Abstract
Background.
Early detection and control of vaccine-derived poliovirus (VDPV) emergences are essential to secure the gains of polio eradication.
Methods.
Serial sewage samples were collected in 4 towns of Mexico before, throughout, and after the May 2010 oral poliovirus vaccine (OPV) mass immunization campaign. Isolation and molecular analysis of polioviruses from sewage specimens monitored the duration of vaccine-related strains in the environment and emergence of vaccine-derived polioviruses in a population partially immunized with inactivated poliovirus vaccine (IPV).
Results.
Sabin strains were identified up to 5–8 weeks after the campaign in all towns; in Aguascalientes, 1 Sabin 3 was isolated 16 weeks after the campaign, following 7 weeks with no Sabin strains detected. In Tuxtla Gutiérrez, type 2 VDPV was isolated from 4 samples collected before and during the campaign, and type 1 VDPV from 1 sample collected 19 weeks afterward. During 2009–2010, coverage in 4 OPV campaigns conducted averaged only 57% and surveillance for acute flaccid paralysis (AFP) was suboptimal (AFP rate <1 per 100 000 population <15 years of age) in Tuxtla Gutierrez.
Conclusions.
VDPVs may emerge and spread in settings with inadequate coverage with IPV/OPV vaccination. Environmental surveillance can facilitate early detection in these settings.
Keywords: environmental surveillance, inactivated poliovirus vaccine, polio, vaccine-derived poliovirus
The trivalent oral poliovirus vaccine (tOPV), containing attenuated polioviruses (Sabin strains) types 1, 2, and 3, has been the vaccine of choice for the global eradication of polio. Both OPV and inactivated poliovirus vaccine (IPV) induce humoral immunity that confers individual protection against paralysis, but OPV also induces local intestinal immunity that reduces re-infection and transmission. In addition, OPV is more affordable and easier to administer in mass campaigns compared with IPV [1, 2].
The attenuated Sabin strains contained in OPV may rarely cause paralytic disease via 3 mechanisms. First, vaccine-associated paralytic poliomyelitis (VAPP) occurs in a very small number of vaccine recipients and close contacts (approximately 1 case per 2.5–2.9 million tOPV doses distributed in the United States), but it becomes the main cause of poliomyelitis in countries that have eliminated wild poliovirus (WPV) [3, 4]. Second, in populations with low poliovirus immunity levels, sustained person-to-person transmission of Sabin strains among susceptible individuals can result in the emergence of drifted revertant strains that have reacquired the neurovirulence and transmissibility characteristics of WPV [5–8]. These circulating vaccine-derived polioviruses (cVDPVs) have been responsible for 646 reported paralytic polio cases from 2000 to 2012, 86% of which were type 2 [9–11]. Finally, prolonged replication of Sabin viruses in patients with primary B-cell immunodeficiencies can result in the emergence of immunodeficient-associated vaccine-derived poliovirus (iVDPV), which may cause paralysis in the individual and, potentially, in close susceptible contacts, although this phenomenon is very rare [12, 13].
Therefore, achieving and sustaining a polio-free world will require stopping all immunization with OPV plus maintenance of strategies that allow rapid detection and control of outbreaks following emergence of VDPVs or potential reintroduction of WPV [14–16]. Detection of poliovirus in stools from children with acute flaccid paralysis (AFP) has been the gold standard to identify poliovirus circulation. However, the sensitivity of AFP surveillance decreases significantly with low prevalence of poliovirus infections and, possibly, in highly immune populations among whom polioviruses may circulate without causing paralysis [17, 18]. Detection of poliovirus in sewage waters (environmental surveillance) can supplement AFP surveillance in the documentation of WPV elimination from endemic reservoirs, and the detection of VDPVs or reintroduced WPV in polio-free countries [19, 20].
Because Mexico has been free of WPV since 1990 [21], the polio immunization schedule was changed from an OPV-only to an IPV-OPV schedule in 2007 [22]. IPV is provided in combination with diphtheria and tetanus toxoids, acellular pertussis, and Haemophilus influenzae type b vaccines (pentavalent) to children 2, 4, 6, and 18 months of age through the routine immunization program. tOPV is administered twice a year through vaccination campaigns (National Health Weeks, NHW) to children aged 6 to 59 months who have previously received at least 2 doses of IPV [22]. Thus, Mexico offers a unique opportunity to evaluate the risk for VDPV emergence following OPV campaigns in a middle-income tropical country with a partially IPV-vaccinated population, which will inform strategies for maintaining population immunity and responding to outbreaks when OPV is phased out from routine immunization [14].
The objectives of this study were to monitor the presence of Sabin strains in the environment following an immunization campaign with OPV in 4 towns in Mexico, and to characterize the molecular genetic evolution of the isolated polioviruses.
MATERIALS AND METHODS
Selection of Sites
One town from each of 4 different Mexico States was selected for the project based on the following criteria: stable population below 1 million persons, existence of a converging sewer network for most of the population, and variable coverage with 4 doses of pentavalent vaccine in the routine immunization program during 2007 (Figure 1).
Figure 1.
