Abstract
Inactivated poliovirus vaccine (IPV) is efficacious against paralytic disease, but its effect on mucosal immunity is debated. We assessed the efficacy of IPV in boosting mucosal immunity. Participants received IPV, bivalent 1 and 3 oral poliovirus vaccine (bOPV), or no vaccine. A bOPV challenge was administered 4 weeks later, and excretion was assessed 3, 7, and 14 days later. Nine hundred and fifty-four participants completed the study. Any fecal shedding of poliovirus type 1 was 8.8, 9.1, and 13.5% in the IPV group and 14.4, 24.1, and 52.4% in the control group by 6- to 11-month, 5-year, and 10-year groups, respectively (IPV versus control: Fisher’s exact test P < 0.001). IPV reduced excretion for poliovirus types 1 and 3 between 38.9 and 74.2% and 52.8 and 75.7%, respectively. Thus, IPV in OPV-vaccinated individuals boosts intestinal mucosal immunity.
Since the beginning of polio vaccine development in the 1950s, the choice of which vaccine to use, either the Salk inactivated poliovirus (IPV) or the Sabin live-attenuated oral poliovirus vaccine (OPV), has been fiercely contested (1, 2). The debate continued even after the goal of global polio eradication was established in 1988 (3). Regardless, the global polio eradication initiative (GPEI) chose OPV to eliminate the final chains of poliovirus transmission (4).
Through massive use of OPV, the number of polio-endemic countries decreased from 125 in 1988 to 3 in 2013, and the incidence declined by >99% (5). Wild poliovirus type 2 transmission was interrupted globally in 1999 (6), and wild poliovirus type 3 was last detected in November 2012 (7) (fig. S1). However, parts of three countries (Afghanistan, Nigeria, and Pakistan) continue to report wild poliovirus type 1 (8), and exportation of this virus causes outbreaks (9, 10).
Despite the advantages of OPV (superior mucosal immunity, secondary spread to contacts, ease of administration, and lower price), the vaccine has limitations: low immunogenicity in some tropical countries (11, 12); and incomplete intestinal immunity that wanes rapidly (13). To interrupt transmission, OPV must be administered to a high proportion of children (1, 4).
The intestinal immunity induced by OPV is incomplete. Even after a full series with OPV, between 10 and 20% of children excrete poliovirus after a challenge with Sabin poliovirus (1, 14). Studies in India demonstrate that a fraction of children excrete Sabin strains after OPV exposure despite having received more than seven doses of OPV (15). To address the low OPV immunogenicity, the GPEI developed new formulations of OPV, including monovalent OPVs (mOPVs; mOPV1 and mOPV3) in 2005 and bivalent types 1 + 3 OPV (bOPV) in 2009 (16, 17).
Although IPV administered after OPV effectively closes the humoral immunity gaps (2, 18, 19), its effect on intestinal mucosal immunity is less-well characterized. Because mucosal immunity appears to wane rapidly (13), we initiated a trial in Northern India to assess whether IPV could boost mucosal immunity. We enrolled infants aged 6 to 11 months and children aged 5 and 10 years. On enrollment, the three age groups were randomized to receive IPV, bOPV, or no vaccine (control group). Four weeks later, all participants received a challenge dose of bOPV, and poliovirus excretion was measured on days 3, 7, and 14.
Enrollment was completed within 6 days (i.e., during 11 to 16 October 2011), and follow-up visits were concluded by 11 December 2011. Nine hundred and ninety children were enrolled in the trial (fig. S2). Of these, 36 (3.6%) were excluded or withdrawn (1 due to wrong group assignment, 22 with incomplete stool samples, and 13 with incomplete serology samples), leaving 954 (96.4%) for analysis. Virus titer could not be determined for 18 poliovirus type 1 and 13 type 3 stool samples.
There were no significant differences between the groups with regard to median age, gender, father’s education, or baseline seroprevalence levels, which was high (≥99%) for type 1 and somewhat lower for types 2 and 3, particularly in the youngest age group (Table 1).
