Abstract
Neuroprotective factors are involved in brain functioning. Although physical exercise has been shown to have a positive influence on these factors, the effect of resistance exercise on them is not well known. This systematic review and meta-analysis aimed to 1) estimate the efficacy of resistance exercise on major neuroprotective factors, such as insulin-like growth factor-1 (IGF-1), brain-derived neurotrophic factor (BDNF), and vascular endothelial growth factor (VEGF), in middle and late life and 2) determine whether the effect is dose dependent. A systematic search was conducted in CINAHL, Cochrane CENTRAL, MEDLINE, Scopus, PEDro, SPORTDiscus, and Web of Science up to November 2022. Random effects models were used to estimate standardized mean differences (SMDs) and their respective 95% confidence intervals (CI) for the effect of resistance exercise on peripheral IGF-1, BDNF or VEGF levels in older adults. Thirty randomized clinical trials with 1247 subjects (53.25% women, 45-92 years) were included in the systematic review, and 27 were selected for the meta-analysis. A significant effect of resistance exercise on IGF-1 levels was observed (SMD: 0.48; 95% CI: 0.27, 0.69), being more effective when performing 3 sessions/week (SMD: 0.55; 95% CI: 0.31, 0.79) but not on BDNF (SMD: 0.33; 95% CI: -0.29, 0.94). The effect on VEGF could not be determined due to the scarcity of studies. Our data support the resistance training recommendation in middle and late life, at a frequency of at least 3 sessions/week, to mitigate the neurological and cognitive consequences associated with aging, mainly through IGF-1.
Keywords: brain-derived neurotrophic factor, insulin-like growth factor type 1, vascular endothelial growth factor, strength exercise, neuroplasticity
1. Introduction
Aging is a natural physiological process associated with cellular and synaptic changes at the brain level related to cognitive processes. Cognitive decline is a slow process that begins in middle life [1] and this is characterized by a loss of volume in the hippocampus, changes in white matter and atrophy and a decrease in gray matter in the prefrontal cortex [2,3], in addition to a reduction in neuroprotective factors [4]. The main substances of a protein nature include insulin-like growth factor type 1 (IGF-1), brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), which, combined with other hormones and neurotransmitters, have an important role in cell proliferation and growth as well as in neuronal development and function [4-6].
IGF-1 is a peptide that regulates the effects of growth hormones, and BDNF belongs to the neurotrophin family. Both are essential proteins in brain development and tissue remodelling [7,8]. They provide great benefits to cognition due to their effects on neuroplasticity [5,9]. Moreover, these factors are expressed in some regions of the central nervous system specific to cognition, supporting the idea that their decrease causes cognitive impairment [5,8]. Finally, VEGF, like BDNF, is a neurotrophin with some neuroprotective effect [10]. It is an angiogenic factor that has the capacity to preserve brain cells and slow the deterioration of spatial memory and cognitive impairment [10-12]. There is sufficient evidence to support that physical exercise has a positive influence on the release of neuroprotective factors and their cerebral effect by increasing their expression in the central nervous system [13]. Considering that these are peptides that cross the blood–brain barrier, the elevation of their peripheral levels as a consequence of exercise has been reported to favor learning, neurogenesis and angiogenesis [14].
The effect of resistance exercise on these factors has not been sufficiently studied in older adults, and the literature thus far has shown inconclusive results for VEFG [16,17], while they seem to be more consistent for IGF-1[18] and BDNF [19]. On the other hand, the importance of training parameters such as exercise frequency or intensity in enhancing neuroprotective factors has been described [20], although the magnitude of influence of these training characteristics has not been sufficiently quantified. Therefore, the objectives of this systematic review and meta-analysis were 1) to update and synthesize the available evidence regarding the effect of resistance exercise on key neuroprotective factors at the peripheral level in middle and late life and 2) to determine whether the effect depends on exercise dose.
2. Methods
The present systematic review and meta-analysis was conducted following the recommendations of the Cochrane Handbook of Systematic Reviews of Interventions [21], and the standards for systematic reviews and meta-analyses of the PRISMA Statement [22] were followed. This review was registered in the PROSPERO database (registration number: CRD420223 02859).
2.1. Search strategy
A systematic search was conducted in the following bibliographic databases: CINAHL (via EBSCOhost), Cochrane Central Register of Controlled Trials, MEDLINE (via PubMed), Embase (via Scopus), Physiotherapy Evidence Database (PEDro), SPORTDiscus (via EBSCOhost) and Web of Science from inception up to November 2022. Search terms included "strength exercise", "resistance exercise", "strength training", "resistance training", "weight training", "weightlifting", “IGF-1”, "insulin-like growth factor 1", “BDNF”, "brain-derived neurotrophic factor", “VEGF”, "vascular endothelial growth factor" and “random* control* trials”. The references of the included studies were also reviewed. The search strategy in the different databases can be found in Supplementary Table 1.
In addition, it was necessary to contact four authors [16,23-25], obtaining a response from only one of them because the data required to carry out the meta-analysis could not be obtained from the articles.
The systematic search was performed independently by two reviewers (E.R.G. and A.T.C.). When there were disagreements, a third researcher made the final decision (V.M.V.).
2.2. Eligibility criteria
The inclusion criteria for the systematic review and meta-analysis were as follows: 1) participants: adults with a mean age ≥ 45 years; 2) intervention: resistance exercise (minimum 1 session); 3) comparator: control group; and 4) outcome: concentration of BDNF, IGF-1 or VEGF in serum and/or plasma. Furthermore, the exclusion criteria were as follows: 1) studies other than randomized clinical trials (RCTs); 2) subjects with cognitive impairment.
Two independent reviewers (E.R.G. and A.T.C.) conducted the study selection. When there were disagreements, a third researcher made the final decision (V.M.V.).
2.3. Data extraction
The full texts of the included studies were reviewed, and the main data were independently extracted from the included studies by 2 reviewers (E.R.G. and A.T.C.) and synthesized in an ad hoc table including 1) author's name, 2) year of publication, 3) country, 4) population characteristics (final number of participants in each group, proportion of women, age of participants, health status), 5) intervention characteristics (type of intervention in each group, frequency, duration, intensity and volume) and 6) outcome (IGF-1, BDNF or VEGF levels in plasma and/or serum). A third reviewer (V.M.V.) was consulted to resolve disagreements between reviewers.
Continuous data were extracted from the studies (including prepost mean IGF-1, BDNF and VEGF values, standard deviation and sample size of the intervention and control groups).
For statistical analysis, all IGF-1, BDNF and VEGF values were transformed to the same unit (ng/mL) (where 1 ng/mL = 1000 pg/mL).