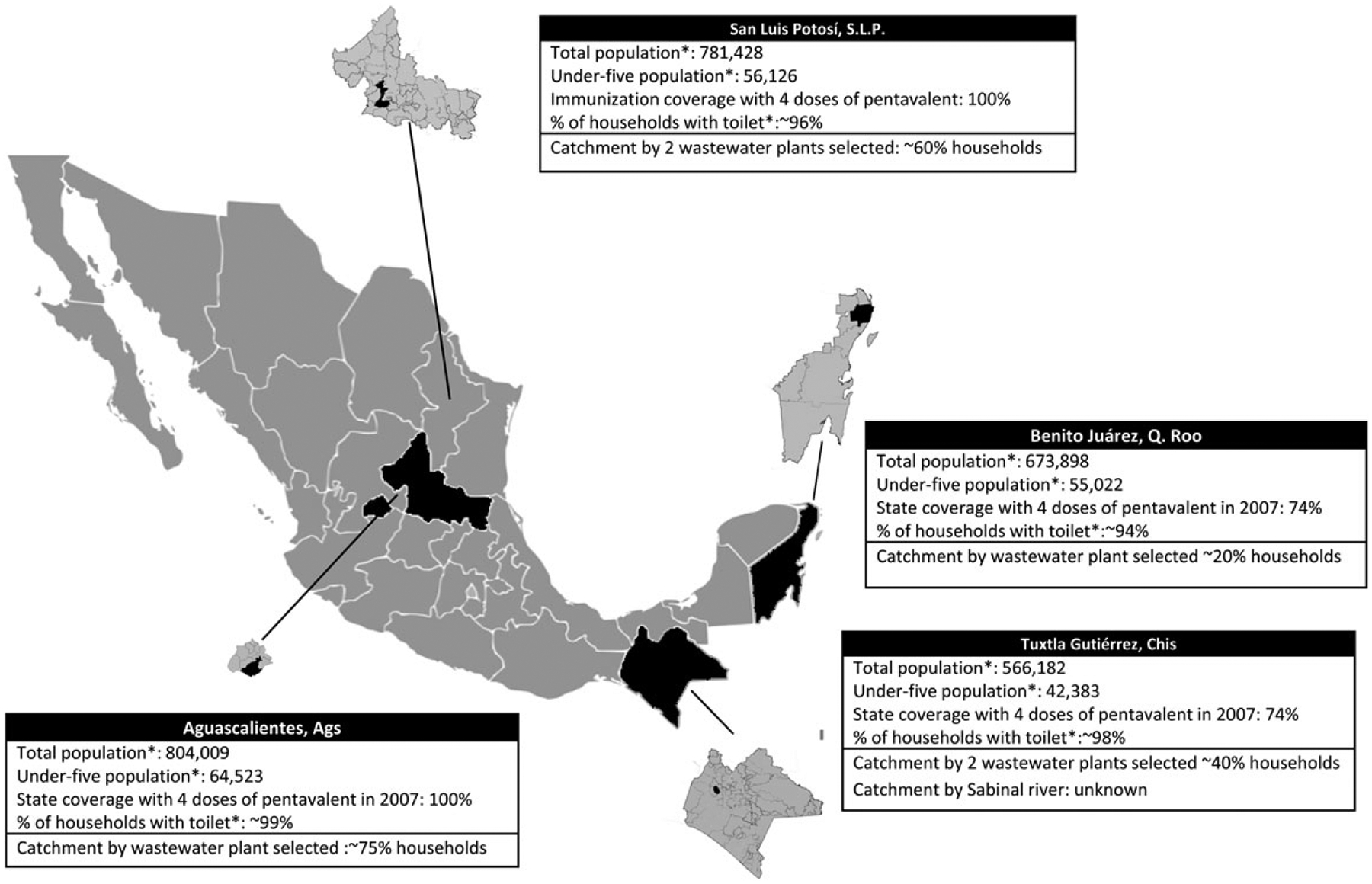
Location and population characteristics of the sampling sites. Source: National Population Council. 2010. Available at: http://www.conapo.gob.mx/es/CONAPO/Municipales.
Sampling Technique and Schedule
One-liter wastewater samples were collected using the grab method from a predefined point, usually located upstream from a location where wastewaters enter into the sedimentation tank in a water treatment plant. In Aguascalientes and Benito Juárez, samples were collected from a single treatment plant; in San Luis Potosí (SLP), from 2 treatment plants; and in Tuxtla Gutiérrez, from a treatment plant and from the Sabinal River, which collects waste from an undetermined number of house-holds in underserved areas (Figure 1).
Samples were collected in 0.5 L containers and transported at 2°C–4°C to the local laboratory for storage at −20°C. In SLP and Tuxtla Gutiérrez, initially 2 samples per day (1 sample from each source) were collected and stored in separate containers; later on, samples from both sources were mixed and redistributed in a single container for storage. At the end of the collection period, frozen samples were shipped for processing to the National Polio Laboratory in the Institute of Epidemiological Diagnosis and Reference (InDRE) in Mexico City, Mexico, and to the Global Poliovirus Reference Laboratory at the Centers for Disease Control and Prevention (CDC), in Atlanta, Georgia.
Prospective collection of samples was planned as follows: (1) twice a week 1 week before the NHW to establish a baseline; (2) no collection during the NHW (official dates, 29 May–5 June), because a high frequency of Sabin viruses was expected; (3) twice a week for 4 weeks after the NHW, then once a week for 6 weeks; and finally (4) once every 2 weeks for 10 additional weeks. This schedule was chosen to optimize laboratory resource use, considering that Sabin viruses were unlikely to be isolated more than 10 weeks after the campaign [23, 24]. However, the schedule was not followed exactly, because the start date and duration of the campaigns varied by town (2–5 weeks), and this information was not readily available for the study staff during sample collection.
Sample Processing and Isolation of Poliovirus
Half-liter aliquots of samples were concentrated using the 2-phase separation method recommended by the World Health Organization (WHO) [25]. The clarified sewage samples were treated with 2 polymers, mixed vigorously, and incubated over-night at 4°C in a separation funnel. The lower portion and interphase were collected and extracted with chloroform [26, 27]. The upper aqueous phase was treated with antibiotics and inoculated onto five 25 cm2 flasks containing monolayers of L20B cells (mouse fibroblast cells that express the human poliovirus receptor) and onto one 25 cm2 flask containing human rhabdomyosarcoma (RD) cells, following the WHO methodology [28, 29].
Characterization of Poliovirus by Real-Time Polymerase Chain Reaction
The presence of poliovirus or other enteroviruses and classification of polioviruses were determined by real-time reverse transcriptase intratypic differentiation polymerase chain reaction (rRT-ITD-PCR) [30]. Sabin isolates were screened by the real-time PCR assay for VDPVs as described previously [31].
Sequencing and Phylogenetic Analysis of VDPVs
Isolates that were discordant in the intratypic differentiation (ITD) and VDPV assays, indicative of possible presence of VDPVs, were selected for viral capsid protein 1 (VP1) sequencing. The VP1 capsid coding region was amplified by RT-PCR and sequenced following standard procedures using an ABI 3130 instrument [32]. Phylogenetic analysis was performed as described previously [33], and ancestral dates of VDPVs were estimated based on the poliovirus molecular clock of approximately 1.1% nucleotide substitutions per year [34]. Assignment of isolates to independent VDPV2 emergence groups was based on pairwise VP1 capsid region sequence differences within and between groups. Sabin isolate and VDPV sequences were submitted to GenBank under accession numbers KF701215 through KF701306. Sequences DOR00016, DOR00041c2, HAI00003, HAI01007, and Mahoney were obtained from Gen-Bank, accession numbers AF405634, AF405625, AF405614, AF405611, and AY082689, respectively.
