Abstract
Background/aim
Data on antibody response following COVID-19 in kidney transplant recipients is scarce. This crosssectional study aims to investigate the antibody response to COVID-19 among kidney transplant recipients.
Materials and methods
We recruited 46 kidney transplant recipients with RT-PCR-confirmed COVID-19 and 45 recipients without COVID-19 history. We also constructed two control groups (COVID-19 positive and negative) from a historical cohort of healthcare workers. We used age and sex-based propensity score matching to select the eligible subjects to the control groups. We measured the SARS-CoV-2 IgG levels quantitatively using the Abbott ARCHITECT system. An antibody level above 1.4 S/C was defined as positivity.
Results
Transplant recipients with COVID-19 had a higher BMI, and COVID-19 history in a household member was more common than that of the transplant recipient without COVID-19. IgG seropositivity rate (69.6% vs. 78.3%, p = 0.238) and the median IgG level (3.28 [IQR: 0.80–5.85] vs. 4.59 [IQR: 1.61–6.06], p = 0.499) were similar in COVID-19-positive transplant recipients and controls. Kidney transplant recipients who had a longer duration between RT-PCR and antibody testing had lower antibody levels (r = −0.532, p < 0.001).
Conclusion
At the early post-COVID-19 period, kidney transplant recipients have a similar antibody response to controls. However, these patients’ antibody levels and immunity should be closely monitored in the long term.
Keywords: COVID-19, immune response, immunosuppression, kidney transplantation, SARS-CoV-2 IgG antibody
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the SARS-CoV-2 virus has affected the global health system in an unprecedented way. The rapid development of various vaccines has become an important milestone in combating the pandemic. Vaccination relies on the production of antibodies via the stimulation of humoral immunity. However, data regarding antibody response to infection in immunosuppressed patients, including kidney transplant recipients, is not well defined.
Different mortality rates for COVID-19 were reported from different countries. According to a recent study in Western countries, mortality rates changed between 4.0% and 16.1%. Higher mortality rates were reported in patients with kidney disease in different studies, specifically in kidney transplantation patients having a mortality rate that ranges between 18.0% and 41.6% [1–6]. Moreover, data on the seropositivity rate following COVID-19 in kidney transplant recipients are scarce and not in agreement across studies. According to a recent study, lower antibody response rates (41.0%) were reported in kidney transplant recipients following recovery from the infection [7,8]. On the other hand, Azzi et al. investigated 69 kidney transplant recipients with reverse transcriptase-polymerase chain reaction (RT-PCR)-confirmed COVID-19 infection and found antinucleocapsid antibodies in 55 (80.0%) of them [9].
Usually, higher antibody response rates were reported for patients from the general population [8]. In a recent study, antinucleoprotein seropositivity rate was found 78.2% among healthcare workers with RT-PCR-confirmed COVID-19 [10].
In this crosssectional study, we aimed to investigate the prevalence of anti-SARS-CoV-2 antibodies in kidney transplant recipients first. Then, we examined the factors associated with the absence of antibody response.
2. Materials and methods
2.1. Study design
We performed a crosssectional study to recruit renal transplant recipients who were under regular follow-up at two university hospital’s transplantation centers (CMF and KMF) and had RT-PCR-confirmed COVID-19. We also recruited consecutive renal transplant recipients who did not have a history of COVID-19 and were attending the transplantation outpatient clinics in those institutions. We constructed two additional control groups from a previously screened cohort of healthcare workers [10]. None of the included patients was vaccinated for COVID-19 prior to the antibody measurement.
We used the descriptive comparative design method to assess the outcomes. The study protocol was approved by the Clinical Research Ethics Committee of İstanbul University-Cerrahpaşa (approval no: 2021-2921) and the Ministry of Health’s Scientific Committee (approval no: 2020-11-30T14_57_30). The study was conducted in accordance with the 1975 Declaration of Helsinki, as revised in 2013.
