Abstract
Video Abstract
OBJECTIVES
Describe clinical and epidemiologic patterns of pediatric allergy using longitudinal electronic health records (EHRs) from a multistate consortium of US practices.
METHODS
Using the multistate Comparative Effectiveness Research through Collaborative Electronic Reporting EHR database, we defined a cohort of 218 485 children (0–18 years) who were observed for ≥5 years between 1999 and 2020. Children with atopic dermatitis (AD), immunoglobulin E–mediated food allergy (IgE-FA), asthma, allergic rhinitis (AR), and eosinophilic esophagitis (EoE) were identified using a combination of diagnosis codes and medication prescriptions. We determined age at diagnosis, cumulative incidence, and allergic comorbidity.
RESULTS
Allergic disease cumulative (and peak age of) incidence was 10.3% (4 months) for AD, 4.0% (13 months) for IgE-FA, 20.1% (13 months) for asthma, 19.7% (26 months) for AR, and 0.11% (35 months) for EoE. The most diagnosed IgE-FAs were peanut (1.9%), egg (0.8%), and shellfish (0.6%). A total of 13.4% of children had ≥2 allergic conditions, and respiratory allergies (ie, asthma, AR) were commonly comorbid with each other, and with other allergic conditions.
CONCLUSIONS
We detail pediatric allergy patterns using longitudinal, health care provider-based data from EHR systems across multiple US states and varied pediatric practice types. Our results support the population-level allergic march progression and indicate high rates of comorbidity among children with food and respiratory allergies.
What’s Known on This Subject:
Analysis of electronic health record–based clinical cohorts is an emerging approach for the longitudinal study of disease patterns that has not been previously applied to broadly study US patterns of pediatric allergy.
What This Study Adds:
Our study uses data from a multistate electronic health record consortium to describe patterns of health care provider-diagnosed pediatric allergy. Our results support the population-level allergic march paradigm and indicate marked comorbidity among children with food and/or respiratory allergies.
Pediatric allergic diseases are associated with impaired quality of life, absenteeism, and significant health care expenses.1–4 The most common allergic manifestations in children include atopic dermatitis (AD), systemic (ie, anaphylactic) immunoglobulin E–mediated food allergy (IgE-FA), asthma, allergic rhinitis (AR), and eosinophilic esophagitis (EoE).5 Together, these conditions are among the most common chronic diseases of childhood, affecting approximately 1 in 5 children.6
Assessing allergic disease patterns is necessary to identify risk factors, detect disparate health outcomes, and inform clinical and research efforts.7–9 To date, allergic disease epidemiology in the United States has been measured largely via retrospective surveys.10–14 A methodologic strength of survey-based approaches is the ability to construct nationally representative cohorts. However, surveys are also subject to reporting bias.15–17 An emerging and complementary strategy is analysis of electronic health record (EHR)-based clinical cohorts. Strengths of EHR-based analyses include their use of provider-based diagnosis and prescription data and their longitudinal nature, enabling examinations of disease onset, risk factors, and comorbidities over time.18–20 To date, EHR-based studies of US pediatric allergy patterns have not been performed using multistate cohorts.5,6,21,22
To address this need, we conducted a detailed examination of pediatric allergy patterns using the Comparative Effectiveness Research through Collaborative Electronic Reporting (CER2) Consortium database, comprising more than 1 million children across multiple independent primary care practices and health systems within the United States.23 For each allergic disease, we determined age at diagnosis, cumulative incidence, and allergic disease comorbidities. Our study details clinical and epidemiologic features of pediatric allergy as managed by a consortium of primary care practices and health systems across the United States.
Methods
Subject Identification and Study Design
Using the American Academy of Pediatrics CER2 EHR database, a Health Insurance Portability and Accountability Act–limited database, we defined a retrospective birth cohort of 218 485 US children who established care before their first birthday and were observed for at least 5 years between their first and last office visits through age 18 years, between 1999 and 2020. Diagnosis codes and medication prescriptions were used to identify children with AD, IgE-FA, asthma, AR, and EoE. Additional clinical descriptors were used to identify individual IgE-FAs, as detailed later. In addition to abstracting birth year and sex (a separate variable for gender was not available), we abstracted data on self-reported racial/ethnic designations to assess for factors that might influence disease rates.24 The race category “Unknown” includes subjects with self-identified unknown race, whereas “Other” comprises all other entries including “American Indian or Alaska native,” “Mixed racial group,” and “Race not available.” Geographic distribution (Supplemental Table 7) was determined using subject’s ZIP codes. Urbanicity (Supplemental Table 8) was determined by merging subject’s ZIP codes with 2010 Rural-Urban Commuting Area codes.25 Statistical analyses were performed using multivariable logistic regression with membership in the allergic disease subgroup of interest as the outcome. The study was approved by the American Academy of Pediatrics institutional review board and deemed not to be human subjects research by the Children’s Hospital of Philadelphia institutional review board.
Disease Definitions and Epidemiologic Analyses
To identify children with AD, IgE-FA, asthma, AR, and EoE (Fig 1), the CER2 database was queried for the presence of relevant International Classification of Diseases Ninth Revision or Tenth Revision diagnosis codes and/or Systematized Nomenclature of Medicine codes (Supplemental Table 9). Search terms were specified to identify individual IgE-FAs (Supplemental Table 10). Disease-specific diagnosis codes were required during at least 2 clinical visits separated by at least 6 months, with the exception of EoE, for which only 1 visit was required as this condition is commonly managed by specialists following initial diagnosis. Codes corresponding to “reactive airway disease” and asthma diagnosis before age 1 year year were not included in our asthma subcohort definition (Supplemental Table 9).5,6 Similarly, to define IgE-FA, we did not include diagnosis codes related to lactose intolerance, celiac disease, or milk protein sensitivity (Supplemental Table 9). Finally, we required presence of relevant prescriptions for the asthma (asthma medications on at least 2 separate calendar days) and IgE-FA (epinephrine on at least 1 calendar day) subcohorts (Supplemental Table 9). Similar disease criteria showed concordance with established practice parameters on chart review in a regional EHR-based analysis.5,6 R software (version 3.6) was used to determine incidence rate (ie, new cases per given age range) and cumulative incidence (ie, total cases per given age range). Descriptive statistics were also obtained in a sensitivity analysis with a minimum observation period of 2 years rather than 5 years.