Collection of Information on AFP Surveillance and Vaccination Coverage
Following the Pan American Health Organization guidelines, healthcare providers report all cases of acute paralytic illness of any cause in a child younger than 15 years of age [35]. Local public health officials conduct a case investigation with collection of at least 1 stool sample that is sent to the polio reference national laboratory (InDRE) for isolation, identification, and characterization of poliovirus using standardized cell culture methods. We collected information available at the InDRE on AFP cases reported during 2009–2010 in the project towns. The Department of Statistics at the National Center for Child and Adolescent Health provided information reported by health facilities for each town during 2009–2010 on the number of infants 12–23 months of age who had received the appropriate number of pentavalent doses for their age through routine immunization, and the number of children 1–4 years of age who received OPV during campaigns. Children in the age groups targeted by routine immunization and campaigns were calculated using midyear population estimates based on the most recent census [36].
RESULTS
Isolation of Sabin and Nonpolio Enterovirus in Sewage Samples
A total of 96 sewage samples were collected in 87 days; 22 from Aguascalientes, 30 from Tuxtla Gutiérrez, 21 from Benito Juárez, and 23 from SLP. Combining the results of samples collected the same day, poliovirus was isolated in 47 of 87 samples (54%, Figure 2). Most of the samples were positive for Sabin poliovirus during and for up to 3 weeks after the end of the campaign in all sites (Figure 2). The frequency of Sabin strain isolation decreased after 3 weeks, but Sabin strains could be detected up to 5–8 weeks after the end of the campaigns. In Aguascalientes, Sabin 3 was isolated 16 weeks after the end of the campaign, after a period of 7 weeks with no Sabin strains detected. The isolation of Sabin virus before the official start date of the May campaign could be due to early start in some health facilities or, less likely, persistent shedding from the previous campaign, which officially ended 12 weeks before, on 5 March 2010. The most common Sabin strain isolated, alone or in combination, was type 3 (36/47 positive samples), followed by type 2 (34 samples), whereas type 1 was isolated much less frequently (14 samples). Poliovirus mixtures were present in 31 (66%) of positive samples, including the 4 samples with VDPV2. Nonpolio enterovirus was isolated in 64 samples (74% of total), alone in 38 samples, and in a mixture with polioviruses in 26 samples (Figure 2).
Figure 2.
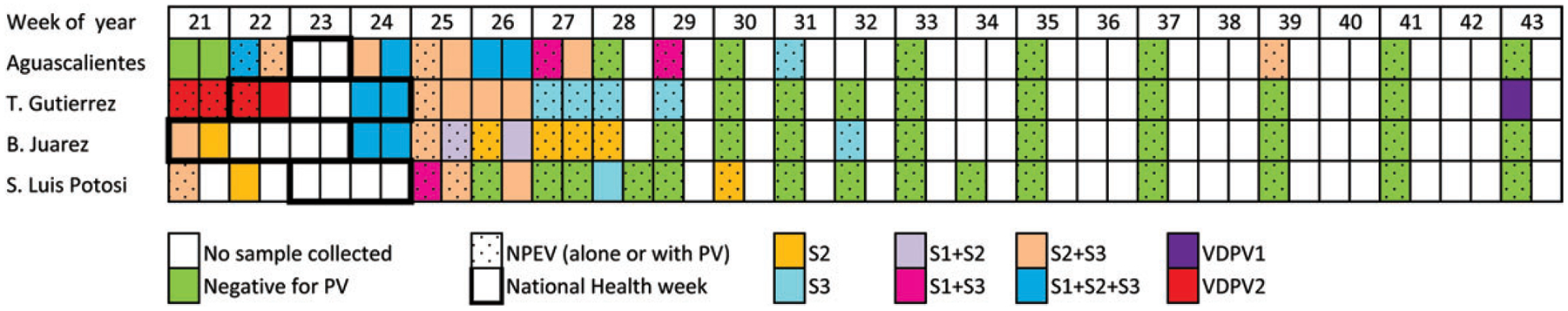
Sabin viruses isolated during May–October 2010 in 4 study sites in Mexico. VDPV2 was isolated in mixtures with Sabin 2 and 3 (3 samples), and in mixture with Sabin 1 and 2 (1 sample). None of the samples had Sabin 1 alone. Abbreviations: NPEV, nonpolio enterovirus; PV, poliovirus; S1, Sabin 1; S2, Sabin 2; S3, Sabin 3; VDPV1, type 1 vaccine-derived poliovirus; VDPV2, type 2 vaccine-derived poliovirus.
Characteristics of VDPV Isolated
Type 2 VDPV was isolated in 4 samples collected before and during the first week of the round in Tuxtla Gutiérrez (Figure 2). The 4 samples contained VDPV2 in a mixture with Sabin viruses; 3 samples were mixed Sabin 2 and 3, and 1 sample with Sabin 1 and 2. Based on the sequencing analysis, 2 of the VDPVs were determined to be genetically linked, and the other 2 appeared to represent independent emergences (Figure 3). Each VDPV VP1 sequence had 6 or 7 nucleotide differences from the Sabin strain, which suggests that the virus circulated for about 8 months before isolation. Previous campaigns distributing OPV were conducted during 29 May–4 June 2009 and 27 February–5 March 2010.
Figure 3.