2.2. Sampling
The study was conducted between December 7, 2020, and February 12, 2021. The date of blood specimen collection for antibody measurement was accepted as the enrollment date to the study. The date of the RT-PCR testing was accepted as the first day of infection.
2.2.1. Transplant recipients
Based on previous studies, we predicted the seropositivity following COVID-19 as 60% for transplant recipients [9,11] and 90% for subjects from the general population [12–14]. We performed a power analysis, and we planned to recruit 42 participants to each group.
A total of 623 patients had attended the outpatient transplantation clinics during the year 2020. Our target population comprised of patients who had COVID-19 following April, 2020. We did not formally screen all patients under follow-up; however, all of our transplant patients who had an RT-PCR-confirmed COVID-19 history were eligible for the study. We located 57 patients who had COVID-19, one of them died before the start of the study, 46 of them accepted to participate in the study.
We recruited transplant patients who gave informed consent in the COVID-19-negative group if they have not received a diagnosis of COVID-19 as of the recruitment day. We also checked if they had any RT-PCR test due to mandatory screening (before hospitalization due to any cause, having a household member with COVID-19) and confirmed that their RT-PCR test was negative. A total of 45 patients met the inclusion criteria for the control group.
Seventy-one of the 91 transplant recipients included in the study received a transplant from a living donor, while all donors were first- or second-degree relatives.
2.2.2. Controls
We recruited control subjects from a cohort of healthcare workers that we examined previously [10]. The details of the recruitment and data collection for those participants were previously described in detail [10].
In that cohort, 116 subjects were RT-PCR positive. We excluded any subjects with malignancy or using immunosuppressive drugs. We transformed the duration between RT-PCR and antibody testing to binomial data based on a cutoff value of 8 weeks. The RT-PCR-positive control group is formed by recruiting subjects, using propensity score matching based on age, sex, and transformed antibody testing duration data with a 1:1 ratio.
Among healthcare workers who did not have a history of COVID-19, we selected the subjects designated as “no risk” (healthcare workers who were not attending the hospital because of administrative changes related to the pandemic) regarding COVID-19. We excluded any subjects with malignancy or using immunosuppressive drugs. Finally, 106 subjects were eligible for selection. We used age and sex-based propensity score matching to select subjects from this cohort with a ratio of 1:1.
We used the same laboratory procedures to measure the antibody levels in those subjects and transplant recipients.
2.3. Data collection
We filled in a standard form for every patient. We used patient interviews, medical records of the patients, the hospital’s electronic database, and the national public health data management system to collect data. Our form consisted of the following parts; demographics, clinical data including transplantation history, drug use, laboratory parameters, history, and clinical data related to COVID-19, and computed tomography (CT) findings. We also used the COVID-19 severity index to classify the patients under five mutually exclusive categories; asymptomatic or presymptomatic, mild, moderate, severe, critical illness [15].
2.4. PCR testing and assessment of antibodies
We used the same methods for RT-PCR testing and SARS-CoV-2- antibody measurement as described in detail previously [10,16]. For the detection of COVID-19 RNA, a commercial RT-PCR kit (Bio-Speedy SARS-CoV-2 RT-qPCR kit; Bioeksen R&D Technologies Ltd., İstanbul, Turkey) was used. For the detection of SARS-CoV-2 IgG (antinucleocapsid protein antibodies), chemiluminescent microparticle immunoassay (Abbott Laboratories, cat. no: 6R86, lot no: 16253FN00) was carried out according to the manufacturer’s instructions, and samples were run on the related instrument (ARCHITECT; Abbott Laboratories, Abbott Park, IL, USA). The qualitative results were reported by the instrument with a cutoff value of 1.40 S/C as recommended.
2.5. Therapeutic approach
Antiviral therapy with favipiravir and prophylactic anticoagulation with low-molecular-weight heparin were considered in all patients unless contraindicated. For patients with systemic inflammation, tocilizumab or anakinra was initiated in addition to high-dose corticosteroids. Regarding immunosuppressive treatment, antiproliferative agents were ceased or reduced, calcineurin inhibitors were maintained stable, dose-reduced or were completely withdrawn, and corticosteroid doses were increased based on the disease severity.
