Abstract
Jena virus (JV) is a noncultivatable bovine enteric calicivirus associated with diarrhea in calves and was first described in Jena, Germany. The virus was serially passaged 11 times in colostrum-deprived newborn calves and caused diarrheal disease symptoms at each passage. The complete JV genome sequence was determined by using cDNA made from partially purified virus obtained from a single stool sample. JV has a positive-sense single-stranded RNA genome which is 7,338 nucleotides in length, excluding the poly(A) tail. JV genome organization is similar to that of the human Norwalk-like viruses (NLVs), with three separate open reading frames (ORFs) and a 24-nucleotide sequence motif located at the 5′ terminus of the genome and at the start of ORF 2. The polyprotein (ORF 1) consists of 1,680 amino acids and has the characteristic 2C helicase, 3C protease, and 3D RNA polymerase motifs also found in the NLVs. However, comparison of the N-terminal 100 amino acids of the JV polyprotein with those of the group 1 and group 2 NLVs showed a considerable divergence in sequence. The capsid protein (ORF 2) at 519 amino acids is smaller than that of all other caliciviruses. JV ORF 2 was translated in vitro to produce a 55-kDa protein that reacted with postinfection serum but not preinfection serum. Phylogenetic studies based on partial RNA polymerase sequences indicate that within the Caliciviridae JV is most closely related to the group 1 NLVs.
Caliciviruses cause a wide spectrum of diseases and are a major cause of gastroenteritis in humans (7, 11, 29, 30). However, little is known about enteric caliciviruses from other species (4). The prototype strain of human caliciviruses is Norwalk virus, which was first described in 1972 associated with an outbreak of gastroenteritis and vomiting involving children and staff at an elementary school in Norwalk, Ohio (31). Norwalk virus and the subsequently described Norwalk-like caliciviruses (NLVs) have also been collectively described as small, round-structured viruses (6). NLVs are so called because they can be clearly distinguished from other enteric viruses on the basis of virion morphology. These viruses are approximately 30 to 35 nm in diameter and have an amorphous structure with a ragged edge. This morphology contrasts with that of the Sapporo-like viruses (SLVs) which are also associated with human gastroenteritis. The SLVs are predominantly associated with pediatric gastroenteritis and display the distinctive morphology typical of other well-defined animal caliciviruses (37).
Complete nucleotide sequences are available for the prototype Norwalk virus (23, 28) and two NLVs, Southampton virus (33, 35) and Lordsdale virus (10). These viruses have single-stranded (ss) positive-sense RNA genomes of 7,500 to 7,700 nucleotides organized into three major open reading frames (ORFs). ORF 1 encodes a large polyprotein (180 kDa) that undergoes proteolytic cleavage by a 3C-like protease, ORF 2 encodes a single capsid protein (60 kDa), and ORF 3 encodes a small, basic protein (Mr, 20 to 25 kDa) of unknown function. Comparisons of the three complete genomic sequences together with phylogenetic analyses of many partial sequences have shown that the NLVs can be divided in to two genetic groups (34). NLVs do not grow in cell culture, and therefore molecular studies have relied on the availability of stool samples from volunteers or clinical specimens. Phylogenetic analyses also suggest that the NLVs are quite distinct from the SLVs and constitute a separate genus of the family Caliciviridae (3, 43).
Enteric caliciviruses have been described for a number of animal species, including cattle, pigs, cats, dogs, and chickens (2, 4, 16, 26, 27, 39, 40, 47, 48, 50, 51). However, these viruses remain candidate caliciviruses because of the absence of definitive sequence evidence linking them to the Caliciviridae (8). Progress in the molecular characterization of these viruses has been severely hampered by the lack of routine in vitro procedures for their isolation. Only a porcine enteric calicivirus isolate from the United States has been shown to replicate in cell culture (14, 44). Early studies with the NLVs indicated that the likely target cells for virus replication for the human enteric viruses are enterocytes of the small intestine (1, 52, 53). Bovine enteric caliciviruses, like the NLVs also replicate in enterocytes of the small intestine (22). This similarity in tissue tropism between viruses from different host species together with the recent observation that porcine enteric caliciviruses from Japan are related to group 2 NLVs (55) led us to investigate whether the bovine enteric viruses might also be related to the NLVs. The bovine enteric caliciviruses would certainly be useful as a model system for investigating the pathogenesis of enteric calicivirus infection because fresh, healthy bovine small intestine tissue is readily available, whereas surgically removed fresh, undiseased human small intestine tissue is exceedingly difficult to obtain. Thus, the primary purpose of this work was to characterize the complete genome of a bovine enteric calicivirus originally isolated in Jena, Germany, in 1980 (20).
A calicivirus has been isolated from cattle (41), but this virus (Tillamook virus; BCV-Bos1) causes respiratory symptoms. Phylogenetic analysis showed that the Tillamook virus is very closely related to the San Miguel sea lion virus and vesicular exanthema of swine virus and can also infect pigs, causing vesicular lesions. However, in contrast, Jena virus (JV) (117/80) was discovered by electron microscopic (EM) examination of diarrheic stools from newborn calves (20, 21).
Molecular analysis of JV.
