ABSTRACT
Emerging evidence indicates that proper mitochondrial dynamics are critical for adipocyte differentiation and functional thermogenic capacity. We found that the mitochondrial fission protein dynamin-related protein 1 (DRP1, also known as DNML1) is highly expressed in brown adipose tissue compared to expression in white adipose tissue, and these expression levels increase during brown adipocyte differentiation. Our results reveal that the inhibition of DRP1 using mdivi-1 mitigates beige adipocyte differentiation and differentiation-associated mitochondrial biogenesis. We found that DRP1 is essential for the induction of the early-phase beige adipogenic transcriptional program. Intriguingly, inhibition of DRP1 is dispensable following the induction of beige adipogenesis and adipogenesis-associated mitochondrial biogenesis. Altogether, we demonstrate that DRP1 in preadipocytes plays an essential role in beige and brown adipogenesis.
This article has an associated First Person interview with the first author of the paper.
Keywords: Adipogenesis, Brown adipocytes, Beige adipocytes, DRP1, Mitochondrial biogenesis
Summary: The mitochondrial fission protein dynamin-related protein 1 (DRP1) plays an essential role in early transcriptional reprogramming during beige and brown adipocyte differentiation.
INTRODUCTION
The adipose tissue acts as a central organ in regulating metabolic homeostasis and is broadly categorized into two types: white adipose tissue (WAT) and classical brown adipose tissue (BAT) (Choe et al., 2016; Kahn et al., 2019). Recent studies have uncovered an inducible form of adipocytes in white fat depots, named beige (or brite) adipocytes (Ikeda et al., 2018; Kajimura et al., 2015; Wu et al., 2012). Beige adipocytes are induced by cold exposure, β3-selective adrenergic agonists and dietary intervention, such as caloric restriction (Barbatelli et al., 2010; Mooli et al., 2020). White adipocytes store energy as triglycerides, whereas the beige and brown adipocytes expend energy via non-shivering thermogenesis (Giralt and Villarroya, 2013; Sidossis and Kajimura, 2015). The metabolic distinction between the adipocytes is partly driven by their mitochondrial characteristics. For example, white adipocytes have fewer mitochondria, limiting their oxidative capacity, whereas brown and beige adipocytes possess abundant mitochondria expressing uncoupling protein 1 (UCP1), which is involved in non-shivering thermogenesis (Sepa-Kishi and Ceddia, 2018; Vosselman et al., 2013). Thus, the mechanisms associated with mitochondrial remodeling have a key role in regulating adipocyte function.
Mitochondrial remodeling is tightly regulated by the mitochondrial dynamics, which includes fission and fusion (Scott and Youle, 2010; Tilokani et al., 2018). Fission is mediated by the cytosolic protein, dynamin-related protein 1 (DRP1, also known as DNML1), which is recruited to the mitochondrial surface with the help of accessory proteins such as mitochondrial fission factor (MFF) and mitochondrial fission protein 1 (FIS1) (Bleazard et al., 1999). Fission is followed by fusion, which is coordinated by inner and outer mitochondrial proteins such as mitofusin (MFN)-1 and -2 and optic atrophy 1 (OPA1) (Westermann, 2002; Zhang and Chan, 2007). Studies have shown that the dysregulation of mitochondrial dynamics has a profound effect on cellular energetics, which in turn could affect their function (Eisner et al., 2018; Mishra and Chan, 2016). For instance, impairment in mitochondrial fragmentation decreases the reprogramming and differentiation efficiency of progenitor cells (Forni et al., 2016). Similarly, inhibition of DRP1-mediated mitochondrial fragmentation mitigates the self-renewal potency or ‘stemness’ of progenitor cells (Khacho et al., 2016). Thus, mitochondrial dynamics play a key role in governing cellular properties and functions.
Although studies have highlighted the importance of adipose tissue mitochondrial dynamics from a metabolic perspective, very little is known about their contribution to beige adipogenesis. We found that DRP1 is highly expressed in the BAT and that the level of DRP1 expression increases during beige and brown adipocyte differentiation. We report that inhibition of DRP1 by pharmacological or genetic approaches attenuates beige and brown adipogenesis. Furthermore, we demonstrate that the inhibition of DRP1 during the early phase of differentiation is sufficient to attenuate beige adipogenesis and adipogenesis-associated mitochondrial biogenesis. Thus, we demonstrate an indispensable role of DRP1 in regulating beige and brown adipocyte differentiation.
RESULTS
DRP1 is highly expressed in brown and beige adipocytes
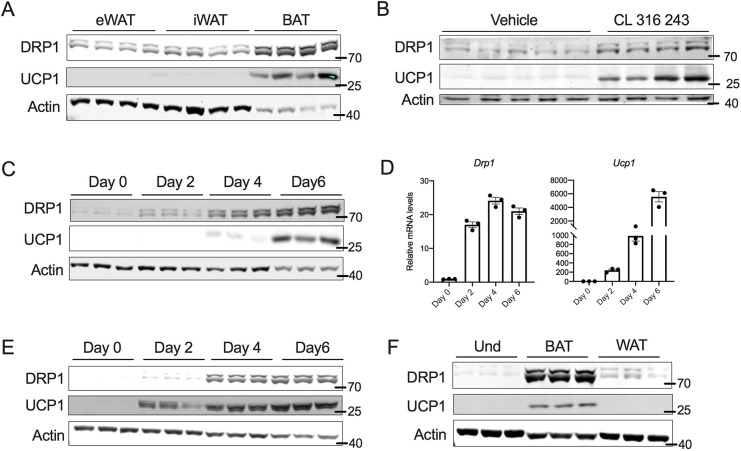
DRP1 plays an essential role in regulating mitochondrial homeostasis by inducing mitochondrial fission (Bleazard et al., 1999). Because brown adipocytes possess an abundance of mitochondria, we explored the expression of DRP1 using western blotting. Our data show that brown adipose tissue (BAT) expresses high levels of DRP1, as compared to expression in the inguinal white adipose tissue (iWAT) or epididymal white adipose tissue (eWAT) (Fig. 1A). Because beige adipocytes have mitochondrial characteristics similar to brown adipocytes, we further determined whether induction of beige adipocyte formation (beigeing) increases DRP1 expression. To this end, C57BL6 mice were injected with β3 adrenergic receptor agonist (CL316,243; CL) for 7 consecutive days, which induced beige adipocytes, as revealed by an increase in the expression of UCP1 in the iWAT. We found that beigeing was associated with an increase in DRP1 in the iWAT (Fig. 1B). We then assessed expression levels of DRP1 in transformed mouse stromal vascular fraction (SVF) cells during differentiation into beige adipocytes. The expression of DRP1 increased as early as 24 h following the start of induction, and we found a time-dependent increase in expression during beige adipocyte differentiation (Fig. 1C). qPCR analysis showed a parallel increase in Drp1 mRNA levels during beige adipocyte differentiation (Fig. 1D). Similar to the transformed SVF cells, differentiation of adipocyte precursor SVF cells led to increased DRP1 expression (Fig. 1E). When we further compared the expression of DRP1 between preadipocytes differentiated to beige or white adipocytes, DRP1 levels were higher in the differentiated beige adipocytes when compared to levels in the white adipocytes (Fig. 1F). Taken together, our data indicate that DRP1 is highly expressed in the brown and beige adipocytes.
