Abstract
Background/aim
Hashimoto thyroiditis (HT) is one of the most prevalent autoimmune diseases. The intestine microbiota is strongly associated with autoimmune diseases. Zonulin, a modulator of tight junctions that controls the selective permeability of the intestine can induce an elevation in gut permeability. We aimed to investigate the association of plasma zonulin levels with HT.
Materials and methods
We compared 77 HT patients with 66 age-gender and BMI-matched healthy individuals in the case of plasma zonulin levels. Plasma zonulin levels were measured by ELISA. The statistical analyses were performed using Student’s t-test and chi-square tests. The predictive power was investigated using univariate and multivariate logistic regression analysis.
Results
We found that the increase in plasma zonulin levels in the HT group was statistically significant compared to the control group (p < 0.001). The regression analysis showed that urea, anti-thyroid peroxidase, aspartate aminotransferase, thyroid-stimulating hormone, free T3, and serum zonulin levels were found to be associated with HT in both univariate and multivariate models (p < 0.05).
Conclusion
Zonulin is a possible biomarker candidate that may link intestinal permeability with the etiology of autoimmune diseases.
Keywords: Hashimoto thyroiditis, autoimmune diseases, microbiota, gut permeability, zonulin
1. Introduction
The first serious description and analyses of autoimmune thyroiditis leading to primary hypothyroidism emerged during the 1910s [1]. Hakaru Hashimoto, in 1912, defined a distinctive hypothyroidism disease in which inflammatory cells infiltrate into thyroid tissue resulting in the production of antibodies against thyroid hormones such as triiodothyronine (T3) and thyroxine (T4) which are synthesized by a pivotal enzyme, namely thyroperoxidase (TPO) [2]. Generally speaking, commonly observed in females (>15% of +60 years females but <2% of males) [3], this disease is a major public health problem which corresponds to 10% of all adult hypothyroiditis patients [4] and is called “Hashimoto’s thyroiditis (HT)” after being discovered. The disease displays its symptoms via the elevation of antibodies (anti-TPO) against enzyme TPO which is observed in nearly 20% of general society and almost always increases in HT [5].
The intestinal epithelium, composed of single-layered epithelial cells lining the small intestine, provides the largest mucosal barrier between the external environment and the human host [6]. The selectively regulated passage of macromolecules is maintained by intestinal paracellular permeability which depends on the integrity of one of the hallmarks of absorptive and secretory epithelia, intercellular tight junctions (TJs) [7]. TJs are highly dynamic structures capable of rapid and coordinated responses that separate the basolateral and apical cellular compartments. In healthy conditions, quantitively small macromolecules with active immunogenicity are allowed to cross the mucosal barrier [8].
The intestinal microbiota displays a substantial function to maintain the homeostasis of the gastrointestinal system and may be at the heart of our understanding of thyroid disorders [9]. Intestinal flora teeming with various microbial organisms may explain the large differences among different world regions in the prevalence of goiter, a thyroid disorder mainly caused by insufficient iodine (I−) intake. For example, in southern India, goiter is not associated with I-intake, whereas hypothyroidism is more common in iodine-rich areas of Japan than in iodine-poor areas [10,11].
The end products of intestinal microbiota such as short-chain fatty acids are reservoirs of energy to be served in the production of thyroid hormones and strengthening intercellular TJs [12]. The cooperation of intestinal content and intercellular TJs stabilizes homeostasis in case of the immune response to nonself antigens thus the presence of an aberrant intestinal gut microbiome triggers the proliferation of auto-reactive T-cells [13].
Considering the possible external inducers of HT, the gut emerges as a key region, as the immune system with mucosa experiences and responds to the outside environment for the first time. Many autoimmune diseases such as diabetes, inflammatory bowel disease (IBD), and neurodegenerative diseases, cardiovascular diseases and cancers are being associated with microbiota [14], while several ones with the aberrant intestinal barrier permeability [15].