EM examination of JV showed a typical NLV morphology, with virions of approximately 30 nm in diameter (21). The virus was passaged 11 times in colostrum-deprived, newborn calves, which were transported to the laboratory soon after birth. Each calf received a 5- to 10-ml oral inoculum of fecal supernatant from the previous calf. Inocula were prepared by centrifugation of fecal samples at 3,000 × g for 30 min followed by dilution with 4 volumes of phosphate-buffered saline (PBS). Two hours after receiving the inoculum, calves were given 2 liters of colostrum and then fed milk at 500 ml/10 kg of body weight twice daily. On each passage, the calves became symptomatic with diarrheal disease and the presence of JV was verified by EM.
For molecular studies, the virus was purified from the first diarrheal sample collected at 12.5 h postinfection from newborn calf 319/92 (10th passage). This sample was diluted with 4 volumes of PBS and centrifuged at 3,000 × g for 30 min. The supernatant was mixed with 2 volumes of 1,1,2-trichlorotrifluoroethane, and the organic and aqueous phases were separated by centrifugation at 3,000 × g for 30 min. The supernatant was extracted with chloroform, and then a sample of the aqueous phase was purified by sedimenting it through a 35% sucrose cushion at 35,000 rpm for 2 h in a Sorvall TH641 rotor. This sample was dialyzed against PBS and used for cDNA synthesis. JV (100 μl) purified through a sucrose cushion was extracted with Trizol (Gibco-BRL, Paisley, United Kingdom), and the genomic RNA was adsorbed to silica particles (RNAid; Bio 101 Inc., La Jolla, Calif.). ss cDNA was synthesized from this RNA by using RNase H− Moloney murine leukemia virus reverse transcriptase (Superscript; Gibco-BRL) by priming with the 3′ terminally degenerate oligo(dT) primer 5′ T25VN 3′ (T25-A/G/C-A/G/C/T) in a standard cDNA synthesis reaction as described by the manufacturer. RNA/DNA hybrids (in a final reaction volume of 50 μl) were treated with 5 μl of 1 M NaOH at 65°C for 10 min and then neutralized with 5 μl of 1 M HCl plus 5 μl of 1 M Tris-HCl (pH 7.5). This cDNA was used as a template for amplification of a small fragment of the RNA-dependent RNA polymerase gene using the primer pair GLPSG1 (5′-GAIGGICTICCATCWGGITTYCC-3′) and YGDD1 (5′-ACIATYTCRTCATCICCRTARAA-3′). These primers were originally designed to amplify cDNA from human group 1 NLVs by using a consensus sequence obtained by aligning the RNA polymerase genes (18). Amplification was performed with a Tetrad Peltier thermal cycler (MJ Research, Watertown, Mass.) and consisted of 35 cycles of 94°C for 15 s, 50°C for 15 s, and 72°C for 15 s, using Bio-X-act polymerase (Bioline, London, United Kingdom) in a reaction volume of 50 μl containing 25 mM TAPS [Tris(hydroxymethyl)-methyl-amino-propanesulfonic acid and sodium salt, pH 9.3 (at 25°C)], 50 mM KCl, 2 mM MgCl2, 1 mM β-mercaptoethanol, 200 μM each dATP, dGTP, dTTP, and dCTP. Following amplification, the PCR product (156 bp) was sequenced directly with primers GLPSG1 and YGDD1.
The sequence obtained was used to build the specific primer JV1 (5′-4450ACTTCCCAAGTTAATTCTATT4470-3′) to amplify a 2,914-bp amplicon with primer 5′ T25VN 3′, using Bio-X-act polymerase for 35 cycles of 94°C for 15 s, 50°C for 15 s, and 72°C for 3 min. This amplicon was purified from the reaction mix by using a Wizard PCR Prep column (Promega, Southampton, United Kingdom) and then digested to completion with the restriction endonucleases HaeIII or RsaI (Promega). The DNA fragments were ligated into SmaI-digested pSP73 (Promega), and the recombinant plasmids were transformed into Escherichia coli DH5α. Analysis of the inserts from these recombinants allowed the rapid accumulation of sequence data from which a set of custom oligonucleotide primers were synthesized and used to amplify and directly sequence a series of PCR fragments.
Sequences upstream of the RNA polymerase region as far as the helicase motif were obtained by reverse transcription-PCR, using primers JV2 (5′-4470AATAGAATTAACTTGGGAAGT4450-3′) and Helicase 3 (5′-1465GGCCMCCCKGGIWKIGGIAAA1685-3′). Primer Helicase 3 was designed from the amino acid sequence motif GXXGXGKT found in the helicase region of all caliciviruses. PCR conditions were the same as those used to amplify the 3′-terminal 3-kb fragment with primer pair JV1 and 5′ T25VN 3′.
The 5′ end of the JV genome was sequenced by the random PCR method (10) adapted from the procedures of Froussard (15) and Grothues et al. (19). Briefly, ss cDNA was generated by using specific primers and then converted into double-stranded (ds) cDNA using a random primer, LinkerN7 (5′-TAGTACATAGTGGATCCAGCTN7-3′), and Klenow polymerase. The randomly primed viral ds cDNA was then amplified in two successive, nested PCRs using specific primers and a primer based on the linker component of LinkerN7. Reaction products were sequenced directly to enable the design of new oligonucleotides for repeated rounds of random primer extension toward the 5′ terminus. This approach enabled sequence data to be collected to within 80 nucleotides of the authentic genomic 5′ terminus.