2.4. Risk of bias assessment
Two reviewers (E.R.G. and A.T.C.) independently assessed the risk of bias of the included studies using the Cochrane Risk of Bias Tool for Randomized Clinical Trials (RoB 2.0) [21]. Any discrepancies were resolved by a third reviewer (V.M.V.). This tool consists of an assessment based on the following domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of reported outcome. Each of these domains can be assessed as "low risk of bias", "some concerns" and "high risk of bias". Therefore, the overall risk for each of the studies was classified as "low risk of bias" when a low risk of bias was determined for all domains; "unclear risk of bias" when at least one domain had unclear risk but no high risk of bias for any specific domain; and "high risk of bias" when at least one domain was assessed as high risk of bias or as unclear risk of bias in multiple domains [26].
2.5. Data analysis
The estimated pooled standardized mean differences (SMDs) of the mean differences for IGF-1, BDNF and VEGF and their 95% confidence intervals (CIs) were calculated using Cohen’s d index [21]. When repeated measurements were reported, we considered only the last measurement as the end point. When a study had two intervention groups that performed resistance exercise, they were taken into account as different studies in the analysis of the results.
A meta-analysis for each factor was performed using a random-effects model with the DerSimonian and Laird method [27] to determine the effect of resistance exercise on neuroprotective factors compared to a control group. Heterogeneity of results between studies was assessed using the I2 statistic, which is classified as unimportant (0% to 30%), moderate (30% to 50%), substantial (50% - 75%) and high (75% - 100%). The corresponding p values were also considered [21]. As recommended by the Cochrane Handbook, when data on standard deviation were not reported, they were estimated using the standard error, the CI or a statistical test (t test, F test or a p value) [21].
A sensitivity analysis was performed to determine the robustness of the estimates by eliminating each study included in the meta-analysis one by one, as well as studies in which the population had any specific health disorder or pathology, to determine whether any represented a large proportion of heterogeneity in the pooled ES. For neuroprotective factors where a significant difference was found after resistance exercise, the dose–response relationship was estimated by subgroup analysis according to frequency (days/week), sets, exercise intensity, considering high intensity >10 repetitions maximum (RM)(28) and ≥70% RM [29] and light-moderate intensity ≤10RM and <70% RM and those studies in which indicate that they perform light and/or moderate intensity exercises, the session time (minutes) of the intervention and duration of the exercise program (months). We also conducted a subgroup analysis by sex and a meta-regression model to determine the influence of body mass index and age on this association.
Publication bias was evaluated using Egger's regression asymmetry test, with p values less than 0.10 considered statistically significant. STATA Statistical software, version 16 (StataCorp LLC, College Station, TX, USA) was used to perform the statistical analyses.
3. Results
3.1. Study selection
The flow chart with the study selection procedure of the systematic review and meta-analysis is shown in detail in Fig. 1. A total of 1066 studies were found (Supplementary Fig. 1), and 179 potentially includable studies were identified through the title and abstract, of which 30 RCTs [17,23-25,30-55] met the eligibility criteria and were included in the systematic review; of these, 27 were included in the meta-analysis [17,23,30-38,40-55] (3 were excluded because no data were available).
Figure 1.
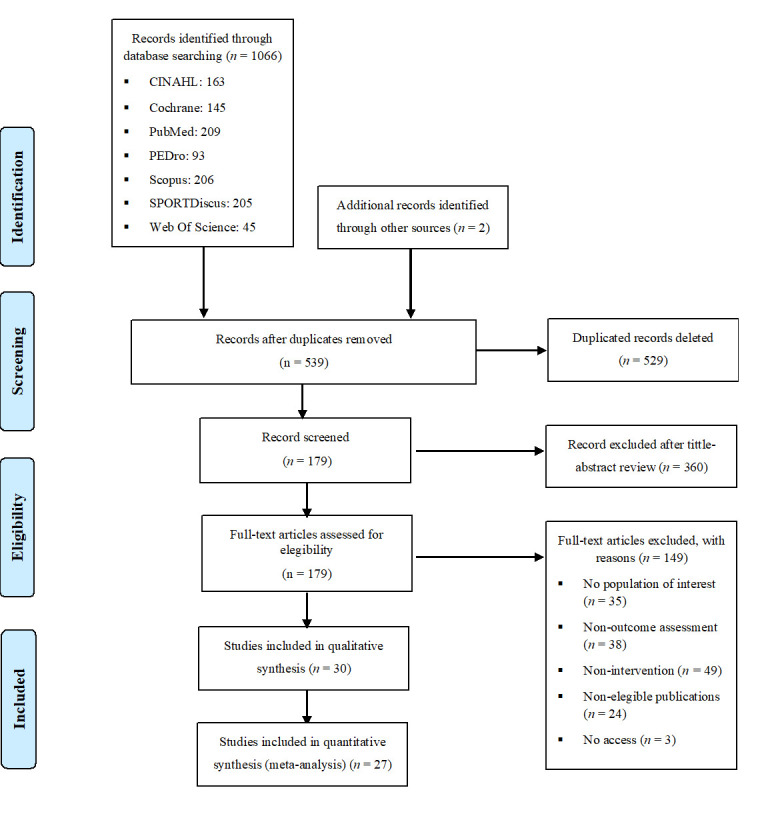
Flow diagram of the studies through the review.
3.2. Study and intervention characteristics
The most significant characteristics of the studies analysed in this systematic review are shown in Table 1. A total of 30 RCTs [16,23-25,30-48] published between 1994 and 2021 involving 1247 subjects (664 women; 53.25%) aged 45 to 92 years were included in the systematic review. The RCTs were conducted in the following continents: Oceania [39], Asia [17,32,33, 42, 44,48,50], Europe [24,31,41,43,45,46,52] and America [23, 25, 30,34-38,40,47,49,53-55]. In addition, 22 studies were performed in a healthy population, described as individuals without specific health disorders or pathologies [23,24,30-32,34-38,40,42-47,49-51,53,55] and 8 in populations suffering from specific diseases or health disorders such as sarcopenic obesity [33], type 2 diabetes [39], limited mobility [41], low bone mineral density [25], hypertension [48], coronary heart disease [17], chronic kidney disease [54] and fibromyalgia [52].
Table 1.
