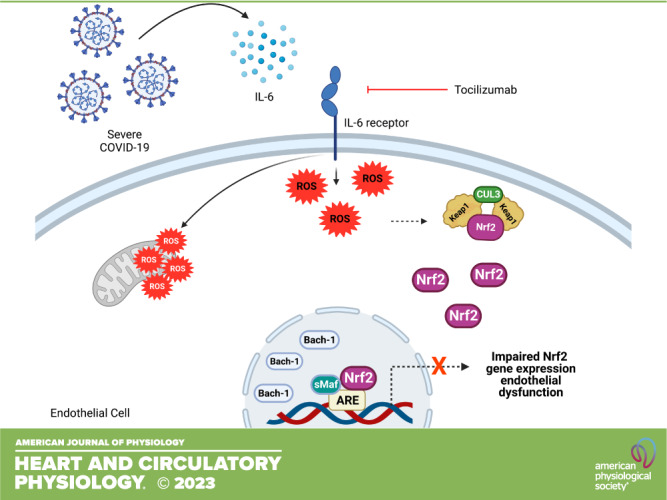
Keywords: COVID-19, endothelial cells, IL-6, Nrf2, vascular function
Abstract
The cytokine storm in SARS-CoV-2 infection contributes to the onset of inflammation and target-organ damage. The endothelium is a key player in COVID-19 pathophysiology and it is an important target for cytokines. Considering that cytokines trigger oxidative stress and negatively impact endothelial cell function, we sought to determine whether serum from individuals with severe COVID-19 decreases endothelial cells’ main antioxidant defense, i.e., the antioxidant transcriptional factor Nrf2. Human umbilical vein endothelial cells (HUVECs) were incubated with serum from patients with severe COVID-19 at different time points and the effects on redox balance and Nrf2 activity were determined. Serum from individuals with COVID-19 increased oxidant species, as indicated by higher DHE (dihydroethydine) oxidation, increased protein carbonylation, and induced mitochondrial reactive oxygen species (ROS) generation and dysfunction. Serum from patients with COVID-19, but not serum from healthy individuals, induced cell death and diminished nitric oxide (NO) bioavailability. In parallel, Nrf2 nuclear accumulation and the expression of Nrf2-targeted genes were decreased in endothelial cells exposed to serum from individuals with COVID-19. In addition, these cells exhibited higher expression of Bach-1, a negative regulator of Nrf2 that competes for DNA binding. All events were prevented by tocilizumab, an IL-6 receptor blocker, indicating that IL-6 is key to the impairment of endothelial antioxidant defense. In conclusion, endothelial dysfunction related to SARS-CoV-2 infection is linked to decreased endothelial antioxidant defense via IL-6-dependent mechanisms. Pharmacological activation of Nrf2 may decrease endothelial cell damage in individuals with severe COVID-19.
NEW & NOTEWORTHY We demonstrate that endothelial cell dysfunction in SARS-CoV-2-infected individuals is linked to decreased activity of the major antioxidant system regulator, the Nrf2 transcription factor. We provide evidence that this phenomenon relies on IL-6, an important cytokine involved in the pathophysiology of COVID-19. Our data support the view that Nrf2 activation is a potential therapeutical strategy to prevent oxidative stress and vascular inflammation in severe cases of COVID-19.
INTRODUCTION
At the end of 2019, the world was afflicted by a pandemic outbreak, the coronavirus disease 2019 (COVID-19) (1), and several deaths were caused mainly by acute respiratory distress syndrome (2). Although the advent of vaccination has resulted in a massive reduction in deaths, the comorbidities found during the illness and after COVID-19 have impacted several clinical scenarios, including those associated with the cardiovascular system (3).
Classically, viral diseases, including respiratory diseases, are marked by a unique profile in the production of inflammatory cytokines (4). Epithelial and endothelial cells, and especially cells of the immune system, contribute to the cytokine storm found in these diseases. Nevertheless, the inflammatory identity in COVID-19 has been described as an important factor in the physiopathogenesis and the deleterious consequences for many systems and organs (5). In addition to the massive cytokine release, the generation of reactive oxygen species (ROS) and coagulation events are commonly reported during COVID-19 (6). In the cardiovascular system, these events culminate in increased vascular permeability, leakage of blood content, and impaired tissue perfusion, contributing to multiple organ failure (7).
The vasculature, mainly the endothelium, is a key component of the systemic effects of COVID-19 (8). After infection by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the endothelium becomes “activated,” displaying increased expression of adhesion molecules, increased ROS generation, procoagulant signaling and vascular leakage, events that increase immune cells chemotaxis and adjacent tissue damage (9).
Oxidative stress, a condition where ROS are produced in amounts that cells are unable to eliminate, leads to cell damage and apoptosis. However, cells also rely on intrinsic counterregulatory mechanisms, which include antioxidant enzymes, aimed to restore redox balance (10). The effectors of the antioxidant system are tightly regulated by the erythroid nuclear factor-related factor 2 (Nrf2), an important antioxidant transcription factor. Decreased Nrf2 activity has been implicated in the pathogenesis of chronic, inflammatory, neurodegenerative, and cardiovascular diseases (11) Experimental and clinical evidence demonstrates that Nrf2 inducers, such as l-sulforaphane (SFN), an isothiocyanate found in cruciferous vegetables, reduce diabetic cardiomyopathy and nephropathy, and improve glucose metabolism (12–14). SFN acts as an indirect antioxidant interacting with the main negative regulator of Nrf2, the Keap1 protein, allowing Nrf2 to translocate into the nucleus and to start the antioxidant response (15).
Nrf2 has also emerged as a novel anti-inflammatory and antiviral transcriptional factor. It regulates the expression of proinflammatory cytokines, such as interleukin IL-1β and IL-6, and inhibits viral replication (16). In this context, decreased Nrf2 signaling/expression has been reported in biopsies from deceased patients with COVID-19, and in vitro analysis showed that 4-octyl-itaconate and dimethyl fumarate (Nrf2 activators) inhibit SARS-CoV-2 replication and the expression of inflammatory genes (17, 18). There are no data on the impact of COVID-19 on the cell antioxidant system or whether the pharmacological activation of Nrf2 reduces ROS generation and inflammation linked to COVID-19.
Our group recently demonstrated that COVID-19 causes endothelial cell damage through activation of the TLR9-mitochondrial DNA axis (19) and that serum of patients with COVID-19 promotes glycocalyx shedding, redox imbalance (20), and dysfunction of umbilical cord arteries (21). In the present study, we hypothesized that the cytokine storm in patients with severe COVID-19 impairs the endothelial cell antioxidant capacity through the downregulation of Nrf2, leading to endothelial cell oxidative stress and dysfunction, which, in turn, may contribute to increased cardiovascular risk in patients with COVID-19.
MATERIALS AND METHODS
Human Samples
The Brazilian National Committee for Ethics in Human Research (CONEP) approved all experimental procedures (CONEP CAAE: 30816620.0.0000.5440). This investigation also followed the principles of the Helsinki declaration and the resolution #466/2012 from the Brazilian Ministry of Health for research involving humans. Written informed consent was obtained from all participants, or their next of kin, before inclusion in the present investigation.
The population consisted of 25 hospitalized individuals in the Hospital das Clínicas de Ribeirão Preto (Ribeirao Preto Medical School) with a positive RT-PCR for SARS-CoV-2 infection (nasopharyngeal samples), and a control group with eight healthy individuals who tested negative for SARS-CoV-2 infection (Supplemental Fig. S1; see https://doi.org/10.6084/m9.figshare.22114838.v2).
Blood from individuals with COVID-19 was collected on the first day of admission to the intensive care unit of Hospital das Clínicas de Ribeirão Preto, between May 2020 and January 2021. For the clinical classification of COVID-19 severity, we used the World Health Organization Clinical Progression Scale (22). Individuals that required intensive care unit admission and mechanical ventilation or oxygen therapy by noninvasive ventilation or high-flow nasal cannula were classified as having severe disease. Blood from healthy individuals was collected after an 8-h fasting period. Blood samples were collected in tubes without anticoagulants to obtain the serum and were stored at −80°C for later analysis. Table 1 summarizes clinical information and biochemical parameters for individuals with COVID-19 and healthy subjects.