Table 1.
Demographic characteristics and baseline prevalence of poliovirus antibodies (n = 954).
| Study group/age group | Median age (years) (IQR)* | Gender [% male (95% CI)] | Father’s education [% illiterate (95% CI)] |
|---|---|---|---|
|
| |||
| IPV | 5.4 (0.9–10.2) | 45.9 (40.3–51.6) | 45.1 (39.5–50.8) |
| bOPV | 5.5 (0.9–10.2) | 47.7 (42.1–53.3) | 44.9 (39.3–50.8) |
| Control | 5.5 (0.9–10.2) | 49.2 (43.6–54.9) | 43.5 (37.9–49.2) |
| 6–11 months | 0.8 (0.7–0.9) | 45.8 (40.1–51.5) | 44.2 (38.5–49.9) |
| 5 years | 5.4 (5.2–5.7) | 48.8 (43.2–54.3) | 44.3 (38.8–49.9) |
| 10 years | 10.4 (10.2–10.6) | 48.1 (42.5–53.8) | 45.0 (39.4–50.6) |
| Seroprevalence |
|||
| Type 1 | Type 2 | Type 3 | |
| % (95% CI) | % (95% CI) | % (95% CI) | |
| Overall | 99.6 (98.9–99.9) | 91.6 (89.7–93.3) | 93.3 (91.5–94.8) |
| IPV | 99.7 (98.2–100) | 92.1 (88.5–94.8) | 94.6 (91.5–96.8) |
| bOPV | 99.4 (97.9–99.9) | 89.1 (85.2–92.3) | 91.0 (87.3–93.9) |
| Control | 99.7 (98.3–100) | 93.7 (90.4–96.1) | 94.3 (91.2–96.6) |
| 6–11 months | 99.0 (97.2–99.8) | 81.8 (77.0–86.0)† | 89.9 (86.0–93.1)‡ |
| 5 years | 100 (98.9–100) | 95.4 (92.5–97.4) | 97.5 (95.2–98.9) |
| 10 years | 99.7 (98.3–100) | 97.2 (94.7–98.7) | 92.2 (88.7–94.9) |
IQR, interquartile range.
P < 0.001 versus 5-year and 10-year age groups.
P < 0.001 versus 5-year age group; P value calculated with Fisher’s exact (two-sided) test; 95% confidence intervals calculated with Clopper-Pearson method.
The prevalence of poliovirus excretion for type 1 and 3 after challenge with bOPV is shown in table S1. For poliovirus type 1, the highest prevalence was 43.8% [95% confidence interval (CI) 34.1–53.8] in 10-year olds in the control group 3 days after challenge compared with 12.5% (95% CI 6.8–20.4) in the IPV group. For poliovirus type 3, the highest prevalence was 44.8% (95% CI 35.0–54.8) 7 days after challenge in the 10-year-olds of the control group compared to 7.7% (95% CI 3.4–14.6) in the IPV group (Fig. 1). Excluding participants excreting virus on day 0 (n = 17) produced similar results.
Fig. 1. Excretion of poliovirus type 1 and type 3 after challenge with bOPV.