The genomic 5′ terminus of JV was defined by homopolymer tailing and PCR, using a commercial kit (5′ RACE; Gibco-BRL) in combination with a number of custom oligonucleotide primers. Separate lots of cDNA were then synthesized from the purified viral RNA and a single primer JV3 (5′-406TGTACCCCTCGTAAAACTC388-3′). The cDNA was tailed with A or C residues at the 3′ terminus by using terminal deoxynucleotide transferase in separate reactions and amplified by two successive rounds of PCR using Pfu polymerase (Stratagene, Cambridge, United Kingdom) with either primers 5′ T25VN 3′ or the 5′ RACE-abridged anchor primer/abridged universal amplification primer (Gibco-BRL) and the specific primers for nested PCR JV4 (5′-82ATTCTGGGTCACTAACTTTG63-3′) and JV5 (5′-55GTGGTCCAGCAACTTTAAC37-3′).
Sequence data obtained from clones were used to synthesize specific primers for direct sequencing of both strands of amplified cDNA with an Applied Biosystems model 373A automated sequencer using Taq cycle dideoxy terminator chemistry. Computer analyses of the sequence data were performed using Lasergene software (DNASTAR Inc., Madison, Wis.). Oligonucleotides were synthesized on a Millipore Expedite 8909 automated synthesizer using β-cyanoethylphosphoramidite chemistry.
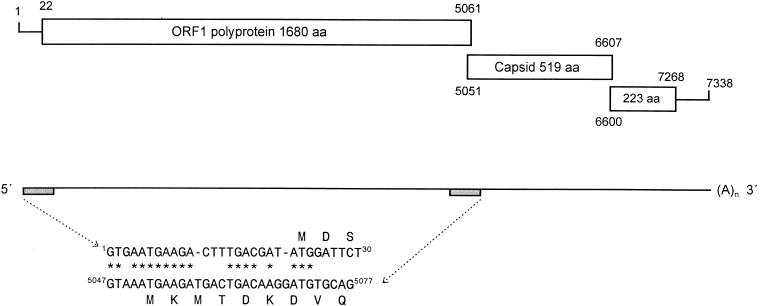
JV has a genome of 7,338 nucleotides, excluding the poly(A) tail, and a nucleotide composition of A (20.7%), G (26.2%), T (23.4%), and C (29.6%), with an overall G:C content of 55.8%. The nucleotide sequence contains characteristic motifs located at the 5′ terminus of the genome and the start of ORF 2 (Fig. 1). Within the first 24 nucleotides at the 5′ terminus of the genome, 18 are conserved when compared to the similar motif at the start of ORF 2. At the 5′ terminus is a guanosine residue which is followed immediately by the translation termination codon (TGA). The first translation initiation codon at nucleotide position 5 aligns with the predicted start codon for ORF 2; however, the reading frame terminates after only 17 amino acids. The first initiation codon for a large uninterrupted ORF that fits the features of ORF 1 from other caliciviruses is located at nucleotide 22 and is situated within a favorable context (GATATGGAT) for translation initiation by the ribosome scanning model (32). Thus, the JV 5′ noncoding region is slightly longer than the 5′ noncoding region of feline calicivirus (19 nt) and rabbit hemorrhagic disease virus (10 nt). This is in striking contrast to those of the human enteric viruses Norwalk, Southampton, and Lordsdale, in which there are just four nucleotides in the 5′ noncoding region. In addition, the first seven amino acids of both ORF 1 and ORF 2 are highly conserved in the human enteric caliciviruses (7). This, together with the location of the first in-frame initiator codon in JV at position 22, strongly suggests that the AUG at nucleotide position 11 in both group 1 and group 2 human NLVs is likely to be the authentic initiator codon, because in both cases it is situated in a favorable context for translation initiation.
FIG. 1.
Diagrammatic representation of reading frame usage in JV. The nucleotide coordinates of the translation products are numbered on the open boxes. The repeat motifs at the 5′ genomic terminus and the predicted 5′ terminus of the subgenomic RNA are aligned beneath the genomic map. Shaded boxes indicate the genomic locations of these motifs.
As expected, JV ORF 1 encodes the 2C-like helicase, 3C-like protease, and 3D-like RNA polymerase motifs characteristic of the caliciviruses (Fig. 2). At 1,680 amino acids, the ORF 1 polyprotein is similar in size to that of the group 2 polyprotein from Lordsdale virus (1,699 amino acids). The polyprotein sequences from group 1 and group 2 NLVs have been compared (10). This analysis showed a similar overall organization, although there was little identity between group 1 and group 2 polyproteins within the first 150 N-terminal amino acids. JV also has a highly divergent amino terminus for the first 100 amino acid residues, but overall it is more closely related to the group 1 viruses. It is notable that the predicted cleavage dipeptide 321QG in both group 1 and 2 human viruses is also present at the N terminus of the 2C-like helicase and is replaced by a 683QA dipeptide in the corresponding C-terminal cleavage site (38). Interestingly, the genomic RNA of JV contains several polypyrimidine tracts that encode highly unusual proline-rich regions in the predicted translation product of the N-terminal region, as previously described for Lordsdale virus (10).
FIG. 2.