Table 1.
Baseline characteristics of healthy individuals and individuals with COVID-19
| Healthy | COVID-19 | P Value | |
|---|---|---|---|
| n | 8 | 25 | |
| Age [interquartile range], yr | 25 [20–35] | 52 [45–70] | <0.05a |
| Male sex, % | 62.5 | 60 | >0.05b |
| Female sex, % | 37.5 | 40 | >0.05b |
| Hypertension, % | 0 | 60 | <0.05b |
| Obesity, % | 0 | 40 | <0.05b |
| Diabetes, % | 0 | 40 | <0.05b |
| Symptom days | 14 ± 3 | ||
| Hospitalization days | 28 ± 7 | ||
| Mechanical ventilation, % | 100 | ||
| Kidney injury, % | 50 | ||
| Mortality rate, % | 20 | ||
| D-dimer, mg/dL [interquartile range] | 0.8 [0.4–1.2] | 1.4 [1.1–3.8] | <0.05a |
| Fibrinogen, mg/dL [interquartile range] | 262 [204–345] | 798 [659–847] | <0.05a |
| / [interquartile range] | 160 [120–190] | ||
| SOFA score [interquartile range] | 3 [2–4.5] | ||
| SAPS-3 [interquartile range] | 46 [36–50] |
Values are means ± SD, percentages, and interquartile ranges; n, number of participants. The criteria for defining hypertension, obesity, diabetes, and kidney injury, respectively, were high blood pressure (>120/80 mmHg), BMI (>24 kg/m2), high fasting blood glucose (>100 mg/dL) and high levels of serum creatinine (>1.3 mg/dL). P < 0.05, comparison between healthy and critically ill individuals with COVID-19. aStudent’s t test; bχ2 test. BMI, body mass index; / ratio, the ratio between arterial oxygen partial pressure ( in mmHg) and fractional inspired oxygen; SAPS-3, simplified acute physiology score III; SOFA, sequential assessment of organ failure.
ELISA Assay
Serum samples collected by venous puncture from healthy individuals and individuals with severe COVID-19 were analyzed for circulating cytokines [IL-6 (Cat. No. DY206), tumor necrosis factor-α (TNF-α, Cat. No. DY210), IL-1β (Cat. No. DY201)] by enzyme-linked immunosorbent assay (ELISA) using Human DuoSet ELISA kits (R&D Systems, Minneapolis). The samples were kept at −80°C until the test was performed. The assays were performed in duplicate according to the manufacturer’s instructions. Values are expressed in pg/mL.
Cultured Endothelial Cells and Experimental Design
Human umbilical vein endothelial cells (HUVECs) were commercially purchased from ATCC (Cat. No. CRL-1730). Cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a CO2 incubator, at 37°C, 5% CO2. After confluence, cells were maintained in the presence of 2% FBS (23). Cells were incubated with serum from healthy individuals or who developed severe COVID-19 (10% vol/vol for different times) (20). To determine the mechanisms involved, cells were preincubated with tocilizumab (a recombinant humanized monoclonal antibody that acts as an IL-6 receptor antagonist, 100 µg/mL for 30 min, Actemra, Roche), l-sulforaphane (Nrf2 activator, 1 µM, for 30 min, Cayman Chemical Company; Cat. No. 14797), or respective vehicles.
Measurement of Reactive Oxygen Species
Dihydroethidium.
HUVECs were incubated in 96-well plates with serum for 15, 30, and 60 min and 6, 12, and 24 h, as previously described. At the end of the incubation periods, the dihydroethidine probe (DHE, 5 µM at 37°C, Biotrend; Cat. No. 17084-50) was added for 30 min; cells were then washed with phosphate-buffered saline (PBS). For each sample, the assay was performed in duplicate. Analyses were performed by fluorimetry, using a Flexstation 3 (Molecular Devices, San Jose), at excitation and emission wavelengths of 358 and 461 nm, respectively. Results are expressed as fluorescence intensity/mg protein. The extracted proteins were quantified by the Bradford method (24).
MitoSOX.
HUVECs were incubated for 24 h, as previously described. Mitochondrial ROS were assessed by the MitoSOX assay (excitation/emission wavelengths at 510/580 nm, Thermo Fisher; Cat. No. M36008) adapted to flow cytometry, according to previous studies (25, 26). Cells were incubated with MitoSOX (5 µM) during 30 min at 37°C. The fluorescence was detected using FACS Melody (BD Biosciences, San Jose) for MitoSOX, excited with a 488 nm blue laser, and collected with a 530/30 nm filter. Analyses were performed in FlowJo software (Becton Dickinson and Company, Franklin Lakes). The frequency of MitoSOX+ cells was evaluated in total single cells (FSC-H vs. FSC-A) and the median of fluorescence intensity (MFI) was measured in MitoSOX+ cells. Nonstained endothelial cells were used as a negative control of fluorescence intensity.
Bioenergetic Measurements Using Seahorse Mitochondrial Flux Analyses
HUVECs were incubated for 24 h in the presence of vehicle or tocilizumab as previously described. To measure the rate of oxidative phosphorylation (OXPHOS) in HUVECs, a Seahorse metabolic flux analyzer was used, according to methods previously described (27, 28). To determine the oxygen consumption rate (OCR), XF Cell Mito Stress Test kit (Agilent Technologies, Santa Clara) was used along with different pharmacological inhibitors to probe the function of individual components in the respiratory chain. Cells were incubated in XF24 culture microplates with culture medium for 24 h and the sensor cartridge was hydrated in XF Calibrant at 37°C in a non-CO2 incubator overnight. One hour before all bioenergetic assays, the culture medium was replaced with unbuffered DMEM (pH 7.4) supplemented with 4 mM l-glutamine. To estimate the basal OCR coupled to ATP synthesis, 1 μM oligomycin was injected to inhibit the ATP synthase (complex V). Typically, the decreased OCR in response to oligomycin indicates the cells were using mitochondria to generate ATP. The remaining OCR can be ascribed to either proton leakage or the demand on the mitochondrial proton gradient for the movement of ions or metabolites. To determine the maximal OCR that the cells could sustain, 0.5 μM carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) was injected, which made the mitochondrial inner membrane permeable to protons. Finally, 0.5 μM antimycin A and rotenone were injected to inhibit electron flux. The remaining OCR can be ascribed to oxygen consumption because of the formation of mitochondrial ROS and non-mitochondrial sources.
Protein Oxidation
HUVECs were incubated with serum from individuals with COVID-19 and healthy individuals for 60 min and 24 h, as previously described. Carbonylated protein levels in endothelial cells were evaluated by the OxyBlot assay (Millipore; Cat. No. S7150). The OxyBlot assay detects carbonyl groups introduced into proteins’ side chains by a site-specific mechanism. All bands in the membranes were quantified considering that many proteins undergo carbonylation (29).
Nrf2 Activity
HUVECs were incubated with serum for 60 min and 24 h. To determine the nuclear accumulation of Nrf2, nuclear extracts were isolated from endothelial cell lysates using the Active Motif nuclear extract kit (Active Motif, Carlsbad) following the manufacturer’s protocol. Cells were resuspended in 1× hypotonic buffer and centrifuged for 30 s (s) at 11,000 rpm at 4°C. Nuclear pellets were resuspended in lysis buffer provided by the manufacturer. The suspension remained for 30 min on ice on a rocking platform set at 150 rpm and then centrifuged for 10 min at 11,000 rpm. The supernatant was transferred to a prechilled microcentrifuge tube. TransAM Nrf2 ELISA kit (Active Motif) was used to measure the nuclear accumulation of Nrf2 at a wavelength of 450 nm.
