Abstract
The core enzyme of protein phosphatase 2A is composed of a regulatory subunit A and a catalytic subunit C. It is controlled by three types of regulatory B subunits (B, B′, and B") and by tumor (T) antigens, which are unrelated by sequence but bind to overlapping regions on the A subunit. To find out whether the different B subunits and T antigens bind to identical or distinct amino acids of the A subunit, mutants were generated and their abilities to bind B subunits and T antigens were tested. We found that some amino acids are involved in the binding of all types of B subunits, whereas others are specifically involved in the binding of one or two types of B subunits. T-antigen-binding specificity does not correlate with that of a particular type of B subunit.
Protein phosphatase 2A (PP2A) is an abundant enzyme constituting approximately 0.3% of the total protein in mammalian cells (17). It exists in cells in two forms—the core enzyme, composed of a 36-kDa catalytic C subunit and a 65-kDa regulatory A subunit, and the holoenzyme, consisting of core enzyme to which one of several B subunits is bound—at approximately equal concentrations (8). The A and C subunits both occur as two isoforms (α and β), whereas the B subunits fall into three families, designated B, B′ (also called B56), and B", that are unrelated by protein sequence (13). The B family has three members, Bα, Bβ, and Bγ, each with a molecular mass of around 55 kDa (5, 10, 14, 23). The B′ family consists of numerous, recently identified isoforms and splice variants whose molecular masses range from 54 to approximately 70 kDa (3, 11, 12, 18, 19). The B" family has two members, which have molecular masses of 72 and 130 kDa and are splice variants of the same gene (7). B subunits are the key regulators of PP2A. They determine not only the activity and substrate specificity but also the intracellular localization of PP2A (11, 19). In addition to the three classes of B subunits, a fourth class of proteins that associate with the PP2A core enzyme are the tumor (T) antigens encoded by polyomaviruses, which are unrelated by sequence to the B subunits (for a review, see reference 22). The association between T antigens and PP2A plays an important role in virus-induced neoplastic cell transformation.
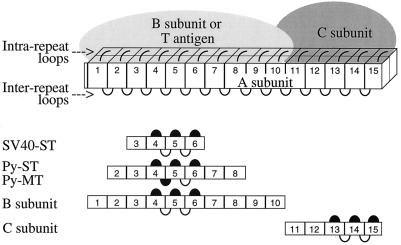
The A subunit polypeptide consists of 15 nonidentical repeats, approximately 40 amino acids long (6, 21), which form a rod-shaped molecule (2). The B subunits from all three families bind to repeats 1 through 10, and the C subunit binds to repeats 11 through 15 of the A subunit. Simian virus 40 (SV40) small t antigen binds to repeats 3 through 6, and polyoma small t and middle T bind to repeats 2 through 8 (15, 16). We have proposed a model according to which each repeat consists of two amphipathic helices that are connected by loops (intrarepeat loops), and we have shown that these loops are involved in the binding of B subunits and T antigens (Fig. 1).
FIG. 1.
Model of the PP2A holoenzyme. The rod-shaped A subunit consists of 15 repeats. Each repeat is composed of two α helices connected by an intrarepeat loop. Adjacent repeats are connected by interrepeat loops. Binding regions for B and C subunits, as well as for T antigens, are indicated by bars. Loops involved in binding are solid; loops not involved in binding are open. (Reprinted from reference 15, with permission.)
An intriguing question is how the three families of B subunits and the T antigens, although unrelated by sequence, are able to bind to the same region of the A subunit. To explain this finding, we considered two models. (i) B, B′, B", and T antigens fold in such a way that a similarly spaced set of identical residues is generated in each case, which binds to a complementary set of residues on the A subunit (over a stretch of 10 repeats). Therefore, mutants of the A subunit that affect binding of one type of B subunit would also affect the binding of all other B subunits and of T antigens. (ii) B subunits and T antigens recognize distinct amino acids. This model predicts that one could generate mutants of the A subunit that are unable to bind certain B subunits or T antigens while still binding others. Our previous data supported the first model, since deletion of any one of repeats 1 to 10 or substitution of intrarepeat loop 4, 5, or 6 abolished binding of all types of B subunits, suggesting that there was no specificity in binding of individual B subunits. On the other hand, the first model seemed unlikely because the same binding site would have to have evolved in four unrelated protein families.