Fig. 1.
DRP1 is highly expressed in brown and beige adipocytes. (A) Immunoblots of DRP1 and UCP1 in various fat depots of 6-week-old C57BL6 mice. (B) Immunoblots of DRP1 and UCP1 in the subcutaneous fat of C57BL6 mice injected intraperitoneally with β3-adrenergic agonist CL316,243 at a dose of 1 mg/kg bodyweight for 7 days. (C) Immunoblots of DRP1 and UCP1 in transformed mouse SVF cells during differentiation into beige adipocytes. (D) mRNA levels of Drp1 and Ucp1 in transformed mouse SVF cells during differentiation into beige adipocytes. β-actin was used to normalize the gene expression. Data are presented as mean±s.e.m. (E) Immunoblots of DRP1 and UCP1 in primary mouse SVF cells from subcutaneous fat of C57BL6 mice during differentiation into beige adipocytes. (F) Immunoblots of DRP1 and UCP1 in transformed mouse SVF cells differentiated into beige or white adipocytes. Data for the differentiation assays was collected after 6 days of differentiation. Und, undifferentiated. In A–C,E,F, actin is shown as a loading control.
DRP1 is essential for the thermogenic program of beige and brown adipocytes
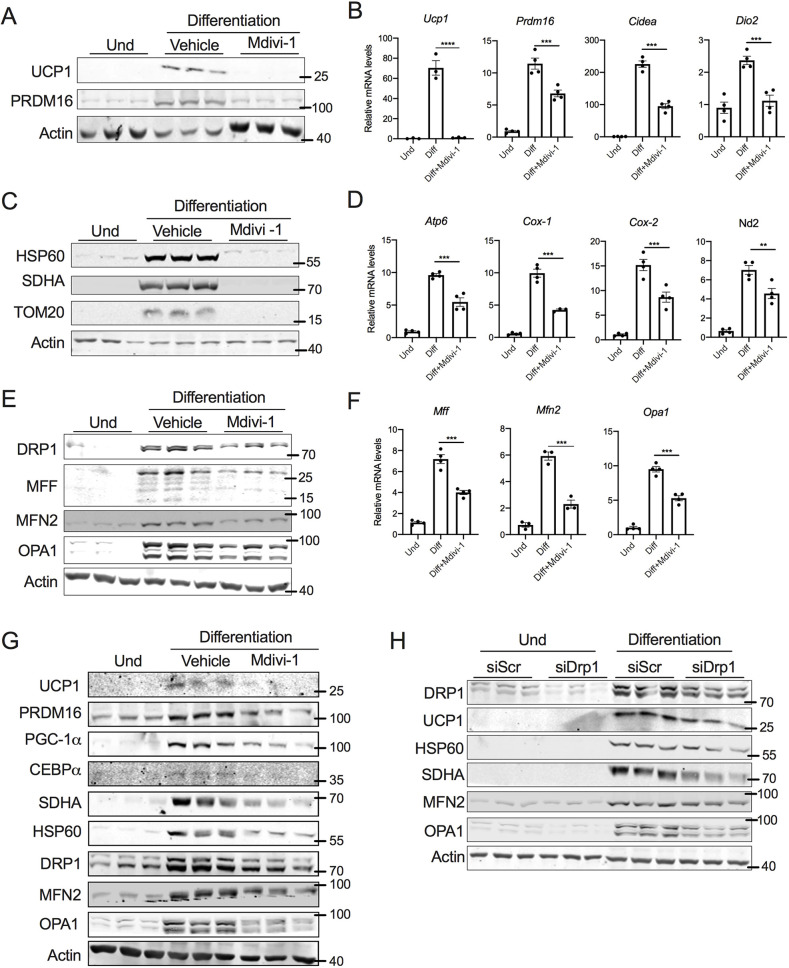
The activity of DRP1 is determined by its GTPase domain, which promotes DRP1 self-assembly into oligomers (Smirnova et al., 2001). To investigate the role of DRP1 in beige adipogenesis, we differentiated SVF cells into beige adipocytes in the presence or absence of mdivi-1, an inhibitor of DRP1 GTPase activity (Bleazard et al., 1999). Inhibition of DRP1 from day 0 of differentiation abolished the induction of UCP1 expression (Fig. 2A). PRDM16, a large zinc finger-containing transcription factor, is the master regulator of thermogenic gene expression (Harms et al., 2014). Inhibition of DRP1 decreased the expression of PRDM16 (Fig. 2A). Moreover, the mRNA levels of other brown fat-specific genes such as Ucp1, Prdm16, Cidea and Dio2 were significantly downregulated in the adipocytes differentiated in the presence of mdivi-1 (Fig. 2B).
Fig. 2.