Quantitative Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from HUVECs using TRIzol. RNA was treated with DNAse I (1 U/μL) and 2 μg of RNA was reverse transcribed in a reaction containing oligo dT (High-Capacity cDNA Reverse Transcription Kit, Thermo Fisher; Cat. No. 4368814). mRNA levels were quantified in triplicate by qPCR (StepOnePlusTM Life Technologies, Foster City). Specific primers (TaqManTM) for RT-qPCR were as follows: HO-1 (Hs01071088_m1), Prdx (Hs01379108_m1), NQO-1 (Hs01045993_g1), SOD-1 (Hs00533490_m1), CAT (Hs00156308_m1) and GAPDH (Hs01060665_g1), all purchased from Life Technologies. qPCR cycling conditions included 10 min at 95°C, followed by 40 cycles at 95°C for 15 s, 60°C for 1 min, and 72°C for 60 s. Dissociation curve analysis confirmed that signals corresponded to unique amplicons. Specific mRNA expression levels were normalized relatively to GAPDH mRNA levels using the comparative ΔΔCt method.
Western Blot Analysis
HUVECs were incubated with serum from healthy individuals or individuals with severe COVID-19 for 24 h. Endothelial cells were frozen in liquid nitrogen and homogenized in a lysis buffer (50 mM Tris/HCl, 150 mM NaCl, 1% Nonidet P40, 1 mM EGTA, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 mM sodium fluoride). The extracted proteins were quantified by the Bradford method. Proteins (30 μg) were separated by electrophoresis on 12% polyacrylamide gel and transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked with 5% bovine serum albumin in tris-buffered saline containing 0.1% Tween 20 (for 1 h at 24°C). Membranes were incubated with antibodies (at the indicated dilutions) overnight at 4°C. Antibodies were used as follows: anti-Keap-1 (1:1,000 dilution; Abcam; Cat. No. 66620), anti-Bach-1 (1:1,000 dilution; Santa Cruz; Cat. No. sc-271211), and anti-β-actin (1:10,000 dilution; Sigma-Aldrich Inc.; Cat. No. A3854). After incubation with the secondary antibody (1:5,000 dilution; Abcam; Cat. No. ab6721), signals were obtained by chemiluminescence and densitometrically quantified.
Cell Viability
HUVECs were incubated with serum for 15 min, 30 min, 60 min, 6 h, 12 h, and 24 h. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to assess endothelial cell viability. Cells were seeded in triplicates, at a density of ∼15,000 cells/well, and were cultivated in a 96-well plate (200 µL). After incubations, the treatment medium was discarded, and the cells were reimmersed in 20 µL of MTT salt solution (5-mg MTT in 1 mL 0.1 M PBS) and 100 µL of culture medium. After a 4-h incubation (37°C), 100-µL dimethyl sulfoxide (DMSO) was added per well, and cells were incubated for 1 h (37°C). The optical density of the formazan product was quantified using spectrophotometry (BioTek, Winooski) at 550 nm. The results were expressed as % of control viability.
Phosphatidylserine Externalization
Phosphatidylserine translocation from the inner to the outer leaflet of the plasma membrane is one of the early features of apoptosis. Cell surface phosphatidylserine was detected by phosphatidylserine-binding protein annexin V conjugated with Cy3 using the commercially available annexin V Cy3 apoptosis detection kit (98% purity, Sigma-Aldrich Inc.). HUVECs were incubated, as previously described, for 60 min and 24 h. Endothelial cells were then processed according to the kit instructions, and fluorescence intensity was detected using excitation and emission wavelengths of 503 and 527 nm, respectively. Endothelial cells stimulated with staurosporine (95% purity, 1 µM, Sigma-Aldrich Inc., Germany) were used as the positive control.
Measurement of Nitric Oxide
HUVECs were incubated in 96-well plates with the serum for 60 min and 24 h, as previously described. At the end of the incubations, cells were incubated with the 5,6-diaminofluorescein diacetate probe (DAF, 5 µM for 30 min at 37°C, Sigma-Aldrich Inc.; Cat. No. 50277) and washed with PBS. For each sample, the assay was performed in duplicate. Analyses were performed by fluorimetry, using Flexstation 3 (Molecular Devices, San Jose), at excitation and emission wavelengths of 485 and 538 nm, respectively. Results are expressed as fluorescence intensity/mg protein. The extracted proteins were quantified by the Bradford method.
Data and Statistical Analyses
Data were assessed for normality with the Shapiro–Wilk test. Medians and interquartile range values were used for the normally distributed descriptive parameters. Student’s t test was performed for comparing the median values between the groups. Categorical variables were presented with numbers and percentages. χ2 test was used to compare categorical variables. The results of the molecular experiments were analyzed by Student’s t test or one-way ANOVA, followed by the Bonferroni posttest. Outliers were analyzed and removed by Grubb’s test (α = 0.05). The Prism software, version 9.0 (GraphPad Software, San Diego, CA) was used to analyze these parameters. Data are presented as means ± SE. The acceptable level of significance was P < 0.05.
RESULTS
A total of 33 individuals were included in the present study: 25 individuals who tested positive for COVID-19 and developed the severe form of the disease and 8 healthy individuals. The use of mechanical ventilation, the / ratio, and d-dimer concentrations were used to identify the severe form of the disease. In all analyses, the clinical profile of individuals with COVID-19 was defined as potentially critical (Table 1).
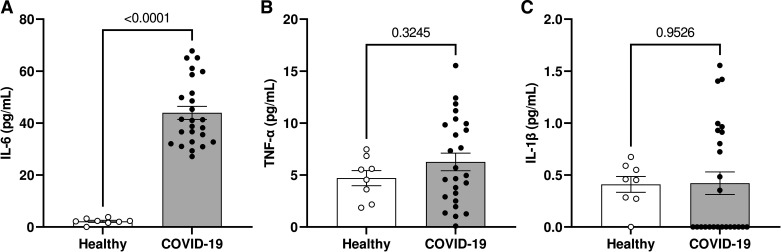
Individuals who developed severe COVID-19 had significantly higher serum levels of IL-6 compared with healthy individuals (Fig. 1A). TNF-α and IL-1β levels did not differ between the groups (Fig. 1, B and C, respectively). In all experiments, there were no differences between cells of the “Basal” group, i.e., cells that were not treated with serum either from control or individuals with COVID-19 (but that remained in the experimental protocols for the same time as cells exposed to serum) and cells that were treated with serum from healthy individuals. Therefore, the “basal” group was chosen for the statistical analyses.
Figure 1.
Individuals with severe COVID-19 exhibit higher serum IL-6 concentrations. The figures show circulating levels (in pg/mL) of IL-6 (A), TNF-α (B), and IL-1β (C) in the serum of healthy individuals and patients with severe COVID-19. Values represent means ± SE (n = 8–25). Student’s t test.