Translation products encoded by the three major ORFs of JV. The conserved motifs defining the 2C helicase (GPPGIGKT), 3C protease (GDCG), and 3D RNA-dependent RNA polymerase (GLPSG.....YGDD) are underlined with asterisks. The putative cleavage sites surrounding the 2C helicase are indicated by shaded boxes. The amino acid sequence coordinates for each of the three reading frames are on the left.
The predicted molecular size of the JV capsid (56 kDa) is in close accordance with the estimated size of the capsid protein from partially purified virions of the candidate bovine enteric calicivirus, Newbury agent 2, described in a recent preliminary report (9) and the capsid of a porcine calicivirus from the United States (45). Alignment of the JV ORF 2 translation product with representative examples of group 1 and group 2 NLVs shows that the capsid protein can be divided into the same three discrete regions as previously reported for the NLVs (11, 17, 36). This observation is consistent with the domain organization proposed for the primary sequence of the Norwalk virus capsid protein (46). The region of most significant homology with the NLVs occurs within the N-terminal 274-amino-acid residues of JV (55.1 to 60.5% amino acid identity). This part of the protein has been predicted to form a β-barrel structure comprising the lower shell domain (S) of the capsid dimer. The C-terminal region of the JV capsid (395 to 519 amino acids) is slightly more variable (48.4 to 53.8%). A central hypervariable region predicted to encode the projecting arched domain of the virus capsomere is located between amino acids 275 and 394. In Norwalk virus, regions 2 and 3 of the molecule contain important antigenic components of the virion capsid recognized by monoclonal antibodies (24). In caliciviruses, the capsid protein is normally in the size range of 58 to 60 kDa. In the case of feline calicivirus, the capsid protein is encoded by ORF 2 and synthesized as a larger precursor protein (671 amino acids) that undergoes cleavage by the 3C-like protease encoded by ORF 1 to yield a final mature capsid product of 547 amino acids (5, 54). However, in the NLVs, there is no evidence that the capsid protein is expressed as a precursor that undergoes modification by the genomically encoded 3C-like protease. Consistent with this observation for enteric caliciviruses, the genome of JV has a smaller reading frame for ORF 2, encoding a capsid of only 519 amino acids. Uniquely among the caliciviruses analyzed to date, the initiation codon for this ORF overlaps the 3′ end of ORF 1 by 11 nucleotides.
The third ORF of 669 nucleotides (ORF 3) encodes a relatively small protein of 223 amino acids and is located in frame −2 relative to ORF 1. This ORF has a counterpart in all other caliciviruses, although the biological function remains unknown. At 223 amino acids, the ORF 3 protein is larger than that of both the rabbit hemorrhagic disease virus and feline calicivirus proteins (117 and 106 amino acids, respectively) and is closer in size to the group 1 (211 amino acids) rather than the group 2 (268 amino acid) NLV ORF 3 proteins. The predicted protein is basic and hydrophilic and contains no cysteine residues. The AUG initiator codon of JV ORF 3 overlaps ORF 2 by eight nucleotides.
ORF 3 protein has been detected in feline calicivirus-infected cells (25), and its counterpart in rabbit hemorrhagic disease virus has been described as a virion-associated protein (57). It has also been suggested as an RNA binding protein (42). JV ORF 3 is closest in size to ORF 3 from the group 1 NLVs, and alignment with ORF 3 from the group 1 and 2 NLVs (data not shown) indicates that the overall primary sequence also correlates most closely to group 1 NLV ORF 3 (36.7% for Norwalk virus to 46% for Southampton virus). The 3′ end of the genome contains 70 untranslated nucleotides prior to the poly(A) tail.
In vitro analysis of the JV capsid protein.
A subgenomic clone containing JV ORF 2 was constructed by amplification of the cDNA. A 2,361-bp amplicon was generated by PCR with the primer pair JVsubF (5′-ACAAACGTTAACTAATACGAC TCACTATA5047GTAAATGAAGATGACTGAC5065-3′) and JVsubR (5′-AACAAACAAGGATCCT(25)-3′), using standard reaction conditions as described previously. Restriction enzyme cleavage sites for HpaI and BamHI, respectively, are italicized, and the T7 promoter is in bold type. The resulting amplicon was digested with the appropriate restriction enzymes and cloned into pSP73 (Promega). This recombinant plasmid (pJVC1) was resequenced to ensure that it was an authentic copy of the JV consensus for ORF 2 under the control of the T7 promoter. Purified plasmid pJVC1 was used as a template for in vitro protein synthesis, using a T7 RNA polymerase-coupled reticulocyte lysate system (TNT; Promega) in accordance with the manufacturer’s instructions. The reaction mixture (total volume, 25 μl) was incubated at 30°C, and the reaction was stopped after 1 h for immunoprecipitation and analysis of reaction products by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Gels were stained and prepared for autoradiography by treatment with 1 M sodium salicylate–50% methanol for 30 min at room temperature. Gels were then dried under vacuum and exposed to Kodak XAR-5 film at −70°C.
