false-negative interpretation on 18F-FDA PET/CT (patient 4) and for the three false-negative interpretations on FDG PET/CT (patients 4, 7, and 14), no explanation was identified at post hoc image review. In all of these cases, the lesion was positive on PET/CT using the other radiotracers (when available).
The four false-negative interpretations on CT (patients 4, 8, 9, and 10) were attributed in part to the presence of only a portal venous phase, limiting assessment. The lesions had been considered to possibly represent adenoma or other adrenal neoplasm.
No false-negative interpretations were present for 18F-FDOPA PET/CT or MRI.
Interreader Agreement
The interreader agreement analysis showed substantial agreement in conspicuity score for 68Ga-DOTATATE PET/CT (κ, 0.69) and FDG PET/CT (κ, 0.67), and moderate agreement for 18F-FDA PET/CT (κ, 0.42), CT (κ, 0.58), and MRI (κ, 0.57) (Table 3). Interreader agreement could not be computed for 18F-FDOPA PET/CT, for which both readers provided a conspicuity score of 5 for all lesions.
TABLE 3:
Interreader Agreement for Conspicuity Scores
| Conspicuity Score | Positivity | |||
|---|---|---|---|---|
| Modality | κ | 95% CI | Agreement (%) | Raw Data |
| 68Ga-DOTATATE PET/CT | 0.69 | 0.53–0.84 | 100.0 | 14/14 |
| FDG PET/CT | 0.67 | 0.43–0.90 | 85.7 | 12/14 |
| 18F-FDOPA PET/CT | NAa | NA | 100.0 | 11/11 |
| 18F-FDA PET/CT | 0.42 | 0.06–0.77 | 85.7 | 6/7 |
| CT | 0.58 | 0.32–0.85 | 100.0 | 12/12 |
| MRI | 0.57 | 0.08–1.00 | 100.0 | 12/12 |
Kappa coefficient could not be computed because both readers gave same confidence score (score of 5) to all 11 cases.
For the dichotomous classification, the readers agreed 100.0% (14/14) for 68Ga-DOTATATE PET/CT, 100.0% (11/11) for 18F-FDOPA PET/CT, 100.0% (11/11) for CT, 100.0% (12/12) for MRI, 85.7% (12/14) for FDG PET/CT, and 85.7% (6/7) for 18F-FDA PET/CT.
For SUVmax measurements, the readers showed excellent agreement (ICC = 0.91–0.99) for 68Ga-DOTATATE PET/CT, good to excellent agreement (ICC = 0.69–1.00) for FDG PET/CT, excellent agreement (ICC = 0.96–1.00) for 18F-FDOPA PET/CT, and fair to excellent agreement (ICC = 0.55–1.00) for 18F-FDA PET/CT (Table 4).
TABLE 4:
Interreader Agreement for SUVmax Measurements
| Modality | Intraclass Correlation Coefficient |
|---|---|
| 68Ga-DOTATATE PET/CT | |
| Adrenal lesion | 0.99 (0.99–1.00) |
| Normal adrenal gland | 0.97 (0.92–0.99) |
| Normal liver | 0.91 (0.78–0.97) |
| FDG PET/CT | |
| Adrenal lesion | 1.00 (0.99–1.00) |
| Normal adrenal gland | 0.69 (0.39–0.89) |
| Normal liver | 0.89 (0.72–0.96) |
| 18F-FDOPA PET/CT | |
| Adrenal lesion | 1.00 (1.00–1.00) |
| Normal adrenal gland | 0.96 (0.88–0.99) |
| Normal liver | 0.97 (0.90–0.99) |
| 18F-FDA PET/CT | |
| Adrenal lesion | 1.00 (1.00–1.00) |
| Normal adrenal gland | 1.00 (0.98–1.00) |
| Normal liver | 0.55 (0.13–0.91) |
Note—Values in parentheses are 95% CI.
Discussion
In this prospective study, we performed an intraindividual comparison of visualization of sporadic primary PHEO using 68Ga-DOTATATE PET/CT, FDG PET/CT, 18F-FDOPA PET/CT, 18F-FDA PET/CT, portal venous phase CT, and MRI. Given the small sample size, the visualization measures were not different among the modalities. Nonetheless, the findings suggest particularly excellent visualization of PHEO for 18F-FDOPA PET/CT, which was the only imaging modality that received a conspicuity score of 5 for all patients for both readers, and also the modality that had highest SUVmax ratios between the adrenal lesion and either the contralateral normal adrenal gland or normal liver. In comparison, the mean conspicuity score and mean SUVmax ratio of adrenal lesion to normal liver was lowest for FDG PET/CT, whereas the mean SUVmax ratio of adrenal lesion to normal contralateral adrenal gland was lowest for 68Ga-DOTATATE PET/CT. The observations are in line with current guidelines that recommend 18F-FDOPA PET/CT followed by 68Ga-DOTATATE PET/CT in this clinical setting, and that deem FDG PET/CT as a third-line modality [4].