General characteristics of the studies
| Study Characteristics | Population characteristics | Intervention characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | n (female) | Age, years | Health status | Intervention | Frequency | Duration | Intensity and volume | Outcome |
| Arazi et al, (2021) (29) | Iran | IG-1: 10 (0) IG-2: 10 (0) CG: 10 (0) |
IG-1: 60.8 ± 1.8 IG-2: 60.7 ± 1.7 GC: 60.9 ± 0.9 |
Healthy | IG-1: resistance exercise IG-2: aerobic exercise CG: no exercise |
1 day | 1 day | 45 min, 2 sets x 10 rep, 65-70% 1RM | BDNF IGF-1 (serum) |
| Bagheri et al (2021) (51) | NR | IG: 10 (10) CG: 10 (10) |
56 ± 3.7 | Healthy, Sedentary | IG: resistance exercise CG: no exercise |
3 days/ week |
8 weeks | 3-4 sets x 10-12 rep, 60-75% 1RM | IGF-1 (serum) |
| Benitalebi, et al (2020) (50) | Iran | IG: 10 (10) CG: 9 (9) |
67.35 ± 1.4 | Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
12 weeks | 50 min, 2-3 sets x 8-16 rep, 40-75% 1RM | IGF-1 (serum) |
| Bermon et al, (1999) (43) | France | IG: 16 (NR) CG: 16 (NR) |
IG: 70.1 ± 1 CG: 70.5 ± 0.9 |
Healthy, Sedentary | IG: resistance exercise CG: no exercise |
3 days/ week | 8 weeks | 3 sets x 8 rep, 80% 1RM | IGF-1 (plasma) |
| Cassilhas et al, (2010) (32) | Brazil | IG: 20 (0) CG: 23 (0) |
IG: 68.4 ± 0.67 CG: 67.04 ± 0.54 |
Healthy, Sedentary | IG: resistance exercise GC: warm-up and stretching |
3 days/ week |
24 weeks | 1 hour, 2 sets x 8 rep, 80% 1RM | IGF-1 (serum) |
| Cassilhas et al, (2007) (31) | Brazil | IG-1: 20 (0) IG-2: 19 (0) CG: 23 (0) |
IG-1: 68.4 ± 0.67 IG-2: 69.01 ± 1.1 CG: 67.04 ± 0.54 |
Healthy, Sedentary | IG-1: high intensity resistance exercise IG-2: low intensity resistance exercise CG: warm-up and stretching |
3 days/ week |
24 weeks | IG-1: 1 hour, 2 sets x 8 rep, 80% 1RM. IG-2: 1 hour, 2 sets x 8 rep, 50% 1RM. |
IGF-1 (serum) |
| Chen et al, (2017) (30) | China | IG-1: 15 (12) IG-2: 15 (14) IG-3: 15 (11) CG: 15 (13) |
IG-1: 68.9 ± 4.4 IG-2: 69.3 ± 3.0 GI-3: 68.5 ± 2.7 GC: 68.6 ± 3.1 |
Sarcopenic obesity | IG-1: resistance exercise IG-2: aerobic exercise IG-3: combined exercise (resistance + aerobic) CG: no exercise |
2 days/ week |
12 weeks | IG-1: 1 hour, 3 sets x 8-12 rep, 60-70% 1RM. IG-2: 1-hour, different exercises of moderate intensity. IG-3: Each day they perform a different exercise program. |
IGF-1 (serum) |
| Chen et al, (2019) (17) | China | IG-1: 19 (8) CG: 18 (7) |
IG-1: 62.84 ± 5.54 CG: 65.89 ± 5.51 |
Coronary heart disease | IG: isometric resistance exercise CG: no exercise |
2 sessions per day, 5 days/ week |
3 months | 1 set x 10 rep, 40-50% 1RM | VEGF (serum) |
| Coelho-Junior et al, (2020) (44) | Brazil | IG-1: 10 (10) IG-2: 12 (12) CG: 14 (14) |
IG-1: 67 ± 6.2 GI-2: 66.7 ± 5.1 GC: 66.7 ± 4.6 |
Healthy | IG-1: traditional resistance exercise IG-2: resistance-power exercise with an elastic band CG: no exercise |
2 days/ week |
22 weeks | IG-1: 1 hour, 3 sets x 8-10 rep, intensity 5-6 (Borg scale). IG-2: 1 hour, 3 sets x 8-10 rep at intensity 3 (Borg scale) performing concentric phase as fast as possible. |
BDNF (serum) |
| Copeland et al, (2014) (55) | Canada | IG-1: 16 (16) CG: 16 (16) |
IG-1: 53.8 ± 5.85 CG: 56.6 ± 5.6 |
Healthy, Sedentary | IG: resistance exercise CG: no exercise |
3 days/ week |
12 weeks | 2-3 sets x 10 rep, 10RM | IGF-1 (serum) |
| Cunha et al, (2020) (33) | Brazil | IG-1: 21 (21) IG-2: 20 (20) CG: 21 (21) |
IG-1: 70.09 ± 5.95 GI-2: 68.6 ± 4.44 GC: 68.04 ± 4.38 |
Healthy | IG-1: resistance exercise IG-2: resistance exercise CG: no exercise |
3 days/ week |
12 weeks | IG-1: 15 min, 1 set x 10-15RM IG-2: 45 min, 3 sets x 10-15RM |
IGF-1 (NR) |
| Daly et al, (2005) (36) | Australia | IG: 14 (NR) CG: 12 (NR) |
IG: 67.6 ± 5.2 GC: 66.9 ± 5.3 |
Diabetes type 2, Sedentary, Overweight | IG: resistance exercise CG: stretching |
3 days/week | 12 months | 55 min, 3 sets x 8-10 rep, 75-85% 1RM (1-6 months) and 60-80% 1RM (6-12 months at home). | IGF-1 (NR) |
| Deus et al, (2021) (54) | Brazil | IG: 81 (NR) CG: 76 (NR) |
IG: 67.27 ± 3.24 GC: 66.33 ± 3.88 |
Chronic kidney disease | IG: resistance exercise CG: no exercise |
3 days/ week |
6 months | 1 hour, 3 sets, 8-12 rep | BDNF (serum) |
| Fragala et al, (2014) (34) | United States | IG: 13 (NR) CG: 12 (NR) |
IG: 70.64 ± 6.11 GC: 70.64 ± 6.11 |
Healthy | IG: resistance exercise CG: no exercise |
2 days/ week |
6 weeks | 3 sets x 8-15 rep, moderate intensity (5-6 OMNI scale) | BDNF (serum) |
| Hofmann et al, (2016) (42) | Austria | IG-1: 26 (26) IG-2: 21 (21) CG: 23 (23) |
GI-1: 82.9 (71.7 - 92.2) GI-2: 83.9 (65 - 92.2) GC: 84.5 (69.4 - 91.8) |
Healthy | IG-1: resistance exercise IG-2: resistance exercise + nutrition CG: cognitive training |
2 days/week | 6 months | IG-1 y IG-2: 60 min, 1-2 sets x 15rep, adapting the resistance of the elastic band. CG: memory and coordination activities |
IGF-1 (serum) |
| Hvid et al, (2017) (38) | Denmark | IG: 20 (NR) CG: 22 (NR) |
IG: 82.7 ± 5.4 CG: 82.2 ± 4.5 |
Limited mobility | IG: resistance-power exercise CG: no exercise |
2 days /week |