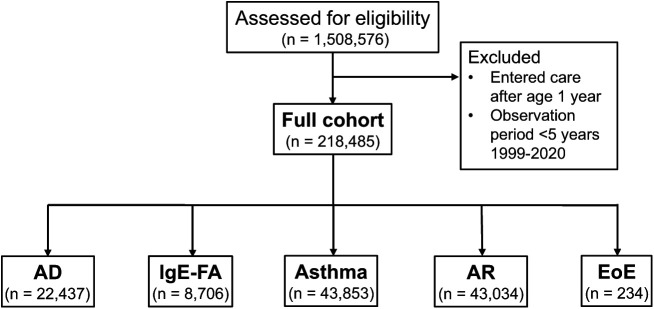
FIGURE 1.
Summary flow diagram of full cohort and allergic disease subcohorts and exclusions. Consort diagram of the generation of the full and disease cohorts. AD, atopic dermatitis; AR, allergic rhinitis; EoE, eosinophilic esophagitis; IgE-FA, immunoglobulin E–mediated food allergy.
Results
Birth and Disease Cohort Demographic Features
Generation of disease cohorts is illustrated in Fig 1. Demographics and geographic distribution of subjects are detailed in Table 1 and Supplemental Table 7, respectively. A total of 78.5% of children were born between 2005 and 2014 (Table 1). The sex breakdown of the total cohort was approximately equal (51.1% male vs. 48.9% female), with a slight male predominance in allergic subcohorts, most notably for EoE (70.9% male vs. 29.1% female). The total cohort was 47.6% White, 31.9% Black, 2.8% Asian or Pacific Islander, and 17.7% Other or Unknown. Regarding ethnicity, the cohort was 10.2% Hispanic, 76.2% non-Hispanic, and 13.6% Other. In allergic subcohorts, compared with the full cohort, there was significantly higher representation of Black children for AD and asthma, significantly higher representation of White children for EoE, and significantly lower representation of Hispanic children for IgE-FA. Most subjects (89.5%) were located in metropolitan cores, a proxy for urbanized areas (Supplemental Table 8).25 The mean observation period for the cohort was 9.3 years (Supplemental Table 11).
TABLE 1.
Demographic Features of Total and Allergic Disease-Specific Cohorts
| Variable | All | AD | IgE-FA | Asthma | AR | EoE | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | P | n | % | P | n | % | P | n | % | P | n | % | P | |
| Sexa | |||||||||||||||||
| Male | 111 577 | 51.1 | 11 983 | 53.4 | — | 5100 | 58.6 | — | 26 015 | 59.3 | — | 24 002 | 55.8 | — | 166 | 70.9 | — |
| Female | 106 907 | 48.9 | 10 454 | 46.6 | <.0001 | 3606 | 41.4 | <.0001 | 17 837 | 40.7 | <.0001 | 19 031 | 44.2 | <.0001 | 68 | 29.1 | <.0001 |
| Raceb | |||||||||||||||||
| White | 103 942 | 47.6 | 6778 | 30.2 | — | 4234 | 48.6 | — | 17 141 | 39.1 | — | 18 725 | 43.5 | — | 141 | 60.3 | — |
| Black | 69 796 | 31.9 | 11 489 | 51.3 | <.0001 | 2861 | 32.9 | <.01 | 19 477 | 44.4 | <.0001 | 18 200 | 42.3 | <.0001 | 54 | 23.1 | <.01 |
| Asian or Pacific Islander | 6210 | 2.8 | 908 | 4.0 | <.0001 | 513 | 5.9 | <.0001 | 1098 | 2.5 | NS | 1092 | 2.5 | NS | 13 | 5.5 | NS |
| Unknown | 14 393 | 6.6 | 1439 | 6.4 | <.0001 | 577 | 6.6 | <.0001 | 2761 | 6.3 | NS | 2457 | 5.7 | <.0001 | 14 | 6.0 | <.05 |
| Other | 24 144 | 11.1 | 1823 | 8.1 | <.0001 | 521 | 6.0 | NS | 3376 | 7.7 | NS | 2560 | 6.0 | <.0001 | 12 | 5.1 | NS |
| Ethnicity | |||||||||||||||||
| Non-Hispanic | 166 521 | 76.2 | 18 074 | 80.5 | — | 7657 | 88.0 | — | 35 125 | 80.1 | — | 36 463 | 84.7 | — | 202 | 86.3 | — |
| Hispanic | 22 195 | 10.2 | 2102 | 9.4 | <.0001 | 516 | 5.9 | <.0001 | 4497 | 10.3 | <.0001 | 3871 | 9.0 | <.0001 | 24 | 10.3 | NS |
| Other | 29 769 | 13.6 | 2261 | 10.1 | <.0001 | 533 | 6.1 | <.0001 | 4231 | 9.6 | <.0001 | 2700 | 6.3 | <.0001 | 8 | 3.4 | <.01 |
| Birth year | |||||||||||||||||
| Pre-2000 | 3337 | 1.5 | 187 | 0.8 | — | 36 | 0.4 | — | 676 | 1.5 | — | 505 | 1.2 | — | 2 | 0.9 | — |
| 2000–2004 | 41 898 | 19.2 | 5226 | 23.3 | <.0001 | 1294 | 14.9 | <.05 | 9877 | 22.5 | <.0001 | 9417 | 21.9 | NS | 30 | 12.8 | NS |
| 2005–2009 | 94 489 | 43.3 | 10 562 | 47.1 | <.0001 | 4139 | 47.5 | <.01 | 20 074 | 45.8 | <.0001 | 21 023 | 48.8 | <.0001 | 127 | 54.3 | NS |
| 2010–2014 | 76 919 | 35.2 | 6286 | 28.0 | NS | 3156 | 36.3 | NS | 12 988 | 29.6 | <.0001 | 11 915 | 27.7 | <.0001 | 74 | 31.6 | NS |
| 2015 or later | 1842 | 0.8 | 176 | 0.8 | NS | 81 | 0.9 | NS | 238 | 0.6 | <.0001 | 174 | 0.4 | <.0001 | 1 | 0.4 | NS |
| Total | 218 485 | 100.0 | 22 437 | 100.0 | — | 8706 | 100.0 | — | 43 853 | 100.0 | — | 43 034 | 100.0 | — | 234 | 100.0 | — |
Race was not available for 20 343 (9.3% of total) subjects. P value presented in boldface if <.05. AD, atopic dermatitis; AR, allergic rhinitis; EoE, eosinophilic esophagitis; IgE-FA, immunoglobulin E–mediated food allergy; NS, not significant.