DRP1 is essential for the thermogenic program of beige and brown adipocytes. (A) Immunoblots and (B) qPCR analysis of thermogenic genes in SVF cells differentiated into beige adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. (C) Immunoblots and (D) qPCR analysis for mitochondrial genes in SVF cells differentiated into beige adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. (E) Immunoblots and (F) qPCR analysis of mitochondrial dynamics-related genes in SVF cells differentiated into beige adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. (G) Immunoblots of mitochondrial dynamics- and adipogenesis-related proteins in the SVF cells of brown adipose tissue differentiated into brown adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. (H) Immunoblots for DRP1, thermogenic-, mitochondrial content- and mitochondrial dynamics-related proteins in SVF cells transfected with scrambled siRNA (siScr) or siRNA against Drp1 (siDRP1) and then differentiated into beige adipocytes. Data for the differentiation assays was collected after 6 days of differentiation. Bar graphs are presented as mean±s.e.m. (**P<0.01 ***P<0.001, ****P<0.0001). Und, undifferentiated. Actin is shown as loading control for immunoblots, and β-actin was used to normalize gene expression.
Brown adipocyte differentiation is strongly associated with mitochondrial biogenesis (Uldry et al., 2006). Because DRP1-mediated fission is part of the mitochondrial biogenesis machinery, and because the thermogenic program is strongly associated with mitochondrial biogenesis (Cannon and Nedergaard, 2008), we assessed whether inhibition of DRP1 affects adipogenesis-mediated mitochondrial biogenesis. Western blot analysis showed that the expression of mitochondrial proteins such as HSP60 (also known as HSPD1), SDHA and TOM20 was decreased in adipocytes differentiated in the presence of mdivi-1 (Fig. 2C). Similarly, staining with MitoTracker Green FM revealed higher staining intensity in differentiated beige adipocytes, compared to staining in mdivi-1-treated adipocytes (Fig. S1A). Furthermore, mdivi-1 treatment decreased the mRNA levels of numerous mitochondrial genes, including Atp6 (Mtatp6), Cox-1 (mt-Co1), Cox-2 (mt-Co2), and Nd2 (Mtnd2) (Fig. 2D). Taken together, this suggests that mitochondrial content is decreased in beige adipocytes differentiated in the presence of ndivi-1. When we assessed mitochondrial-dynamics-related proteins, we found that the expression of MFF, OPA1 and MFN2, were decreased both at the mRNA and protein levels in mdivi-1-treated adipocytes (Fig. 2E,F). We also found that DRP1 expression was induced by differentiation of SVF cells from BAT tissue to brown adipocytes (Fig. S1B). Furthermore, mdivi-1 treatment attenuated brown adipocyte differentiation in the BAT SVF cells (Fig. 2G; Fig. S1C), suggesting that DRP1 is also essential for brown adipocyte differentiation.
Mdivi-1 has been shown to exert a DRP1-independent effect on mitochondrial homeostasis (Ruiz et al., 2018). Therefore, to further exclude the off-target effects of pharmacological inhibition of DRP1, we knocked down DRP1 in SVF cells using siRNA. As Fig. 2H shows, both basal levels and the differentiation-mediated increase of DRP1 expression were decreased in cells transfected with siDRP1. Similar to mdivi-1 treatment, knockdown of DRP1 decreased the expression of UCP1, HSP60, SDHA, MFN2 and OPA1 (Fig. 2H). Taken together, the data demonstrate that inhibition of DRP1 mitigates the thermogenic mitochondrial remodeling of the beige and brown adipocytes.
DRP1 inhibition ablated the beige adipogenic program
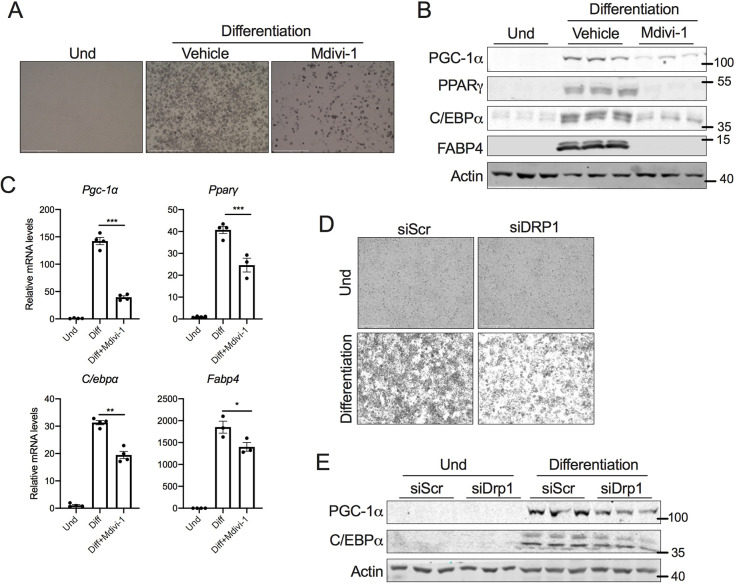
The morphological assessment of differentiated adipocytes showed a dramatic decrease in lipid droplets when DRP1 was inhibited (Fig. 3A). Therefore, we investigated whether DRP1 inhibition attenuated beige adipocyte differentiation, which is regulated by a complex network of transcriptional factors (Farmer, 2006). During the early phase of adipocyte differentiation, the activation of CCAAT/enhancer-binding protein-β and -δ (C/EBPβ and C/EBPδ) is followed by the induction of CCAAT/enhancer-binding protein-α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ) (Guo et al., 2015). The late phase of adipogenesis involves C/EBPα- and PPARγ-mediated expression of PGC-1α (also known as PPARGC1a) and other genes involved in adipocyte differentiation (Guo et al., 2015; Rosen and MacDougald, 2006; Rosen et al., 2000). Inhibition of DRP1 from day 0 of differentiation decreased the expression of key transcription factors involved in both the early and late phases of beige adipocyte differentiation (Fig. 3B). Consistent with this, the mRNA levels of various adipogenesis-specific genes showed a similar decrease in their expression upon DRP1 inhibition (Fig. 3C). Furthermore, the genetic knockdown of DRP1 resulted in decreased lipid accumulation (Fig. 3D) and expression of PGC-1α and C/EBPα (Fig. 3E). Collectively, the data show that DRP1 plays an essential role in beige adipocyte differentiation.