12 weeks | 3 sets x 8-10 rep, 70-80% 1RM performing concentric phase as fast as possible. | BDNF (serum) |
| Nunes et al, (2019) (49) | Brazil | IG-1: 12 (12) IG-2: 10 (10) CG: 12 (12) |
IG-1: 59.7 (55.9 - 63.5) IG-2: 64.2 (58.4 - 69.9) CG: 59.0 (55.4 - 62.5) |
Healthy | IG-1: resistance exercise IG-2: resistance exercise CG: no exercise |
3 days/ week |
16 weeks | IG-1: 90 min, 3-6 sets x 8.12 rep, 70% 1RM IG-2: 45 min, 3 sets x 8.12 rep, 70% 1RM |
IGF-1 (NR) |
| Orsatti et al, (2008) (53) | Brazil | IG: 21 (21) CG: 22 (22) |
IG: 57.8 ± 8.0 CG: 59.3 ± 6.2 |
Healthy, Sedentary | GI: resistance exercise CG: no exercise |
3 days/ week |
16 weeks | 50-60 min, 1-3 sets, 8-15 rep, 60-80% 1RM | IGF-1 (serum) |
| Parkhouse et al, (2000) (24) | Canada | IG: 13 (13) CG: 9 (9) |
IG: 67 ± 1 GC: 70 ± 2 |
Low bone mineral density, Sedentary | GI: resistance exercise CG: no exercise |
3 days/ week |
8 months | 3 sets x 8-10 rep, 75-80% 1RM | IGF-1 (serum) |
| Pyka et al, (1993) (22) | United States | IG: 8 (NR) CG: 6 (NR) |
GI: 69.6 ± 1.1 GC: 69.6 ± 1.1 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
52 weeks | 3 sets x 8 rep, 65-75% 1RM | IGF-1 (serum) |
| Ruiz et al, (2015) (40) | Spain | IG: 20 (16) CG: 20 (16) |
IG: 92.3 ± 2.3 CG: 92.1 ± 2.3 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
8 weeks | 40-45 min, 1-3 sets x 8-10 rep, 30-70% 1RM | BDNF (serum) |
| Sartorio et al, (2015) (23) | Italy | IG: 16 (0) CG:14 (0) |
IG: 72.9 ± 0.95 CG: 73.3 ± 1.04 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
16 weeks | 6 sets x 10 rep, 50-80% 1RM (MMII) and 40-65% (MMSS). | IGF-1 (NR) |
| So et al, (2013) (39) | South Korea | IG: 18 (12) CG: 22 (15) |
IG: 71.6 ± 5.5 CG: 68.4 ± 5.8 |
Healthy | IG: resistance exercise with elastic bands CG: no exercise |
3 days/ week |
12 weeks | 60 min, 2-3 sets x 15-25 rep with red elastic band (low intensity). | IGF-1 (serum) |
| Son et al, (2020) (45) | South Korea | IG: 10 (10) CG: 10 (10) |
IG: 67.8 ± 1.1 CG: 67.6 ± 1.3 |
Stage 1 hypertension, Sedentary | IG: resistance exercise CG: no exercise |
3 days/week | 12 weeks | 60 min, 2-4 sets x 10-20 rep, 40-70% 1RM. | IGF-1 (plasma) |
| Tomelari et al, (2020) (37) | Brazil | IG-1: 15 (15) IG-2: 14 (14) CG: 15 (15) |
GI-1: 71.4 ± 6 GI-2: 69.7 ± 5.7 CG: 68.6 ± 5.1 |
Healthy | IG-1: mono-joint resistance exercise IG-2: multijoint resistance exercise CG: no exercise |
3 days/ week |
12 weeks | 3 sets x 10-15RM. | IGF-1 (serum) |
| Tsai et al, (2015) (41) | China | IG: 21 (0) CG: 20 (0) |
IG: 66.05 ± 6.64 CG: 64.5 ± 6.95 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
12 months | 60 min, 3 sets x 10 rep, 75-80% 1RM. | IGF-1 (serum) |
| Urzi et al, (2019) (28) | Eslovenia | IG: 11 (11) CG: 9 (9) |
IG: 84.4 ± 7.7 CG: 88.9 ± 5.3 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
12 weeks | 45-50 min, 1-3 sets x 5-12 rep, mild-moderate intensity. | BDNF (plasma) |
| Vale et al, (2017) (27) | Brazil | IG-1: 10 (10) IG-2: 10 (10) CG: 10 (10) |
GI-1: 66.1 ± 2.77 IG-2: 67.1 ± 3.54 GC: 68.8 ± 5.41 |
Healthy | IG-1: resistance exercise on land IG-2: resistance exercise in water CG: no exercise |
3 days/ week |
12 weeks | IG-1: 50 min, 3 sets x 15 rep, 50% 1RM (1-4 week) y 3 sets x 8-10 rep, 75-85% 1RM (5-12 week). IG-2: 50 min, 3 sets x 15-20 rep without aquatic accessories (1-4 week) y 3 sets x 8-10 rep aquatic accessories (5-12 week). |
IGF-1 (serum) |
| Vale et al, (2014) (35) | Brazil | IG: 14 (14) CG: 10 (10) |
IG: 69 ± 5.1 CG: 69 ± 5.9 |
Healthy | IG: resistance exercise CG: no exercise |
3 days/ week |
20 weeks | 50 min, 2-3 sets x 15-20 rep, mild-moderate intensity (3-5 on OMNI-RES scale) | IGF-1 (serum) |
| Valkeinen et al, (2005) (52) | Finland | IG: 13 (13) CG: 13 (13) |
IG: 60 ± 2 CG: 59 ± 4 |
Fibromyalgia | IG: resistance exercise CG: no exercise |
2 days/ week |
21 weeks | 10-20 rep, 40-80% 1RM | IGF-1 (serum) |
Abbreviations: BDNF = brain-derived neurotrophic factor, CG = control group, IG = intervention group, IGF-1 = insulin-like growth factor type 1, min = minutes, MMII = lower limbs, MMSS = upper limbs, NR = No reported, OMNI-RES = OMNI-Resistance Exercise Scale, rep = repetition, RM = maximum repetition, VEGF = vascular endothelial growth factor
Resistance training ranged from 1 to 6 sets and 5 to 25 repetitions at 30-85% one repetition maximum (1RM). The length of the exercise programs was up to 12 months and the frequency ranged from 2 to 3 days/week, prevailing 3 days/week. In most studies, the control group did not exercise. In three studies, they performed warm-up and/or stretching [34,35,39], and in one, they performed cognitive training [45] (Table 1). IGF-1 was analysed in 23 articles [23-25,30,32-36,38-40,42,44-46,48-53,55], in 2 measured through its concentration in plasma [46,48], in 16 through serum [23, 30, 32-35, 38, 40,42,44,45,50-53,55] and the remaining 5 did not reported these data [24,25,36,39,49]; BDNF was analysed in 7 articles [23,24,32,33,37,45,54], in all of them, except one, through serum [31]; finally, VEGF was only measured in one article through its concentration in serum with significant results [17].