2.6. Statistical analysis
Descriptive data were presented as mean ± standard deviation (SD) and median and interquartile range (IQR) for the continuous variables and frequency and percentages (%) for the categorical variables. Continuous variables were evaluated for normality using the Shapiro-Wilk test. Kidney transplant recipients and control groups were compared with an independent samples t-test for normally distributed variables and the Mann-Whitney U test for nonnormally distributed variables. Categorical variables were compared using the chi-square or Fisher’s exact test for proportion. Multivariate analysis was applied to determine the association between the antibody level, the groups, and postinfection duration. All significance tests were two-tailed, and values of p <0.05 were considered statistically significant. All statistical analyses were performed using the SPSS software version 21 (IBM Corporation, Armonk, NY, USA).We employed propensity score matching to balance in the observed baseline covariates and reduce the bias of treatment effect between the kidney transplant recipients and control groups. We assumed a ratio of 1:1 on age and sex with the nearest neighbor matching method. The propensity score matching was performed using the RStudio v.4.0.2 software.
Based on previous studies, we accepted the seropositivity rate following COVID-19 as 60% for transplant patients and 90% for the general population. Therefore, considering the percentage from the previous studies, we performed power analysis (G*Power software version 3.1; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) with a power of 90% and an error of 0.05 to determine the minimum sample size for the RT-PCR-positive kidney transplant recipients and control groups. A minimum sample size of 42 was estimated for each group.
3. Results
3.1. Demographic, clinical and laboratory data
We recruited a total of 91 kidney transplant recipients. Of them, 46 had RT-PCR-confirmed COVID-19, whereas 45 did not have a history of COVID-19 (Table 1). Demographic, clinical, and laboratory data of the transplant recipients grouped according to their COVID-19 status are shown in Table 1. Both groups were similar regarding age and sex. The etiology of CKD, donor type, and posttransplant duration were also similar between the two groups. However, patients with COVID-19 had a higher BMI, and COVID-19 history in a household member was more common among them. Other parameters were similar between the two groups.
Table 1.
Demographic, clinical, and laboratory parameters of the kidney transplant recipients according to their COVID-19 status.
| COVID-19 (+) (n = 46) | COVID-19 (−) (n = 45) | p | |
|---|---|---|---|
| Age (years) | 46.5 (36.8–55.0) | 37.0 (32.0–55.0) | 0.194 |
| Male, n (%) | 32 (69.6) | 27 (60.0) | 0.231 |
| Etiology of CKD | |||
| Diabetes mellitus, n (%) | 5 (10.9) | 3 (6.7) | 0.599 |
| Glomerulonephritis, n (%) | 8 (17.4) | 11 (24.4) | |
| Others, n (%) | 33 (71.7) | 31 (68.9) | |
| Living donor, n (%) | 36 (78.3) | 35 (77.8) | 0.600 |
| Transplantation duration (months) | 108.0 (48.0–147.0) | 84.0 (24.0–132.0) | 0.378 |
| COVID-19 in a household member, n (%) | 29 (64.4) | 3 (6.8) | <0.001 |
| Comorbidities | |||
| Diabetes, n (%) | 13 (28.3) | 11 (24.4) | 0.431 |
| Hypertension, n (%) | 31 (67.4) | 26 (57.8) | 0.232 |
| BMI (kg/m2) | 27.4 ± 4.5 | 24.7 ± 4.2 | 0.004 |
| Serum creatinine (mg/dL) | 1.28 (1.01–1.53) | 1.23 (1.02–1.72) | 0.778 |
| e-GFR* (mL/min/1.73 m2) | 64.9 ± 24.1 | 64.6 ± 31.6 | 0.955 |
| Baseline immunosuppression | |||
| Steroids, n (%) | 46 (100.0) | 42 (93.3) | 0.117 |
| Calcineurin inhibitor, n (%) | 43 (93.5) | 43 (95.6) | 0.511 |
| Mycophenolic acid derivatives, n (%) | 41 (89.1) | 35 (77.8) | 0.119 |
| m-TOR inhibitors, n (%) | 4 (8.7) | 4 (8.9) | 0.631 |
| Azathioprine, n (%) | 3 (6.5) | 7 (15.6) | 0.149 |
Values are presented as mean ± SD or as median and IQR.