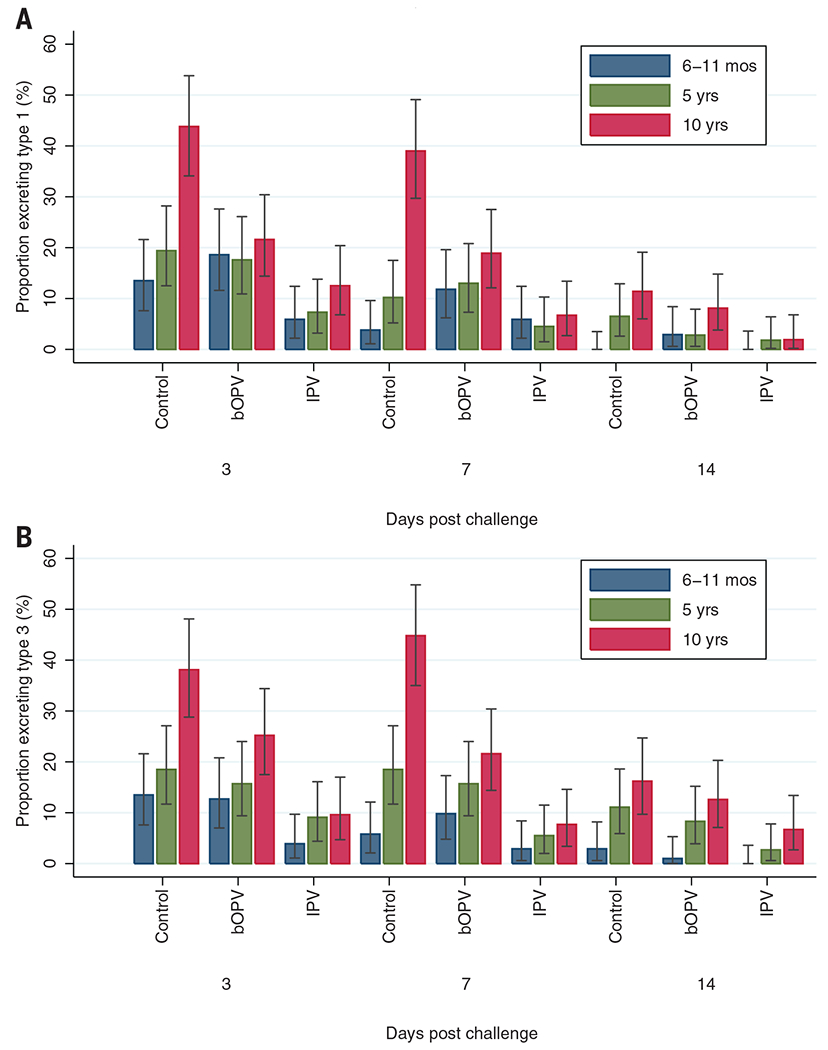
The prevalence of poliovirus type 1 (A) and type 3 (B) excretions is plotted for each time point after challenge, for each study arm and age group. All subjects (n = 954) are included. Bars represent 95% confidence intervals calculated with the Clopper-Pearson method.
The relative reduction in excretion between IPV group and control group was markedly consistent across the poliovirus types. For poliovirus type 1, the decrease in excretion in any stool sample after challenge in the IPV group compared to the control group was 38.9% (8.8% versus 14.4%) in the 6- to 11-month, 62.2% (9.1% versus 24.1%) in the 5-year, and 74.2% (13.5% versus 52.4%) in the 10-year age group; the corresponding decreases for poliovirus type 3 were 71.1% (3.9% versus 13.5%) in the 6- to 11-month, 52.8% (11.8% versus 25.0%) in the 5-year, and 75.7% (12.5% versus 51.4%) in the 10-year age group (table S1).
bOPV significantly decreased excretion of type 1 by 51.9% and type 3 by 40.5% only in the 10-year age group (Fisher’s exact test P < 0.001 for type 1; P = 0.002 for type 3).
For poliovirus type 1, the median duration of excretion was estimated to be 7.6 days (95% CI 6.0–10.1 days) for the IPV group, 8.7 days (95% CI 7.3–10.5) in the control group, and 9.1 days (95% CI 7.4–11.4) in the bOPV group. For poliovirus type 3, the median duration was 10.4 days (95% CI 7.4–15.4) for the IPV group, 13.1 days (95% CI 10.1–17.4) in the control group, and 11.6 days (95% CI 9.0–15.4) in the bOPV group. None of these differences were statistically significant (table S2).