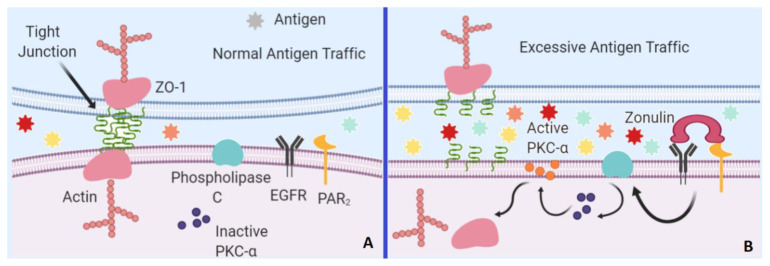
Zonulin is a paracrine signaling protein largely secreted by small intestine epithelial cells that coordinate the opening of intestinal TJs [16]. In normal antigenic trafficking within the small intestine, TJs’ main component, zonula occludens 1 (ZO-1) form the TJs complex with the help of actin filaments binding. However, zonulin binding to two transmembrane receptors, namely protease-activated receptor 2 and epidermal growth factor receptor triggers a cascade in which phospholipase C activates protein kinase C alpha, which in turn catalyzes the phosphorylation of ZO-1 and other target proteins and the depolymerization of actins [17] which eventually results in a loose conformation of TJs, increased permeability, and excessive-abnormal antigenic trafficking (Figure 1).
Figure 1.
ZO-1 is the main part of the TJs. A. During normal antigen trafficking, ZO-1 is strictly bound to TJs complex with the help of actin filaments leading to selectively permeability of the intestinal barrier. B. In the presence of zonulin, the activated EGFR and PAR2 receptors trigger phospholipase C activation which in turn activates PKC-α. Activated PKC-α then triggers the release of ZO-1 and actin filaments leading to dysfunction of TJs and aberrant barrier permeability. PAR2: protease-activated receptor 2, PKC-α: Protein Kinase C Alpha, ZO-1: Zona Occludens 1, EGFR: Epidermal growth factor receptor (the figure was drawn using biorender.com).
Based on the relationship between the intestinal microbiome, intestinal permeability, and autoimmunity, we sought to explore whether elevated levels of plasma zonulin contributed to HT. Here, we discuss the crosstalk between plasma zonulin levels and HT susceptibility, which may improve target-specific therapy for HT.
2. Materials and methods
2.1. Study subjects
The study enrolled newly diagnosed 77 Hashimoto thyroiditis patients (75 of females and 2 of males) and age-gender-BMI matched 66 healthy subjects (65 of females and 1 of male) at the Internal Medicine Department outpatient clinic of Kanuni Sultan Süleyman Traning and Research Hospital, İstanbul, Turkey.
Generally speaking, the individuals with an increased thyroid-stimulating hormone (TSH) and low levels of free thyroxine (FT4), with elevated antithyroid peroxidase (TPO) antibodies demonstration coupled with the physical examination and differential diagnosis from many other thyroid gland related disorders or autoimmune diseases are included within the study as the patients’ group [18]. Individuals with no systemic or autoimmune disease history are included as the control group. The mean age was 36.8 ± 9.3 years in the patients and 34.6 ± 11.4 in control groups. All procedures were performed following the ethical standards of the Institutional Ethics Committee of our hospital (Ethics Committee decision no: KAEK-2019.04.87) and with the 1964 Helsinki Declaration ethical standards. All study subjects were inhabitants of İstanbul having Turkish origin and provided signed informed consent prior to the sample and data collection.
2.2. Sample preparation
After the patient or the control had fasted for at least 8 h, peripheral blood samples were taken from antecubital veins in the morning, thereafter, samples were collected in tubes with EDTA for biochemical analysis and without additives for serum zonulin level. The following parameters were run simultaneously. In biochemical analysis, complete blood count, liver function parameters such as gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT) and aspartate aminotransferase (AST), potassium and sodium levels, fasting blood glucose, HbA1c, thyroid-stimulating hormone, free T3, free T4, anti-thyroid peroxidase, triglyceride, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), total cholesterol, urea, uric acid, and creatinine were measured. An Abbot Aeroset 2.0 (Abbot Diagnostic, USA) was used for spectrophotometric calculation of the amount of liver enzymes ALT, AST, and GGT, lipid profiles, uric acid and glucose levels, whereas Sysmex XN 9000 hematology analyzer (Symex Europe GmbH, Germany) was for the complete blood count, Cobas 8000 C702 (Roche Diagnostics, USA) chemistry analyzer was for Na and K electrolytes and Variant 2 Turbo (Biorad, USA) was for HbA1c%. Plasma zonulin concentrations were determined by an enzyme-linked immunosorbent assay (Human Zonulin ELISA kit, Cat no: E3704Hu, Bioassay Technology Laboratory, Shanghai, CHINA). The intra-assay precision of the kit is <8%, whereas the interassay precision is <10%. The precision (%) is calculated by the equation of SD/mean × 100.