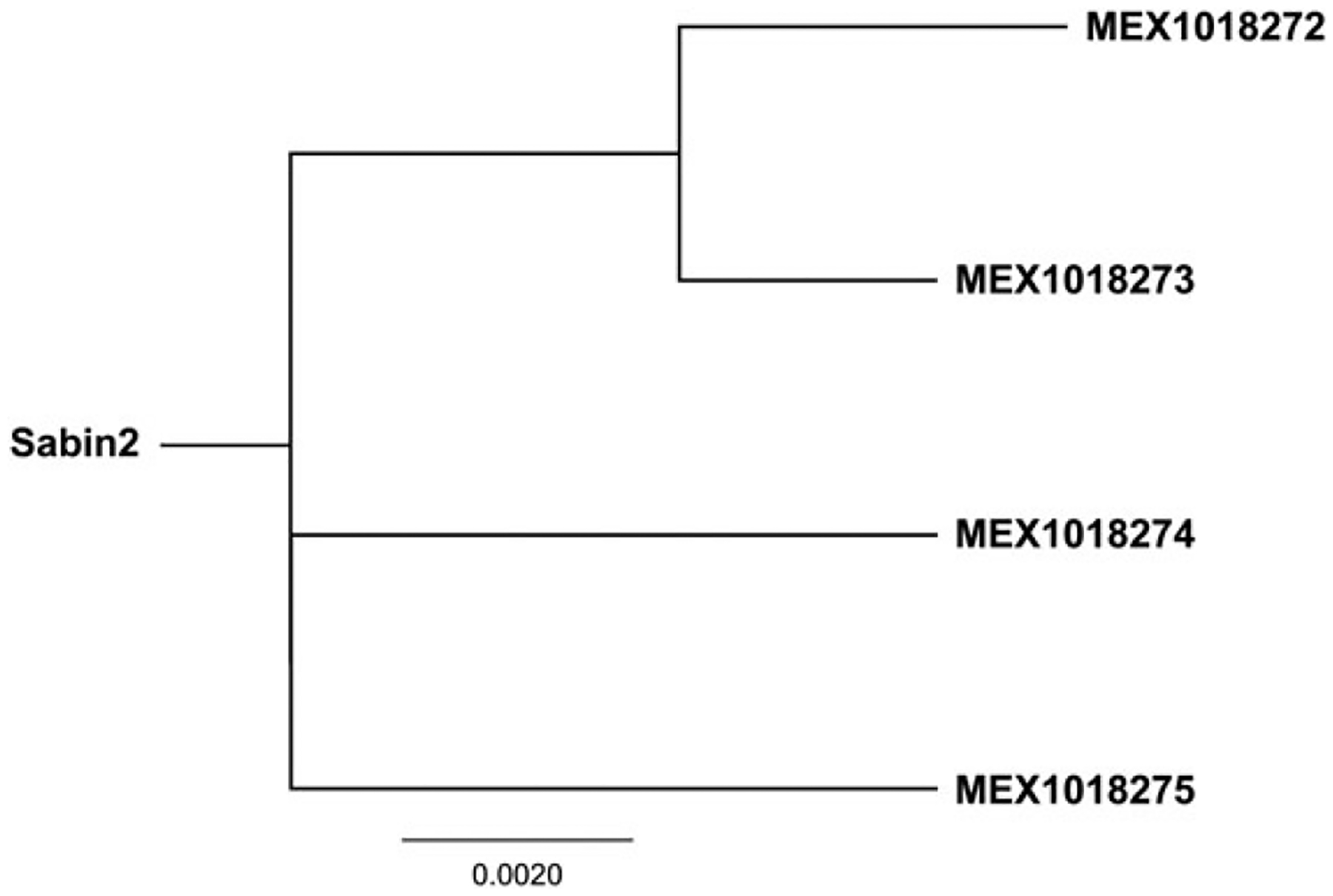
Phylogenetic relationships among type 2 VDPVs isolated in sewage water samples from Tuxtla Gutiérrez, Chiapas. Neighbor-joining tree of VP1 capsid nucleotide sequences. Abbreviations: VDPV2, type 2 vaccine-derived poliovirus; VP1, viral capsid protein 1.
A type 1 VDPV was isolated from another sample collected in Tuxtla Gutiérrez 19 weeks after the end of the May 2010 campaign. The capsid protein VP1 sequence had 12–13 nucleotide differences from Sabin 1 (data not shown), which is more than twice the expected changes for a virus emerging from the recent May 2010 campaign (3–4 nucleotide changes), and implies a date of origin around June 2009. An amino acid alignment of neutralizing antigenic (NAg) sites of the Mexico VDPV, 4 other cVDPV1 isolates from an outbreak on the island of Hispaniola, and the reference Mahoney strain is presented in Figure 4. Amino acid substitutions were found at 1 position in the NAg site 1 and 1 position in NAg site 3a of the Mexico VDPV1. The VDPV1 was not likely to be an iVDPV because the VP1 sequence did not have a high number of nonsynonymous substitutions (Figure 4), which are commonly found in iVDPVs [12].
Figure 4.
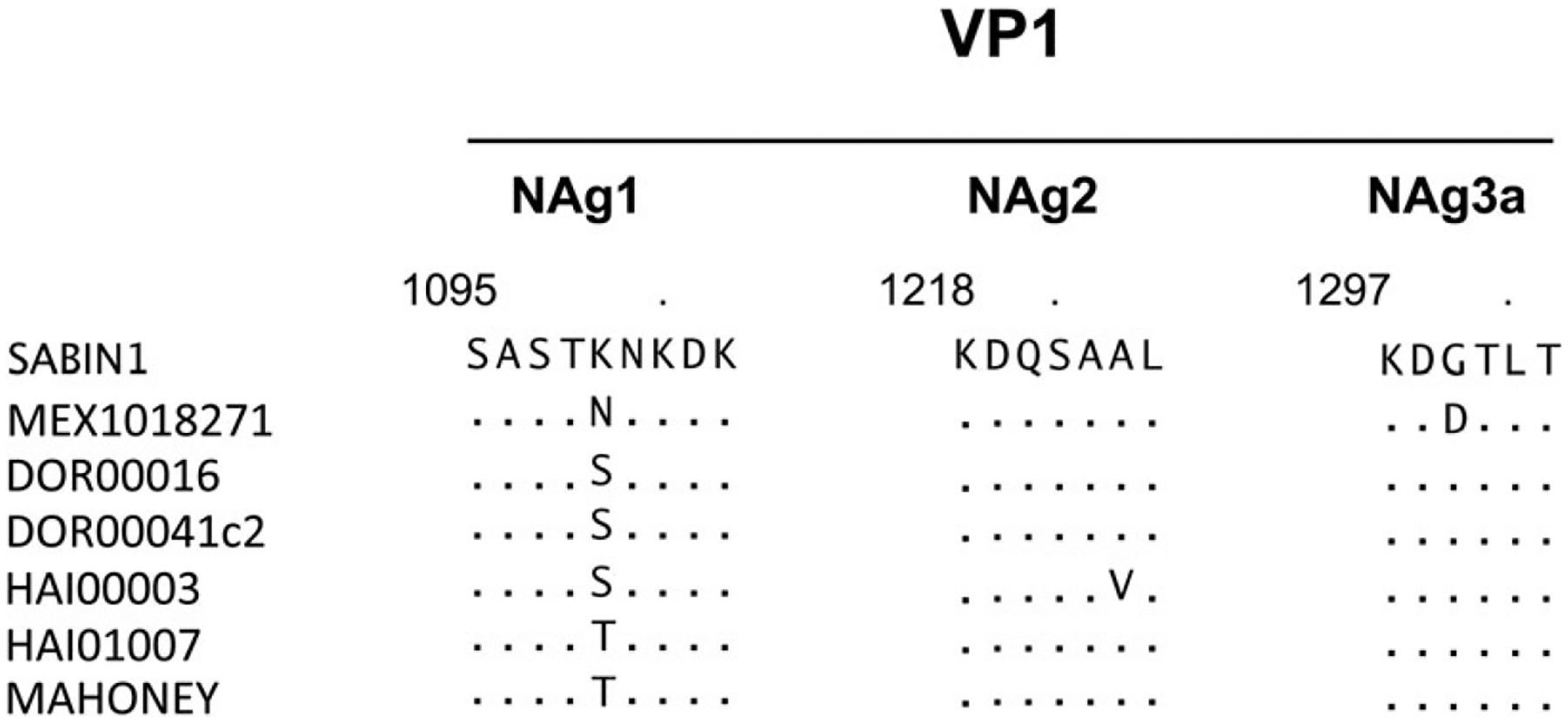
Amino acid sequence within or near the NAg sites of the capsid protein VP1 in the type 1 VDPV isolated in sewage water samples in Tuxtla Gutiérrez, in comparison with other VDPVs, Sabin oral poliovirus vaccine virus, and wild poliovirus (Mahoney). The type 1 VDPV sequence is aligned with the Sabin 1 oral poliovirus vaccine, Mahoney (the neurovirulent wild-type poliovirus type 1 that is used as a seed strain for inactivated poliovirus), and 4 cVDPV isolates from the cVDPV1 outbreak in the Dominican Republic (DOR00016 and DOR00041c2) and Haiti (HIA00003 and HAI01007) in 2000 and 2001. Single-letter codes are used for amino acid residues. The numbers above the alignment reflect the amino acid locations; the first digit indicates the capsid protein VP1 and the next 3 digits indicate the amino acid position in the VP1 protein. Abbreviations: cVDPV, circulating vaccine-derived polioviruses; Nag, neutralizing antigenic; VDPV, vaccine-derived poliovirus; VP1, viral capsid protein 1.