The median virus titers among participants excreting poliovirus type 1 were highest 7 days after challenge, ranging from 4.1 log10 CCID50 (50% cell culture infectious dose) per gram of stool (95% CI 3.5–4.7) in the control group to 3.8 log10 CCID50 (95% CI 2.1–5.4) in the IPV group, a decrease of 49.9% (Wilcoxon rank-sum test P = 0.872). The median titers to poliovirus type 3 were 4.1 log10 CCID50 (95% CI 3.6–4.5) in the control group compared to 3.4 log10 CCID50 (95% CI 2.6–4.2) in the IPV group, a decrease of 80.0% (P = 0.143) (table S3). The relative reduction in the prevalence of excretion in the IPV group compared to the control group was between 38.9 and 75.7%, the absolute decrease in median titer of virus in stool specimens was between 0.3 and 0.7 log10 CCID50 (at day 7 after challenge), and the absolute decline in length of excretion was between 1.1 and 2.7 days (Fig. 2).
Fig. 2. Magnitude of excretion of poliovirus type 1 and type 3 after bOPV challenge for each study arm and age group.
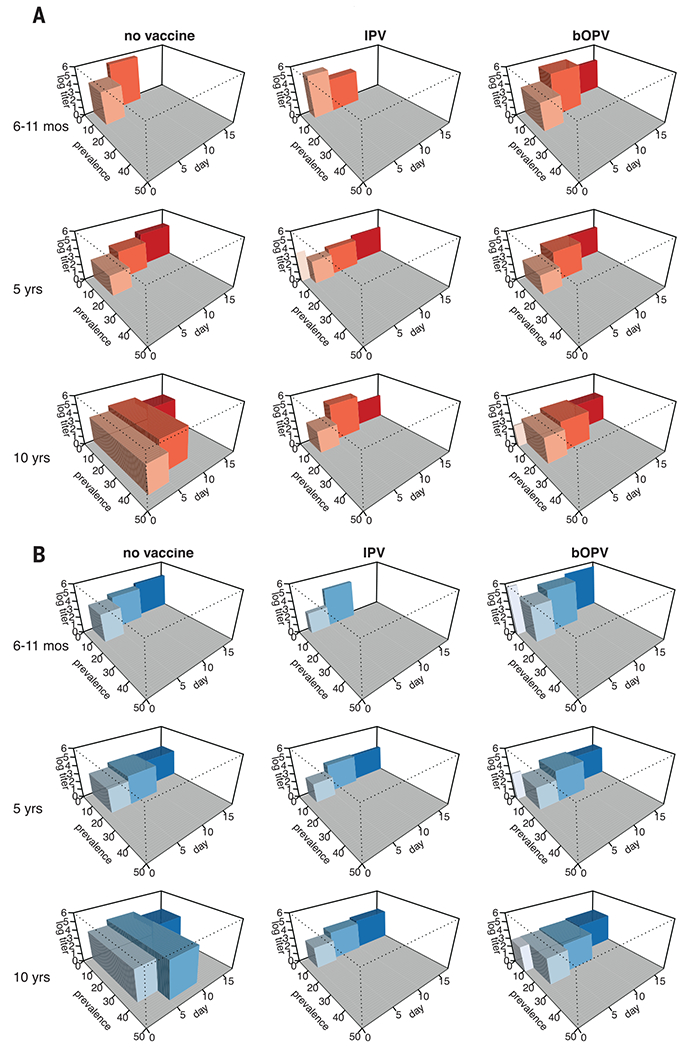
The prevalence of poliovirus type 1 (A) and type 3 (B) excretion and the mean log10 titer are plotted for each time point after challenge, with the width of the boxes spanning the midpoints between these time points (except for those boxes representing shedding on the day of challenge, where the plot is truncated at day 0). The volume of the boxes therefore roughly represents the total quantity of poliovirus that is excreted (prevalence × quantity × duration). Prevalence measure includes 954 participants. Titer measure includes 303 participants excreting virus after bOPV challenge.