2.3. Statistical analysis
In the descriptive statistics of the data, the median, standard deviation, average, frequency, lowest-highest values, and ratio were determined. The distribution of variables was measured by the Kolmogorov Smirnov test. In the analysis of quantitative independent data, normal distribution between groups was evaluated using Student’s t-test, while the Mann–Whitney U test was used for nonnormally distributed data. The qualitative independent data were evaluated by the chi-square test. In the cases the chi-square test conditions were not met, the Fischer test was applied. The predictive power was investigated using univariate and multivariate logistic regression analysis. The predictive power and cut-off value were analyzed with the ROC curve. The Statistical Package for Social Sciences statistical software release 22 (IBM Corporation, Armonk, NY) was used for the statistical calculations.
3. Results
The demographics, laboratory parameters and clinical features of both control and HT patients are summarized in Table 1 and Table 2.
Table 1.
The comparison of demographic characteristics between Hashimoto’s thyroiditis and control groups.
| Parameters | Control group | Thyroiditis group | p | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD/n-% | Mean ± SD/n-% | |||||||
| Age, years | 34.6 | ± | 11.4 | 36.8 | ± | 9.3 | 0.202 | |
| Gender | Female | 65 | 98.5 % | 75 | 97.4% | 1.000 | ||
| Male | 1 | 1.5 % | 2 | 2.6% | 1.000 | |||
| Height, m | 1.6 | ± | 0.0 | 1.6 | ± | 0.1 | 0.717 | |
| Weight, kg | 66.3 | ± | 13.4 | 69.1 | ± | 13.6 | 0.316 | |
| BMI; kg/m 2 | 25.0 | ± | 4.9 | 25.9 | ± | 5.0 | 0.352 | |
BMI, Body mass index; s.d, Standard deviation; m, Meter; n, Number; m2, Square meter; kg, Kilogram
Table 2.
The comparison of laboratory parameters between Hashimoto’s thyroiditis and control groups.
| Parameters | Control group | Thyroiditis group | p | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD Median | Mean ± SD Median | ||||||
| Glucose, mg/dL | 88.7 | ± | 8.5 | 91.2 | ± | 9.5 | 0.111 |
| Urea, mg/dL | 22.9 | ± | 7.2 22 | 27.8 | ± | 24.3 25 | 0.010 |
| Creatinine, mg/dL | 0.6 | ± | 0.1 | 0.6 | ± | 0.1 | 0.367 |
| AST, U/L | 15.5 | ± | 2.6 | 18.0 | ± | 7.8 | 0.005 |
| ALT, U/L | 14.8 | ± | 4.9 14 | 16.8 | ± | 11.0 14 | 0.498 |
| LDL, mg/dL | 92.5 | ± | 28.5 | 101.4 | ± | 38.5 | 0.186 |
| HDL, mg/dL | 59.5 | ± | 14.6 | 56.4 | ± | 11.5 | 0.335 |
| TG, mg/dL | 86.7 | ± | 49.7 | 92.1 | ± | 44.3 | 0.371 |
| T. Chol, mg /dL | 168.3 | ± | 35.0 | 174.9 | ± | 45.1 | 0.579 |
| Uric acid, mg/dL | 3.7 | ± | 0.9 | 3.8 | ± | 0.9 | 0.481 |