Evaluation of the AFP Surveillance System in Study Towns
An annual proportion of AFP cases negative for poliovirus (nonpolio AFP [NPAFP]) higher than 1 per 100 000 (for polio-free countries) or 2 per 100 000 (for polio-infected countries) suggests adequate sensitivity of the surveillance system to detect poliovirus circulation [35]. Because excretion of poliovirus stops 2 weeks after paralysis onset in more than 75% of children, and maintenance of a cold chain is essential for virus survival, having ≥80% of AFP cases with 2 stool specimens collected ≥24 hours apart, within 14 days of paralysis onset, and arriving in good condition at the laboratory is also used as an indicator to assess quality of specimens.
A total of 24 AFP cases were reported and specimens sent to the InDRE, during 2009–2010 from the 4 towns participating in the study. None of the stool samples were positive for poliovirus, but AFP performance indicators were within expectations only in SLP (Table 1). In Tuxtla Gutiérrez and Benito Juárez, a single AFP case was detected during the 2-year period instead of the 3 or more cases expected, and Aguascalientes had an appropriate NP-AFP rate, but a high proportion of samples classified as “inadequate” (Table 1).
Table 1.
Performance Indicators of Acute Flaccid Paralysis Surveillance by Study Town, Mexico, 2009–10
| Town, State | Population <15 Years | NPAFP Cases | NPAFP Ratea | Number of Stool Samples Collected | Cases With Adequate Stools, %b | Samples With NPEV Isolation, %c |
|---|---|---|---|---|---|---|
| 2009 | ||||||
| Aguascalientes, Aguascalientes | 229 444 | 4 | 1.7 | 7 | 50 | 14 |
| Tuxtla Gutiérrez, Chiapas | 137 002 | 0 | 0.0 | 0 | … | … |
| Benito Juárez, Quintana Roo | 189 345 | 1 | 0.5 | 2 | 0 | 0 |
| San Luis Potosí, San Luis Potosí | 213 444 | 6 | 2.8 | 12 | 100 | 0 |
| 2010 | ||||||
| Aguascalientes, Aguascalientes | 243 799 | 4 | 1.6 | 8 | 75 | 0 |
| Tuxtla Gutiérrez, Chiapas | 155 349 | 1 | 0.6 | 2 | 0 | 0 |
| Benito Juárez, Quintana Roo | 193 871 | 1 | 0.5 | 0 | 0 | 0 |
| San Luis Potosí, San Luis Potosí | 219 289 | 7 | 3.2 | 14 | 100 | 0 |
Abbreviations: NPAFP, nonpolio acute flaccid paralysis; NPEV, nonpolio enterovirus.
Target for annual nonpolio AFP rate per 100 000 children <15 years of age is ≥1/100 000.
Adequate stools defined as at least 1 specimen collected ≤14 days after paralysis onset, shipped in cold chain, and arrival to the laboratory in good condition (without leakage or desiccation). Target is ≥80%.
Target is ≥10% of samples [35].
Administrative Coverage With Poliovirus Vaccines
The proportion of children 12–23 months of age who were up to date on IPV vaccination (eg, had received 3–4 doses of IPV depending on age) varied by locality and by year, as shown on Figure 5A. In Tuxtla Gutiérrez and SLP, only 71%–73% had received their scheduled IPV doses in 2009, with an increase to approximately 80% during 2010–2011. Coverage increased from 78% in 2009 to 92% in 2011 in Benito Juárez, and decreased from 95% in 2009 to 83% during 2010–2011 in Aguascalientes.
Figure 5.