Fig. 3.
DRP1 inhibition attenuates the beige adipogenic program. (A) Representative images of SVF cells differentiated to beige adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. Scale bars: 500 µm. (B) Immunoblots and (C) qPCR analysis of key transcription factors involved in adipogenesis using differentiated beige adipocytes in the presence or absence of 10 µM mdivi-1 added from day 0 of differentiation. β-actin was used to normalize the gene expression, and actin is shown as a loading control for the immunoblots. (D) Representative images of SVF cells transfected with scrambled siRNA (siScr) or siRNA against Drp1 (siDRP1) and differentiated into beige adipocytes. Scale bars: 275 µm. (E) Immunoblots for proteins involved in adipogenesis in SVF cells with or without knockdown of Drp1 that are differentiated to beige adipocytes. Actin is shown as a loading control. Data for the differentiation assays was collected after 6 days of differentiation. Bar graphs are presented as mean±s.e.m. (*P<0.05, **P<0.01, ***P<0.001). Und, undifferentiated.
DRP1 is indispensable for the induction of beige adipocyte differentiation
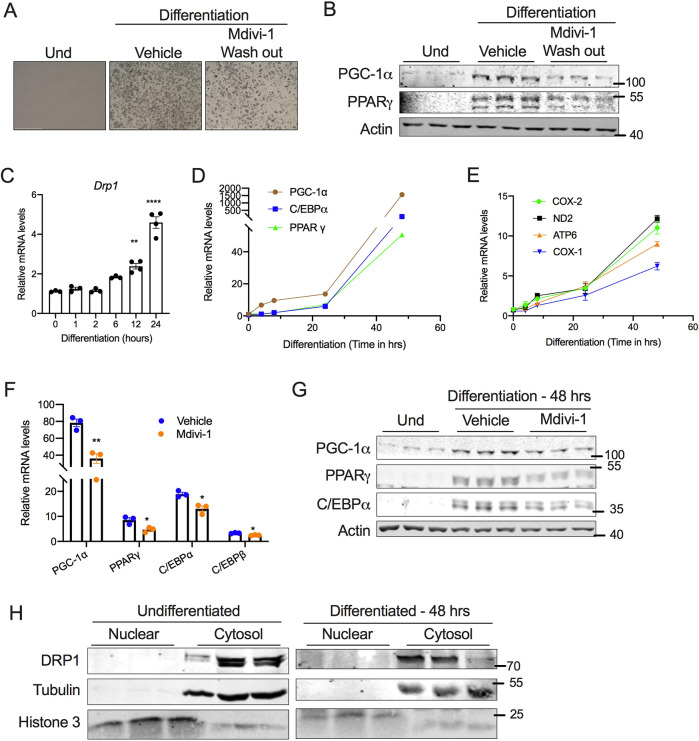
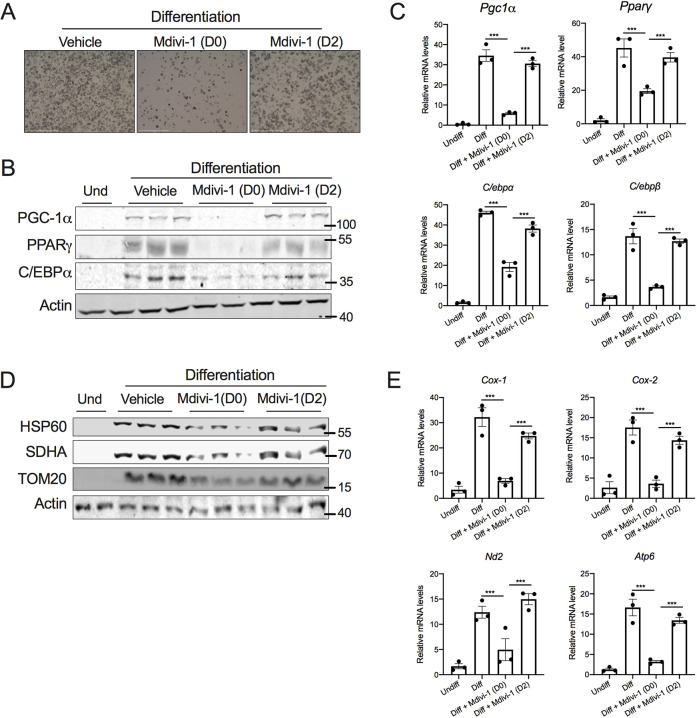
To further delineate the requirement of DRP1 for beige adipocyte differentiation, we inhibited DRP1 during the induction period alone by treating SVF cells with mdivi-1 on day 2 of differentiation (D2) and then washing the wells with medium before continuing the differentiation in maintenance medium without mdivi-1. Restoring DRP1 activity by washing out mdivi-1 did not rescue the differentiation potential of the SVF cells, as revealed by the morphological assessment of lipid droplets (Fig. 4A). Moreover, the expression of adipogenesis-related proteins was not restored in mdivi-1-washout cells (Fig. 4B). Further analysis showed that the mRNA levels of Drp1 were significantly increased as early as 6 h following differentiation (Fig. 4C), indicating that the increase in DRP1 during the induction period is essential to promote beige adipocyte differentiation. We then sought to determine the mechanism by which inhibition of DRP1 during the induction period attenuates beige adipogenesis. The expression of genes encoding adipogenesis-related proteins (PGC-1α, PPARγ and C/EBPα) and mitochondria-related proteins (COX1, COX2, ND2, ATP6) were induced as early as 24 h following differentiation (Fig. 4D,E). We found that the inhibition of DRP1 significantly attenuated the early induction of beige adipogenesis- and mitochondria-related genes both at the mRNA and protein level (Fig. 4F,G), suggesting that DRP1 plays a critical role in the transcriptional programming of beige adipocyte differentiation. Because nuclear expression of DRP1 has been demonstrated (Chiang et al., 2009), we questioned whether adipogenesis induces nuclear translocation of DRP1. However, no detectable levels of DRP1 were observed in the nucleus of SVF cells with or without differentiation (Fig. 4H), suggesting that cytosolic DRP1 regulates the transcriptional programming of beige adipocyte differentiation.