| GGT, U/L | 12.9 | ± | 5.2 11 | 14.8 | ± | 15.7 11 | 0.887 |
| Hb, g/dL | 12.6 | ± | 1.3 | 12.3 | ± | 1.5 | 0.338 |
| WBC, (×10 3 ) | 7.2 | ± | 1.9 | 7.1 | ± | 1.6 | 0.934 |
| Platelets (×10 9 ) | 269.5 | ± | 63.4 | 278.5 | ± | 55.6 | 0.382 |
| MPV, fL | 10.8 | ± | 0.9 | 11.3 | ± | 7.5 | 0.092 |
| Sodium, mmol /L | 140.7 | ± | 2.0 | 140.3 | ± | 1.5 | 0.381 |
| Potassium, mmol /L | 4.4 | ± | 0.3 | 4.5 | ± | 0.4 | 0.051 |
| HbA1c, g/dL | 5.2 | ± | 0.4 | 5.3 | ± | 0.4 | 0.358 |
| TSH, uIU/mL | 2.0 | ± | 1.3 1.6 | 4.9 | ± | 11.3 3.3 | ≤0.001 |
| FT3, pg/mL | 3.2 | ± | 0.5 | 3.0 | ± | 0.5 | 0.008 |
| FT4, ng / dL | 1.3 | ± | 0.7 | 1.2 | ± | 0.5 | 0.935 |
| ANTI-TPO, IU/mL | 11.9 | ± | 5.0 | 259.5 | ± | 179.0 | ≤0.001 |
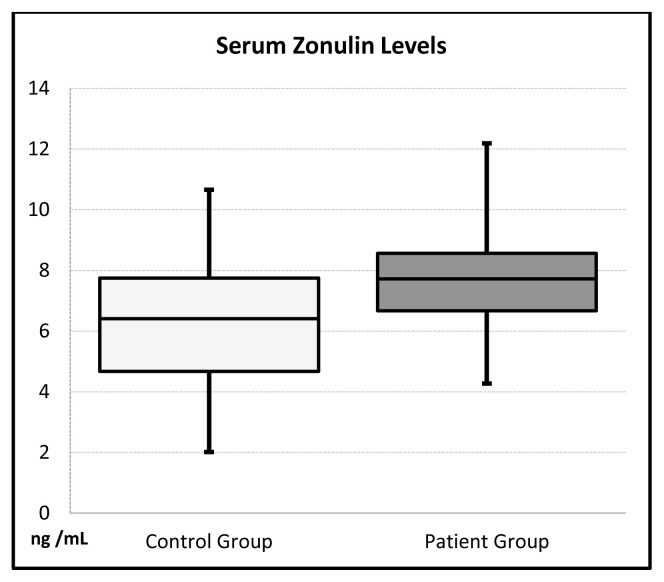
| Zonulin, ng/mL | 6.4 | ± | 1.9 | 7.7 | ± | 1.5 | ≤0.001 |
HbA1C, Hemoglobin A1C; TG, Triglyceride; AST, Aspartate aminotransferase; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; T. Chol, Total cholesterol; Hb, Hemoglobin; ALT, Alanine aminotransferase; GGT, Gamma-glutamyl transferase; WBC; White blood counts, MPV; Mean platelets volume; FT4, Free thyroxine; FT3, Free triiodothyronine; TSH, Thyroid stimulating hormone; ANTI-TPO, Anti-thyroid peroxidase; mg, Milligram; U, Unit; s.d, Standard deviation; L, Liter; n, Number; dL, Deciliter; g, Gram; fL, Femtoliter; mmol, Milimol; mL, Milliliter; pg, Picogram; ng, Nanogram.
There was no significant difference between the Hashimoto thyroiditis and control groups concerning age, gender, height, weight, and body mass index (p > 0.05) for all comparisons (Table 1).
The fasting glucose, total cholesterol, LDL, HDL, uric acid, creatinine, TG, HgA1c, ALT, GGT, Na, K, FT4, WBC, PLT, MPV, and Hgb levels were not significant statistically between the patient and control groups. The level of ure (p = 0.010), anti-TPO (p ≤ 0.001), AST (p = 0.005), TSH (p ≤ 0.001), and serum zonulin (p≤0.001) (Figure 2) elevated significantly whereas the FT3 level decreased (p = 0.008) in patient groups compared to control group (Table 2).
Figure 2.
The comparison of plasma zonulin levels between control and patient groups.
Uni/multivariate regression analysis was performed to determine the possible influence of urea, anti-TPO, AST, TSH, FT3, and serum zonulin on the presence of HT. Urea, anti-TPO, AST, TSH, FT3, tri-iodothyronine, and serum zonulin levels were found to be associated with HT in both univariate and multivariate models (p < 0.05) (Table 3).
Table 3.
The regression analysis of univariate and multivariate models within the patient group.