In the full cohort and asthma and AR subcohorts, sex was unknown for 1 subject.
The race category “Unknown” represents subjects with self-identified unknown race, whereas “Other” comprises all other entries including “American Indian or Alaska Native,” “Mixed racial group,” and “Race not available.”
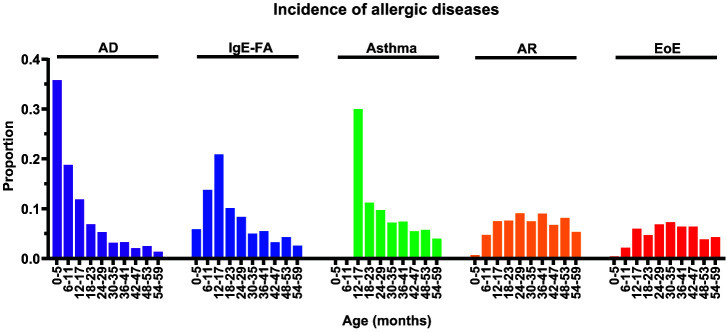
Age at Diagnosis, Incidence, and Cumulative Incidence of Allergic Diseases
For each allergic manifestation, we determined age at diagnosis (Table 2), distribution of incidence rates (Fig 2), and cumulative incidence (Table 3). The median (and interquartile range) age at diagnosis was 10 (4–25) months for AD, 23 (13–50) months for IgE-FA, 29 (16–52) months for asthma, 45 (27–69) months for AR, and 62 (33–97) months for EoE. The peak incidence for AD, IgE-FA, asthma, AR, and EoE occurred at 4 months, 13 months, 13 months, 26 months, and 35 months, respectively (Fig 2; Table 2). We also determined the cumulative incidence, a proxy for disease prevalence, for each allergic manifestation (Table 3). For all children (0–18 years), the cumulative incidence was 10.3% for AD, 4.0% for IgE-FA, 20.1% for asthma, 19.7% for AR, and 0.11% for EoE (Table 3). A total of 61.1% (26 800 of 43 853) of children with asthma retained their diagnosis in the last year of their observation period. We note that a sensitivity analysis with a minimum observation period of 2 rather than 5 years (n = 366 056 subjects) yielded comparable results for cumulative incidences and peak age of onset (data not shown).
TABLE 2.
Age at Diagnosis of Allergic Conditions
| Age Range (mo) | AD | IgE-FA | Asthma | AR | EoE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| 0–<6 | 8029 | 35.8 | 510 | 5.9 | 0 | 0.0 | 302 | 0.7 | 1 | 0.4 |
| 6–<12 | 4216 | 18.8 | 1199 | 13.8 | 0 | 0.0 | 2031 | 4.7 | 5 | 2.1 |
| 12–<18 | 2659 | 11.9 | 1819 | 20.9 | 13 143 | 30.0 | 3231 | 7.5 | 14 | 6.0 |
| 18–<24 | 1540 | 6.9 | 883 | 10.1 | 4914 | 11.2 | 3284 | 7.6 | 11 | 4.7 |
| 24–<30 | 1188 | 5.3 | 726 | 8.3 | 4272 | 9.7 | 3910 | 9.1 | 16 | 6.8 |
| 30–<36 | 704 | 3.1 | 437 | 5.0 | 3165 | 7.2 | 3214 | 7.5 | 17 | 7.3 |
| 36–<42 | 741 | 3.3 | 479 | 5.5 | 3247 | 7.4 | 3883 | 9.0 | 15 | 6.4 |
| 42–<48 | 466 | 2.1 | 283 | 3.3 | 2400 | 5.5 | 2898 | 6.7 | 15 | 6.4 |
| 48–<54 | 559 | 2.5 | 371 | 4.3 | 2512 | 5.7 | 3505 | 8.1 | 9 | 3.8 |
| 54–<60 | 303 | 1.4 | 225 | 2.6 | 1747 | 4.0 | 2297 | 5.3 | 10 | 4.3 |
| ≥60 | 2032 | 9.1 | 1774 | 20.4 | 8453 | 19.3 | 14 479 | 33.6 | 121 | 51.7 |
| Total | 22 437 | 100.0 | 8706 | 100.0 | 43 853 | 100.0 | 43 034 | 100.0 | 234 | 100.0 |
AD, atopic dermatitis; AR, allergic rhinitis; EoE, eosinophilic esophagitis; IgE-FA, immunoglobulin E–mediated food allergy.
FIGURE 2.
Distribution of allergic condition incidence by age. Proportions of initial diagnoses of atopic dermatitis (AD), immunoglobulin E–mediated food allergy (IgE-FA), asthma, allergic rhinitis (AR), or eosinophilic esophagitis (EoE) by age intervals (months). For asthma, incidence before age 1 year was excluded as per the disease definition criteria.
TABLE 3.
Cumulative Incidence of Allergic Conditions
| Age Range (y) | AD | IgE-FA | Asthma | AR | EoE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| 0–<3 | 19 543 | 8.9 | 6336 | 2.9 | 31 141 | 14.3 | 22 753 | 10.4 | 94 | 0.04 |
| 0–<5 | 21 051 | 9.6 | 7412 | 3.4 | 38 312 | 17.5 | 32 982 | 15.1 | 139 | 0.06 |
| 0–<11 | 22 264 | 10.2 | 8500 | 3.9 | 43 219 | 19.8 | 42 154 | 19.3 | 210 | 0.10 |
| 0–<14 | 22 415 | 10.3 | 8665 | 4.0 | 43 778 | 20.0 | 42 930 | 19.6 | 229 | 0.10 |
| 0–18 | 22 437 | 10.3 | 8706 | 4.0 | 43 853 | 20.1 | 43 034 | 19.7 | 234 | 0.11 |
All reported percentages indicate the percentage of total subjects in the full cohort (n = 218 485) with the stated allergic disease. AD, atopic dermatitis; AR, allergic rhinitis; EoE, eosinophilic esophagitis; IgE-FA, immunoglobulin E–mediated food allergy.