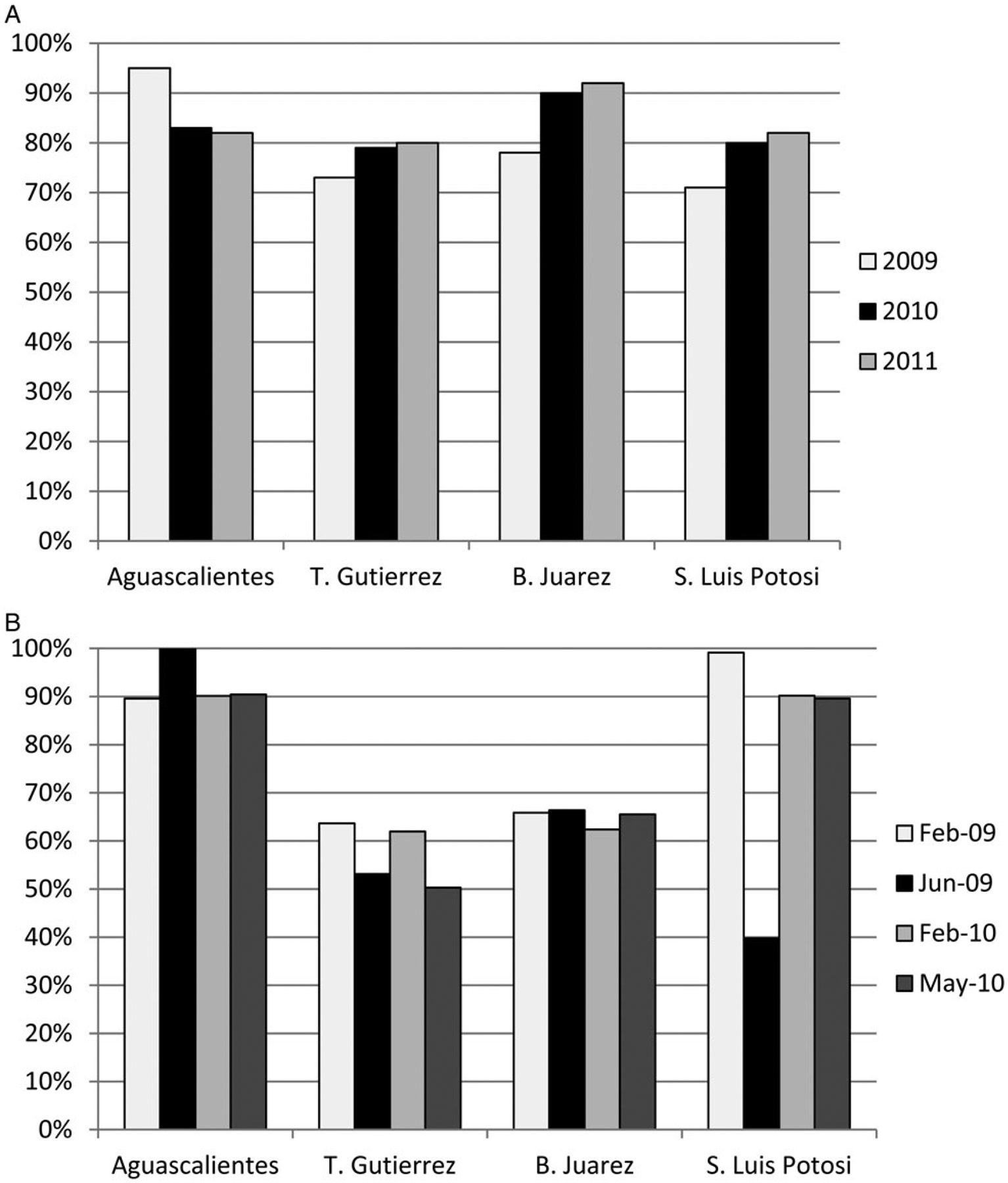
Coverage with polio vaccines by year and study site, Mexico, 2009–2011. A, Percent of children aged 12–23 months who had completed immunization with pentavalent vaccine containing inactivated polio vaccine, according to their age (eg, received 3–4 doses). B, Percent of children 1–4 years of age administered OPV during the campaigns conducted in February 2009, June 2009, February 2010, and May 2010. Abbreviation: OPV, oral polio vaccine.
Suboptimal quality of the 4 OPV campaigns conducted during 2009–2010 was observed in Tuxtla Gutiérrez and Benito Juárez, with average coverage among children 1–4 years of age of 57% and 65%, respectively (Figure 5B). Average coverage for Aguascalientes was 93%, and for SLP, 80%.
DISCUSSION
This study brings new insight on the potential duration of Sabin strain circulation following mass campaigns and the risk for VDPV emergence in a tropical setting with a population partially vaccinated with IPV.
The duration of Sabin virus detection in the environment following the OPV mass campaign was not very different in Mexico than in Cuba, despite different vaccination schedules. IPV is not provided in Cuba, and children receive OPV only through campaigns conducted twice per year with very high coverage, which increases the number of susceptible children receiving their first OPV dose. Polioviruses were detected in 100% of sewage samples up to 5 weeks after a campaign in Cuba. After that, the frequency decreased rapidly to 0% by week 7, but a single type 1 isolate was found 15 weeks after the campaign [23]. In Mexico, 100% of sewage samples were positive for 4 weeks after the campaign in 3 towns (for 1 week in SLP). Sabin strains were isolated with lower frequency from 5–8 weeks, and 1 isolate was found 16 weeks after the campaign. On the other hand, we observed differences in serotype prevalence. In Cuba, Sabin 1 was the most commonly isolated strain after a campaign (27% of all samples), followed by type 3 (16%) and type 2 (9%) [23]. In Mexico, Sabin types 2 and 3 were isolated more frequently than type 1. These disparities could be due to differences in population immunity profiles and variable sensitivity of molecular assays used in each study to identify specific poliovirus types.
During the short duration of our project, we detected emergence of VDPVs of 2 serotypes in Tuxtla Gutiérrez, Chiapas State. Genetic analysis of the isolates suggest that the type 2 VDPVs were the result of at least 3 independent emergences from original Sabin strains, and the initiating doses occurred up to 8 months beforehand. For the VDPV1, the degree of divergence from the Sabin 1 strain suggests circulation for about 15 months. Therefore, the VDPV1 could have emerged from the campaign conducted in June 2009. The VDPV2 appears to have emerged between the campaigns conducted in June 2009 and February 2010, perhaps due to contact with a vaccinee from a country using OPV in routine immunization or contact with a child who received OPV during a small mop-up response conducted around an AFP case. During 2010–2011, 8 mop-ups were conducted in the study municipalities, administering a range of 7 to 386 doses of OPV to children younger than 5 living in the neighborhoods of AFP cases. Finally, the VDPVs could have been excreted by 1 or more immunodeficient individuals, although the VDPV sequences did not have characteristics associated with immunodeficiency, such as a high frequency of nonsynonymous substitutions (amino acid changes) [12]. The disappearance of type 2 VDPVs after the May 2010 campaign is more indicative of circulating than of immunodeficient VDPVs.
VDPVs isolated in sewage without any paralytic cases detected in the community and without a genetic linkage to other VDPVs are classified as ambiguous VDPVs (aVDPVs) [37]. No vaccination response is recommended in settings with high levels of vaccination-induced immunity and when the viruses show genetic characteristics of iVDPVs or early divergence from Sabin strains. Investigation of ambiguous VDPVs (aVDPVs) in Israel and several countries in Europe has suggested that chronic shedding of virus by immunodeficient individuals for months or years or sporadic shedding of VDPVs by healthy individuals may be detected with sensitive environmental surveillance, but high herd immunity appears to prevent persistent transmission [19, 26, 27, 38, 39]. On the other hand, aVDPVs from environmental samples may be an indication of elevated risk for a cVDPV outbreak in settings with low population immunity and insensitive AFP surveillance, as observed in Nigeria, Pakistan, or Yemen [10, 38, 39]. Implementation of tOPV mass campaigns is recommended to close immunity gaps in these settings [14].