Four weeks after vaccination, an IPV dose induced a humoral immune response (i.e., seroconversion or fourfold increase in antibody titer) to poliovirus type 1 in 82.9% (95% CI 67.9–92.8), 98.2% (95% CI 90.6–100), and 95.6% (89.1–98.8) of participants in the 6- to 11-month, 5-year, and 10-year age groups, respectively, compared to 2.9% (95% CI 0–15.3), 3.1% (95% CI 0.3–10.8), and 0% (95% CI 0–4.2) in the control group (Fisher’s exact test P < 0.001 for all age groups). A bOPV dose induced a humoral immune response in 14.3% (95% CI 4.8–30.3), 12.9% (95% CI 5.7–23.9), and 42.4% (32.1–53.1) of participants (P = 0.198 for the 6- to 11-month group, P = 0.052 for the 5-year group, and P < 0.001 for 10-year group, compared to control group; significantly lower than the IPV group, P < 0.001 for all age groups). Similar levels in humoral response were noted against poliovirus type 3 (Table 2).
Table 2.
Seroconversion or fourfold increase in poliovirus antibody titer between days 0 and 28.
| Type 1 |
Type 2 |
Type 3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention groups | n | % (95% CI) | P value | n | % (95% CI) | P value | n | % (95% CI) | P value | |
| 6–11 months | Control | 1/34 | 2.9 (0.0–15.3) | Ref | 3/73 | 4.1 (0.9–11.5) | Ref | 3/69 | 4.3 (0.9–12.2) | Ref |
| IPV | 34/41 | 82.9 (67.9–92.8 | <0.001 | 55/67 | 82.1 (70.8–90.4) | <0.001 | 66/72 | 91.7 (82.7–96.9) | <0.001 | |
| bOPV | 5/35 | 14.3 (4.8–30.3) | 0.198 | 5/74 | 6.8 (2.2–15.1) | 0.719 | 10/71 | 14.1 (7.0–24.4) | 0.078 | |
| 5 years | Control | 2/64 | 3.1 (0.3–10.8) | Ref | 1/103 | 1.0 (0.0–5.3) | Ref | 2/81 | 2.5 (0.3–8.6) | Ref |
| IPV | 56/57 | 98.2 (90.6–100) | <0.001 | 90/95 | 94.7 (88.1–98.3) | <0.001 | 80/82 | 97.6 (91.5–99.7) | <0.001 | |
| bOPV | 8/62 | 12.9 (5.7–23.9) | 0.052 | 7/101 | 6.9 (2.8–13.8) | 0.034 | 14/88 | 15.9 (9.0–25.2) | 0.003 | |
| 10 years | Control | 0/87 | 0.0 (0.0–4.2) | Ref | 2/101 | 2.0 (0.2–7.0) | Ref | 3/102 | 2.9 (0.6–8.4) | Ref |
| IPV | 87/91 | 95.6 (89.1–98.8) | <0.001 | 99/103 | 96.1 (90.4–98.9) | <0.001 | 98/98 | 100.0 (96.3–100) | <0.001 | |
| bOPV | 39/92 | 42.4 (32.1–53.1) | <0.001 | 36/105 | 34.3 (25.3–44.2) | <0.001 | 54/101 | 53.5 (43.3–63.5) | <0.001 | |
P values calculated with Fisher’s exact (two-tailed) test; 95% confidence intervals calculated with Clopper-Pearson method.
We divided the antibody titers into quartiles and assessed the excretion prevalence in each quartile. Because the antibody titers were high, we collapsed quartiles 3 and 4 (where the median titers were at the final dilution tested, ≥1:1448). The overall excretion of poliovirus type 1 was 0 (n = 1), 18.2, and 9.9% in quartiles 1, 2, and 3-4, respectively, in the IPV group compared to 40.8, 26.5, and 12.9% in the control group and 30.7, 28.4, and 13.0% in the bOPV group, respectively (test for overall trend: Fisher’s exact test P < 0.001). Similar trends were found for poliovirus type 3.