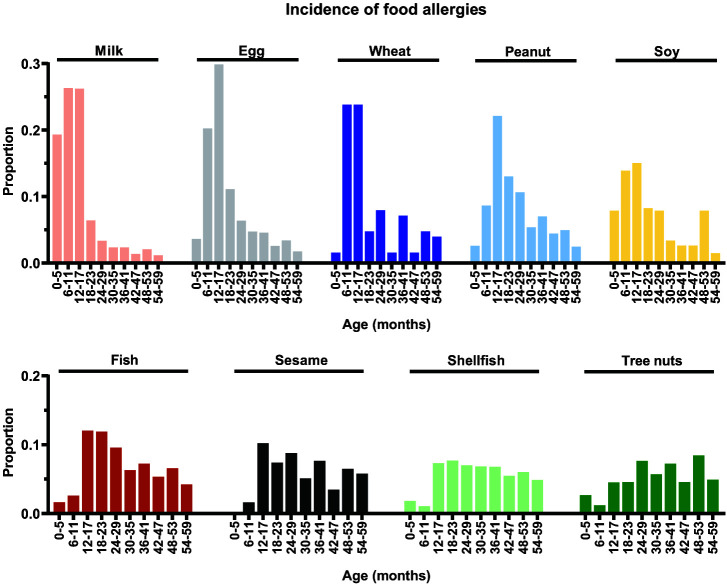
Age at Diagnosis, Incidence, and Cumulative Incidence of IgE-Mediated Food Allergies
We next determined age at diagnosis (Table 4; Supplemental Table 13), distribution of incidence rates (Fig 3), and cumulative incidence (Table 5; Supplemental Table 14) for individual IgE-FA allergens. The median (and interquartile range) age at diagnosis was 12 (7–19) months for milk, 16 (12–34) months for egg, 18 (11–49) months for wheat, 25 (15–49) months for peanut, 26 (13–65) months for soy, 40 (22–72) months for fish, 52 (28–83) months for sesame, 54 (30–86) months for shellfish, and 58 (34–86) months for tree nuts. The peak age of incidence was 12 months for milk, 13 months for egg and wheat, 15 months for peanut and soy, 22 months for fish, 26 months for sesame, 28 months for shellfish, and 52 months for tree nuts (Fig 3).
TABLE 4.
Age at Diagnosis of IgE-Mediated Food Allergies by Food Allergen
| Age Range (mo) | Egg | Milk | Soy | Wheat | Fish | Shellfish | Peanut | Tree Nut | Sesame | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 0–<6 | 62 | 3.6 | 196 | 19.3 | 21 | 7.9 | 2 | 1.6 | 12 | 1.6 | 24 | 1.8 | 109 | 2.6 | 33 | 2.7 | 1 | 0.2 |
| 6–<12 | 346 | 20.3 | 267 | 26.3 | 37 | 13.9 | 30 | 23.8 | 19 | 2.6 | 14 | 1.1 | 364 | 8.7 | 15 | 1.2 | 7 | 1.6 |
| 12–<18 | 510 | 29.9 | 266 | 26.2 | 40 | 15.0 | 30 | 23.8 | 88 | 12.0 | 96 | 7.3 | 930 | 22.1 | 56 | 4.5 | 44 | 10.2 |
| 18–<24 | 190 | 11.1 | 65 | 6.4 | 22 | 8.3 | 6 | 4.8 | 87 | 11.9 | 101 | 7.7 | 548 | 13.0 | 57 | 4.6 | 32 | 7.4 |
| 24–<30 | 109 | 6.4 | 34 | 3.4 | 21 | 7.9 | 10 | 7.9 | 70 | 9.6 | 92 | 7.0 | 447 | 10.6 | 95 | 7.6 | 38 | 8.8 |
| 30–<36 | 81 | 4.7 | 24 | 2.4 | 9 | 3.4 | 2 | 1.6 | 46 | 6.3 | 90 | 6.9 | 226 | 5.4 | 71 | 5.7 | 22 | 5.1 |
| 36–<42 | 78 | 4.6 | 24 | 2.4 | 7 | 2.6 | 9 | 7.1 | 53 | 7.3 | 89 | 6.8 | 295 | 7.0 | 90 | 7.2 | 33 | 7.6 |
| 42–<48 | 44 | 2.6 | 14 | 1.4 | 7 | 2.6 | 2 | 1.6 | 39 | 5.3 | 72 | 5.5 | 187 | 4.4 | 57 | 4.6 | 15 | 3.5 |
| 48–<54 | 58 | 3.4 | 21 | 2.1 | 21 | 7.9 | 6 | 4.8 | 48 | 6.6 | 79 | 6.0 | 208 | 4.9 | 105 | 8.4 | 28 | 6.5 |
| 54–<60 | 30 | 1.8 | 12 | 1.2 | 4 | 1.5 | 5 | 4.0 | 31 | 4.2 | 64 | 4.9 | 104 | 2.5 | 61 | 4.9 | 25 | 5.8 |
| ≥60 | 199 | 11.7 | 91 | 9.0 | 77 | 28.9 | 24 | 19.0 | 238 | 32.6 | 592 | 45.1 | 785 | 18.7 | 603 | 48.5 | 187 | 43.3 |
| Total | 1707 | 100.0 | 1014 | 100.0 | 266 | 100.0 | 126 | 100.0 | 731 | 100.0 | 1313 | 100.0 | 4203 | 100.0 | 1243 | 100.0 | 432 | 100.0 |
IgE, immunoglobulin E.
FIGURE 3.
Distribution of individual immunoglobulin E–mediated food allergy incidence by age. Proportions of initial diagnoses of individual food allergies for milk, egg, wheat, peanut, soy, fish, sesame, shellfish, and tree nuts by age intervals (months).
TABLE 5.