2.3. Risk of bias assessment
According to the RoB 2.0. tool [26], 11 out of 30 were classified as "high risk of bias", and 19 out of 30 were classified as "unclear risk of bias". The most affected domains were randomization process, deviations from intended interventions and selection of the reported result. The assessment of risk is shown in Supplementary Fig. 2.
2.4. Meta-analysis
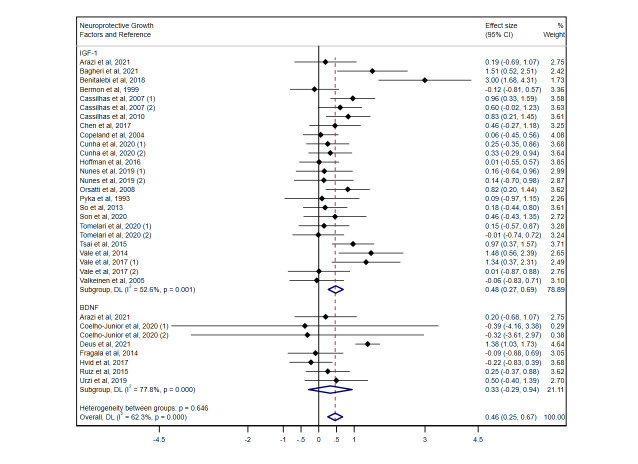
The pooled SMD of resistance exercise on IGF-1 levels was 0.48 (95% CI: 0.27, 0.69; I2 = 52.6%, P=0.001) (Fig. 2). In relation to training characteristics, exercise frequency showed significant results with at least 3 days per week (SMD: 0.55; 95% CI: 0.31, 0.79). In relation to the number of sets of the program, both performing 2 or fewer and more than 2 sets showed significant benefits, as well as light-moderate and high-intensity exercises, sessions of more or less than 60 minutes and exercise programs of more or less than 3 months (Table 2). In both men and women, IGF-1 levels increased significantly after resistance exercise (Supplementary Table 2). Meta-regression analysis showed that neither body mass index nor age influenced peripheral IGF-1 levels (P>0.05) in older adults (Supplementary Table 3).
Figure 2.
Standardized mean difference (95% CI) of the effect of resistance exercise vs. the control group on neuroprotective factors after intervention, with subgroup analysis by factors.
Table 2.
Subgroup analysis of the effect of resistance exercise dose (frequency, sets, intensity, session time and duration of exercise program) on IGF-1
| Subgroup | n | SMD (95% CI) | I2 | p |
|---|---|---|---|---|
| Frequency of exercise | ||||
| < 3 days | 4 | 0.13 (-0.22, 0.48) | 0.0% | 0.750 |
| ≥ 3 days | 21 | 0.55 (0.31, 0.79) | 56.4% | 0.001 |
| Sets of exercises | ||||
| ≤ 2 sets | 6 | 0.48 (0.16, 0.79) | 30.9% | 0.204 |
| > 2 sets | 18 | 0.52 (0.24, 0.80) | 59.2% | 0.001 |
| Intensity | ||||
| Light-moderate | 11 | 0.31 (0.11, 0.52) | 0.0% | 0.475 |
| High | 14 | 0.62 (0.26, 0.97) | 65.8% | 0.000 |
| Session time | ||||
| < 60 min | 8 | 0.73 (0.18, 1.28) | 71.2% | 0.001 |
| ≥ 60 min | 10 | 0.56 (0.33, 0.79) | 17.7% | 0.280 |
| Duration of exercise program | ||||
| ≤ 3 months | 15 | 0.38 (0.10, 0.67) | 55.7% | 0.005 |
| > 3 months | 12 | 0.53 (0.25, 0.88) | 42.1% | 0.061 |
Abbreviations: SMD = standardized mean differences
BDNF levels were not significantly different when performing resistance exercise versus the control group (SMD: 0.33; 95% CI: -0.29, 0.94; I2 = 77.8%, P=0.000) (Fig. 2). Subgroup analyses on sex could not be performed due to the limited number of studies.
VEGF was only analysed by one study [17], so it was not included in the meta-analysis. Significant differences were observed between the resistance exercise group and the control group.
3.5. Sensitivity analysis
The pooled ES estimates for the effect of resistance exercise on IGF-1 and BDNF were not significantly changed in magnitude or direction when removing each study included in the meta-analysis one by one, as well as when eliminating studies in which the population had a specific health disorder or pathology.
3.6. Publication bias
There was evidence of publication bias, as seen in the funnel plots and Egger's tests for IGF-1 (P=0.052) and BDNF (P=0.100) (Supplementary Fig. 3).
4. Discussion
Although several studies have reported the effectiveness of physical exercise on the main neuroprotective factors, there was no updated review comparing the effect of resistance exercise on these factors and determining the dose necessary to achieve this effect in middle and late life. Our data support that resistance exercise increases IGF-1 levels, being more effective when performed at least three sessions per week, independent of the number of sets, the intensity of the exercise, the time of the session and the duration of the exercise program. However, no significant effects were estimated for peripheral BDNF levels, and in the case of VEGF, the scarce number of articles made it impossible to analyse the effect on this factor.
The progressive decline in IGF-1 levels experienced by the population [56,57] is associated with impaired brain function and risk of vascular dementia [56,58]. The available evidence seems to indicate that resistance exercise could mitigate these adverse effects [18,59]. Several factors could justify the effect of resistance exercise on IGF-1 levels, such as the fact that this type of exercise induces anabolic hormonal responses, allowing the direct release of IGF-1 by the liver or indirectly when induced by growth hormone [57]. Furthermore, a positive relationship has been established between IGF-1 levels, strength, and muscle mass because this factor is capable of increasing the proliferation capacity of muscle satellite cells, thereby preventing the loss of muscle mass related to aging [60].
Regarding the dose necessary to achieve this beneficial effect, our data corroborate that three or more sessions per week would be necessary to increase IGF-1 levels, independent of the number of series, the intensity of the exercise, the duration of the sessions or the duration of the exercise program. However, contrary to what has been reported in previous reviews [18,59], which indicated that the increase in IGF-1 levels would only occur in women, our data show that this association is also positive in men, which seems logical considering that as a consequence of exercise, there are increases in growth hormone levels in both sexes, and this is closely related to IGF-1 synthesis [61].