In Tuxtla Gutiérrez, the suboptimal quality of the OPV mass campaign rounds, which only reached 50%–60% of the target age group, the low coverage with IPV through routine immunization, and withholding vaccine to children 0–6 months of age or older children who had not received 2 IPV doses, may have created an accumulation of susceptible individuals sufficient to sustain transmission of Sabin strains after a campaign or after introduction of OPV by vaccinees from other countries or mop-ups. The May 2010 campaign appeared to interrupt the cVDPV2 circulation, but our study period was too short to provide evidence of the duration of the VDPV1 circulation. Persistent low coverage with OPV and IPV may create new opportunities for future emergences that could be missed by the low sensitivity of the AFP surveillance in Tuxtla Gutiérrez and could result in a large cVDPV outbreak.
An additional consideration in Mexico is the potential for IPV vaccinees participating in silent asymptomatic transmission of VDPVs, which may result in widespread circulation of the virus before it is detected through AFP surveillance; however, this has only been observed with WPV. In the Netherlands, coverage with 3 doses of IPV is >95%, but certain geographically clustered religious groups reject vaccination [9, 40]. During an outbreak in 1994, only unvaccinated individuals in the religious group were paralyzed, but a stool survey found WPV in 10.2 children and 5.2 adults per 1000 persons among the general population compared with 70.7 per 1000 among the religious group [40]. In Israel, which reports >90% coverage with IPV in routine immunization, a WPV type 1 that originated in Pakistan was isolated in sewage samples collected in February 2013 [20]. In the following months, the same WPV was isolated in additional sewage sampling sites throughout the country as well as in stools from healthy individuals, but no WPV cases were reported as of April 2014. Israel conducted an OPV mass campaign to boost mucosal immunity levels and stop circulation [9]. Experience with environmental detection of VDPVs in temperate IPV-using countries suggests that high coverage is sufficient to prevent VDPV emergences, despite intermittent introduction of OPV from vaccinees from other countries, but new lessons could be learned as IPV-only schedules are introduced in countries with tropical climates, substandard sanitation, and variable coverage.
The observation of multiple VDPV emergences in Tuxtla Gutiérrez but not in Benito Juárez, despite similarly low levels of coverage with polio vaccines, agrees with prior observations of repeated VDPV2 emergences in countries such as Ethiopia, Madagascar, or Yemen but not in countries such as Mali, with similarly low coverage in routine immunization [5, 8, 11, 41]. Chiapas State (where T. Gutiérrez is located) borders countries that provide OPV through the routine immunization program, and has a number of communities that reject government services, including immunization, but we could not ascertain the potential role of these or other factors in VDPV emergence. Additional limitations for the interpretation of our results include the following: (1) sample collection was conducted in a few study sites not selected to be representative of the whole country or state; (2) collection occurred for a limited time, not allowing for adequate comparisons among towns; and (3) samples may exclude virus excretion from children wearing diapers or living in areas with sewage converging into treatment plants not included in this project.
In conclusion, this study proved the effectiveness of environmental surveillance for the early detection of VDPV emergence, especially in settings with suboptimal AFP surveillance performance. Because environmental surveillance is less reliable in areas without a confluent sewage system, requires an additional investment in laboratory resources and is labor-intensive, each country must evaluate the areas where its implementation would be most cost effective. New techniques under development by the Global Poliovirus Laboratory Network may reduce cost and facilitate its introduction in more locations and laboratories [42]. Implementation of environmental surveillance also requires the development of guidelines to respond to the potential isolation of VDPVs. In settings like Tuxtla Gutiérrez, detection of repeated VDPV emergences should trigger responses that strengthen routine immunization and identify and target high-risk groups for vaccination to ensure higher effectiveness of campaigns. Considerations should also be given to strategies that reduce the risk for OPV introduction in a population with many susceptible children, such as reducing the minimum age for receipt of OPV during campaigns and canceling or expanding the scope of mop-ups in response to AFP cases. Finally, as we progress to the end-game of polio eradication, further research is necessary to better understand the potential role of IPV vaccinees in contributing to the emergence of VDPVs in settings with low vaccination coverage with IPV and/or OPV.
Acknowledgments.
We are grateful to the local field and laboratory staff in Aguascalientes, B. Juárez, T. Gutiérrez and SL Potosí for their contribution to the collection and initial processing of the environmental samples. We also would like to acknowledge Dr Luis Sarmiento and Dr Howard Gary for their contribution to the study design; and Ana Maria Hernández Martínez for her extraordinary effort in the collection and analysis of immunization coverage data.
Footnotes
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine - live. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia: Saunders, 2008:631–85. [Google Scholar]
- 2.Estívariz CF, Pallansch MA, Anand A, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol 2013; 3:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander LN, Seward JF, Santibanez TA, et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States. JAMA 2004; 292:1696–701. [DOI] [PubMed] [Google Scholar]
- 4.Strebel PM, Sutter RW, Cochi SL, et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis 1992; 14:568–79. [DOI] [PubMed] [Google Scholar]
- 5.Kew OM, Wright PF, Agol VI, et al. Circulating vaccine-derived polioviruses: current state of knowledge. Bull World Health Organ 2004; 82:16–23. [PMC free article] [PubMed] [Google Scholar]
- 6.Minor P Vaccine-derived poliovirus (VDPV): Impact on poliomyelitis eradication. Vaccine 2009; 27:2649–52. [DOI] [PubMed] [Google Scholar]
- 7.Estívariz CF, Watkins MA, Handoko D, et al. A large vaccine-derived poliovirus outbreak on Madura Island—Indonesia, 2005. J Infect Dis 2008; 197:347–54. [DOI] [PubMed] [Google Scholar]
- 8.Burns CC, Shaw J, Jorba J, et al. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in northern Nigeria. J Virol 2013; 87:4907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Global Polio Eradication Initiative. Data and monitoring. http://www.polioeradication.org. Accessed 7 August 2013.
- 10.Wassilak S, Pate MA, Wannemuehler K, et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: emergence and widespread circulation in an underimmunized population. J Infect Dis 2011; 203:898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wringe A, Fine PE, Sutter RW, Kew OM. Estimating the extent of vaccine-derived poliovirus infection. PLOS ONE 2008; 3:e3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Ann Rev Microbiol 2005; 59:587–635. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JP, Ehresmann K, Seward J, et al. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 2009; 199:391–7. [DOI] [PubMed] [Google Scholar]
- 14.Global Polio Eradication Initiative. Polio Eradication & Endgame Strategic Plan 2013–2018 2013. WHO/POLIO/13.02. http://www.polioeradication.org/Portals/0/Document/Resources/StrategyWork/PEESP_EN_US.pdf. Accessed 15 November 2013.