To resolve this question, we generated smaller mutations by substituting for only 1 to 4 amino acids in intrarepeat loops 3 (amino acids 100 to 105), 4 (amino acids 139 to 144), 5 (amino acids 177 to 182), and 7 (amino acids 255 to 260). Some mutations outside the loop regions were also generated. The expectation was that smaller mutations might have a better chance of revealing differences in binding specificity between different B subunits and T antigens. Site-directed mutagenesis was performed with the Gene Editor system from Promega. The plasmid used for mutagenesis, pcDNA3-AEE, is a eukaryotic expression vector encoding the wild-type A subunit tagged at the C terminus with the EE tag (EEEEYMPME) (9). The tag was introduced into the A subunit within Bluescript, p16-1A (21), by insertion of a fragment generated by PCR and encoding a BglII site, the EE tag, and a HindIII site. AEE was then moved from Bluescript to pcDNA3 as an EcoRI-XhoI fragment. To assay whether A subunit mutants bind B subunits, both were synthesized separately in vitro and labeled with [35S]methionine by using Promega’s TNT T7 Quick coupled transcription/translation system, as previously described (8). Five microliters of each reaction mixture was combined and incubated for 4 h at 30°C to allow complex formation between the labeled A subunit mutant, endogenous C subunit (unlabeled), and labeled B subunit, as previously described (15, 16). Mutations in repeats 1 to 10 have no effect on the binding of C subunit to repeats 11 to 15 (15, 16). Anti-EE monoclonal antibodies were used to immunoprecipitate the tagged A subunit mutants. The precipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to identify coprecipitated B subunits. Since in vitro-synthesized middle T does not form a complex with core enzyme (1), we used a previously described assay to determine whether A subunit mutants bind T antigens (16). The mutants were synthesized in vitro as described above. SV40 small t was added as a bacterially expressed purified protein (20), polyomavirus small t was added as a baculovirus-expressed protein in Sf9 cell lysate, and middle T was added as an overexpressed protein in 293 cell lysate. Hamster antitumor serum was added to immunoprecipitate SV40 small t, and rat antitumor ascitic fluid was used to precipitate polyomavirus small t and middle T (16). The mixtures were incubated at 4°C for 16 h. The immunoprecipitates were washed with buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 3 mM MgCl2, 0.5% Triton X-100) and analyzed by SDS-PAGE. Coprecipitated A subunit mutants were visualized by autoradiography. Radioactive bands were quantitated with a Molecular Dynamics PhosphorImager and ImageQuant software.
The results demonstrate that some mutations affect binding of all B subunits, whereas others affect binding of specific B subunits (Fig. 2 and 3). The mutants fall into four categories.
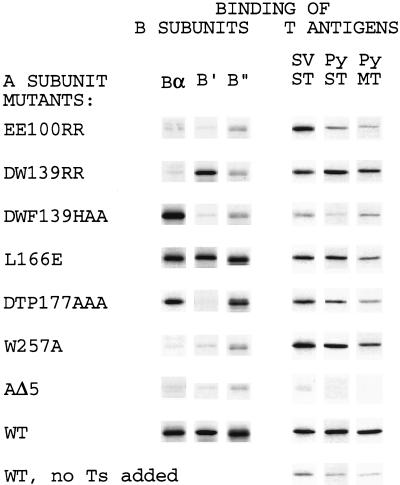
FIG. 2.
Binding of B subunits and T antigens to mutants of the A subunit. Amino acid sequences of repeats 1 to 10 of the A subunit and locations of mutations are shown. Binding of A subunit mutants to Bα, B′, B", and T antigens is expressed as a percentage of wild-type A subunit binding. Values shown are averages from 2 to 4 experiments. Amino acids conserved between repeats are underlined. The locations of α helices and intrarepeat loops are indicated by lines at the top of the figure.
FIG. 3.
Examples of mutants with distinct binding properties. Shown are examples of the four categories of mutants described in the text. Mutant L166E has reduced middle T binding compared to the wild type (WT) but full-strength B subunit binding; mutant W257A binds T antigens but little of the B subunits. AΔ5 is a negative control that does not bind any B subunit or T antigen (8, 15). An additional control for T-antigen binding, in which T antigens were not added before immunoprecipitation, is shown at the bottom. SV, SV40; Py, polyomavirus; ST, small t; MT, middle T.