Different meta-analyses have evaluated the effects of exercise interventions on BDNF, reporting significant effects in adolescents and children and in neurodegenerative disorders [62-64]. In older adults, Marinus et al. [19] evaluated the impact of resistance exercise on BDNF before and after exercise, observing a significant increase in peripheral levels of this neuroprotective factor. However, our results do not show this effect when compared against a control group, which could be because the BDNF generated in skeletal muscle with contraction is not released into the circulation but would be used to enhance muscle oxidation [65]. This could also be because the release and synthesis of this factor occurs immediately after exercise [66]. However, when exercise ceases, this effect disappears, and the peripheral concentration of BDNF normalizes [65,67], which could indicate that circulating BDNF would be transported to the brain through the blood circulation, where it would cross the blood–brain barrier, achieving greater neuronal survival and synaptogenesis and, therefore, greater brain function and structural changes [66].
Moreover, several studies have shown that aerobic exercise has contradictory effects on VEGF [68,69]. Resistance exercise seems to be effective in the adult population when it is performed with blood flow restriction [70]. However, the lack of studies has not allowed a synthesis of the effect of resistance exercise on this factor.
Some limitations of our systematic review and meta-analysis should be acknowledged. First, the lack of studies evaluating the different neuroprotective factors, particularly VEGF, limited the possibility of determining the effect of resistance exercise on this factor. Second, and in relation to the methodological quality of the studies, although in general the quality of the studies was acceptable, a large proportion of them did not provide information about some domains of the RoB 2.0., and the risk of bias was rated as high risk or some concerns. However, to overcome these limitations, sensitivity analyses were performed by eliminating each study included in the meta-analysis one by one. This same process was carried out with those studies in which the population had some pathology or health disorder to provide evidence of the robustness of the results. Third, there was a great heterogeneity of the interventions in terms of type of exercises, volume, frequency, and intensity; however, they have been pooled in a way that can provide conclusive results in relation to the dose–response.
5. Conclusion
Our data support a neuroprotective effect of resistance exercise in middle and late life, mainly through its influence on IGF-1. Therefore, physical activity programs targeted to this population should emphasize the promotion of this type of training, with a frequency of at least 3 days/week, to mitigate the neurological and cognitive consequences associated with aging. Because of the scarcity of studies, more clinical trials are needed to consistently establish the neuroprotective effect of resistance exercise, particularly in neuroprotective factors that have not yet been sufficiently studied.
Supplementary Materials
The Supplementary data can be found online at: www.aginganddisease.org/EN/10.14336/AD.2022.1207.
Footnotes
Authors’ contributions
ERG participated in the design of the study and contributed to data collection and data reduction/analysis; ATC and CPM participated in the design of the study; DPM participated in the design of the study and contributed to data collection; MGM contributed to data reduction/analysis; VMV contributed to data analysis and interpretation of results. All authors contributed to the manuscript writing. All authors have read and approved the final version of the manuscript and agree with the order of presentation of the author.
Competing interests
The authors declare that they have no competing interests.
References
- [1].Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet, 396(10248):413-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Borrás Blasco C, Viña Ribes J (2016). Neurofisiología y envejecimiento. Concepto y bases fisiopatológicas del deterioro cognitivo. Rev Esp Geriatr Gerontol, 51:3-6. [DOI] [PubMed] [Google Scholar]
- [3].Morrison JH, Baxter MG (2012). The Aging Cortical Synapse: Hallmarks and Implications for Cognitive Decline. Nat Rev Neurosci, 13(4):240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tsai CL, Erickson KI, Sun HS, Kuo YM, Pai MC (2021). A cross-sectional examination of a family history of Alzheimer’s disease and ApoE epsilon 4 on physical fitness, molecular biomarkers, and neurocognitive performance. Physiol Behav, 230:113268. [DOI] [PubMed] [Google Scholar]
- [5].Erickson KI, Miller DL, Roecklein KA (2012). The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. Neuroscientist, 18(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tyndall A v, Clark CM, Anderson TJ, Hogan DB, Hill MD, Longman RS, et al. (2018). Protective Effects of Exercise on Cognition and Brain Health in Older Adults. Exerc Sport Sci Rev, 46(4):215-23. [DOI] [PubMed] [Google Scholar]
- [7].Tapia-Arancibia L, Rage F, Givalois L, Arancibia S (2004). Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol, 25(2):77-107. [DOI] [PubMed] [Google Scholar]
- [8].Frater J, Lie D, Bartlett P, McGrath JJ (2018). Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing Res Rev, 42:14-27. [DOI] [PubMed] [Google Scholar]
- [9].Deak F, Sonntag WE (2012). Aging, Synaptic Dysfunction, and Insulin-Like Growth Factor (IGF)-1. J. Gerontol. A Biol. Sci. Med, 67A(6):611-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Calvo P, Pastor A, de La Cruz R (2018). Vascular endothelial growth factor: an essential neurotrophic factor for motoneurons? Neural Regen Res, 13(7):1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopez-Lopez C, LeRoith D, Torres-Aleman I (2004). Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A, 101(26):9833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao Y, Xiao W, Chen K, Zhan Q, Ye F, Tang X, et al. (2019). Neurocognition and social cognition in remitted first-episode schizophrenia: correlation with VEGF serum levels. BMC Psychiatry, 19(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].El-Sayes J, Harasym D, Turco C v, Locke MB, Nelson AJ (2019). Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist, 25(1):65-85. [DOI] [PubMed] [Google Scholar]
- [14].Cotman CW, Berchtold NC, Christie LA (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci, 30(9):464-72. [DOI] [PubMed] [Google Scholar]