CKD: chronic kidney disease, BMI: body mass index, m-TOR inhibitors: mechanistic target of rapamycin inhibitors.
Calculated using the CKD-EPI formula.
The majority (95.6%) of the patients with COVID-19 were symptomatic, and according to computed tomography of the thorax, 30 patients (65.2%) had findings compatible with COVID-19. There was no need for hospitalization in 12 patients (26.1%); the remaining 34 patients (73.9%) were hospitalized, 16 patients (34.8%) needed oxygen, and three patients (6.5%) were followed up in the intensive care unit. Two patients (4.3%) needed intubation. Except for one patient who died on the 26th day of the infection, all patients recovered from COVID-19. The hospitalization duration was 11.7 ± 7.9 days (median: 9 days, range: from 3 to 38 days). According to the COVID-19 severity index, two patients (4.3%) were asymptomatic or presymptomatic, 15 (32.6%) had a mild illness, 16 (34.8%) had a moderate illness, 10 (21.7%) had a severe illness, and three (6.5%) had a critical illness.
3.2. Seropositivity
The seropositivity rates and IgG levels among kidney transplant recipients and controls stratified by the COVID-19 status are shown in Table 2. Among the subjects with COVID-19 history, the SARS-Cov-2 IgG positivity rate (69.6% vs. 78.3%) and IgG level (3.28 vs. 4.59 S/C) of kidney transplant recipients were similar to those of the control group. The frequency of COVID-19 related symptoms was more common among kidney transplant recipients than that of the controls; however, the frequency of pulmonary involvement assessed by computed tomography was similar between the two groups. There was no statistically significant difference between the kidney transplant recipients and controls in terms of the duration between RT-PCR and antibody testing (Table 2).
Table 2.
Seropositivity among the kidney transplant recipients and controls stratified by their COVID-19 status.
| COVID-19 (+) | p | COVID-19 (–) | p | |||
|---|---|---|---|---|---|---|
| Kidney transplant recipients (n = 46) | Controls (n = 46) | Kidney transplant recipients (n = 45) | Controls (n = 45) | |||
| Age (years) | 45.9 ± 12.1 | 41.3 ± 10.2 | 0.053 | 37.0 (32.0–55.0) | 37.0 (27.5–53.0) | 0.534 |
| Male, n (%) | 32 (69.6) | 32 (69.6) | 0.589 | 27 (60.0) | 27 (60.0) | 0.585 |
| Symptoms, n (%) | 44 (95.7) | 36 (78.3) | 0.013 | NA | NA | NA |
| CT result, n (%) | 30 (65.2) | 30 (65.2) | 0.558 | NA | NA | NA |
| IgG positivity rate, n (%) | 32 (69.6) | 36 (78.3) | 0.238 | 3 (6.7) | 3 (6.7) | 0.662 |
| IgG level (S/C) | 3.28 (0.80–5.85) | 4.59 (1.61–6.06) | 0.499 | 0.03 (0.02–0.05) | 0.03 (0.02–0.09) | 0.997 |
| Days following RT-PCR test | 49.5 (25.8–70.6) | 55.0 (49.3–61.0) | 0.392 | NA | NA | NA |
CT: computed tomography, Ig: immunoglobulin, RT-PCR: reverse transcriptase-polymerase chain reaction.
Values are presented as mean ± SD or as median and IQR.
Among the subjects without COVID-19 history, three kidney transplant recipients had positive IgG antibodies, and two of them had a history of COVID-19 in a household member. The SARS-Cov-2 IgG antibody positivity rate (6.7% vs. 6.7%) and IgG level (0.03 vs. 0.03 S/C) of kidney transplant recipients were similar to that of the control group (Table 2).