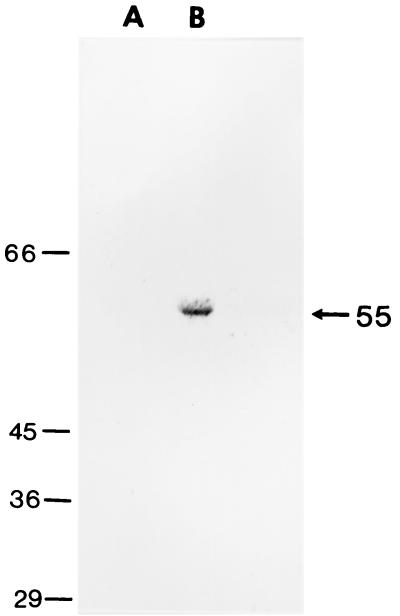
Calf 321/93 (11th passage) was infected with a fecal suspension from calf 319/92 (10th passage) as described above. Blood samples for serum analysis were collected from calf 321/93 before infection with JV and then at weekly intervals. Calf 321/93 was reinfected orally with aliquots of the same inoculum at 1, 2, and 4 weeks after the first infection. Translation products from the TNT-coupled transcription-translation reaction of pJVC1 (5 μl) were incubated with 2 μl of undiluted bovine antiserum in 600 μl of radioimmune precipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 0.15 mM NaCl, 0.1% SDS, 0.5% Empigen BB, 0.1 mM phenylmethylsulfonyl fluoride) and incubated at 37°C for 1 h. Immune complexes formed with bovine antisera were captured by using protein G immobilized on Sepharose 4B Fast Flow beads (Sigma, Poole, United Kingdom). The beads were washed three times with RIPA buffer and with a final wash in PBS before derivitization in sample-dissociating buffer and separation by SDS-PAGE. pJVC1 used as a template in a coupled transcription-translation reaction gave a single product of 55 kDa when analyzed by SDS-PAGE, indicating that in this system only ORF 2 is translated. Preimmune serum obtained from colostrum-deprived, newborn calf 321/93 before experimental infection with JV was used in an immunoprecipitation reaction with labelled JV capsid. This serum showed that antibodies to the JV capsid protein were not present prior to infection. However, postinfection antiserum (4 weeks) was able to immunoprecipitate the JV capsid protein (Fig. 3). All 11 calves used in the serial passage of JV developed diarrheal symptoms following oral administration of the JV inoculum. The presence of JV in the postinfection diarrheal stool samples of each calf and the seroconversion of calf 321/93 (11th passage) to the JV capsid protein indicates the highly infectious and immunogenic nature of this agent.
FIG. 3.
RIPA of the JV capsid protein produced by in vitro transcription/translation. Lane A, preinfection serum from a colostrum-deprived, newborn calf. Lane B, serum taken at 4 weeks postinfection with JV. The position of the immunoprecipitated capsid protein is marked (arrow). Molecular size markers (in kilodaltons) are on the left.
Phylogenetic analysis.
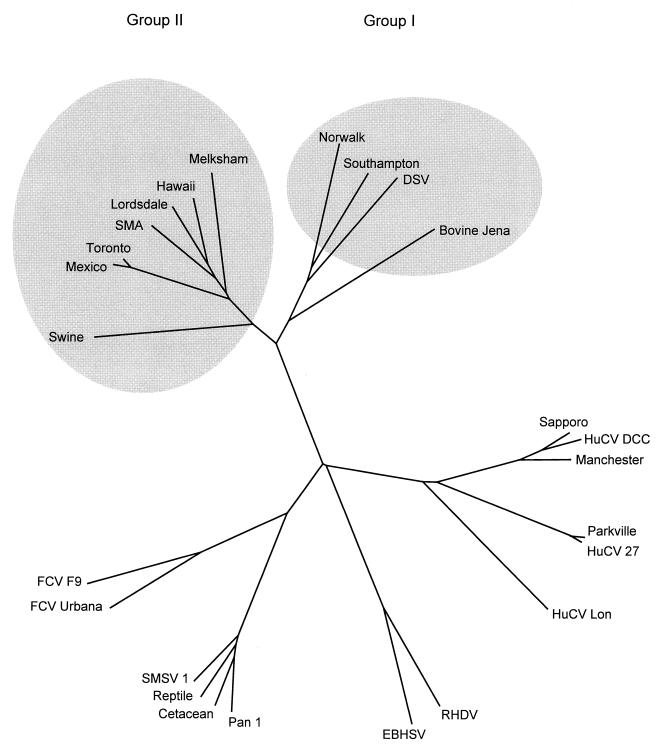
Unrooted phylogenetic trees were constructed for a region of the 3D RNA polymerase for caliciviruses as previously defined (3). Multiple alignments were performed with CLUSTAL X (56), and unrooted trees were generated using the neighbor-joining method (49). Trees were subjected to a bootstrap analysis (12) using 1,000 data sets and output as a graphic representation using DRAWTREE in the PHYLIP package (13). Phylogenetic studies using the RNA polymerase (Fig. 4) and the hypervariable capsid region (data not shown) revealed that JV is most closely related to the group 1 NLVs. This group of human enteric caliciviruses includes Norwalk virus, Southampton virus, and Desert Shield virus.
FIG. 4.