- [15].Kivimäki M, Singh-Manoux A, Pentti J, Sabia S, Nyberg ST, Alfredsson L, et al. (2019). Physical inactivity, cardiometabolic disease, and risk of dementia: an individual-participant meta-analysis. BMJ, 365:1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsai CL, Ukropec J, Ukropcová B, Pai MC (2017). An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. Neuroimage Clin, 17:272-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen W, Ni J, Qiao Z, Wu Y, Lu L, Zheng J, et al. (2019). Comparison of the clinical outcomes of two physiological ischemic training methods in patients with coronary heart disease. Open Med, 14(1):224-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Amiri N, Fathei M, Mosaferi Ziaaldini M (2021). Effects of resistance training on muscle strength, insulin-like growth factor-1, and insulin-like growth factor-binding protein-3 in healthy elderly subjects: a systematic review and meta-analysis of randomized controlled trials. Hormones, 20(2):247-57. [DOI] [PubMed] [Google Scholar]
- [19].Marinus N, Hansen D, Feys P, Meesen R, Timmermans A, Spildooren J (2019). The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports Med, 49(10):1529-46. [DOI] [PubMed] [Google Scholar]
- [20].Knaepen K, Goekint M, Heyman EM, Meeusen R (2010). Neuroplasticity - exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med, 40(9):765-801. [DOI] [PubMed] [Google Scholar]
- [21].Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- [22].Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. (2021). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. The BMJ, 372:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pyka G, Taaffe DR, Marcus R (1994). Effect of a sustained program of resistance training on the acute growth hormone response to resistance exercise in older adults. Horm Metab Res2, 26(7):330-3. [DOI] [PubMed] [Google Scholar]
- [24].Sartorio A, Lafortuna C, Capodaglio P, Vangeli V, Narici M, Faglia G (2001). Effects of a 16-week progressive high-intensity strength training (HIST) on indexes of bone turnover in men over 65 years: a randomized controlled study. J Endocrinol Invest, 24(11):882-6. [DOI] [PubMed] [Google Scholar]
- [25].Parkhouse WS, Li C, Vanderhoek KJ, Coupland DC (2000). IGF-1 bioavailability is increased by resistance training in older women with low bone mineral density. Mech Ageing Dev, 113(2):75-83. [DOI] [PubMed] [Google Scholar]
- [26].Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J, 366:4898. [DOI] [PubMed] [Google Scholar]
- [27].DerSimonian R, Kacker R (2007). Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials, 28(2):105-14. [DOI] [PubMed] [Google Scholar]
- [28].Baechle TR, Earle RW. Principios del entrenamiento de la fuerza y del acondicionamiento físico. Colorado Springs: National Strength and Conditioning Association; 2007. [Google Scholar]
- [29].Serra-Rexach JA, Bustamante-Ara N, Hierro Villarán M, González Gil P, Sanz Ibáñez MJ, Blanco Sanz N, et al. (2011). Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: a randomized controlled trial. J Am Geriatr Soc, 59(4):594-602. [DOI] [PubMed] [Google Scholar]
- [30].Vale R, Ferrão MLD, de Alkmim Moreira Nunes R, da Silva JB, Nodari RJ, Dantas EHM (2017). Muscle strength, GH and IGF-1 in older women submitted to land and aquatic resistance training. Rev Bras Med Esporte, 23(4):274-9. [Google Scholar]
- [31].Urzi F, Marusic U, Ličen S, Buzan E (2019). Effects of Elastic Resistance Training on Functional Performance and Myokines in Older Women-A Randomized Controlled Trial. J Am Med Dir Assoc, 20(7):830-834.e2. [DOI] [PubMed] [Google Scholar]
- [32].Arazi H, Babaei P, Moghimi M, Asadi A (2021). Acute effects of strength and endurance exercise on serum BDNF and IGF-1 levels in older men. BMC Geriatr, (1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen HT, Chung YC, Chen YJ, Ho SY, Wu HJ (2017). Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J Am Geriatr Soc, 65(4):827-32. [DOI] [PubMed] [Google Scholar]
- [34].Cassilhas RC, Viana VAR, Grassmann V, Santos RT, Santos RF, Tufik S, et al. (2007). The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc, 39(8):1401-7. [DOI] [PubMed] [Google Scholar]
- [35].Cassilhas RC, Tufik S, Antunes HKM, de Mello MT (2010). Mood, anxiety, and serum IGF-1 in elderly men given 24 weeks of high resistance exercise. Percept Mot Skills, 110(1):265-76. [DOI] [PubMed] [Google Scholar]
- [36].Cunha PM, Nunes JP, Tomeleri CM, Nascimento MA, Schoenfeld BJ, Antunes M, et al. (2020). Resistance Training Performed with Single and Multiple Sets Induces Similar Improvements in Muscular Strength, Muscle Mass, Muscle Quality, and IGF-1 in Older Women: A Randomized Controlled Trial. J Strength Cond Res, 34(4):1008-16. [DOI] [PubMed] [Google Scholar]
- [37].Fragala MS, Beyer KS, Jajtner AR, Townsend JR, Pruna GJ, Boone CH, et al. (2014). Resistance exercise may improve spatial awareness and visual reaction in older adults. J Strength Cond Res, 28(8):2079-87. [DOI] [PubMed] [Google Scholar]
- [38].Vale R, Ferreira Rodrigues V (2014). Effects of strength training on IGF-1 and functional autonomy in eldely women. Revista Ciencias de la Actividad Física UCM, 15(2):35-42. [Google Scholar]
- [39].Daly RM, Dunstan DW, Owen N, Jolley D, Shaw JE, Zimmet PZ (2005). Does high-intensity resistance training maintain bone mass during moderate weight loss in older overweight adults with type 2 diabetes? Osteoporos Int, 16(12):1703-12. [DOI] [PubMed] [Google Scholar]
- [40].Tomeleri CM, Ribeiro AS, Nunes JP, Schoenfeld BJ, Souza MF, Schiavoni D, et al. (2020). Influence of Resistance Training Exercise Order on Muscle Strength, Hypertrophy, and Anabolic Hormones in Older Women: A Randomized Controlled Trial. J Strength Cond Res, 34(11):3103-9. [DOI] [PubMed] [Google Scholar]
- [41].Hvid LG, Nielsen MKF, Simonsen C, Caserotti P, Andersen M, Caserotti P (2017). Brain-derived neurotrophic factor (BDNF) serum basal levels is not affected by power training in mobility-limited older adults — A randomized controlled trial. Exp Gerontol, 93:29-35. [DOI] [PubMed] [Google Scholar]
- [42].So WY, Song M, Park YH, Cho BL, Lim JY, Kim SH, et al. (2013). Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: A randomized controlled trial. Aging Clin Exp Res, 25(2):167-74. [DOI] [PubMed] [Google Scholar]
- [43].Ruiz JR, Gil-Bea F, Bustamante-Ara N, Rodríguez-Romo G, Fiuza-Luces C, Serra-Rexach JA, et al. (2015). Resistance training does not have an effect on cognition or related serum biomarkers in nonagenarians: A randomized controlled trial. Int J Sports Med, 36(1):54-60. [DOI] [PubMed] [Google Scholar]