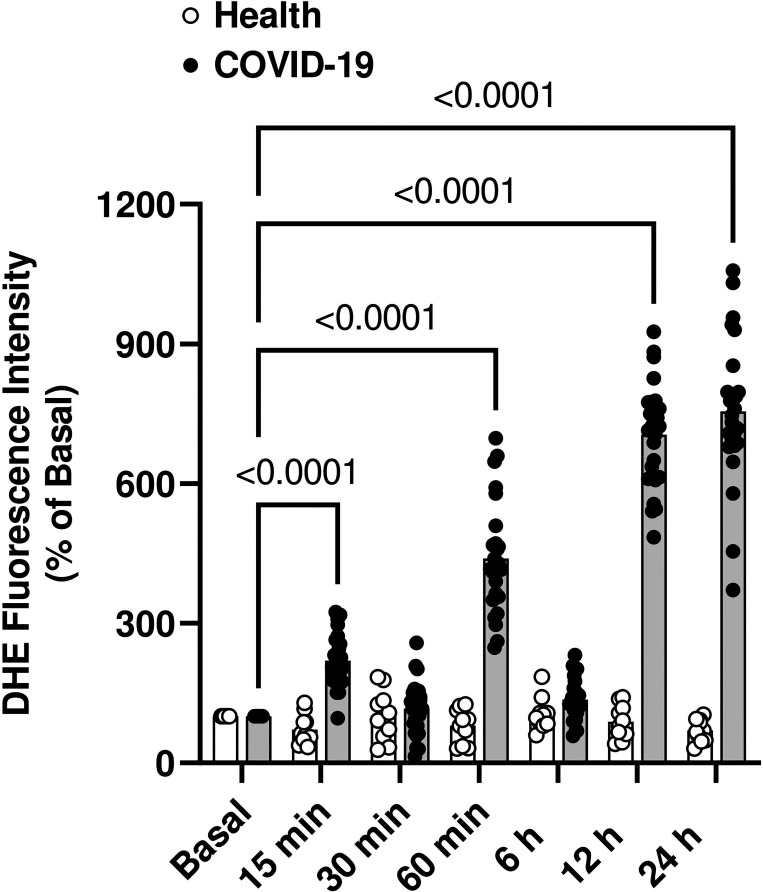
HUVECs were incubated at different time points with serum from healthy individuals and from patients with severe COVID-19. After 15 and 60 min, HUVECs exposed to serum from individuals with severe COVID-19 showed increased ROS generation, measured using the DHE probe. Increased ROS generation was also observed after 12 and 24 h of incubation (Fig. 2).
Figure 2.
Serum from patients with severe COVID-19 has prooxidant effects. HUVECs were exposed to serum from patients with severe COVID-19 or healthy individuals at different time points. Serum from healthy individuals and individuals with COVID-19 was used at a 10%, vol/vol concentration. Fluorescence intensity from dihydroethidium (DHE) oxidation. Values represent means ± SE (n = 8–25). ANOVA test. HUVECs, human umbilical vein endothelial cells.
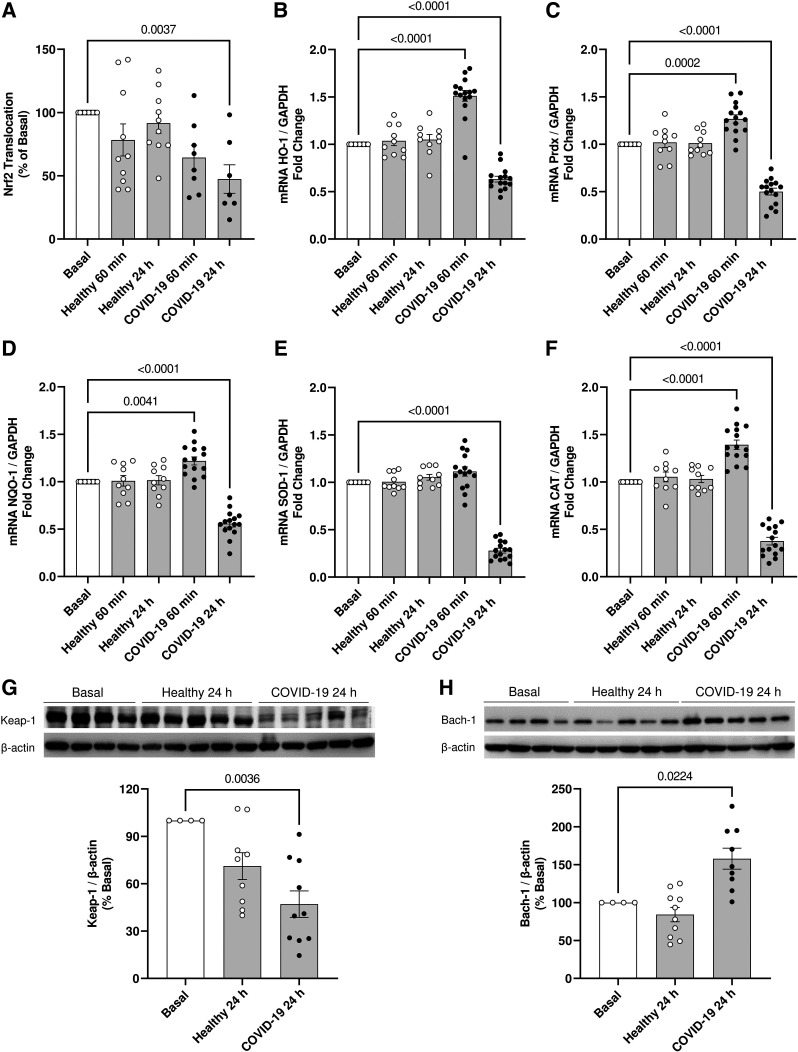
The Nrf2 antioxidant system is crucial to maintain redox balance. HUVECs incubated with serum from individuals with severe COVID-19 showed decreased Nrf2 activity after 24 h (Fig. 3A). HUVECs exposed to serum from individuals with severe COVID-19 for 24 h also exhibited decreased gene expression of the antioxidant enzymes HO-1 (Fig. 3B), Prdx (Fig. 3C), NQO-1 (Fig. 3D), SOD-1 (Fig. 3E), and CAT (Fig. 3F). Except for SOD-1, serum from individuals with severe COVID-19 increased the HUVEC gene expression of antioxidant enzymes after 60 min of incubation. After 24 h of incubation with serum from individuals with severe COVID-19, HUVECs showed decreased Keap-1 protein content (Fig. 3G) and increased Bach-1 protein expression (Fig. 3H).
Figure 3.
Serum from individuals with severe COVID-19 downregulates Nrf2 transcriptional factor activity in endothelial cells. The figure shows the nuclear translocation of Nrf2 (A) and key expression of Nrf2-targeted genes HO-1 (B), Prdx (C), NQO-1 (D), SOD1 (E), and CAT (F) in HUVECs exposed to serum from healthy individuals and patients with severe COVID-19 (10%, vol/vol concentration). Representative Western blot membranes and densitometric analysis of Keap-1 (G) and Bach-1 (H), Nrf2 negative regulators. Values represent means ± SE (n = 4–15). ANOVA test. HUVECs, human umbilical vein endothelial cells.
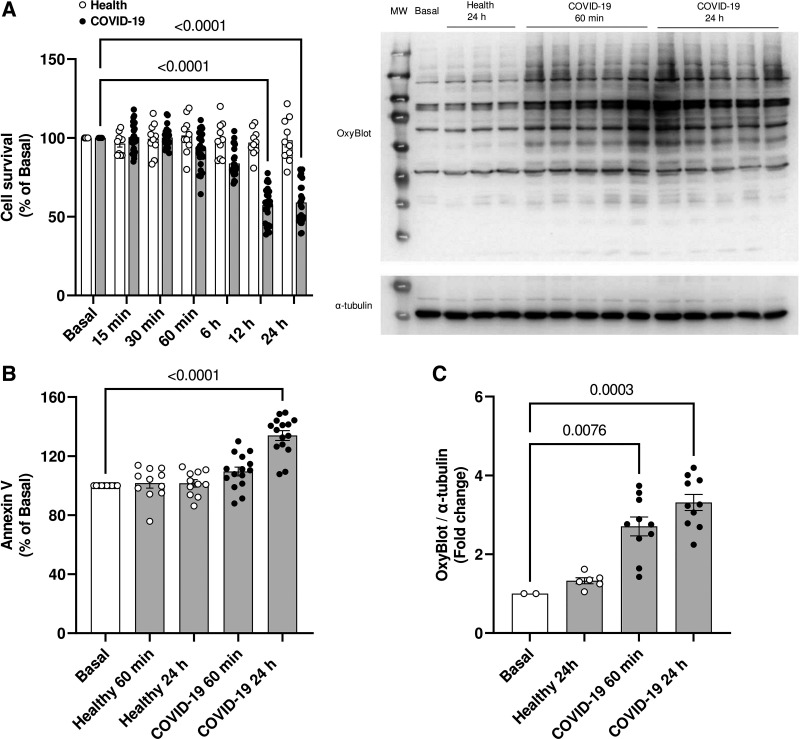
Considering that oxidative stress promotes permanent cell damage, we evaluated whether serum from individuals with severe COVID-19 decreases cell viability. In fact, after 12 and 24 h of incubation, there was a decrease in cell viability (Fig. 4A), accompanied by an increase in the apoptosis marker annexin V (Fig. 4B). After 60 min and 24 h of incubation with serum from individuals with severe COVID-19, HUVECs also showed a considerable increase in the amount of oxidized proteins (Fig. 4C).