Cumulative Incidences of IgE-Mediated Food Allergies by Food Allergen
| Age Range (y) | Egg | Milk | Soy | Wheat | Fish | Shellfish | Peanut | Tree Nut | Sesame | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 0–<3 | 1420 | 0.65 | 890 | 0.41 | 164 | 0.07 | 91 | 0.04 | 414 | 0.19 | 578 | 0.26 | 3106 | 1.42 | 474 | 0.22 | 192 | 0.09 |
| 0–<5 | 1567 | 0.71 | 953 | 0.43 | 211 | 0.10 | 108 | 0.05 | 546 | 0.25 | 860 | 0.39 | 3666 | 1.67 | 789 | 0.36 | 287 | 0.13 |
| 0–<11 | 1685 | 0.77 | 1001 | 0.46 | 261 | 0.12 | 125 | 0.06 | 695 | 0.32 | 1220 | 0.56 | 4136 | 1.89 | 1151 | 0.52 | 399 | 0.18 |
| 0–<14 | 1702 | 0.78 | 1013 | 0.46 | 265 | 0.12 | 126 | 0.06 | 725 | 0.33 | 1294 | 0.59 | 4190 | 1.91 | 1236 | 0.56 | 427 | 0.19 |
| 0–18 | 1707 | 0.78 | 1014 | 0.46 | 266 | 0.12 | 126 | 0.06 | 731 | 0.33 | 1313 | 0.60 | 4205 | 1.92 | 1244 | 0.57 | 432 | 0.20 |
All reported percentages (%) indicate the percentage of total subjects in the full cohort (n = 218 485) with the stated food allergy. IgE, immunoglobulin E.
During the first year of life, the most frequently diagnosed IgE-FA allergens were milk, egg, and peanut (45.6%, 23.9%, and 11.3% of initial diagnoses, respectively) (Table 4). Peanut, egg, and milk were the most prevalent food allergens during the first 5 years of life (cumulative incidences of 1.7%, 0.7%, and 0.4%, respectively) (Table 5). For children older than age 5 years, the most frequently diagnosed food allergens were peanut, tree nuts, and shellfish (18.7%, 48.5%, and 45.1% of initial diagnoses, respectively). The food allergens with the highest cumulative incidence overall were peanut (1.9%), egg (0.8%), and shellfish (0.6%). Of children with documented food allergens, 37.4% had allergy to two or more distinct foods (Table 6; Supplemental Table 12). Age at diagnosis and cumulative incidence data for individual tree nuts are shown in Supplemental Table 13 and Supplemental Table 14, respectively.
TABLE 6.
Number of Documented Food Allergens in Subjects With IgE-Mediated Food Allergy
| Food Allergen(s) (n) | Subjects | |
|---|---|---|
| n | % | |
| 1 | 4290 | 62.6 |
| 2 | 1546 | 22.5 |
| 3 | 638 | 9.3 |
| 4 | 227 | 3.3 |
| ≥5 | 155 | 2.3 |
| Total | 6856 | 100.0 |
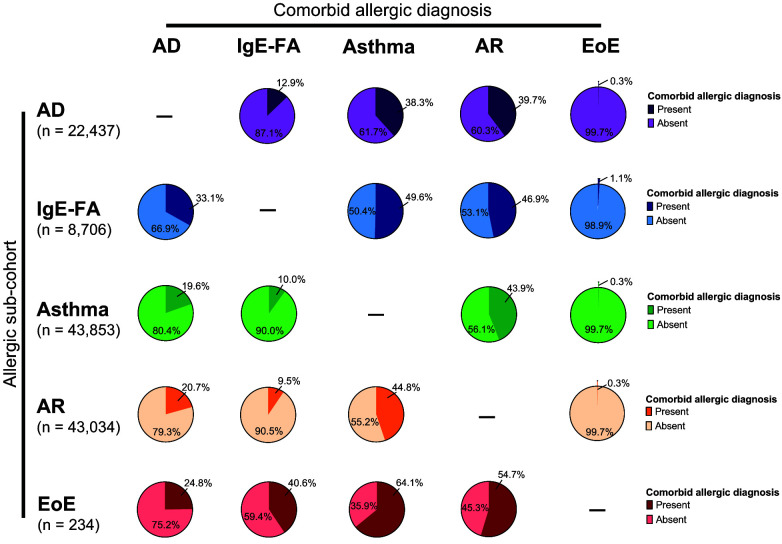
Allergic Comorbidities
Because a personal history of atopy is a risk factor for allergy progression in children, we next determined the portion of children with a specific allergy who were diagnosed with a comorbid allergic manifestation during the observation period (Fig 4). We found that the respiratory allergies (ie, asthma and AR) were markedly comorbid with each other (43.9% of asthma subjects had AR, and 44.8% of AR subjects had asthma) as well as with other allergic manifestations (38.3%–39.7% of AD subjects, 46.9%–49.6% of IgE-FA subjects, and 54.7%–64.1% of EoE subjects had asthma and/or AR). Of subjects with IgE-FA, 33.1% were also diagnosed with AD. Furthermore, comorbidity with EoE was notably higher in subjects with IgE-FA (1.1%) compared with other allergic subcohorts (0.3% for AD, asthma, and AR). Notably, 40.6% of those with EoE were also diagnosed with IgE-FA. In the entire birth cohort, 23.3%, 9.9%, 2.8%, 0.6%, and 0.01% of children had 1, 2, 3, 4, or 5 allergic manifestations, respectively (Supplemental Figure 5). Allergic multimorbidity for each allergic subcohort is shown in Supplemental Figure 5.
FIGURE 4.
Allergic comorbidity. Percentage of subjects in allergic subcohorts who did or did not develop a comorbid allergic diagnosis. AD, atopic dermatitis; IgE-FA, immunoglobulin E–mediated food allergy; AR, allergic rhinitis; EoE, eosinophilic esophagitis.
Discussion
Our analysis uses health care provider diagnosis data to inform our understanding of patterns of pediatric allergic disease across a multistate network of practices and health systems.23 As practices and health systems shift to exclusive electronic documentation, the EHR represents a compendium of medical data that can be leveraged to define large patient cohorts, detect disease patterns, and identify disease-related risk factors to guide clinical and public health interventions.18,19 To our knowledge, our study is the first to determine pediatric allergic disease patterns across multiple US pediatric practices and health systems. This cohort is also unique in that it includes longitudinal data entered by primary care providers including pediatricians, family physicians, and advanced practice providers practicing in urban, suburban, and rural sites.23 In using exclusively health care provider-based diagnostic data, our study minimizes bias (eg, recall bias) that is inherent to survey-based analyses, which rely on caregiver or patient-reported data.