(i) B− B′− B"−.
An example is mutant EE100RR, which bound none of the B subunits.
(ii) B− B′+ B"−.
An example is mutant DW139RR, which showed almost no binding of Bα and B" whereas binding of B′ was reduced only by one-third.
(iii) B+ B′− B"−.
An example is DWF139HAA, which bound Bα as well as or better than the wild type but did not bind B′ or B". Note the dramatic difference in specificities between W140A (B− B′+ B"−) and DWF139HAA (B+ B′− B"−), indicating that W140 is essential for B and B" binding and F141 is required for B′ binding. D139 had little if any effect, since the binding patterns of DW139RR and W140A are very similar. It is intriguing that mutation of W140 destroyed binding of Bα and that the additional mutation of F141 restored binding of Bα.
(iv) B+ B′− B"+.
An example is DTP177AAA, which showed a 25-fold reduction in binding of B′ whereas binding of Bα was normal and that of B" was reduced approximately 50%. This mutant is most specific because its deficiency is limited mainly to one type of B subunit (B′). In view of the fact that the binding regions for all B subunits extend over 10 repeats, it is remarkable that a point mutation such as W257A in repeat 7 completely destroyed the binding capacity for all B subunits (but not for T antigens).
With regard to T antigens, our data demonstrate that, with the exception of EE100RR, mutations that reduced binding of one T antigen also reduced binding of the others. Polyomavirus middle T was more affected than the small t antigens. This is consistent with our previous findings (15, 16). In general, the amino acids involved in binding T antigens were also located in intrarepeat loops. However, one mutant, L166E, located outside of intrarepeat loop 5, showed reduced binding of T antigens but had no effect on B subunit binding. We previously identified a region (interrepeat loop 4/5, amino acids 158 to 165) very close to L166 that is also involved in binding polyomavirus middle T and small t only (15) (Fig. 1). Our results reveal no apparent correlation between the binding of T antigens and the binding of a particular B subunit.
The reason why EE100RR binds SV40 small t but not polyomavirus small t and middle T is probably related to the fact that the binding region for SV40 small t involves repeats 3 through 6 and that for polyomavirus T antigens involves repeats 2 through 8. Probably, the binding region for SV40 small t starts downstream of E100.
We conclude that the binding sites on the A subunit for the different types of B subunits are composed of both distinct and common amino acids. Our results also indicate that all mutations that affect B subunit binding are located in intrarepeat loops, since no mutation outside of these regions had a significant effect on binding. This finding further supports the role of intrarepeat loops in binding, as suggested by our model of the A subunit (Fig. 1) (15, 16). Mutants of the A subunit defective in the binding of specific B subunits are potential tools for studying the role of PP2A in vivo. For example, substitution of the wild-type A subunit by gene replacement with a mutant that is defective in B′ but normal in B and B" binding may shed light on the function(s) of B′ subunits. Furthermore, the mutant L166E, which is partially defective in binding middle T but is normal in B subunit binding, could be used to study the role of the middle T-PP2A interaction in tumor formation. Whereas the binding of PP2A to middle T is a prerequisite for transformation of cultured fibroblasts (1, 4), its importance for tumor formation has not yet been demonstrated. We plan to generate mice in which the wild-type A subunit is replaced by the mutant L166E. If the binding of middle T to PP2A is important, these mice should be immune to polyomavirus- or middle T-induced tumors.
Acknowledgments
This work was supported by Public Health Service grant CA-36111.
We thank Kathy Rundell for providing SV40 small t and Anders Berqvist and Göran Magnusson for supplying polyomavirus small t.