The findings highlight the different receptors or metabolic pathways of PHEO/PGLs that are targeted by the various radiopharmaceuticals. The agent 68Ga-DOTATATE binds to somatostatin receptors (SSTR), which are overexpressed in PHEO/PGLs, especially the SSTR2 subtype [13]. In comparison, 18F-FDOPA targets tumors via the large neutral amino acid transporter system [6, 14], and 18F-FDA targets the norepinephrine transporter system specifically found on on PHEO/PGLs [6]. FDG enters tumors through glucose transporters and is a widely used radiopharmaceutical for oncologic imaging [15]. All cases of a false-negative PHEO for a given agent were positive for the other agents, reflecting these distinct pathways (i.e., loss of SSTR2 expression for 68Ga-DOTATATE PET/CT and of the normal norepinephrine transporter system for 18F-FDA PET/CT). Indeed, this loss of SSTR2 expression is a more likely explanation for the photopenia observed for the three false-negative cases using 68Ga-DOTATATE PET/CT than is possible tumor necrosis, because the uptake observed by the other tracers for all three such lesions confirms the presence of viable tumor.
These findings build on the study by Archier et al. [14] in which 18F-FDOPA PET/CT detected all 10 sporadic PHEOs (both primary and recurrent tumors), whereas 68Ga-DOTATATE PET/CT and conventional imaging (contrast-enhanced CT and MRI) detected only 8 of 10 tumors. The mean SUVmax of 34.6 for PHEO using 18F-FDOPA in our study is greater than a median SUVmax of 12.0 reported by Amodru et al. [16] in a study of 56 patients with PHEO (both sporadic and hereditary). This difference may be explained in part by that study’s lack of carbidopa use, as was administered in our study and which resulted in elevated uptake in our study [17].
Our findings reaffirm 18F-FDOPA PET/CT as a preferred imaging test in the evaluation of sporadic primary PHEO, as supported by the 2019 EANM/SNMMI guidelines for radionuclide imaging of PHEO/PGL. Our findings are also favorable for 18F-FDA PET/CT, though this investigational agent currently has limited availability. At centers where 18F-FDOPA and 18F-FDA are both unavailable, we feel that 68Ga-DOTATATE PET/CT is preferable to FDG PET/CT given the more specific association of SSTR2 expression with PHEO, in comparison with the spectrum of benign and malignant adrenal lesions that exhibit increased FDG activity [18]. Nonetheless, despite much recent interest in the use of 68Ga-DOTATATE PET/CT for a workup of PHEO [4, 19], caution remains warranted with this agent. Our observation of several false-negatives for 68Ga-DOTATATE PET/CT among the 14 patients is consistent with results of earlier studies [7, 14]. Further, given our study’s favorable results for MRI compared with single-phase CT, as well as numerous cases in which MRI but not all of the PET examinations were positive for PHEO, consideration should be given to performing PET examinations for any of these agents by PET/MRI (when available) rather than PET/CT.
Our study has limitations. First, the sample size was small, reflecting the prospective recruitment of patients with an uncommon tumor to undergo a series of imaging tests. Second, not all patients underwent both 18F-FDOPA PET/CT and 18F-FDA PET/CT given logistical challenges in scheduling these examinations. Third, 123I-MIBG scintigraphy was not performed, even though it is recommended by Endocrine Society practice guidelines for sporadic PHEO [20]. At our institution, 123I-MIBG scintigraphy is not routinely performed as part of the diagnostic workup for PHEO given its poor sensitivity for small PHEOs [7, 21]. Rather, the test is used only to determine eligibility for 131I-MIBG therapy in patients with metastatic or inoperable disease. Fourth, the CT examinations were performed as whole-body staging examinations per institutional protocol. At our institution, a dedicated adrenal-protocol CT is not commonly performed but is reserved for when an adrenal lesion remains inconclusive after assessment by other imaging modalities. Fifth, the patients were not tested for somatic mutations that can be present in a minority of patients with sporadic PHEO/PGL. Finally, because the analysis only included patients with histologically confirmed PHEO, the specificity of the six imaging tests was not explored.
In conclusion, this small prospective study of patients with sporadic PHEO who underwent six different imaging tests supports current guidelines in deeming 18F-FDOPA PET/CT as a first-line imaging modality in the workup of sporadic PHEO. The results are also encouraging for 18F-FDA PET/CT, which is investigational along with 18F-FDOPA for PHEO/PGL imaging. When these agents are unavailable, 68Ga-DOTATATE PET/CT remains favored over FDG PET/CT. For all of these agents, PET/MRI, when available, may be preferred over PET/CT, given the strong results for MRI. Larger multicenter studies are warranted for continued insights into the role of these imaging tests in the evaluation of patients with suspected sporadic PHEO.
Supplementary Material
HIGHLIGHTS.