| Univariate model | Multivariate model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||||
| Urea | 1.05 | 1.01 | - | 1.10 | 0.047 | |||||
| Anti-TPO | 1.49 | 1.06 | - | 2.08 | 0.022 | 1.21 | 1.07 | - | 1.36 | 0.002 |
| AST | 1.16 | 1.04 | - | 1.29 | 0.007 | 1.21 | 1.07 | - | 1.36 | 0.002 |
| TSH | 1.62 | 1.29 | - | 2.04 | ≤0.001 | 1.62 | 1.25 | - | 2.11 | ≤ 0.001 |
| FT3 | 0.42 | 0.21 | - | 0.86 | 0.017 | 0.35 | 0.15 | - | 0.81 | 0.015 |
| Serum Zonulin | 1.58 | 1.26 | - | 1.98 | ≤ 0.001 | 1.55 | 1.21 | - | 2.00 | ≤ 0.001 |
| OR: Odds ratio, Cl: Confidence interval | ||||||||||
ANTI-TPO, Anti–thyroid peroxidase; AST, Aspartate aminotransferase; TSH, Thyroid stimulating hormone; FT3, Free triiodothyronine
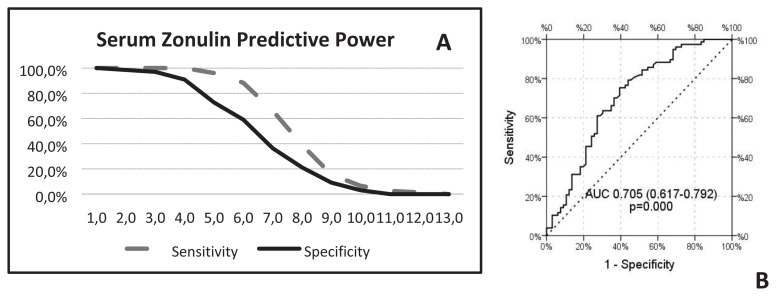
To determine the predictive power of plasma zonulin levels in the case of HT, a ROC analysis was performed (Figure 3-A,B) (Table 4). The plasma zonulin level of ≥4 ng/mL had a sensitivity (100%) so the positive predictive value (PPV) of 100% whereas a specificity (90.6%) so the negative predictive value (NPV) of 90.9% (Table 4). In the discrimination of the patient and control group, we observed a statistically significant efficiency of plasma zonulin levels [area under the curve 0.705 (0.617–0.792)] (Figure 3-B).
Figure 3.
A-The diagrammatic illustration of serum zonulin predictive power in case of sensitivity and specificity. B-The efficiency of plasma zonulin levels is found statistically significant [area under the curve 0.705 (0.617–0.792)].
Table 4.
The sensitivity and specificity of different cut-off values of plasma zonulin levels.
| Zonulin | Sensitivity | Specifity |
|---|---|---|
| 1.0 | 100.0% | 100.0% |
| 2.0 | 100.0% | 98.5% |
| 3.0 | 100.0% | 97.0% |
| 4.0 | 100.0% | 90.9% |
| 5.0 | 96.1% | 72.7% |
| 6.0 | 88.3% | 59.1% |
| 7.0 | 66.2% | 36.4% |
| 8.0 | 39.0% | 21.2% |
| 9.0 | 15.6% | 9.1% |
| 10.0 | 6.5% | 3.0% |
| 11.0 | 2.6% | 0.0% |
| 12.0 | 1.3% | 0.0% |
| 13.0 | 0.0% | 0.0% |
4. Discussion
The most important result of our study is that plasma zonulin levels were higher in the HT group than in the control group. In this context, zonulin as a marker of impaired intestinal permeability may play a role in the pathogenesis of autoimmune diseases.
Humans come to earth from a sterile nature fulfilled with amniotic fluids and afterward the surfaces layered by epithelia such as skin, genital, nasal, and respiratory system tracts are covered systemically by microorganisms [19]. For human survival, it is mandatory to maintain tissue homeostasis. The homeostasis is balanced by the interaction between the host and the microorganisms living inside it. This is the code for a multicellular organism’s existence as both the symbiotic commensal microbiota inside the host and the macroscopic host are the two sides of the same coin. This substantial task is succeeded by a complex but coordinated set of both adaptive and innate reactions that modulate responses optimally against nutrients, commensal microorganisms, and probable pathogens [20]. Although the breakthrough in understanding the role of microbiota in the gut was Do derlein’s work which reported a specific bacteria type as the gate-keeper of the vaginal ecosystem in 1892, more than two thousand years ago, Hippocrates the father of medicine, claimed that all disease begins in the gut.
Autoimmune disorders are described as self-tissue damage presence in diverse regions of the human body. Innate cells, circulating autoantibodies, and eventually autoreactive lymphocytes are the three arms of the inflammation, however, the mechanisms responsible for the triggering and maintaining the process remain to be figured out completely. Gut commensal microorganisms as being the target of the adaptive immune system display a connective role in homeostasis [21]. Thus, any alteration in the population of these microorganisms is directly connected to autoimmune diseases. Generally speaking, patients having autoimmunity disorders represent proof of aberrant gut barriers which in turn lead to an immune response to commensal gut bacteria [7].