Fig. 4.
DRP1 is indispensable for the induction of beige adipocyte differentiation. (A) Representative images for SVF cells differentiated into beige adipocytes in the absence or presence of 10 µM mdivi-1 during the induction period (48 h), followed by wash out and continued differentiation on maintenance medium without mdivi-1 for the next 6 days. Scale bars: 500 µm. (B) Immunoblots for expression of adipogenesis-related proteins in cells treated as described in A. (C–E) qPCR analysis assessing the time-dependent change in expression of (C) Drp1, (D) adipogenesis-related genes and (E) mitochondrial markers during the early phase of beige adipocyte differentiation. (F) qPCR and (G) western blot analysis of genes involved in adipogenesis assessed at 48 h following the induction of SVF cell differentiation in the presence or absence of 10 µM mdivi-1. (H) Immunoblots of DRP1 in nuclear and cytosolic fractions of SVF cells assessed at 48 h following induction of differentiation. In C–F, β-actin was used to normalize gene expression. In B and G, actin is shown as a loading control. In H, tubulin and histone 3 are shown as cytosolic and nuclear loading controls, respectively. Quantitative data are presented as mean±s.e.m. (*P<0.05, **P<0.01, ****P<0.0001). Und, undifferentiated.
DRP1 is dispensable during the late phase of beige adipocyte differentiation
Thus far, we have demonstrated that inhibition of DRP1 during differentiation suppressed beige adipocyte differentiation. The acquisition of the mature adipocyte phenotype by precursor cells involves sequential steps, which is reflected by the appearance of early, intermediate and late mRNA and protein markers of adipocyte differentiation (Kelly and Scarpulla, 2004). To further elucidate the role of DRP1 in beige adipogenesis, we took an alternative approach where we inhibited DRP1 during the maintenance period alone. To this end, beige adipocyte differentiation was induced in the inguinal SVF cells, and on day 2 of differentiation the cells were switched to maintenance medium with or without mdivi-1 and continued with the respective treatments throughout the differentiation period. Interestingly, inhibition of DRP1 during the maintenance period (D2) did not affect beige adipogenesis, as revealed by visual examination of lipid droplets (Fig. 5A). Moreover, inhibition of DRP1 during the maintenance period did not affect the mRNA and protein levels of beige adipogenesis- or mitochondria-related genes (Fig. 5B–E). Collectively, the data emphasize that DRP1 is dispensable during the maintenance period of beige adipocyte differentiation.
Fig. 5.
DRP1 is dispensable during the late phase of beige adipocyte differentiation. SVF cells were differentiated into beige adipocytes in the presence or absence of 10 µM mdivi-1 added either from day 0 (D0) or day 2 (D2) of differentiation and continued until 6 days of differentiation. (A) Representative images of the differentiated beige adipocytes. Scale bars: 500 µm. (B) Immunoblots and (C) qPCR analysis of adipogenesis-related genes. (D) Immunoblots and (E) qPCR analysis of mitochondria-related genes in the differentiated beige adipocytes. Data for the differentiation assays was collected after 6 days of differentiation. β-actin was used to normalize gene expression and actin immunoblots are shown as a loading control. Bar graphs are presented as mean±s.e.m. (***P<0.001). Und, undifferentiated.
DISCUSSION
Mitochondrial dynamics play a pivotal role in diverse cellular processes, such as brown adipocyte differentiation, lipid homeostasis and oxidative metabolism. Adipose tissue mitochondrial dysfunction contributes significantly to the pathogenesis of obesity-associated metabolic diseases (Cedikova et al., 2016; Chouchani and Kajimura, 2019; Dai and Jiang, 2019; Lee et al., 2019). Therefore, it is imperative to identify the mechanisms involved in the biogenesis and functional maintenance of mitochondria in the adipocytes. In the present study, we explored the role of the mitochondrial fission protein DRP1 in the adipose tissue. We found that DRP1 is highly expressed in BAT and that DRP1 expression levels increase during beigeing and brown adipocyte differentiation. We demonstrated that inhibition of DRP1 during the induction period is sufficient to attenuate beige and brown adipogenesis and adipogenesis-associated mitochondrial biogenesis. However, we found that DRP1 is dispensable during the post-induction period (i.e. after induction of differentiation). This is consistent with a previous study, where knockdown of DRP1 in mature adipocytes did not affect white-to-beige adipocyte conversion (Pisani et al., 2018). We demonstrated that DRP1 plays an essential role in the early phase of differentiation by regulating numerous genes involved in the transcriptional programming of brown adipogenesis through unclear mechanisms.
The adipogenic program is a highly regulated process that involves the formation of mature adipocytes from the precursor cells (Fève, 2005). It is evident from the literature that adipogenesis not only involves the activation of the transcriptional network but also requires mitochondrial remodeling (Wilson-Fritch et al., 2003). During the early phase of adipogenesis, precursor cells are characterized by distinct mitochondrial dynamics and network reorganization, compared to mature adipocytes (Chen and Chan, 2017; Seo et al., 2018). Our results reveal that the inhibition of DRP1 in adipocyte precursor cells attenuates the early induction of the adipogenic transcriptional factors such as C/EBPα, PPARγ, and PGC-1α.