Unrooted phylogenetic tree constructed for a region of the 3D RNA-dependent RNA polymerase gene showing the relationship of JV to other caliciviruses. Shaded ellipses have been added to highlight the distinction between the group 1 and group 2 NLVs. Accession numbers (in parentheses) for caliciviruses are as follows; swine (AB009412); Mexico (U22498); Toronto (U02030); SMA (L23831); Lordsdale (X86557); Hawaii (U07611); Melksham (X81879); Norwalk (M87661); Southampton (L07418); Desert Shield virus (DSV) (U04469); bovine Jena (AJ011099); Sapporo (S77903); human calicivirus (HuCV) DCC (U67856); Manchester (X86559); Parkville (U73124); HuCV 27 (U67859); HuCV Lon (U67858); rabbit hemorrhagic disease virus (RHDV) (M67473); European brown hare syndrome virus (EBHSV) (Z69620); Pan 1 (U52086); cetacean (U52091); reptile (U52092); San Miguel sea lion virus (SMSV 1) (M87481); feline calicivirus (FCV) Urbana (L40021); FCV F9 (M86379).
A recent large Japanese survey for swine caliciviruses identified four closely related but unique sequences by reverse transcription-PCR. Molecular phylogenetic studies linked these porcine calicivirus sequences to the group 2 NLVs (55). However, in contrast to our study with JV, none of the pigs from which viral sequences were obtained had symptoms of enteric disease. The close similarity of the porcine and bovine sequences to the NLVs suggests that these viruses shared a relatively recent common ancestor and raises the intriguing possibility of an animal reservoir for human infection. The complete nucleotide sequence of JV unequivocally establishes this virus as a member of the Caliciviridae. The sequence will enable the development of specific primers to study the molecular epidemiology of bovine enteric disease and also investigation into the nature and extent of variation in the bovine enteric caliciviruses. Furthermore, it will be possible to produce JV capsid antigen by expression of ORF 2 in insect cells using baculovirus vectors, thus facilitating the development of the materials required to investigate the disease caused in cattle and possibly other species by JV.
Nucleotide sequence accession number.
The sequence of JV has been deposited in the EMBL and GenBank databases under accession no. AJ011099.
REFERENCES
- 1.Agus S G, Dolin R, Wyatt R G, Tousimis A J, Northrup R S. Acute infectious nonbacterial gastroenteritis: intestinal histopathology. Histologic and enzymatic alterations during illness produced by the Norwalk agent in man. Ann Intern Med. 1973;79:18–25. doi: 10.7326/0003-4819-79-1-18. [DOI] [PubMed] [Google Scholar]
- 2.Almeida J D, Craig C R, Hall T E. Multiple viruses present in the faeces of a scouring calf. Vet Rec. 1978;102:170–171. doi: 10.1136/vr.102.8.170. [DOI] [PubMed] [Google Scholar]
- 3.Berke T, Golding B, Jiang X, Cubitt D W, Wolfaardt M, Smith A W, Matson D O. Phylogenetic analysis of the caliciviruses. J Med Virol. 1997;52:419–424. doi: 10.1002/(sici)1096-9071(199708)52:4<419::aid-jmv13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Bridger J C. Small viruses associated with gastroenteritis in animals. In: Saif L J, Theil K W, editors. Viral diarrheas of man and animals. Boca Raton, Fla: CRC Press; 1990. pp. 161–182. [Google Scholar]
- 5.Carter M J, Milton I D, Turner P C, Meanger J, Bennett M, Gaskell R M. Identification and sequence determination of the capsid protein gene of feline calicivirus. Arch Virol. 1992;122:223–235. doi: 10.1007/BF01317185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caul E O, Appleton H. The electron microscopical and physical characteristics of small round human fecal viruses: an interim scheme for classification. J Med Virol. 1982;9:257–265. doi: 10.1002/jmv.1890090403. [DOI] [PubMed] [Google Scholar]
- 7.Clarke I N, Lambden P R, Caul E O. Human enteric RNA viruses: caliciviruses and astroviruses. In: Mahy B W J, Collier L, editors. Topley & Wilson’s microbiology and microbial infections. London, United Kingdom: Arnold; 1998. pp. 511–535. [Google Scholar]
- 8.Cubitt D, Bradley D W, Carter M J, Chiba S, Estes M K, Saif L J, Schaffer F L, Smith A W, Studdert M J, Thiel H J. Viral taxonomy, classification and nomenclature; sixth report of the International Committee on the Taxonomy of Viruses. Arch Virol Suppl. 1995;10:359–363. [Google Scholar]
- 9.Dastjerdi A M, Bridger J C, Snodgrass D R, Bredl J C, Plummer J M. Proceedings of the First International Symposium on Caliciviruses ESVV 77-82, Reading, United Kingdom. 1997. Characterisation of bovine enteric calici-like viruses. [Google Scholar]
- 10.Dingle K E, Lambden P R, Caul E O, Clarke I N. Human enteric Caliciviridae: the complete genome sequence and expression of virus-like particles from a genetic group II small round structured virus. J Gen Virol. 1995;76:2349–2355. doi: 10.1099/0022-1317-76-9-2349. [DOI] [PubMed] [Google Scholar]