There is a growing body of evidence regarding the microbiota and its possible role in the autoimmune response. As reported in the Mu survey, a significant alteration in gut microbiota is found between susceptible, healthy or resistant samples in the case of collagen-induced arthritis murine model [22]. Several autoimmune diseases such as systemic lupus erythematosus [23], inflammatory bowel disease [24], systemic sclerosis [25], rheumatoid arthritis [26], type 1 diabetes (T1D) [27] and spondyloarthritis [28] are linked to gut microbiota alteration.
Zonulin, as the key to forcing the intestinal barrier to be more permeable, manages TJs regulation so the reciprocal movement of macromolecules, fluid, and the leukocytes [15].
This research examines the emerging role of the plasma zonulin levels in the context of an autoimmune disease, namely Hashimoto’s thyroiditis. Increased plasma zonulin levels may allow pathogenic antigens to pass the intestinal gut barrier loosening the TJs which can explain the auto-response of innate immunity. Our findings are in agreement with other autoimmune diseases, as plasma zonulin concentrations elevated in celiac disease [17], T1D [29], and inflammatory bowel disease such as Crohn’s disease and ulcerative colitis [30].
Without a shadow of doubt, the discovery of zonulin which is the only physiological gut permeability regulator depicted so far has been the breakpoint to understand the role of intestinal permeability in both disease and health. This study was the first to represent the association between HT and plasma zonulin levels.
The zonulin may be used as a marker in the future to predict clinical response in HT. The small sample size, absence of power analysis before the study, and the lack of additional markers that can affect the gut permeability and the nutritional status that can alter plasma zonulin levels measured are the limiting factors of our study. Additional work with larger sample size is needed not only to confirm our results but also to uncover whether alterations in intestinal microbiota in the case of zonulin are the consequences or the causes of autoimmune diseases.
Acknowledgment/Disclaimers/Conflict of interest
The authors declare no conflict of interest. The authors declared that this study received no financial support.
Footnotes
Informed consent
A signed informed consent was provided from all participants of the study. All procedures were performed following the ethical standards of the Institutional Ethics Committee (Ethics committee decision number: KAEK-2019.04.87) and with the 1964 Helsinki Declaration ethical standards.
References
- 1.Shoenfeld Y, Cervera R, Gershwin ME. Diagnostic criteria in autoimmune diseases. Berlin: Springer Science & Business Media; 2010. [Google Scholar]
- 2. Fröhlich E, Wahl R. Microbiota and Thyroid Interaction in Health and Disease. Trends Endocrinol Metabolism. 2019;30( 8):479–490. doi: 10.1016/j.tem.2019.05.008. Epub 2019 Jun 27. [DOI] [PubMed] [Google Scholar]
- 3. Nacamulli D, Petricca D, Mian C, Betterle C. Selenium and autoimmune thyroiditis. J Endocrinol Investigation. 2013;36( 10):8–14. [PubMed] [Google Scholar]
- 4. Aghini-Lombardi F, Antonangeli L, Martino E, Vitti P, Maccherini D, et al. The spectrum of thyroid disorders in an iodine-defcient community: the Pescopagano survey. The Journal of Clinical Endocrinology and Metabolism. 1999;84( 2):561–566. doi: 10.1210/jcem.84.2.5508. [DOI] [PubMed] [Google Scholar]
- 5. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) The Journal of Clinical Endocrinology and Metabolism. 2002;87(2):489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 6. Serena G, Camhi S, Sturgeon C, Yan S, Fasano A. The role of gluten in celiac disease and type 1 diabetes. Nutrients. 2015;7( 9):7143–7162. doi: 10.3390/nu7095329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. The American Journal of Pathology. 2008;173( 5):1243–1252. doi: 10.2353/ajpath.2008.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee V. Membrane transporters. European Journal of Pharmaceutical Sciences. 2000;11( 2):41–50. doi: 10.1016/s0928-0987(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 9. Young VB. The intestinal microbiota in health and disease. Current Opinion in Gastroenterology. 2012;28( 1):63–69. doi: 10.1097/MOG.0b013e32834d61e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kerakada N, Ganesh M, Meer MC. Prevalence of iodine deficiency among multinodular goiter patients: a South Indian study. International Surgery Journal. 2017;4( 2):680–684. doi: 10.18203/2349-2902.isj20170213. [DOI] [Google Scholar]