Figure 4.
Serum from individuals with severe COVID-19 induces permanent endothelial cell damage. Cell viability (A), phosphatidylserine externalization (B), and protein oxidation levels (C) in HUVECs incubated with serum from healthy individuals or patients with severe COVID-19 (10%, vol/vol concentration). Representative membrane from Western blot depicting the degree of protein oxidation (top) and densitometric analysis (bottom) (C). Values represent means ± SE (n = 6–15). ANOVA test. HUVECs, human umbilical vein endothelial cells.
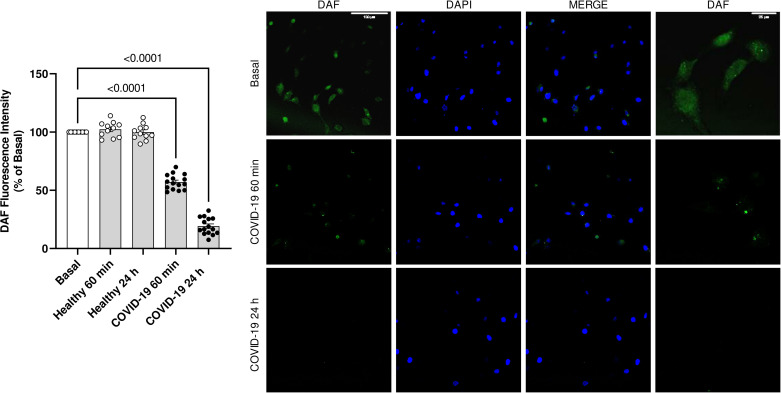
Endothelial cells are specialized in the production of nitric oxide (NO). Serum from individuals with severe COVID-19 drastically decreased NO production in HUVECs after 60 min and 24 h of incubation (Fig. 5).
Figure 5.
NO bioavailability in HUVECs decreases in response to serum from individuals with severe COVID-19. The graph (left) shows the 5,6-diaminofluorescein diacetate (DAF) fluorescence intensity. Representative immunostaining images (right). HUVECs were incubated with DAF 5 µM for 30 min after exposure to serum from COVID-19 or healthy individuals (10%, vol/vol concentration). Values represent means ± SE (n = 6–15). ANOVA test. HUVECs, human umbilical vein endothelial cells.
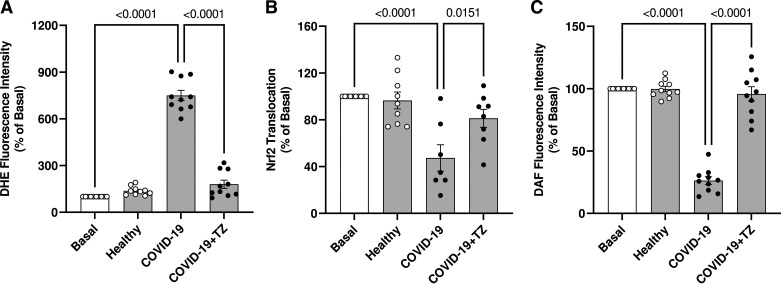
Considering that IL-6 is a potent ROS inducer (30, 31), we determined ROS generation in HUVECs exposed to serum from individuals with severe COVID-19 for 24 h, in the presence of vehicle or tocilizumab, an IL-6 receptor antibody that blocks IL-6-mediated signaling. ROS generation was decreased in the presence of tocilizumab (Fig. 6A), indicating that IL-6 contributes to ROS generation induced by serum from individuals with severe COVID-19. The presence of tocilizumab also prevented the decreased Nrf2 activity (Fig. 6B) and decreased NO production (Fig. 6C).
Figure 6.
IL-6 contributes to oxidative stress and decreases Nrf2 activity in endothelial cells. The figures show DHE fluorescence intensity (A), Nrf2 nuclear translocation (B), and DAF fluorescence intensity (C). HUVECs were treated with TZ (100 μg/mL for 30 min) before exposure to serum from severe COVID-19 and healthy individuals (10%, vol/vol concentration). Values represent means ± SE (n = 6–10). ANOVA test. DAF, 5,6-diaminofluorescein; DHE, dihydroethydine; HUVECs, human umbilical vein endothelial cells; TZ, tocilizumab.
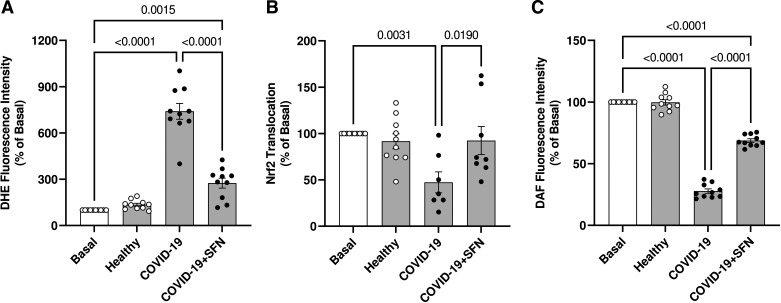
To determine whether Nrf2 plays a role in the redox imbalance induced by serum from individuals with severe COVID-19, HUVECs were incubated with the Nrf2 activator, l-sulforaphane. The presence of l-sulforaphane decreased ROS generation stimulated by the serum from individuals with severe COVID-19 (Fig. 7A). Furthermore, l-sulforaphane increased Nrf2 activity (Fig. 7B) and partially recovered NO production (Fig. 7C).
Figure 7.
Activation of Nrf2 with SFN restores redox balance in HUVECs exposed to serum from individuals with severe COVID-19. The figures show DHE fluorescence intensity (A), Nrf2 nuclear translocation (B), and DAF fluorescence intensity (C). HUVECs were pretreated with SFN (1 µM for 30 min) before exposure to serum from severe COVID-19 and healthy individuals (10%, vol/vol concentration). Values represent means ± SE (n = 6–10). ANOVA test. DAF, 5,6-diaminofluorescein; DHE, dihydroethydine; HUVECs, human umbilical vein endothelial cells; SFN, l-sulforaphane.
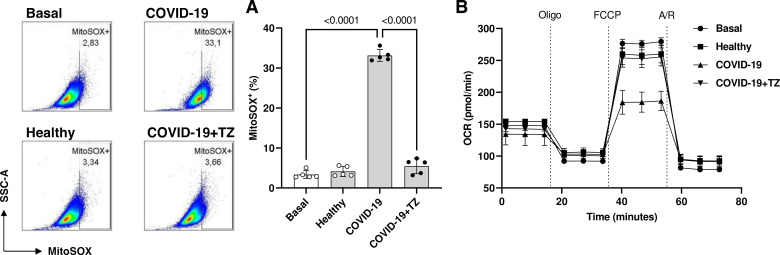
The contribution of mitochondria to ROS production induced by the serum from individuals with severe COVID-19 was also determined using a specific probe (MitoSOX). After 24 h, HUVECs exposed to serum from individuals with severe COVID-19 showed increased production of mitochondrial ROS. This increase was prevented by tocilizumab (Fig. 8A), indicating that IL-6 participates in mitochondrial ROS production. To investigate whether serum from individuals with severe COVID-19 changes mitochondrial aerobic metabolism in HUVECs, a Cell Mito Stress assay kit was used to detect the OCR. HUVECs were incubated with serum from individuals with severe COVID-19 in the presence of vehicle or tocilizumab for 24 h before exposure to oligomycin, FCCP, rotenone, and antimycin A. As shown in Fig. 8B, serum from individuals with severe COVID-19 decreased OCR in HUVECs, which was prevented by tocilizumab, suggesting that IL-6 inhibits mitochondrial aerobic respiration and potentially contributes to mitochondrial ROS generation.