Our trial provides the following insights: (i) A single dose of IPV boosts intestinal mucosal immunity against polioviruses in infants and children with a history of multiple doses of OPV; (ii) the magnitude of this effect is substantially larger after IPV compared to bOPV; and (iii) the oldest age group displayed the highest degree of waning intestinal mucosal immunity manifested by highest prevalence of excretion after challenge.
Although IPV reliably induces humoral immunity (18–20) that is highly efficacious in preventing paralytic poliomyelitis (21, 22), the ability of IPV alone to induce intestinal mucosal immunity is limited (23, 24). Studies in countries that do not use OPV show that >90% of IPV-vaccinated children excrete challenge poliovirus (25, 26). Limited data are available on IPV effectiveness in boosting intestinal mucosal immunity in children with a history of multiple doses of OPV (19, 27, 28). Live virus contact to mucosal surfaces appears necessary to induce a specific secretory immunoglobulin A response (29, 30).
The most important comparisons are between the IPV and the control group, where a decrease in the prevalence of excretion of 39 to 76% was found in the IPV group compared to the control group. The decrease after a dose of bOPV was considerably lower, not significant in the 6- to 11-month and 5-year age groups, but 41 to 52% among 10-year-olds.
The study also demonstrated that higher humoral antibody titers are associated with decreased challenge virus excretion, as has been shown previously (4, 19, 27). bOPV increased antibody titers to poliovirus type 2, presumably because of boosting of cross-reacting neutralizing antibodies (31), since we did not isolate poliovirus type 2 from any stool samples. The data support the use of IPV (or OPV) to boost intestinal immunity for travelers to and from polio-infected countries.
Our study had a limitation. We conducted the study in Moradabad district in Uttar Pradesh State of India where the immunogenicity of OPV is low (11, 12). Therefore, extrapolation or generalization of these findings to other areas in India or elsewhere must be done with caution.
The Strategic Advisory Group of Experts (SAGE) recommended in 2012 the introduction of ≥1 dose of IPV in all routine immunization programs for risk mitigation before OPV2 withdrawal (32). A dose of IPV is expected to induce seroconversion or priming in close to 100% of naïve infants (33). Should poliovirus type 2 be reintroduced, a second IPV dose would rapidly boost antibody titers and prevent, or decrease the magnitude of, an outbreak. Furthermore, IPV would be expected to close the remaining type-specific immunity gaps.
Our study provides strong evidence that IPV boosts intestinal immunity among children with a history of multiple OPV doses more effectively than an additional OPV dose. These data provided the scientific foundation for the development of the new polio endgame plan (34) and are guiding the development of strategies to hasten the elimination of the final poliovirus reservoirs and to decrease the vulnerability of polio-free areas to epidemic transmission after poliovirus importations.
Thus, more than 25 years after the World Health Assembly resolution to eradicate poliomyelitis, the answer to the vaccine controversy is apparent––both vaccines, IPV and OPV, should be used. As a result, the Word Health Organization (WHO) is no longer recommending an all-OPV schedule; rather, it recommends that all OPV-using countries introduce ≥1 dose of IPV into routine vaccine schedules (35).