The CER2 database represents a nonrandom sample of health systems making comparisons with nationally representative surveys challenging. However, the allergic disease rates we measured are in general agreement with previously reported rates. For example, the overall cumulative incidence of AD in our study is in line with previous reports,5,10,26,27 and the cumulative incidence of IgE-FA is within the lower end of previously reported rates.6,10,11,12 We speculate that the latter finding reflects our intentional exclusion of multiple non–IgE-mediated (nonanaphylactic) food allergies. These are sometimes conflated with IgE-FAs, which are the primary cause of anaphylaxis in the pediatric population and that necessitate treatment with epinephrine in the case of systemic reactions.12,28 For individual food allergens, our reported rates were dependent on a child’s age, consistent with the known natural histories of specific food allergies.29 In our cohort, peanut, egg, and shellfish were the most common food allergens overall. Of note, although milk was recently reported as the second most common allergen using survey-based methods,28 milk was fifth most common in our analysis, possibly because of our exclusion of non–IgE-mediated cow’s milk allergies and intolerances from our IgE-FA disease subcohort.
The cumulative incidence for asthma and AR aligns with figures reported in recent regional, EHR-based studies.5,6 Although the 2018 National Health Interview Survey estimates for the prevalence of hay fever, asthma, or respiratory allergy are 7.2% to 9.6%,10 considerably higher rates have been reported elsewhere, likely because of differences in definitions and populations studied. For example, studies of predominantly urban cohorts, like ours, have reported asthma rates as high as 25%.30 Likewise, AR prevalence as high as 43% has been reported.31,32 We note that the peak incidence of asthma in our cohort occurs during a period when 50% or more children may experience the clinically distinct and transient entity of reactive airway disease.33–35 Further, the majority of children with asthma in our cohort retained their asthma diagnosis in the last year of their observation period; however, the possibility that some subjects experienced transient wheeze cannot be excluded. With regard to rhinitis, given the common occurrence of rhinitis symptoms in children, it is possible that some clinicians might not formally code cases of mild disease. Last, our observed cumulative incidence of EoE is similar to recent reports.5,36 This finding, and the later peak age of diagnosis of EoE relative to other allergic conditions, are of particular clinical relevance because EoE is among the least recognized allergic morbidities.
Our study lays the foundation for large-scale, longitudinal study of pediatric allergic disease patterns and potential associated risk factors that are readily available in the EHR. This is especially important as risk of allergy is modified by a complex and incompletely understood interplay of genetics and environmental factors, such as early life exposures, climate, air pollutants, and exposure to indoor and outdoor aeroallergens.37–39 Although there is variability with respect to the number and sequence of allergic conditions that children develop over time at the individual level, prior population-level studies have shed light on predictable sequences of allergy, a paradigm known as the allergic march.37 In general, the incidence patterns in our study support the allergic march, with peak incidence of AD occurring first, consistent with the hypothesis that allergic sensitization via the skin is one mechanism by which allergy may develop later in life.37 Furthermore, more than 13% of children in our cohort had at least 2 allergic manifestations, with several remarkable comorbidity patterns: (1) asthma and AR (ie, respiratory allergies) were markedly comorbid with each other, as were IgE-FA and EoE (ie, food allergies); (2) respiratory allergies were markedly (>38%) comorbid with AD and with food allergies; and (3) particularly high rates of comorbidity (>24%) with all allergic conditions were seen in the EoE subcohort. These patterns are relevant to both the primary care and specialist settings, because they encourage maintenance of clinical suspicion for possible development of 1 or more allergies later in life in children who have a single allergic condition. Future studies are needed to quantify risk relationships and define high-risk allergy populations that may benefit from screening.
Because disproportionate rates of adverse health outcomes occur in children from specific racial and ethnic minority backgrounds, it is imperative that studies of disease patterns are attuned to the complex interplay of factors that underlie these differences; for example, racism embedded in housing, employment, and education can adversely impact access to and delivery of care.9,40,41 Although it was not possible to determine specific insurance types as a proxy for socioeconomic status, a larger limitation of our dataset is that it does not capture those children who ultimately could not access care. Although it is impossible to delineate specific causes of disparities, we recognize several notable trends among the allergic subcohorts. First, we observed higher representation of Black children within the AD and respiratory allergy (ie, asthma and AR) subcohorts, consistent with previously reported higher prevalence and severity of AD, aeroallergen sensitization, and asthma prevalence in Black compared with White children.10,42,43 With the exception of asthma, we also detected lower rates of food, skin, and respiratory allergy in Hispanic children compared with non-Hispanic White and non-Hispanic Black children, consistent with prior reports.10 Last, we observed a higher representation of White male children in the EoE subcohort though with notably higher rates of EoE in non-White subjects than previously reported.5,44 In light of evolving knowledge about differences in clinical symptoms and endoscopic features of EoE in White versus non-White racial groups,45 this could indicate that prior studies of EoE racial predominance were impacted by diagnostic and/or other biases. We note that, compared with the general US population, our cohort contains a higher representation of self-identified Black children (31.9% vs. 14%) and a lower representation of self-identified Hispanic children (10.2% vs. 26%), underscoring the caveat that this cohort is not nationally representative.46
There are additional limitations of our study that should be considered. First, diagnosis codes do not necessarily indicate the presence of disease but may rather reflect clinical suspicion. To minimize the effects of these limitations, we required at least 2 consecutive visits with a respective diagnosis code as well as prescriptions for relevant medications. We also note that in prior regional EHR-based studies, chart review of longitudinal medical records demonstrated good correlation between provider documentation and diagnosis code utilization.6 In addition, by requiring an observation period of at least 5 years, some children may be lost to follow-up. However, a sensitivity analysis of a 2-year observation period yielded comparable age-at-diagnosis and cumulative incidence findings, suggesting that subject loss is not a significant confounder. We further note that our requirement of a minimum 5-year observation period precludes rigorous analysis of annual incidence patterns, in particular during the final 5 years of the observation period (ie, 2016–2020). Although most allergic diagnoses considered (ie, AD, IgE-FA, asthma, and AR) are made based on clinical criteria that have been largely unchanged during our observation period,47–51 future studies should elucidate annual disease patterns, for example IgE-FA patterns following early food introduction guidelines.52
In conclusion, EHR-based clinical cohorts are a valuable tool for the study of allergy disease rates and clinical patterns. Our study builds on prior, region-limited EHR analyses to describe features of provider-diagnosed allergy in a multistate cohort of pediatric practices and health systems. Our results support the population-level allergic march progression, and reveal high rates of allergic comorbidity among children, including those with food and respiratory allergies. Although direct comparisons with nationally representative surveys cannot be performed, we note that allergic disease prevalence rates in our cohort were within previously reported ranges, with notably lower rates of IgE-FA and higher rates of asthma and AR. Relevant future directions for the field include developing nationally representative EHR-based cohorts and identifying potentially modifiable disparities in disease outcomes.