REFERENCES
- 1.Campbell K S, Auger K R, Hemmings B A, Roberts T M, Pallas D C. Identification of regions in polyomavirus middle T and small t antigens important for association with protein phosphatase 2A. J Virol. 1995;69:3721–3728. doi: 10.1128/jvi.69.6.3721-3728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S-C, Kramer G, Hardesty B. Isolation and partial characterization of an Mr 60,000 subunit of a type 2A phosphatase from rabbit reticulocytes. J Biol Chem. 1989;264:7267–7275. [PubMed] [Google Scholar]
- 3.Csortos C, Zolnierowicz S, Bako E, Durbin S D, DePaoli-Roach A A. High complexity in the expression of the B′ subunit of protein phosphatase 2A0. Evidence for the existence of at least seven novel isoforms. J Biol Chem. 1996;271:2578–2588. doi: 10.1074/jbc.271.5.2578. [DOI] [PubMed] [Google Scholar]
- 4.Glenn G M, Eckhart W. Amino-terminal regions of polyomavirus middle T antigen are required for interactions with protein phosphatase 2A. J Virol. 1995;69:3729–3736. doi: 10.1128/jvi.69.6.3729-3736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Healy A M, Zolnierowicz S, Stapleton A E, Goebl M, DePaoli-Roach A A, Pringle J R. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmings B A, Adams-Pearson C, Maurer F, Muller P, Goris J, Merlevede W, Hofsteenge J, Stone S R. α- and β-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990;29:3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- 7.Hendrix P, Mayer-Jaekel R E, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings B A. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. J Biol Chem. 1993;268:15267–15276. [PubMed] [Google Scholar]
- 8.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol Cell Biol. 1997;17:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacArthur H, Walter G. Monoclonal antibodies specific for the carboxyl terminus of simian virus 40 large T antigen. J Virol. 1984;52:483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer R E, Hendrix P, Cron P, Matthies R, Stone S R, Goris J, Merlevede W, Hofsteenge J, Hemmings B A. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991;30:3589–3597. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- 11.McCright B, Rivers A M, Audlin S, Virshup D M. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 12.McCright B, Virshup D M. Identification of a new family of protein phosphatase 2A regulatory subunits. J Biol Chem. 1995;270:26123–26128. doi: 10.1074/jbc.270.44.26123. [DOI] [PubMed] [Google Scholar]
- 13.Mumby M C, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- 14.Pallas D C, Weller W, Jaspers S, Miller T B, Lane W S, Roberts T M. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992;66:886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruediger R, Hentz M, Fait J, Mumby M, Walter G. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J Virol. 1994;68:123–129. doi: 10.1128/jvi.68.1.123-129.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruediger R, Roeckel D, Fait J, Bergqvist A, Magnusson G, Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992;12:4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruediger R, van Wart Hood J E, Mumby M, Walter G. Constant expression and activity of protein phosphatase 2A in synchronized cells. Mol Cell Biol. 1991;11:4282–4285. doi: 10.1128/mcb.11.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanabe O, Nagase T, Murakami T, Nozaki H, Usui H, Nishito Y, Hayashi H, Kagamiyama H, Takeda M. Molecular cloning of a 74-kDa regulatory subunit (B" or delta) of human protein phosphatase 2A. FEBS Lett. 1996;379:107–111. doi: 10.1016/0014-5793(95)01500-0. [DOI] [PubMed] [Google Scholar]
- 19.Tehrani M A, Mumby M C, Kamibayashi C. Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J Biol Chem. 1996;271:5164–5170. doi: 10.1074/jbc.271.9.5164. [DOI] [PubMed] [Google Scholar]
- 20.Turk B, Porras A, Mumby M C, Rundell K. Simian virus 40 small-t antigen binds two zinc ions. J Virol. 1993;67:3671–3673. doi: 10.1128/jvi.67.6.3671-3673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter G, Ferre F, Espiritu O, Carbone-Wiley A. Molecular cloning and sequence of cDNA encoding polyoma medium tumor antigen-associated 61-kDa protein. Proc Natl Acad Sci USA. 1989;86:8669–8672. doi: 10.1073/pnas.86.22.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter G, Mumby M. Protein serine/threonine phosphatases and cell transformation. Biochim Biophys Acta. 1993;1175:207–226. doi: 10.1016/0304-419x(93)90005-w. [DOI] [PubMed] [Google Scholar]
- 23.Zolnierowicz S, Csortos C, Bondor J, Verin A, Mumby M C, DePaoli-Roach A A. Diversity in the regulatory B subunits of protein phosphatase 2A: identification of a novel isoform highly expressed in brain. Biochemistry. 1994;33:11858–11867. doi: 10.1021/bi00205a023. [DOI] [PubMed] [Google Scholar]