Figure 8.
IL-6 is involved in mitochondria-related oxidative burst caused by the serum from individuals with severe COVID-19. A: representative dot plot (left) and respective frequency of MitoSOX+ (%) endothelial cells. B: HUVECs were treated TZ (100 μg/mL for 30 min) before exposure to serum from individuals with COVID-19 or healthy individuals for 24 h. Then, HUVECs were incubated with MitoSOX (5 µM, 30 min) to determine mitochondrial ROS. The oxygen consumption rate (OCR) was analyzed using Seahorse XF-24 Extracellular Flux analyzer after injections of Oligomycin (1 μM), FCCP (0.5 μM), and antimycin A/rotenone (0.5 μM). The values represent means ± SE (n = 5). ANOVA test. FCCP, carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone; HUVECs, human umbilical vein endothelial cells; ROS, reactive oxygen species; TZ, tocilizumab.
DISCUSSION
Cardiovascular complications resulting from COVID-19 are found in a massive part of the population, especially in individuals who have developed the severe form of the disease. This study demonstrated that the cytokine storm found in severe COVID-19 contributes to endothelial cell damage, mainly through events that impair redox balance. Our efforts were focused on understanding the role of IL-6 in endothelial oxidative stress, resulting from increased generation of ROS and decreased activity of the Nrf2 antioxidant system. We used human endothelial cells incubated with serum from individuals with severe COVID-19 as an experimental model. This model provided important information on how the components of blood circulation directly affect endothelial and vascular function.
For many researchers in vascular biology, endothelial cells not only play a significant role in vascular homeostasis, recruitment of inflammatory cells, myelopoiesis, and thromboembolism but also resemble innate immune cells, by expressing several regulatory genes of the innate immune system, such as receptors for virus infections, receptors for DAMPs and PAMPs, cytokines, regulators for metabolic, and epigenetic reprogramming related to adaptive immunity (32–34). In this context, it is evident that endothelial cells are important players in the cardiovascular damage associated with severe COVID-19.
Although many proinflammatory and prooxidant components are present in the circulation during COVID-19, we directed special attention to the actions of IL-6. Initially, we confirmed that serum from individuals with severe COVID-19 induces ROS generation. We observed specific increases in ROS generation after 15 and 60 min of incubation. ROS levels were decreased after these time points. However, after 12 and 24 h of incubation, there was a sustained increase in endothelial ROS generation. Classically, all cells have antioxidant defense mechanisms, which aim to detoxify free radicals (35). The fluctuation in ROS generation is associated with this process, and intermittent prooxidant stimuli culminate in antioxidant system failure and permanent cellular damage (36).
Based on the ROS generation profile and the sustained levels of ROS at 24 h of incubation with serum from individuals with severe COVID-19, we questioned whether the activity of the Nrf2 antioxidant transcription system was impaired. Reanalysis of public transcriptomics data generated from blood, immune cells, and lung specimens from subjects with COVID-19 showed increased gene expression of prooxidant markers and decreased expression patterns of antioxidant genes (6, 37). The use of Nrf2 activators in the treatment of COVID-19-infected subjects (16, 38, 39), mainly related to the resolution of inflammation and focusing on Nrf2 as an antiviral transcriptional factor (17, 40, 41).
Expression of Nrf2-targeted genes, HO-1, Prdx, NQO-1, SOD-1, and CAT, was decreased after 24 h of exposure to serum from patients with COVID-19, in comparison with basal conditions and exposure for 24 h to serum from healthy individuals. Regarding the mechanisms, we evaluated the protein content of two key regulators of Nrf2 signaling, the Keap-1 and Bach-1 proteins. Keap-1, a cytosolic protein that, by interacting with Nrf2, targets the complex to ubiquitination and degradation in the ubiquitin-proteasome system under basal conditions, was first analyzed. The protein content of Keap-1 in HUVECs was decreased after treatment with serum from patients with COVID-19, indicating potential Nrf2 activation in response to COVID-19. On the other hand, Bach-1, a transcriptional factor that dimerizes with the family of proteins called small Maf proteins (Smaf F, G, or K), competes for the antioxidant-responsive element with Nrf2 on the DNA (42). As Keap-1 protein content was decreased in our experiments but less Nrf2 was found in the nucleus, one can speculate that Nrf2 is being exported from the nucleus and directed to degradation by the ubiquitin-proteasome system. Nrf2 nuclear export and subsequent degradation is facilitated by the active form of glycogen synthase kinase 3 β (GSK3-β) (Tyr216), which allows Nrf2 to be exported and sequestered by other kinases leading to its degradation in the ubiquitin-proteasome system. Although we did not check GSK3-β activation, transcriptome analysis of SARS-CoV-2-infected lung cells reveals upregulation of GSK3-β (43), which could also be happening in HUVECs exposed to serum from patients with COVID-19.
ROS play a significant role in viral infections as they favor viral replication and subsequent cell damage (44). Once oxidative stress sets in, permanent cellular damage can be observed. Thus, serum from individuals with severe COVID-19 decreased endothelial cell viability, increasing apoptosis and the number of oxidized proteins. These events are linked to the vicious cycle where ROS promotes cell damage and cell damage increases ROS generation.
Endothelial cells are specialized in the production of NO, an important regulator of vascular tone and blood pressure (45). Endothelial function can be assessed by the endothelial cell’s ability to generate NO. In this study, serum from individuals with severe COVID-19 decreased the bioavailability of NO in HUVECs. The balance between NO levels and ROS generation is crucial to determine the bioavailability of NO (46) since ROS sequester NO and form more toxic species, such as peroxynitrite and hydroxyl radical (47).
Since the main aim of our work was to determine whether the cytokine storm affects the endothelial cell antioxidant defense, our next experiments focused on the effects of tocilizumab, a humanized antibody against the IL-6 receptor (48). IL-6 correlates with many viral infections and serves as a marker of prognostic and indicator of the severity of COVID-19 and other inflammatory diseases (49, 50). Tocilizumab prevented the oxidative stress in HUVECs caused by the serum of patients with COVID-19, restored Nrf2 translocation to basal levels, and increased NO bioavailability. Our group recently demonstrated that inhibition of IL-6 signaling prevents dysfunction of umbilical cord arteries induced by serum from women with severe COVID-19 (21), further supporting a key role for IL-6 on COVID-19-associated vascular dysfunction.
l-Sulforaphane, a well-known Nrf2 pharmacological activator, prevented increases in ROS generation and partially restored NO bioavailability (51). SFN treatment also abolished the impairment in Nrf2 translocation caused by serum from patients with COVID-19 in HUVECs. Since blockade of IL-6 with tocilizumab prevented increases in ROS generation and restored Nrf2 activity in HUVECs treated with serum from subjects with COVID-19 and similar results were observed using SFN, it is plausible that serum from subjects with COVID-19, at least in part due to increased IL-6 levels, increases ROS generation and impairs the antioxidant response in HUVECs by reducing Nrf2 activity.
There are several sources of ROS production, including NADPH oxidase, xanthine oxygenase, cyclooxygenase, and the mitochondrial respiratory chain (47). We sought to understand whether IL-6 present in the serum of individuals with severe COVID-19 induces the generation of mitochondrial ROS and whether this cytokine interferes with mitochondrial metabolism. Serum from individuals with severe COVID-19 increased ROS generation and mitochondrial metabolism in HUVECs. These effects were prevented by tocilizumab. Little is known about the actions of IL-6 on the mitochondria of endothelial cells; however, it is known that IL-6 reduces mitochondrial dynamics via the FOXO1 transcription factor (52). Our study brings new horizons on the role of IL-6 in severe COVID-19, in terms of mitochondrial damage and oxidative stress.