Adipocyte differentiation is associated with the nuclear translocation of various proteins involved in the regulation of adipogenic transcription factors (Singh et al., 2006). Recent studies have demonstrated a nuclear localization of DRP1 (Chiang et al., 2009), raising the possibility of a transcriptional regulatory role for DRP1 in beige and brown adipogenesis. However, we were unable to detect nuclear localization of DRP1 in precursor or mature adipocytes. DRP1 has been shown to interact with numerous proteins that are involved in cell cycle progression, differentiation and metabolism (Chou et al., 2012; Ganesan et al., 2019). Therefore, assessing the binding partners of DRP1 will provide novel insights on the mechanism by which cytosolic DRP1 induces nuclear reprogramming of the adipocyte transcriptional network. We recently demonstrated that caloric restriction-mediated induction of beige adipocytes is associated with an increase in the adipose tissue expression of DRP1 (Mooli et al., 2020). Therefore, our future studies will be aimed at understanding the molecular underpinnings of DRP-1 regulation of beige and brown adipogenesis using mouse models with DRP1 deletion in adipocyte precursor cells.
Obesity and other metabolic diseases are associated with adipose tissue dysfunction and attenuated beigeing and browning efficiencies (Dai and Jiang, 2019). However, the molecular mechanisms are not completely understood. Here, we propose that DRP1 plays an essential role in the commitment of adipocyte precursor cells by regulating the induction of adipogenic transcriptional factors. Further investigation of the expression and post-translational modifications of DRP1 in the adipocyte precursors will provide novel insights on the role of mitochondrial remodeling in obesity and metabolic diseases.
MATERIALS AND METHODS
Animals
Male C57BL6 mice (10 weeks old) were purchased from Jackson Laboratories, Bar Harbor, ME. Upon arrival, all the mice were acclimatized in a specific pathogen-free facility in 12-h day and night cycles and continued on their chow diet (D12450J, Research Diets, New Brunswick, NJ). For β3-adrenergic agonist treatment, CL316,243 was injected intraperitoneally at a dose of 1 mg/kg bodyweight once a day for 7 days. All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Isolation, culture, differentiation and treatment of primary SVF cells
Primary mouse inguinal adipose stromal vascular fraction (SVF) cells were isolated as described previously (Liu et al., 2017). Briefly, the inguinal white and brown adipose tissues were quickly collected and minced in a Petri dish containing freshly prepared digestion buffer with collagenase type D (for inguinal white fat) and type B (for brown fat) and dispase II. The tissues were incubated at 37°C for 20–30 min with constant agitation. The digestion was neutralized with DMEM/F12 medium (Cat. No. 10-090-CV, Corning) and filtered using a 100-µm cell strainer. The filtrate was centrifuged at 500 g for 5 min at room temperature and the pellet was suspended in DMEM/F12 medium and further filtered using a 40-µm cell strainer then centrifuged at 500 g for 5 min at room temperature. Finally, the SVF cell pellet was resuspended and plated in a 10 cm plate in DMEM/F12 medium supplemented with 10% FBS and 1% penicillin/streptomycin. The preadipocytes were cultured in DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C. For beige or brown differentiation, cells were plated in a 12-well plate and, once cells reached 70–80% confluency (designated as day 0), differentiation was induced using induction medium consisting of DMEM/F12 containing 10% FBS, 0.5 mM isobutylmethylxanthine, 125 nM indomethacin, 1 μM dexamethasone, 850 nM insulin, 1 nM thyroid hormone T3, and 1 μM rosiglitazone (Lu et al., 2018). 2 days after induction, cells were switched to maintenance medium consisting of DMEM/F12 containing 10% FBS, 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone and were maintained for another 4–6 days. Maintenance medium was refreshed every 48 h.
Culture, differentiation, siRNA transfection and treatment of SVF cells
Unless mentioned as primary SVF cells in the figure legends, all the experiments using SVF cells were performed using a transformed mouse inguinal SVF preadipocyte cell line (EVC005) purchased from Kerafast, Inc. (Boston, MA). Transformed SVF cells were cultured and differentiated to beige adipocytes as explained above for the primary SVF cells. For DRP1 inhibition, cells were treated with 10 µM mdivi-1 (Cassidy-Stone et al., 2008) (Cat no A4472; APExBIO) from day 0 (D0; induction period) or day 2 (D2; post-induction). For mdivi-1 washout experiments, 10 µM mdivi-1 was added during the induction period (D0) and on D2 the wells were washed and the differentiation process was continued without mdivi-1. For knockdown experiments, 60–70%-confluent SVF cells were transfected with 25 pmol scrambled or pre-designed siRNA against Drp1 (DNM1L Silencer select #s92135; Thermo Fisher Scientific; sense, 5′-CGAUUGAAGGAACCGCAAtt-3′; antisense, 5′-UUUGCGGUUCCUUCAAUCGtg-3′) using Lipofectamine RNAiMAX transfection reagent (Thermofisher Scientific). At 24-h post-transfection, SVF cells were differentiated to beige adipocytes as detailed above.
MitoTracker Green FM assay
Undifferentiated and differentiated beige adipocytes in the presence or absence of mdivi-1 (at day 6) were incubated with 50 nM MitoTracker Green FM (Thermo Fisher Scientific) for 30 min at 37°C. The cells were briefly washed with 1× PBS and images were captured using an Evos fluorescence microscope. The background noise was corrected equally for all the images using Adobe Photoshop.
Reverse transcription qPCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) as per the manufacturer's instructions. 1 μg of total RNA was reverse transcribed using Mu-MLV reverse transcriptase (Promega, Madison, WI). The gene expression was evaluated by quantitative real-time PCR using SYBR Green Mastermix (Radiant Molecular Tools, Fort Lauderdale, FL) with a Quant Studio 3 Station qPCR machine (Applied Biosystems). The results were expressed as fold-change using the 2−ΔΔCT method, and β-actin was used as an internal normalization control (A list of the primer sequences used is provided in Table S1).
Western blotting
Protein lysates were prepared using RIPA lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 5 mM EDTA and 1% NP-40) containing protease and phosphatase inhibitors (Sigma Aldrich). The protein lysates were separated on 8–12% polyacrylamide gels and transferred to nitrocellulose or PVDF membranes. Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.01% Tween20 and probed with primary antibodies (see Table S2) overnight at 4°C. Membranes were then probed with DyLight-conjugated secondary antibodies (Cell Signaling Technology) and visualized using an Odyssey CLx Imaging System (LI-COR).