- 11. Leung AM, Braverman LE. Consequences of excess iodine. Nature Reviews Endocrinology. 2014;10( 3):136–142. doi: 10.1038/nrendo.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kunc M, Gabrych A, Witkowski JM. Microbiome impact on metabolism and function of sex, thyroid, growth and parathyroid hormones. Acta Biochimica Polonica. 2016;63( 2):189–201. doi: 10.18388/abp.2015_1093. [DOI] [PubMed] [Google Scholar]
- 13. Rook GAW, Brunet LR. Microbes, immunoregulation, and the gut. Gut. 2005;54( 3):317–320. doi: 10.1136/gut.2004.053785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Castillo V, Sanhueza Enrique, McNerney Eileen, Onate Sergio A, García Apolinaria. Microbiota dysbiosis: a new piece in the understanding of the carcinogenesis puzzle. Journal of Medical Microbiology. 2016;65(12):1347–1362. doi: 10.1099/jmm.0.000371. [DOI] [PubMed] [Google Scholar]
- 15.Fasano A. In: Pathological and therapeutic implications of macromolecule passage through the tight junction. 2nd ed. Cereijido M, Anderson JM, editors. Boca Raton: CRC Press Inc; 2001. pp. 697–722. [Google Scholar]
- 16. Tripathi A, Lammers KM, Goldblum S, Donohue TZ, Netzel-Arnett S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106( 39):16799–16804. doi: 10.1073/pnas.0906773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Annals of the New York Academy of Sciences. 2012;1258( 1):25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muller I, Moran C, Lecumberri B, Decallonne B, Robertson N, et al. 2019 European Thyroid Association Guidelines on the Management of Thyroid Dysfunction following Immune Reconstitution Therapy. European Thyroid Journal. 2019;8( 4):173–185. doi: 10.1159/000500881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Opazo MC, Ortega-Rocha EM, Coronado-Arrázola I, Bonifaz LC, Boudin H, et al. Intestinal microbiota influences non-intestinal related autoimmune diseases. Frontiers in Microbiology. 2018;12( 9):432. doi: 10.3389/fmicb.2018.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157( 1):121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330( 6012):1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mu Q, Tavella VJ, Kirby JL, Cecere TE, Chung M, et al. Antibiotics ameliorate lupus-like symptoms in mice. Scientific Reports. 2017;7( 1):13675. doi: 10.1038/s41598-017-14223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo XM, Edwards MR, Mu Q, Yu Y, Vieson MD, et al. Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Applied and Environmental Microbiology. 2018;84( 11):e02288–17. doi: 10.1128/AEM.02288-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rivas MA, Beaudoin M, Gardet A, Stevens C, Sharma Y, et al. National Institute of Diabetes and Digestive Kidney Diseases Inflammatory Bowel Disease Genetics Consortium (NIDDK IBDGC); United Kingdom Inflammatory Bowel Disease Genetics Consortium; International Inflammatory Bowel Disease Genetics Consortium. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nature Genetics. 2011;43( 11):1066–1073. doi: 10.1038/ng.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellocchi C, Volkmann ER. Update on the gastrointestinal microbiome in systemic sclerosis. Current Rheumatology Reports. 2018;20( 8):49. doi: 10.1007/s11926-018-0758-9. [DOI] [PubMed] [Google Scholar]
- 26. Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32( 6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, et al. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proceedings of the National Academy of Sciences of the United States of America USA. 2011;108( 28):11548–11553. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rehaume LM, Matigian N, Mehdi AM, Lachner N, Bowerman KL, et al. IL-23 favours outgrowth of spondyloarthritis-associated pathobionts and suppresses host support for homeostatic microbiota. Annals of the Rheumatic Diseases. 2019;78( 4):494–503. doi: 10.1136/annrheumdis-2018-214381. [DOI] [PubMed] [Google Scholar]
- 29. Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scandinavian Journal of Gastroenterology. 2006;41( 4):408–419. doi: 10.1080/00365520500235334. [DOI] [PubMed] [Google Scholar]
- 30. Caviglia GP, Dughera F, Ribaldone DG, Rosso C, Abate ML, et al. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Medica. 2019;110( 2):95–100. doi: 10.23736/S0026-4806.18.05787-7. [DOI] [PubMed] [Google Scholar]