Supplementary Material
Acknowledgments
The authors thank Jonathan Spergel, MD, PhD, for critical reading and feedback on this manuscript.
Glossary
- AD
atopic dermatitis
- AR
allergic rhinitis
- CER2
Comparative Effectiveness Research through Collaborative Electronic Reporting
- EHR
electronic health record
- EoE
eosinophilic esophagitis
- IgE-FA
immunoglobulin E–mediated food allergy
Footnotes
Drs Gabryszewski and Hill conceptualized the study, performed data analysis, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Dudley and Drs Shu, Faerber, Grundmeier, and Fiks were involved in study design, data collection and analysis, and review and revision of the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: This work was supported by the National Institutes of Health (NIH; grants K08DK116668 and R01HL162715 to Dr Hill; T32DK0070665 and T32HD043021 to Dr Gabryszewski), the Pennsylvania Allergy and Asthma Association (Pennsylvania Allergy Educational and Research Fund Grant to Dr Gabryszewski), and a Children’s Hospital of Philadelphia Food AllergyPilot Award (to Drs Hill and Gabryszewski). Allergy research in the Hill laboratory is also supported by the Hartwell Foundation, the American Academy of Allergy Asthma and Immunology, the American College of Allergy Asthma and Immunology, the American Partnership for Eosinophilic Disorders, the Food Allergy Fund, and the Children’s Hospital of Philadelphia Research Institute. Additional infrastructure funding was provided by the American Academy of Pediatrics and the Health Resources and Services Administration (HRSA) of the US Department of Health and Human Services (HHS) under UA6MC15585 - National Research Network to Improve Children’s Health and U5DMC39344 - Pediatric Research Network Program. The contents are those of the authors and do not necessarily represent the official views of, nor an endorsement, by NIH, HRSA/HHS, or the US government. None of the funding sources had a role in the design or conduct of the study.
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1. Annesi-Maesano I, Fleddermann M, Hornef M, et al. Allergic diseases in infancy: I - epidemiology and current interpretation. World Allergy Organ J. 2021;14(11):100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patel DA, Holdford DA, Edwards E, Carroll NV. Estimating the economic burden of food-induced allergic reactions and anaphylaxis in the United States. J Allergy Clin Immunol. 2011;128(1):110–115 [DOI] [PubMed] [Google Scholar]
- 3. Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson LB, Arroyo AC, Faridi MK, Rudders SA, Camargo CA Jr. Trends in US hospitalizations for anaphylaxis among infants and toddlers: 2006 to 2015. Ann Allergy Asthma Immunol. 2021;126(2):168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. 2018;6(5):1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr. 2016;16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881–1887 [DOI] [PubMed] [Google Scholar]
- 8. Noordzij M, Dekker FW, Zoccali C, Jager KJ. Measures of disease frequency: prevalence and incidence. Nephron Clin Pract. 2010;115(1):c17–c20 [DOI] [PubMed] [Google Scholar]
- 9. Davis CM, Apter AJ, Casillas A, et al. Health disparities in allergic and immunologic conditions in racial and ethnic underserved populations: a Work Group Report of the AAAAI Committee on the Underserved. J Allergy Clin Immunol. 2021;147(5):1579–1593 [DOI] [PubMed] [Google Scholar]
- 10. National Center for Health Statistics . Interactive summary health statistics for children. Available at: https://www.cdc.gov/nchs/nhis/KIDS/www/index.htm. Accessed March 29, 2022
- 11. Liu AH, Jaramillo R, Sicherer SH, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2010;126(4):798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta RS, Springston EE, Warrier MR, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–e17 [DOI] [PubMed] [Google Scholar]
- 13. Dunlop JH, Keet CA. Epidemiology of food allergy. Immunol Allergy Clin North Am. 2018;38(1):13–25 [DOI] [PubMed] [Google Scholar]
- 14. Lopes JP, Sicherer S. Food allergy: epidemiology, pathogenesis, diagnosis, prevention, and treatment. Curr Opin Immunol. 2020;66:57–64 [DOI] [PubMed] [Google Scholar]
- 15. Starr S. Survey research: we can do better. J Med Libr Assoc. 2012;100(1):1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soller L, Ben-Shoshan M, Harrington DW, et al. Adjusting for nonresponse bias corrects overestimates of food allergy prevalence. J Allergy Clin Immunol Pract. 2015;3(2):291–293.e2 [DOI] [PubMed] [Google Scholar]
- 17. Greiwe J, Oppenheimer J, Bird JA, Fleischer DM, Pongracic JA, Greenhawt M; AAAAI Adverse Reactions to Foods Committee . AAAAI Work Group Report: Trends in Oral Food Challenge Practices Among Allergists in the United States. J Allergy Clin Immunol Pract. 2020;8(10):3348–3355 [DOI] [PubMed] [Google Scholar]
- 18. Sutherland SM, Kaelber DC, Downing NL, Goel VV, Longhurst CA. Electronic health record-enabled research in children using the electronic health record for clinical discovery. Pediatr Clin North Am. 2016;63(2):251–268 [DOI] [PubMed] [Google Scholar]
- 19. Birkhead GS, Klompas M, Shah NR. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 2015;36:345–359 [DOI] [PubMed] [Google Scholar]
- 20. Casey JA, Schwartz BS, Stewart WF, Adler NE. Using electronic health records for population health research: a review of methods and applications. Annu Rev Public Health. 2016;37:61–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fulton RL, Mitra N, Chiesa-Fuxench Z, Sockler PG, Margolis DJ. Untapping the potential of utilizing electronic medical records to identify patients with atopic dermatitis: an algorithm using ICD-10 codes. Arch Dermatol Res. 2022;314(5):439–444 [DOI] [PubMed] [Google Scholar]