Nuclear and cytosolic protein lysate preparations
The nuclear and cytosolic protein lysates were prepared using the NE-PER Nuclear and Cytoplasmic Extraction kit (ThermoFisher Scientific) according to the manufacturer's instructions.
Statistical analysis
The experimental data were analyzed using GraphPad Prism 8 (Version 8.1.2) software. The data are presented as mean±s.e.m. Significance between experimental groups was derived using an unpaired two-tailed t-test for two groups or a one-way ANOVA (with Tukey's multiple comparison test) for multiple groups. P<0.05 was considered significant. All the in vitro experiments were carried out in triplicate and repeated at least twice.
Supplementary Material
Footnotes
Author contributions
Conceptualization: R.G.R.M., S.K.R.; Methodology: R.G.R.M.; Validation: R.G.R.M.; Formal analysis: R.G.R.M.; Investigation: R.G.R.M., D.M., N.B., Z.C.; Writing - original draft: R.G.R.M., S.K.R.; Writing - review & editing: R.G.R.M., D.M., N.B., S.K.R.; Supervision: S.K.R.; Funding acquisition: S.K.R.
Funding
This work was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK110537) and a Pittsburgh Liver Research Center Pilot and Feasibility grant (P30DK120531) to S.K.R.
Contributor Information
Raja Gopal Reddy Mooli, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA .
Dhanunjay Mukhi, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA .
Zhonghe Chen, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA .
Nia Buckner, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA .
Sadeesh K. Ramakrishnan, Division of Endocrinology and Metabolism, Department of Medicine, University of Pittsburgh, Pittsburgh, PA 15261, USA .
Peer review history
The peer review history is available online at https://jcs.biologists.org/lookup/doi/10.1242/jcs.247593.reviewer-comments.pdf
References
- Barbatelli, G. , Murano, I. , Madsen, L. , Hao, Q. , Jimenez, M. , Kristiansen, K. , Giacobino, J. P. , De Matteis, R. and Cinti, S. (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244-E1253. 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- Bleazard, W. , McCaffery, J. M. , King, E. J. , Bale, S. , Mozdy, A. , Tieu, Q. , Nunnari, J. and Shaw, J. M. (1999). The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat. Cell Biol. 1, 298-304. 10.1038/13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon, B. and Nedergaard, J. (2008). Studies of thermogenesis and mitochondrial function in adipose tissues. Methods Mol. Biol. 456, 109-121. 10.1007/978-1-59745-245-8_8 [DOI] [PubMed] [Google Scholar]
- Cassidy-Stone, A. , Chipuk, J. E. , Ingerman, E. , Song, C. , Yoo, C. , Kuwana, T. , Kurth, M. J. , Shaw, J. T. , Hinshaw, J. E. , Green, D. R. , et al. (2008). Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell 14, 193-204. 10.1016/j.devcel.2007.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedikova, M. , Kripnerová, M. , Dvorakova, J. , Pitule, P. , Grundmanova, M. , Babuska, V. , Mullerova, D. and Kuncova, J. (2016). Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016, 6067349. 10.1155/2016/6067349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H. and Chan, D. C. (2017). Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 26, 39-48. 10.1016/j.cmet.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, Y.-Y. , Chen, S.-L. , Hsiao, Y.-T. , Huang, C.-H. , Lin, T.-Y. , Chiang, I.-P. , Hsu, W.-H. and Chow, K.-C. (2009). Nuclear expression of dynamin-related protein 1 in lung adenocarcinomas. Mod. Pathol. 22, 1139-1150. 10.1038/modpathol.2009.83 [DOI] [PubMed] [Google Scholar]
- Choe, S. S. , Huh, J. Y. , Hwang, I. J. , Kim, J. I. and Kim, J. B. (2016). Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 7, 30. 10.3389/fendo.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, C.-H. , Lin, C.-C. , Yang, M.-C. , Wei, C.-C. , Liao, H.-D. , Lin, R.-C. , Tu, W.-Y. , Kao, T.-C. , Hsu, C.-M. , Cheng, J.-T. , et al. (2012). GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress. PLoS ONE 7, e49112. 10.1371/journal.pone.0049112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani, E. T. and Kajimura, S. (2019). Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 1, 189-200. 10.1038/s42255-018-0021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, W. and Jiang, L. (2019). Dysregulated mitochondrial dynamics and metabolism in obesity, diabetes, and cancer. Front. Endocrinol. (Lausanne) 10, 570. 10.3389/fendo.2019.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner, V. , Picard, M. and Hajnóczky, G. (2018). Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat. Cell Biol. 20, 755-765. 10.1038/s41556-018-0133-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer, S. R. (2006). Transcriptional control of adipocyte formation. Cell Metab. 4, 263-273. 10.1016/j.cmet.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fève, B. (2005). Adipogenesis: cellular and molecular aspects. Best Pract. Res. Clin. Endocrinol. Metab. 19, 483-499. 10.1016/j.beem.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Forni, M. F. , Peloggia, J. , Trudeau, K. , Shirihai, O. and Kowaltowski, A. J. (2016). Murine mesenchymal stem cell commitment to differentiation is regulated by mitochondrial dynamics. Stem Cells 34, 743-755. 10.1002/stem.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan, V. , Willis, S. D. , Chang, K.-T. , Beluch, S. , Cooper, K. F. and Strich, R. (2019). Cyclin C directly stimulates Drp1 GTP affinity to mediate stress-induced mitochondrial hyperfission. Mol. Biol. Cell 30, 302-311. 10.1091/mbc.E18-07-0463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt, M. and Villarroya, F. (2013). White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154, 2992-3000. 10.1210/en.2013-1403 [DOI] [PubMed] [Google Scholar]