- 15.Thompson KM, Duintjer Tebbens RJ, Pallansch MA, et al. Development and consideration of global policies for managing the future risks of poliovirus outbreaks: insights and lessons learned through modeling. Risk Anal 2006; 26:1571–80. [DOI] [PubMed] [Google Scholar]
- 16.Aylward B, Yamada T. The polio endgame. N Engl J Med 2011; 364:2273–5. [DOI] [PubMed] [Google Scholar]
- 17.Schaap GJ, Bijkerk H, Coutinho RA, Kapsenberg JG, Van Wezel AL. The spread of wild poliovirus in the well-vaccinated Netherlands in connection with the 1978 epidemic. Prog Med Virol 1984; 29: 124–40. [PubMed] [Google Scholar]
- 18.Sutter RW, Patriarca PA, Brogan S, et al. Outbreak of paralytic poliomyelitis in Oman: evidence for widespread transmission among fully vaccinated children. Lancet 1991; 338:715–20. [DOI] [PubMed] [Google Scholar]
- 19.Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, De Gourville EM. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol Infect 2012; 140:1–13. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Global Alert and Response (GAR). Poliovirus detected from environmental samples in Israel—update 15 July 2013, 2013. http://www.who.int/csr/don/2013_07_15/en/. Accessed 18 October 2013
- 21.de Quadros CA, Hersh BS, Olive JM, Andrus JK, da Silveira CM, Carrasco PA. Eradication of wild poliovirus from the Americas: acute flaccid paralysis surveillance, 1988–1995. J Infect Dis 1997; 175 (suppl 1):S37–42. [DOI] [PubMed] [Google Scholar]
- 22.National Immunization Council. Manual for Immunization 2008–2009. Mexico City, Mexico: Secretariat of Health, 2008. [Google Scholar]
- 23.Mas Lago P, Gary HE Jr., Perez LS, et al. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol 2003; 32:772–7. [DOI] [PubMed] [Google Scholar]
- 24.Poyry T, Stenvik M, Hovi T. Viruses in sewage waters during and after a poliomyelitis outbreak and subsequent nationwide oral poliovirus vaccination campaign in Finland. App Environ Microbiol 1988; 54:371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Department of Immunization, Vaccines and Biologicals. Guidelines for Environmental Surveillance of Poliovirus Circulation. WHO/V&B/03.03. Geneva, Switzerland: 2003. [Google Scholar]
- 26.Blomqvist S, Savolainen C, Laine P, et al. Characterization of a highly evolved vaccine-derived poliovirus type 3 isolated from sewage in Estonia. J Virol 2004; 78:4876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shulman L, Manor Y, Sofer D, et al. Neurovirulent vaccine-derived polioviruses in sewage from highly immune populations. PLOS ONE 2006; 1:e69–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Department of Immunization, Vaccines and Biologicals. Polio laboratory manual. 4th Edition. WHO/IVB/04.10. World Health Organization ed. Geneva, World Health Organization, 2004. [Google Scholar]
- 29.World Health Organization. Department of Immunization, Vaccines and Biologicals. Supplement 1to the WHO Polio Laboratory Manual. An alternative test algorithm for poliovirus isolation and characterization. http://www.who.int/immunization_monitoring/Supplement_polio_lab_manual.pdf. Accessed 15 November 2013.
- 30.Kilpatrick DR, Yang CF, Ching K, et al. Rapid group-, serotype-, and vaccine strain-specific identification of poliovirus isolates by real-time reverse transcription-PCR using degenerate primers and probes containing deoxyinosine residues. J Clin Microbiol 2009; 47:1939–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilpatrick DR, Ching K, Iber J, et al. Identification of vaccine-derived polioviruses using udal-stage real-time RT-PCR. J Virol Methods 2014; 197:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilpatrick DR, Iber JC, Chen Q, et al. Serotype-specific sequencing primers. J Virol Methods 2011; 174:128–130. [DOI] [PubMed] [Google Scholar]
- 33.El Bassioni L, Barakat I, Nasr E, et al. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am J Epidemiol 2003; 158:807–15. [DOI] [PubMed] [Google Scholar]
- 34.Jorba J, Campagnoli R, De L, Kew O. Calibration of multiple poliovirus molecular clocks covering an extended evolutionary range. J Virol 2008; 82:4429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan American Health Organization. Poliomyelitis eradication. In:Field Guide. Third Edition. Pan American Health Organization ed. Washington DC, Pan American Health Organization, 2006. http://www2.paho.org/hq/dmdocuments/2010/FieldGuide_Polio_3rdEd_e.pdf. Accessed 15 November 2013. [Google Scholar]
- 36.Consejo Nacional del Población. Proyecciones de la Poblacion de los Municipios de Mexico 2010–2030. http://www.conapo.gob.mx/es/CONAPO/De_los_municipios_de_Mexico_2010_-_2030. Accessed 30 June 2013.
- 37.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses. MMWR Morb Mortal Wkly Rep 2006; 55:1093–7. [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Update on Vaccine-Derived Polioviruses—Worldwide, January 2008–June 2009. MMWR Morb Mortal Wkly Rep 2009; 58:1002–6. [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Update on vaccine-derived polioviruses—worldwide, April 2011–June 2012. MMWR Morb Mortal Wkly Rep 2012; 61:741–6. [PubMed] [Google Scholar]
- 40.Conyn-van Spaendonck MA, Oostvogel PM, van Loon AM, van Wijngaarden JK, Kromhout D. Circulation of poliovirus during the poliomyelitis outbreak in The Netherlands in 1992–1993. Am J Epidemiol 1996; 143:929–35. [DOI] [PubMed] [Google Scholar]
- 41.Rakoto-Andrianarivelo M, Gumede N, Jegouic S, et al. Reemergence of recombinant vaccine-derived poliovirus outbreak in Madagascar. J Infect Dis 2008; 197:1427–35. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Summary of discussions and recommendations of the 18th Informal Consultation of the Global Polio Laboratory Network 28th–29th June 2012. http://www.polioeradication.org/Portals/0/Document/Resources/GPLN_publications/GPLN_Meeting_recommendations_2012.pdf. Accessed 15 November 2013.