- 11.Estes M K, Hardy M E. Norwalk virus and other enteric caliciviruses. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press Ltd; 1995. pp. 1009–1034. [Google Scholar]
- 12.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein J. PHYLIP (Phylogeny Inference Package), version 3.5c. 1993. Distributed by author. [Google Scholar]
- 14.Flynn W T, Saif L J. Serial propagation of porcine enteric calicivirus-like virus in primary porcine kidney-cell cultures. J Clin Microbiol. 1988;26:206–212. doi: 10.1128/jcm.26.2.206-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froussard P. A random-PCR method (rPCR) to construct whole cDNA library from low amounts of RNA. Nucleic Acids Res. 1992;20:2900. doi: 10.1093/nar/20.11.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granzow H, Schirrmeier H. Identification of 32nm viruses in faeces of diarrhoeic calves by electron microscopy. Monatsh Vetmed. 1985;40:228–229. [Google Scholar]
- 17.Green S M, Lambden P R, Caul E O, Ashley C R, Clarke I N. Capsid diversity in small round-structured viruses: molecular characterisation of an antigenically distinct human enteric calicivirus. Virus Res. 1995;37:271–283. doi: 10.1016/0168-1702(95)00041-n. [DOI] [PubMed] [Google Scholar]
- 18.Green S M, Lambden P R, Deng Y, Lowes J A, Lineham S, Bushell J, Rogers J, Caul E O, Ashley C R, Clarke I N. Polymerase chain reaction detection of small round-structured viruses from two related hospital outbreaks of gastroenteritis using inosine-containing primers. J Med Virol. 1995;45:197–202. doi: 10.1002/jmv.1890450215. [DOI] [PubMed] [Google Scholar]
- 19.Grothues D, Cantor C R, Smith C L. PCR amplification of megabase DNA with tagged random primers (T-PCR) Nucleic Acids Res. 1993;21:1321–1322. doi: 10.1093/nar/21.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Günther H, Otto P. Studies into diarrhoea of young calves. Seventh communication: “Zackenvirus” (Jena-Agens 117/80)—a new diarrhoea pathogen to calf. Arch Exp Vet Med Leipzig. 1987;41:934–938. [PubMed] [Google Scholar]
- 21.Günther H, Otto P, Heilman P. Studies into diarrhoea of young calves. Sixth communication: Detection and determination of pathogenicity of a bovine corona virus and an undefined icosahedric virus. Arch Exp Vet Med Leipzig. 1984;38:781–792. [PubMed] [Google Scholar]
- 22.Hall G A, Bridger J C, Brooker B E, Parsons K R, Ormerod E. Lesions of gnotobiotic calves experimentally infected with a calicivirus-like (Newbury) agent. Vet Pathol. 1984;21:208–215. doi: 10.1177/030098588402100213. [DOI] [PubMed] [Google Scholar]
- 23.Hardy M E, Estes M K. Completion of the Norwalk virus genome sequence. Virus Genes. 1996;12:287–290. doi: 10.1007/BF00284649. [DOI] [PubMed] [Google Scholar]
- 24.Hardy M E, Tanaka T N, Kitamoto N, White L J, Ball J M, Jiang X, Estes M K. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology. 1996;217:252–261. doi: 10.1006/viro.1996.0112. [DOI] [PubMed] [Google Scholar]
- 25.Herbert T P, Brierley I, Brown T D K. Detection of the ORF3 polypeptide of feline calicivirus in infected cells and evidence for its expression from a single, functionally bicistronic, subgenomic mRNA. J Gen Virol. 1996;77:123–127. doi: 10.1099/0022-1317-77-1-123. [DOI] [PubMed] [Google Scholar]
- 26.Herbst W, Lange H, Krauss H. 27-nm virus particles found in the faeces of a cat with vomiting and diarrhoea. J Vet Med B. 1987;34:314–316. doi: 10.1111/j.1439-0450.1987.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 27.Herbst W, Lange H, Krauss H. Demonstration of calicivirus-like particles in faeces of diarrheic calves by electron microscopy. Dtsch Tieraerztl Wochenschr. 1987;94:406–407. [PubMed] [Google Scholar]
- 28.Jiang X, Wang M, Wang K, Estes M K. Sequence and genomic organization of Norwalk virus. Virology. 1993;195:51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 29.Kapikian A Z. Norwalk and Norwalk-like viruses. In: Kapikian A Z, editor. Viral infections of the gastrointestinal tract. New York, N.Y: Marcel Dekker; 1994. pp. 471–518. [Google Scholar]
- 30.Kapikian A Z, Estes M K, Chanock R M. Norwalk group of viruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 783–810. [Google Scholar]
- 31.Kapikian A Z, Wyatt R G, Dolin R, Thornhill T S, Kalica A R, Chanock R M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10:1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 33.Lambden P R, Caul E O, Ashley C R, Clarke I N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993;259:516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- 34.Lambden P R, Clarke I N. Genome organization in the Caliciviridae. Trends Microbiol. 1995;3:261–265. doi: 10.1016/s0966-842x(00)88940-4. [DOI] [PubMed] [Google Scholar]
- 35.Lambden P R, Liu B L, Clarke I N. A conserved sequence motif at the 5′ terminus of the Southampton virus genome is characteristic of the Caliciviridae. Virus Genes. 1995;10:149–152. doi: 10.1007/BF01702595. [DOI] [PubMed] [Google Scholar]