- Guo, L. , Li, X. and Tang, Q.-Q. (2015). Transcriptional regulation of adipocyte differentiation: a central role for CCAAT/enhancer-binding protein (C/EBP) beta. J. Biol. Chem. 290, 755-761. 10.1074/jbc.R114.619957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms, M. J. , Ishibashi, J. , Wang, W. , Lim, H.-W. , Goyama, S. , Sato, T. , Kurokawa, M. , Won, K.-J. and Seale, P. (2014). Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 19, 593-604. 10.1016/j.cmet.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, K. , Maretich, P. and Kajimura, S. (2018). The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 29, 191-200. 10.1016/j.tem.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn, C. R. , Wang, G. and Lee, K. Y. (2019). Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Invest. 129, 3990-4000. 10.1172/JCI129187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura, S. , Spiegelman, B. M. and Seale, P. (2015). Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546-559. 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D. P. and Scarpulla, R. C. (2004). Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357-368. 10.1101/gad.1177604 [DOI] [PubMed] [Google Scholar]
- Khacho, M. , Clark, A. , Svoboda, D. S. , Azzi, J. , MacLaurin, J. G. , Meghaizel, C. , Sesaki, H. , Lagace, D. C. , Germain, M. , Harper, M.-E. , et al. (2016). Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19, 232-247. 10.1016/j.stem.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Lee, J. H. , Park, A. , Oh, K.-J. , Lee, S. C. , Kim, W. K. and Bae, K.-H. (2019). The role of adipose tissue mitochondria: regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 20, 4924. 10.3390/ijms20194924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Zheng, L. D. , Donnelly, S. R. , Emont, M. P. , Wu, J. and Cheng, Z. (2017). Isolation of mouse stromal vascular cells for monolayer culture. Methods Mol. Biol. 1566, 9-16. 10.1007/978-1-4939-6820-6_2 [DOI] [PubMed] [Google Scholar]
- Lu, X. , Altshuler-Keylin, S. , Wang, Q. , Chen, Y. , Henrique Sponton, C. , Ikeda, K. , Maretich, P. , Yoneshiro, T. and Kajimura, S. (2018). Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 11, eaap8526. 10.1126/scisignal.aap8526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, P. and Chan, D. C. (2016). Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379-387. 10.1083/jcb.201511036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooli, R. G. R. , Mukhi, D. , Watt, M. , Edmunds, L. , Xie, B. , Capooci, J. , Reslink, M. , Eze, C. , Mills, A. , Stolz, D. B. , et al. (2020). Sustained mitochondrial biogenesis is essential to maintain caloric restriction-induced beige adipocytes. Metabolism 107, 154225. 10.1016/j.metabol.2020.154225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani, D. F. , Barquissau, V. , Chambard, J.-C. , Beuzelin, D. , Ghandour, R. A. , Giroud, M. , Mairal, A. , Pagnotta, S. , Cinti, S. , Langin, D. , et al. (2018). Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Mol. Metab. 7, 35-44. 10.1016/j.molmet.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, E. D. and MacDougald, O. A. (2006). Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885-896. 10.1038/nrm2066 [DOI] [PubMed] [Google Scholar]
- Rosen, E. D. , Walkey, C. J. , Puigserver, P. and Spiegelman, B. M. (2000). Transcriptional regulation of adipogenesis. Genes Dev. 14, 1293-1307. [PubMed] [Google Scholar]
- Ruiz, A. , Alberdi, E. and Matute, C. (2018). Mitochondrial division inhibitor 1 (mdivi-1) protects neurons against excitotoxicity through the modulation of mitochondrial function and intracellular Ca2+ signaling. Front. Mol. Neurosci. 11, 3. 10.3389/fnmol.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, I. and Youle, R. J. (2010). Mitochondrial fission and fusion. Essays Biochem. 47, 85-98. 10.1042/bse0470085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, B. J. , Yoon, S. H. and Do, J. T. (2018). Mitochondrial dynamics in stem cells and differentiation. Int. J. Mol. Sci. 19, 3893. 10.3390/ijms19123893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepa-Kishi, D. M. and Ceddia, R. B. (2018). White and beige adipocytes: are they metabolically distinct? Horm. Mol. Biol. Clin. Investig. 33, 1868-1891. 10.1515/hmbci-2018-0003 [DOI] [PubMed] [Google Scholar]
- Sidossis, L. and Kajimura, S. (2015). Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest. 125, 478-486. 10.1172/JCI78362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, R. , Artaza, J. N. , Taylor, W. E. , Braga, M. , Yuan, X. , Gonzalez-Cadavid, N. F. and Bhasin, S. (2006). Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 147, 141-154. 10.1210/en.2004-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova, E. , Griparic, L. , Shurland, D.-L. and van der Bliek, A. M. (2001). Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245-2256. 10.1091/mbc.12.8.2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilokani, L. , Nagashima, S. , Paupe, V. and Prudent, J. (2018). Mitochondrial dynamics: overview of molecular mechanisms. Essays Biochem. 62, 341-360. 10.1042/EBC20170104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldry, M. , Yang, W. , St-Pierre, J. , Lin, J. , Seale, P. and Spiegelman, B. M. (2006). Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 3, 333-341. 10.1016/j.cmet.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Vosselman, M. J. , van Marken Lichtenbelt, W. D. and Schrauwen, P. (2013). Energy dissipation in brown adipose tissue: from mice to men. Mol. Cell. Endocrinol. 379, 43-50. 10.1016/j.mce.2013.04.017 [DOI] [PubMed] [Google Scholar]
- Westermann, B. (2002). Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep. 3, 527-531. 10.1093/embo-reports/kvf113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch, L. , Burkart, A. , Bell, G. , Mendelson, K. , Leszyk, J. , Nicoloro, S. , Czech, M. and Corvera, S. (2003). Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell. Biol. 23, 1085-1094. 10.1128/MCB.23.3.1085-1094.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Boström, P. , Sparks, L. M. , Ye, L. , Choi, J. H. , Giang, A.-H. , Khandekar, M. , Virtanen, K. A. , Nuutila, P. , Schaart, G. , et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366-376. 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. and Chan, D. C. (2007). New insights into mitochondrial fusion. FEBS Lett. 581, 2168-2173. 10.1016/j.febslet.2007.01.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.