Key Finding
In this prospective intraindividual study, the positivity rate for PHEO was 100.0% (11/11) for 18F-FDOPA PET/CT, 100.0% (12/12) for MRI, 85.7% (6/7) for 18F-FDA PET/CT, 78.6% (11/14) for FDG PET/CT, 78.6% (11/14) for 68Ga-DOTATATE PET/CT, and 66.7% (8/12) for portal venous phase CT.
Importance
The study provides data supporting current guidelines that recommend 18F-FDOPA PET/CT as a first-line imaging modality in the workup of suspected sporadic PHEO.
Acknowledgments
We thank the patients and their families for participating in the study and all of the people who participated in this project, especially the technologists in the NIH Clinical Center PET and radiology department.
Supported in part by Intramural Research Program of the National Institutes of Health (NIH) (grant no. Z1AHD008735 to K. Pacak), NIH/National Cancer Institute (NCI) Cancer Center (grant no. P30 CA008748 to J. A. Carrasquillo), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
The authors declare that they have no disclosures relevant to the subject matter of this article.
Based on a presentation at the Society of Nuclear Medicine and Molecular Imaging 2019 annual meeting, Anaheim, CA.
An electronic supplement is available online at doi.org/10.2214/AJR.21.26071.
References
- 1.Crona J, Taïeb D, Pacak K. New perspectives on pheochromocytoma and paraganglioma: toward a molecular classification. Endocr Rev 2017; 38:489–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam AK. Update on adrenal tumours in 2017 World Health Organization (WHO) of endocrine tumours. Endocr Pathol 2017; 28:213–227 [DOI] [PubMed] [Google Scholar]
- 3.Alrezk R, Suarez A, Tena I, Pacak K. Update of pheochromocytoma syndromes: genetics, biochemical evaluation, and imaging. Front Endocrinol (Lausanne) 2018; 9:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taïeb D, Hicks RJ, Hindié E, et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 2019; 46:2112–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han S, Suh CH, Woo S, Kim YJ, Lee JJ. Performance of 68Ga-DOTA-conjugated somatostatin receptor-targeting peptide PET in detection of pheochromocytoma and paraganglioma: a systematic review and metaanalysis. J Nucl Med 2019; 60:369–376 [DOI] [PubMed] [Google Scholar]
- 6.Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2009; 94:4757–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gild ML, Naik N, Hoang J, et al. Role of DOTATATE-PET/CT in preoperative assessment of phaeochromocytoma and paragangliomas. Clin Endocrinol (Oxf) 2018; 89:139–147 [DOI] [PubMed] [Google Scholar]
- 8.Blake MA, Cronin CG, Boland GW. Adrenal imaging. AJR 2010; 194:1450–1460 [DOI] [PubMed] [Google Scholar]
- 9.McDermott S, McCarthy CJ, Blake MA. Images of pheochromocytoma in adrenal glands. Gland Surg 2015; 4:350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsayes KM, Elmohr MM, Javadi S, et al. Mimics, pitfalls, and misdiagnoses of adrenal masses on CT and MRI. Abdom Radiol (NY) 2020; 45:982–1000 [DOI] [PubMed] [Google Scholar]
- 11.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174 [PubMed] [Google Scholar]
- 12.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994; 6:284–290 [Google Scholar]
- 13.Reubi JC, Waser B, Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001; 28:836–846 [DOI] [PubMed] [Google Scholar]
- 14.Archier A, Varoquaux A, Garrigue P, et al. Prospective comparison of (68) Ga-DOTATATE and (18)F-FDOPA PET/CT in patients with various pheochromocytomas and paragangliomas with emphasis on sporadic cases. Eur J Nucl Med Mol Imaging 2016; 43:1248–1257 [DOI] [PubMed] [Google Scholar]
- 15.Belhocine T, Spaepen K, Dusart M, et al. 18FDG PET in oncology: the best and the worst. (review) Int J Oncol 2006; 28:1249–1261 [PubMed] [Google Scholar]
- 16.Amodru V, Guerin C, Delcourt S, et al. Quantitative 18F-DOPA PET/CT in pheochromocytoma: the relationship between tumor secretion and its biochemical phenotype. Eur J Nucl Med Mol Imaging 2018; 45:278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmers HJ, Hadi M, Carrasquillo JA, et al. The effects of carbidopa on uptake of 6-18F-Fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma. J Nucl Med 2007; 48:1599–1606 [DOI] [PubMed] [Google Scholar]
- 18.Dong A, Cui Y, Wang Y, Zuo C, Bai Y. (18)F-FDG PET/CT of adrenal lesions. AJR 2014; 203:245–252 [DOI] [PubMed] [Google Scholar]
- 19.Taïeb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr Relat Cancer 2019; 26:R627–R652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenders JW, Duh QY, Eisenhofer G, et al. ; Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014; 99:1915–1942 [DOI] [PubMed] [Google Scholar]
- 21.van Berkel A, Pacak K, Lenders JW. Should every patient diagnosed with a phaeochromocytoma have a 123I-MIBG scintigraphy? Clin Endocrinol (Oxf) 2014; 81:329–333 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.