Supplementary Material
ACKNOWLEDGMENTS
Funding was provided by Rotary International Polio Plus Program (through a grant approved by the Polio Research Committee of the World Health Organization). The study was approved by the Drugs Controller General (India), the Indian Council of Medical Research, and the Ethics Review Committees of the WHO. The trial was registered with the Indian Clinical Trials Registry (www.ctri.nic.in) (registration number CTRI/2011/09/002018). We acknowledge the contributions of Uttar Pradesh State Government health staff, social mobilization network of the United Nations Children’s Fund and CORE, and the operations team from WHO India-National Polio Surveillance Project who were instrumental in the setup and implementation of the study. We also acknowledge the extensive work performed by the laboratory staff at ERC Mumbai. Finally, we express our sincere thanks to the Government of India Ministry of Health and Family Welfare and the GPEI partners. The data set is available in the supplementary materials. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, or other participating organizations.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencemag.org/content/345/6199/922/suppl/DC1
Data set
References (36–40)
REFERENCES AND NOTES
- 1.Sutter RW et al. , in Vaccines, Plotkin SA, Orenstein WA, Eds. (Saunders, Philadelphia, PA, ed. June, 2012), vol. 28, p. 598. [Google Scholar]
- 2.Paul JR, History of Poliomyelitis (Yale Univ. Press, Princeton, NJ, 1978. [Google Scholar]
- 3.World Health Organization, World Health Assembly (WHA) resolution, 1988. (resolution 41.28). [Google Scholar]
- 4.Hull HF, Ward NA, Hull BP, Milstien JB, de Quadros C, Lancet 343, 1331–1337 (1994). [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, Wkly. Epidemiol. Rec 88, 153–160 (2013).23620907 [Google Scholar]
- 6.Centers for Disease Control and Prevention, Morb. Mortal. Wkly. Rep 50, 222–224 (2001). [Google Scholar]
- 7.World Health Organization, Wkly. Epidemiol. Rec 88, 385–388 (2013).24052955 [Google Scholar]
- 8.World Health Organization, Wkly. Epidemiol. Rec 87, 381–388 (2012).23074736 [Google Scholar]
- 9.World Health Organization, Wkly. Epidemiol. Rec 88, 349–355 (2013).24040675 [Google Scholar]
- 10.Arie S, BMJ 347, f6682 (2013). [DOI] [PubMed] [Google Scholar]
- 11.John TJ, Jayabal P, Am. J. Epidemiol 96, 263–269 (1972). [DOI] [PubMed] [Google Scholar]
- 12.Grassly NC et al. , Science 314, 1150–1153 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Grassly NC et al. , J. Infect. Dis 205, 1554–1561 (2012). [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine, J. Infect. Dis 171, 1097–1106 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Grassly NC et al. , J. Infect. Dis 200, 794–801 (2009). [DOI] [PubMed] [Google Scholar]
- 16.El-Sayed N. et al. , N. Engl. J. Med 359, 1655–1665 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Sutter RW et al. , Lancet 376, 1682–1688 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Morinière BJ et al. , Lancet 341, 1545–1550 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Sutter RW et al. , N. Engl. J. Med 343, 767–773 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Estívariz CF et al. , Lancet Infect. Dis 12, 128–135 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Robertson SE et al. , Lancet 331, 897–899 (1988). [DOI] [PubMed] [Google Scholar]
- 22.Francis T Jr., J. Am. Med. Assoc 158, 1266–1270 (1955). [DOI] [PubMed] [Google Scholar]
- 23.Hird TR, Grassly NC, PLOS Pathog. 8, e1002599 (2012). v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anis E. et al. , Euro Surveill. 18, 20586 (2013). [DOI] [PubMed] [Google Scholar]
- 25.The Cuba IPV Study Collaborative Group, N. Engl. J. Med 356, 1536–1544 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Piirainen L. et al. , Vaccine 17, 1084–1090 (1999). [DOI] [PubMed] [Google Scholar]
- 27.WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines, J. Infect. Dis 175 (suppl. 1), S215–S227 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Parent I. du Châtelet et al. , Vaccine 21, 1710–1718 (2003).12639494 [Google Scholar]
- 29.Herremans TM, Reimerink JH, Buisman AM, Kimman TG, Koopmans MP, J. Immunol 162, 5011–5018 (1999). [PubMed] [Google Scholar]
- 30.Herremans T. et al. , Clin. Infect. Dis 34, 1067–1075 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Puligedda RD et al. , Antiviral Res. 108, 36–43 (2014). [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization, Wkly. Epidemiol. Rec 88, 1–6 (2013).23311010 [Google Scholar]
- 33.Resik S. et al. , N. Engl. J. Med 368, 416–424 (2013). [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization, WHO, Geneva: (WHO/POL/13.02) (2013). [Google Scholar]
- 35.World Health Organization, Wkly. Epidemiol. Rec 89, 73–92 (2014).24707513 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