- 36.Lew J F, Kapikian A Z, Valdesuso J, Green K Y. Molecular characterization of Hawaii virus and other Norwalk-like viruses: evidence for genetic polymorphism among human caliciviruses. J Infect Dis. 1994;170:535–542. doi: 10.1093/infdis/170.3.535. [DOI] [PubMed] [Google Scholar]
- 37.Liu B L, Clarke I N, Caul E O, Lambden P R. Human enteric caliciviruses have a unique genome structure and are distinct from the Norwalk-like viruses. Arch Virol. 1995;140:1345–1356. doi: 10.1007/BF01322662. [DOI] [PubMed] [Google Scholar]
- 38.Liu B L, Clarke I N, Lambden P R. Polyprotein processing in Southampton virus: identification of 3C-like protease cleavage sites by in vitro mutagenesis. J Virol. 1996;70:2605–2610. doi: 10.1128/jvi.70.4.2605-2610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mochizuki M, Kawanishi A, Sakamoto H, Tashiro S, Fujimoto R, Ohwaki M. A calicivirus isolated from a dog with fatal diarrhoea. Vet Rec. 1993;132:221–222. doi: 10.1136/vr.132.9.221. [DOI] [PubMed] [Google Scholar]
- 40.Nagy B, Nagy G, Meder M, Mocsari E. Enterotoxigenic Escherichia coli, rotavirus, porcine epidemic diarrhoea virus, adenovirus and calici-like virus in porcine postweaning diarrhoea in Hungary. Acta Vet Hung. 1996;44:9–19. [PubMed] [Google Scholar]
- 41.Neill J D, Meyer R F, Seal B S. Genetic relatedness of the caliciviruses: San Miguel sea lion and vesicular exanthema of swine viruses constitute a single genotype within the Caliciviridae. J Virol. 1995;69:4484–4488. doi: 10.1128/jvi.69.7.4484-4488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neill J D, Reardon I M, Heinrikson R L. Nucleotide sequence and expression of the capsid protein gene of feline calicivirus. J Virol. 1991;65:5440–5447. doi: 10.1128/jvi.65.10.5440-5447.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noel J S, Liu B L, Humphrey C D, Rodriguez E M, Lambden P R, Clarke I N, Dwyer D M, Ando T, Glass R I, Monroe S S. Parkville virus: a novel genetic variant of human calicivirus in the Sapporo virus clade, associated with an outbreak of gastroenteritis in adults. J Med Virol. 1997;52:173–178. [PubMed] [Google Scholar]
- 44.Parwani A V, Flynn W T, Gadfield K L, Saif L J. Serial propagation of porcine enteric calicivirus in a continuous cell-line effect of medium supplementation with intestinal contents or enzymes. Arch Virol. 1991;120:115–122. doi: 10.1007/BF01310954. [DOI] [PubMed] [Google Scholar]
- 45.Parwani A V, Saif L J, Kang S Y. Biochemical characterization of porcine enteric calicivirus—analysis of structural and nonstructural viral proteins. Arch Virol. 1990;112:41–53. doi: 10.1007/BF01348984. [DOI] [PubMed] [Google Scholar]
- 46.Prasad, B. V. V., M. E. Hardy, X. Jiang, and M. K. Estes. 1996. Structure of Norwalk virus. Arch. Virol. 12(Suppl.):237–242. [DOI] [PubMed]
- 47.Reckling K F. Use of electron microscopy for observation of small round viruses in faecal samples collected from calves and animals with diarrhoea. Monatsh Vetmed. 1987;42:272–275. [Google Scholar]
- 48.Reynolds D J, Morgan J H, Chanter N, Jones P W, Bridger J C, Debney T G, Bunch K J. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986;119:34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- 49.Saitou N, Nei M. The neighbor joining method—a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 50.San Gabriel M C S, Tohya Y, Mochizuki M. Isolation of a calicivirus antigenically related to feline caliciviruses from feces of a dog with diarrhea. J Vet Med Sci. 1996;58:1041–1043. doi: 10.1292/jvms.58.10_1041. [DOI] [PubMed] [Google Scholar]
- 51.Schaffer F L, Soergel M E, Black J W, Skilling D E, Smith A W, Cubitt W D. Characterization of a new calicivirus isolated from feces of a dog. Arch Virol. 1985;84:181–195. doi: 10.1007/BF01378971. [DOI] [PubMed] [Google Scholar]
- 52.Schreiber D S, Blacklow N R, Trier J S. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N Engl J Med. 1973;288:1318–1323. doi: 10.1056/NEJM197306212882503. [DOI] [PubMed] [Google Scholar]
- 53.Schreiber D S, Blacklow N R, Trier J S. The small intestinal lesion induced by Hawaii agent acute infectious nonbacterial gastroenteritis. J Infect Dis. 1974;129:705–708. doi: 10.1093/infdis/129.6.705. [DOI] [PubMed] [Google Scholar]
- 54.Sosnovtsev S V, Sosnovtseva S A, Green K Y. Cleavage of the feline calicivirus capsid precursor is mediated by a virus-encoded proteinase. J Virol. 1998;72:3051–3059. doi: 10.1128/jvi.72.4.3051-3059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugieda M, Nagaoka H, Kakishima Y, Ohshita T, Nakamura S, Nakajima S. Detection of Norwalk-like virus genes in the caecum contents of pigs. Arch Virol. 1998;143:1–7. doi: 10.1007/s007050050369. [DOI] [PubMed] [Google Scholar]
- 56.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wirblich C, Thiel H-J, Meyers G. Genetic map of the calicivirus rabbit hemorrhagic disease virus as deduced from in vitro translation studies. J Virol. 1996;70:7974–7983. doi: 10.1128/jvi.70.11.7974-7983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]