Although this study expands on existing data sets in vascular biology and COVID-19, it has some limitations. During the period in which the samples were recruited, there were no distinctions or knowledge about the possible variants of the SARS-CoV-2 virus and only a significant possibility that the Gamma P.1 variant is higher in the selected individuals. It is possible that other variants, with different degrees of pathogenicity, may generate different effects on endothelial cells. Donors were recruited before the vaccination began, so the potential effect that vaccines may have on endothelial function should also be evaluated in future studies.
Conclusions
Using a cell-based model to test the hypothesis that the cytokine storm present in COVID-19 decreases the antioxidant defense of endothelial cells, we demonstrated that serum derived from severe SARS-CoV-2-infected individuals causes oxidative stress, cell damage, and apoptosis in HUVECs. These findings strongly correlate with impaired Nrf2 activity and defective antioxidant defense. Mechanistically, we also showed that serum from individuals with COVID-19 increases protein expression of Bach-1, a nuclear protein that competes with Nrf2 for the DNA. This study also provides evidence that this phenomenon in endothelial cells happens through IL-6-dependent mechanisms. Our studies indicate that oxidative stress contributes to the endothelitis in COVID-19 disease, further aggravating the cardiovascular risk of SARS-CoV-2 infection.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
SUPPLEMENTAL DATA
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.22114838.v2.
GRANTS
This study was funded by São Paulo Research Foundation (FAPESP) Grant 2013/08216-2 [Center for Research in Inflammatory Diseases (CRID), to R.C.T.] and FAPESP Grant Fellowships 2021/08847-9 (to R.M.C.), 2021/09273-6 (to D.R.), and 2019/11213-1 (to V.L.D.B.); (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)); and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.C. and R.C.T. conceived and designed research; D.R., R.M.C., M.R.M., J.V.A., T.F.C.F.-S., R.B.M., L.C.B.C., and D.F.F. performed experiments; D.R., M.R.M., and R.M.C. analyzed data; D.R., M.R.M., and R.M.C. interpreted results of experiments; D.R., M.R.M., and R.M.C. prepared figures; D.R. drafted manuscript; D.R., M.R.M., C.B., R.M.C., and R.C.T. edited and revised manuscript; D.R., M.R.M., J.V.A., T.F.C.F.-S., R.B.M., L.C.B.C., D.F.F., A.E.S.C., V.L.D.B., E.A., C.B., P.L.-J., M.A.-M., R.M.C., and R.C.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Carla Pavan Manzato for excellent technical support. We are grateful to the personnel who collected the consent forms. Graphical abstract was created using a licensed version of BioRender.
REFERENCES
- 1. Ciotti M, Ciccozzi M, Terrinoni A, Jiang WC, Wang CB, Bernardini S. The COVID-19 pandemic. Crit Rev Clin Lab Sci 57: 365–388, 2020. doi: 10.1080/10408363.2020.1783198. [DOI] [PubMed] [Google Scholar]
- 2. Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 53: 66–70, 2020. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siripanthong B, Asatryan B, Hanff TC, Chatha SR, Khanji MY, Ricci F, Muser D, Ferrari VA, Nazarian S, Santangeli P, Deo R, Cooper LT Jr, Mohiddin SA, Chahal CAA. The pathogenesis and long-term consequences of COVID-19 cardiac injury. JACC Basic Transl Sci 7: 294–308, 2022. doi: 10.1016/j.jacbts.2021.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guidotti LG, Chisari FV. Cytokine-mediated control of viral infections. Virology 273: 221–227, 2000. doi: 10.1006/viro.2000.0442. [DOI] [PubMed] [Google Scholar]
- 5. Hu B, Huang S, Yin L. The cytokine storm and COVID‐19. J Med Virol 93: 250–256, 2021. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res 51: 384–387, 2020. doi: 10.1016/j.arcmed.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zanoli L, Briet M, Empana JP, Cunha PG, Mäki-Petäjä KM, Protogerou AD, Tedgui A, Touyz RM, Schiffrin EL, Spronck B, Bouchard P, Vlachopoulos C, Bruno RM, Boutouyrie P; Association for Research into Arterial Structure, Physiology (ARTERY) Society, the European Society of Hypertension (ESH) Working Group on Vascular Structure and Function, and the European Network for Noninvasive Investigation of Large Arteries. Vascular consequences of inflammation: a position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J Hypertens 38: 1682–1698, 2020. doi: 10.1097/HJH.0000000000002508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J 41: 3038–3044, 2020. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, Guignabert C, Humbert M. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J 56: 2001634, 2020. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev 2016: 1245049, 2016. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Costa RM, Rodrigues D, Pereira CA, Silva JF, Alves JV, Lobato NS, Tostes RC. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front Pharmacol 10: 382, 2019. doi: 10.3389/fphar.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Axelsson AS, Tubbs E, Mecham B, Chacko S, Nenonen HA, Tang Y, Fahey JW, Derry JMJ, Wollheim CB, Wierup N, Haymond MW, Friend SH, Mulder H, Rosengren AH. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci Transl Med 9: eaah4477, 2017. doi: 10.1126/scitranslmed.aah4477. [DOI] [PubMed] [Google Scholar]
- 13. Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol 57: 82–95, 2013. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 14. Li Z, Guo H, Li J, Ma T, Zhou S, Zhang Z, Miao L, Cai L. Sulforaphane prevents type 2 diabetes-induced nephropathy via AMPK-mediated activation of lipid metabolic pathways and Nrf2 antioxidative function. Clin Sci (Lond) 134: 2469–2487, 2020. doi: 10.1042/CS20191088. [DOI] [PubMed] [Google Scholar]
- 15. James D, Devaraj S, Bellur P, Lakkanna S, Vicini J, Boddupalli S. Novel concepts of broccoli sulforaphanes and disease: induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr Rev 70: 654–665, 2012. doi: 10.1111/j.1753-4887.2012.00532.x. [DOI] [PubMed] [Google Scholar]
- 16. Cuadrado A, Manda G, Hassan A, Alcaraz MJ, Barbas C, Daiber A, Ghezzi P, León R, López MG, Oliva B, Pajares M, Rojo AI, Robledinos-Antón N, Valverde AM, Guney E, Schmidt HHHW. Transcription factor NRF2 as a therapeutic target for chronic diseases: a systems medicine approach. Pharmacol Rev 70: 348–383, 2018. doi: 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- 17. Olagnier D, Farahani E, Thyrsted J, Blay-Cadanet J, Herengt A, Idorn M, et al. SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat Commun 11: 4938, 2020. [Erratum in Nat Commun 11: 5419, 2020]. doi: 10.1038/s41467-020-18764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuadrado A, Pajares M, Benito C, Jiménez-Villegas J, Escoll M, Fernández-Ginés R, Garcia Yagüe AJ, Lastra D, Manda G, Rojo AI, Dinkova-Kostova AT. Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci 41: 598–610, 2020. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costa TJ, Potje SR, Fraga-Silva TFC, da Silva-Neto JA, Barros PR, Rodrigues D, Machado MR, Martins RB, Santos-Eichler RA, Benatti MN, de Sá KSG, Almado CEL, Castro ÍA, Pontelli MC, Serra L, Carneiro FS, Becari C, Louzada-Junior P, Oliveira RDR, Zamboni DS, Arruda E, Auxiliadora-Martins M, Giachini FRC, Bonato VLD, Zachara NE, Bomfim GF, Tostes RC. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vascul Pharmacol 142: 106946, 2022. doi: 10.1016/j.vph.2021.106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potje SR, Costa TJ, Fraga-Silva TFC, Martins RB, Benatti MN, Almado CEL, de Sá KSG, Bonato VLD, Arruda E, Louzada-Junior P, Oliveira RDR, Zamboni DS, Becari C, Auxiliadora-Martins M, Tostes RC. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci 276: 119376, 2021. doi: 10.1016/j.lfs.2021.119376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Almeida CR, Lima JF, Machado MR, Alves JV, Couto AES, Campos LCB, Avila-Mesquita CD, Auxiliadora-Martins M, Becari C, Louzada-Júnior P, Tostes RC, Lobato NS, Costa RM. Inhibition of IL-6 signaling prevents serum-induced umbilical cord artery dysfunction from patients with severe COVID-19. Am J Physiol Regul Integr Comp Physiol 324: R435–R445, 2023. doi: 10.1152/ajpregu.00154.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20: e192–e197, 2020. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodrigues D, Costa TJ, Silva JF, Neto JTO, Alves JV, Fedoce AG, Costa RM, Tostes RC. Aldosterone negatively regulates Nrf2 activity: an additional mechanism contributing to oxidative stress and vascular dysfunction by aldosterone. Int J Mol Sci 22: 6154, 2021. doi: 10.3390/ijms22116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 25. Pokrass MJ, Regot S. 3D time-lapse microscopy paired with endpoint lineage analysis in mouse blastocysts. STAR Protoc 2: 100446, 2021. doi: 10.1016/j.xpro.2021.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kauffman ME, Kauffman MK, Traore K, Zhu H, Trush MA, Jia Z, Li YR. MitoSOX-based flow cytometry for detecting mitochondrial ROS. React Oxyg Species (Apex) 2: 361–370, 2016. doi: 10.20455/ros.2016.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M, Dimmeler S, Boon RA. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol 35: 137–145, 2015. doi: 10.1161/ATVBAHA.114.304277. [DOI] [PubMed] [Google Scholar]
- 28. Reymond S, Vujić T, Schvartz D, Sanchez JC. Morphine-induced modulation of Nrf2-antioxidant response element signaling pathway in primary human brain microvascular endothelial cells. Sci Rep 12: 4588, 2022. doi: 10.1038/s41598-022-08712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsiropoulou S, Touyz RM. Assessment of protein carbonylation and protein tyrosine phosphatase (PTP) oxidation in vascular smooth muscle cells (VSMCs) using immunoblotting approaches. Methods Mol Biol 1614: 31–46, 2017. doi: 10.1007/978-1-4939-7030-8_3. [DOI] [PubMed] [Google Scholar]
- 30. Wassmann S, Stumpf M, Strehlow K, Schmid A, Schieffer B, Böhm M, Nickenig G. Interleukin-6 induces oxidative stress and endothelial dysfunction by overexpression of the angiotensin II type 1 receptor. Circ Res 94: 534–541, 2004. doi: 10.1161/01.RES.0000115557.25127.8D. [DOI] [PubMed] [Google Scholar]
- 31. Youn JY, Zhang Y, Wu Y, Cannesson M, Cai H. Therapeutic application of estrogen for COVID-19: attenuation of SARS-CoV-2 spike protein and IL-6 stimulated, ACE2-dependent NOX2 activation, ROS production and MCP-1 upregulation in endothelial cells. Redox Biol 46: 102099, 2021. doi: 10.1016/j.redox.2021.102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells—conditional innate immune cells. J Hematol Oncol 6: 61, 2013. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amersfoort J, Eelen G, Carmeliet P. Immunomodulation by endothelial cells—partnering up with the immune system? Nat Rev Immunol 22: 576–588, 2022. doi: 10.1038/s41577-022-00694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sturtzel C. Endothelial cells. Adv Exp Med Biol 1003: 71–91, 2017. doi: 10.1007/978-3-319-57613-8_4. [DOI] [PubMed] [Google Scholar]
- 35. Priya Dharshini LC, Vishnupriya S, Sakthivel KM, Rasmi RR. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal 72: 109670, 2020. doi: 10.1016/j.cellsig.2020.109670. [DOI] [PubMed] [Google Scholar]
- 36. Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radic Res 44: 1267–1288, 2010. doi: 10.3109/10715762.2010.507670. [DOI] [PubMed] [Google Scholar]
- 37. Saheb Sharif-Askari N, Saheb Sharif-Askari F, Mdkhana B, Hussain Alsayed HA, Alsafar H, Alrais ZF, Hamid Q, Halwani R. Upregulation of oxidative stress gene markers during SARS-COV-2 viral infection. Free Radic Biol Med 172: 688–698, 2021. doi: 10.1016/j.freeradbiomed.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zinovkin RA, Grebenchikov OA. Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry (Mosc) 85: 833–837, 2020. doi: 10.1134/S0006297920070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calabrese EJ, Kozumbo WJ, Kapoor R, Dhawan G, Lara PC, Giordano J. Nrf2 activation putatively mediates clinical benefits of low-dose radiotherapy in COVID-19 pneumonia and acute respiratory distress syndrome (ARDS): novel mechanistic considerations. Radiother Oncol 160: 125–131, 2021. doi: 10.1016/j.radonc.2021.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khan H, Patel S, Majumdar A. Role of NRF2 and Sirtuin activators in COVID-19. Clin Immunol 233: 108879, 2021. doi: 10.1016/j.clim.2021.108879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herengt A, Thyrsted J, Holm CK. NRF2 in viral infection. Antioxidants (Basel) 10: 1491, 2021. doi: 10.3390/antiox10091491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang X, Guo J, Wei X, Niu C, Jia M, Li Q, Meng D. Bach1: function, regulation, and involvement in disease. Oxid Med Cell Longev 2018: 1347969, 2018. doi: 10.1155/2018/1347969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singh K, Chen YC, Hassanzadeh S, Han K, Judy JT, Seifuddin F, Tunc I, Sack MN, Pirooznia M. Network analysis and transcriptome profiling identify autophagic and mitochondrial dysfunctions in SARS-CoV-2 infection. Front Genet 12: 599261, 2021. doi: 10.3389/fgene.2021.599261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Z, Xu X, Leng X, He M, Wang J, Cheng S, Wu H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch Virol 162: 603–610, 2017. doi: 10.1007/s00705-016-3130-2. [DOI] [PubMed] [Google Scholar]
- 45. Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333: 664–666, 1988. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 46. Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci 21: 3, 2014. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295: C849–C868, 2008. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sebba A. Tocilizumab: the first interleukin-6-receptor inhibitor. Am J Health Syst Pharm 65: 1413–1418, 2008. doi: 10.2146/ajhp070449. [DOI] [PubMed] [Google Scholar]
- 49. Domingo P, Mur I, Mateo GM, Gutierrez M, del M, Pomar V, de Benito N, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA 326: 499–518, 2021. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angriman F, Ferreyro BL, Burry L, Fan E, Ferguson ND, Husain S, Keshavjee SH, Lupia E, Munshi L, Renzi S, Ubaldo OGV, Rochwerg B, Del Sorbo L. Interleukin-6 receptor blockade in patients with COVID-19: placing clinical trials into context. Lancet Respir Med 9: 655–664, 2021. doi: 10.1016/S2213-2600(21)00139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wagner AE, Ernst I, Iori R, Desel C, Rimbach G. Sulforaphane but not ascorbigen, indole-3-carbinole and ascorbic acid activates the transcription factor Nrf2 and induces phase-2 and antioxidant enzymes in human keratinocytes in culture. Exp Dermatol 19: 137–144, 2010. doi: 10.1111/j.1600-0625.2009.00928.x. [DOI] [PubMed] [Google Scholar]
- 52. Dudek M, Lohr K, Donakonda S, Baumann T, Lüdemann M, Hegenbarth S, Dübbel L, Eberhagen C, Michailidou S, Yassin A, Prinz M, Popper B, Rose-John S, Zischka H, Knolle PA. IL-6-induced FOXO1 activity determines the dynamics of metabolism in CD8 T cells cross-primed by liver sinusoidal endothelial cells. Cell Rep 38: 110389, 2022. doi: 10.1016/j.celrep.2022.110389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1: https://doi.org/10.6084/m9.figshare.22114838.v2.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.