- [44].Tsai CL, Wang CH, Pan CY, Chen FC (2015). The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front Behav Neurosci, 9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hofmann M, Schober-Halper B, Oesen S, Franzke B, Tschan H, Bachl N, et al. (2016). Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: the Vienna Active Ageing Study (VAAS). Eur J Appl Physiol, 116(5):885-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bermon S, Ferrari P, Bernard P, Altare S, Dolisi C (1999). Responses of total and free insulin-like growth factor-I and insulin-like growth factor binding protein-3 after resistance exercise and training in elderly subjects. Acta Physiol Scand, 165(1):51-6. [DOI] [PubMed] [Google Scholar]
- [47].Coelho-Júnior HJ, Gonçalves I de O, Sampaio RAC, Sampaio PYS, Cadore EL, Calvani R, et al. (2020). Effects of Combined Resistance and Power Training on Cognitive Function in Older Women: A Randomized Controlled Trial. Int J Environ Res Public Health, 17(10):3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Son WM, Pekas EJ, Park SY (2020). Twelve weeks of resistance band exercise training improves age-associated hormonal decline, blood pressure, and body composition in postmenopausal women with stage 1 hypertension: a randomized clinical trial. Menopause, 27(2):199-207. [DOI] [PubMed] [Google Scholar]
- [49].Nunes PRP, Barcelos LC, Oliveira AA, Furlanetto R Jr., Martins FM, Resende EAMR, et al. (2019). Muscular strength adaptations and hormonal responses after two different multiple-set protocols of resistance training in postmenopausal women. J Strength Cond Res, 33(5): 1276-1285. [DOI] [PubMed] [Google Scholar]
- [50].Banitalebi E, Faramarzi M, Bagheri L, Kazemi AR. (2018). Comparison of performing 12 weeks’ resistance training before, after and/or in between aerobic exercise on the hormonal status of aged women: a randomized controlled trial. Horm Mol Biol Clin Investig, 35(3). [DOI] [PubMed] [Google Scholar]
- [51].Bagheri R, Forbes SC, Candow DG, Wong A (2021). Effects of branched-chain amino acid supplementation and resistance training in postmenopausal women. Exp Gerontol, 144:111185. [DOI] [PubMed] [Google Scholar]
- [52].Valkeinen H, Häkkinen K, Pakarinen A, Hannonen P, Häkkinen A, Airaksinen O, et al. (2005). Muscle hypertrophy, strength development, and serum hormones during strength training in elderly women with fibromyalgia. Scand J Rheumatol, 34(4): 309-314. [DOI] [PubMed] [Google Scholar]
- [53].Orsatti FL, Nahas EAPP, Maesta N, Nahas-Neto J, Burini RC (2008). Plasma hormones, muscle mass and strength in resistance-trained postmenopausal women. Maturitas, 59(4): 394-404. [DOI] [PubMed] [Google Scholar]
- [54].Deus LA, Corrêa HL de L, Neves RVP, Reis AL, Honorato FS, Silva VL, et al. (2021). Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle-Brain-Renal Axis. Int J Environ Res Public Health, 18(21):11299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Copeland JL, Tremblay MS (2004). Effect of HRT on hormone responses to resistance exercise in post-menopausal women. Maturitas, 48(4):360-71. [DOI] [PubMed] [Google Scholar]
- [56].Galle SA, Geraedts IK, Deijen JB, Milders M v, Drent ML (2020). The Interrelationship between Insulin-Like Growth Factor 1, Apolipoprotein E ε4, Lifestyle Factors, and the Aging Body and Brain. J Prev Alzheimers Dis, 7(4):265-73. [DOI] [PubMed] [Google Scholar]
- [57].Gharahdaghi N, Phillips BE, Szewczyk NJ, Smith K, Wilkinson DJ, Atherton PJ (2021). Links Between Testosterone, Oestrogen, and the Growth Hormone/Insulin-Like Growth Factor Axis and Resistance Exercise Muscle Adaptations. Front Physiol, 11:621226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Quinlan P, Horvath A, Nordlund A, Wallin A, Svensson J (2017). Low serum insulin-like growth factor-I (IGF-I) level is associated with increased risk of vascular dementia. Psychoneuroendocrinology, 86:169-75. [DOI] [PubMed] [Google Scholar]
- [59].Jiang Q, Lou K, Hou L, Lu Y, Sun L, Tan SC, et al. (2020). The effect of resistance training on serum insulin-like growth factor 1(IGF-1): A systematic review and meta-analysis. Complement Ther Med, 50:102360. [DOI] [PubMed] [Google Scholar]
- [60].Yuan S, Larsson SC (2021). Genetically predicted insulin-like growth factor-I in relation to muscle mass and strength. Clin Endocrinol, 95(5):800-5. [DOI] [PubMed] [Google Scholar]
- [61].Hatfield DL, Kraemer WJ, Volek JS, Nindl BC, Caldwell LK, Vingren JL, et al. (2021). Hormonal stress responses of growth hormone and insulin-like growth factor-I in highly resistance trained women and men. Growth Horm IGF Res, 59:101407. [DOI] [PubMed] [Google Scholar]
- [62].de Menezes-Junior FJ, Jesus ÍC, Brand C, Mota J, Leite N (2021). Physical Exercise and Brain-Derived Neurotrophic Factor Concentration in Children and Adolescents: A Systematic Review with Meta-Analysis. Pediatr Exerc Sci, 34(1):44-53. [DOI] [PubMed] [Google Scholar]
- [63].de Azevedo KPM, de Oliveira VH, de Medeiros GCBS, Mata ÁN de S, García DÁ, Martínez DG, et al. (2020). The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int J Environ Res Public Health, 17(17):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A (2021). Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci Biobehav Rev, 128:394-405. [DOI] [PubMed] [Google Scholar]
- [65].Matthews VB, Åström MB, Chan MHS, Bruce CR, Krabbe KS, Prelovsek O, et al. (2009). Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia, 52(7):1409-18. [DOI] [PubMed] [Google Scholar]
- [66].Dinoff A, Herrmann N, Swardfager W, Lanctôt KL (2017). The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci, 46(1):1635-46. [DOI] [PubMed] [Google Scholar]
- [67].Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, et al. (2003). Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol, 138(1-2):99-105. [DOI] [PubMed] [Google Scholar]
- [68].Ratajczak M, Skrypnik D, Bogdański P, Madry E, Walkowiak J, Szulińska M, et al. (2019). Effects of Endurance and Endurance-Strength Training on Endothelial Function in Women with Obesity: A Randomized Trial. Int J Environ Res Public Health, 16(21):4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kraus RM, Stallings HW, Yeager RC, Gavin TP (2004). Circulating plasma VEGF response to exercise in sedentary and endurance-trained men. J Appl Physiol, 96(4):1445-50. [DOI] [PubMed] [Google Scholar]
- [70].Li S, Li S, Wang L, Quan H, Yu W, Li T, et al. (2022). The Effect of Blood Flow Restriction Exercise on Angiogenesis-Related Factors in Skeletal Muscle Among Healthy Adults: A Systematic Review and Meta-Analysis. Front Physiol, 13:14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplementary data can be found online at: www.aginganddisease.org/EN/10.14336/AD.2022.1207.