- 22. Singer AG, Kosowan L, Nankissoor N, Phung R, Protudjer JLP, Abrams EM. Use of electronic medical records to describe the prevalence of allergic diseases in Canada. Allergy Asthma Clin Immunol. 2021;17(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiks AG, Grundmeier RW, Steffes J, et al. ; Comparative Effectiveness Research Through Collaborative Electronic Reporting (CER2) Consortium . Comparative effectiveness research through a collaborative electronic reporting consortium. Pediatrics. 2015;136(1):e215–e224 [DOI] [PubMed] [Google Scholar]
- 24. Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine - a time for reckoning with racism. N Engl J Med. 2021;384(5):474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. U.S. Department of Agriculture . 2010 Rural-Urban Commuting Area (RUCA) Codes. Available at: https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation/. Accessed March 9, 2023
- 26. Adams PF, Kirzinger WK, Martinez M. Summary health statistics for the U.S. population: National Health Interview Survey, 2012. Vital Health Stat 10. 2013;(259):1–95 [PubMed] [Google Scholar]
- 27. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123(2):173–178.e1 [DOI] [PubMed] [Google Scholar]
- 28. Gupta RS, Warren CM, Smith BM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142(6):e20181235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savage J, Sicherer S, Wood R. The natural history of food allergy. J Allergy Clin Immunol Pract. 2016;4(2):196–204 [DOI] [PubMed] [Google Scholar]
- 30. Clark NM, Brown R, Joseph CLM, et al. Issues in identifying asthma and estimating prevalence in an urban school population. J Clin Epidemiol. 2002;55(9):870–881 [DOI] [PubMed] [Google Scholar]
- 31. Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 suppl):S43–S70 [DOI] [PubMed] [Google Scholar]
- 32. Wright AL, Holberg CJ, Martinez FD, Halonen M, Morgan W, Taussig LM. Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics. 1994;94(6 Pt 1):895–901 [PubMed] [Google Scholar]
- 33. Martinez FD. New insights into the natural history of asthma: primary prevention on the horizon. J Allergy Clin Immunol. 2011;128(5):939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al-Shamrani A, Bagais K, Alenazi A, Alqwaiee M, Al-Harbi AS. Wheezing in children: approaches to diagnosis and management. Int J Pediatr Adolesc Med. 2019;6(2):68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinez FD. What have we learned from the Tucson Children’s Respiratory Study? Paediatr Respir Rev. 2002;3(3):193–197 [DOI] [PubMed] [Google Scholar]
- 36. Cianferoni A, Warren CM, Brown-Whitehorn T, Schultz-Matney F, Nowak-Wegrzyn A, Gupta RS. Eosinophilic esophagitis and allergic comorbidities in a US-population-based study. Allergy. 2020;75(6):1466–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gabryszewski SJ, Hill DA. One march, many paths: Insights into allergic march trajectories. Ann Allergy Asthma Immunol. 2021;127(3):293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cecchi L, D’Amato G, Annesi-Maesano I. External exposome and allergic respiratory and skin diseases. J Allergy Clin Immunol. 2018;141(3):846–857 [DOI] [PubMed] [Google Scholar]
- 39. Zhang Y, Steiner AL. Projected climate-driven changes in pollen emission season length and magnitude over the continental United States. Nat Commun. 2022;13(1):1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warren CM, Brown E, Wang J, Matsui EC. Increasing representation of historically marginalized populations in allergy, asthma, and immunologic research studies: challenges and opportunities. J Allergy Clin Immunol Pract. 2022;10(4):929–935 [DOI] [PubMed] [Google Scholar]
- 41. Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013; (121):1–8 [PubMed] [Google Scholar]
- 42. Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29(6):749–755 [DOI] [PubMed] [Google Scholar]
- 43. Wegienka G, Johnson CC, Zoratti E, Havstad S. Racial differences in allergic sensitization: recent findings and future directions. Curr Allergy Asthma Rep. 2013;13(3):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Franciosi JP, Tam V, Liacouras CA, Spergel JM. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7(4):415–419 [DOI] [PubMed] [Google Scholar]
- 45. Moawad FJ, Dellon ES, Achem SR, et al. Effects of race and sex on features of eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2016;14(1):23–30 [DOI] [PubMed] [Google Scholar]
- 46. POP3 Race and Hispanic Origin Composition . Percentage of U.S. children ages 0–17 by race and Hispanic origin, 1980–2021 and projected 2022–2050. Available at: https://www.childstats.gov/americaschildren/tables/pop3.asp. Accessed October 30, 2022
- 47. Li JT, Pearlman DS, Nicklas RA, et al. Algorithm for the diagnosis and management of asthma: a practice parameter update: Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81(5):415–420 [DOI] [PubMed] [Google Scholar]
- 48. Sampson HA, Aceves S, Bock SA, et al. ; Joint Task Force on Practice Parameters; Practice Parameter Workgroup . Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134(5):1016–25 [DOI] [PubMed] [Google Scholar]
- 49. Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131(2):295–299 [DOI] [PubMed] [Google Scholar]
- 50. Dykewicz MS, Wallace DV, Amrol DJ, et al. ; Chief Editor(s); Joint Task Force on Practice Parameters; Workgroup Contributors . Rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146(4):721–767 [DOI] [PubMed] [Google Scholar]
- 51. Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC), Cloutier MM, Baptist AP, et al. 2020 Focused Updates to the Asthma Management Guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146(6):1217–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Togias A, Cooper SF, Acebal ML, et al. Addendum Guidelines for the Prevention of Peanut Allergy in the United States: summary of the National Institute of Allergy and Infectious Diseases-Sponsored Expert Panel. J Acad Nutr Diet. 2017;117(5):788–793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.