3.3. Predictors of antibody positivity
We compared the demographic, clinical, laboratory, and treatment-related data of the transplant patients who developed antibodies with those who did not (Table 3). The median duration between RT-PCR and antibody testing was shorter (37.5 days [IQR: 20.5–57.8] vs. 82.5 days [IQR: 52.3–105.0], p = 0.01) in patients who had SARS-Cov-2 IgG antibodies compared to that of the patients who did not have IgG antibodies. There were no statistically significant differences between the two groups regarding demographic, clinical, and laboratory parameters. Additionally, the cessation rate of different immunosuppressive drugs was also similar between the two groups.
Table 3.
Comparison of the transplant recipients with and without IgG positivity following RT-PCR-confirmed COVID-19.
| IgG-positive (n = 32) | IgG-negative (n = 14) | p | |
|---|---|---|---|
| Age (years) | 47.5 ± 11.1 | 42.2 ± 13.8 | 0.172 |
| Male, n (%) | 23 (71.9) | 9 (64.3) | 0.427 |
| Etiology of CKD | |||
| Diabetes mellitus, n (%) | 4 (12.5) | 1 (7.1) | 0.364 |
| Glomerulonephritis, n (%) | 7 (21.9) | 1 (7.1) | |
| Others, n (%) | 21 (65.6) | 12 (85.7) | |
| Living donor, n (%) | 24 (75.0) | 11 (84.6) | 0.392 |
| Transplantation duration (months) | 108.9 ± 61.9 | 88.0 ± 78.8 | 0.337 |
| Comorbidities | |||
| Diabetes, n (%) | 10 (31.3) | 3 (21.4) | 0.381 |
| Hypertension, n (%) | 22 (68.8) | 9 (64.3) | 0.511 |
| Coronary artery disease, n (%) | 7 (21.9) | 5 (35.7) | 0.264 |
| Chronic obstructive pulmonary disease, n (%) | 2 (6.3) | 1 (7.1) | 0.673 |
| BMI (kg/m2) | 27.6 ± 4.3 | 26.9 ± 5.1 | 0.529 |
| Serum creatinine (mg/dL) | 1.33 (1.03–1.48) | 1.15 (0.87–1.83) | 0.867 |
| e-GFR* (ml/min/1.73 m2) | 63.5 ± 19.0 | 68.3 ± 33.6 | 0.537 |
| Disease severity index | |||
| Asymptomatic - moderate, n (%) | 21 (66.0) | 12 (85.0) | 0.286 |
| Severe - critical, n (%) | 11 (34.0) | 2 (15.0) | |
| Days following RT-PCR test | 37.5 (20.5–57.8) | 82.5 (52.3–105.0) | 0.001 |
| Lowest white blood cell count, (/mm3) | 4925.7 ± 1748.0 | 4596.8 ± 1667.2 | 0.582 |
| Lowest lymphocytes count, (/mm3) | 791.0 ± 603.0 | 665.4 ± 314.7 | 0.875 |
| Peak CRP, (mg/dL) | 75.8 ± 66.5 | 74.7 ± 58.0 | 0.813 |
| Peak ferritin, (ng/mL) | 625.0 (249.5–1468.3) | 670.1 (236.0–1245.0) | 0.839 |
| Fibrinogen, (mg/dL) | 447.8 (4.98–649.8) | 329.1 (4.76–515.5) | 0.439 |
| Peak D-dimer, (μg/mL) | 1.03 (0.36–2.94) | 0.91 (0.66–2.78) | 0.868 |
| Peak procalcitonin, (ng/mL) | 1.72 (0.92–127.0) | 92.5 (1.13–191.3) | 0.146 |
| Peak uric acid, (mg/dL) | 8.1 ± 2.1 | 7.7 ± 2.3 | 0.622 |
| Stopping MPA, n (%) | 17 (53.1) | 7 (50.0) | 0.549 |
| Stopping CNI, n (%) | 3 (9.4) | 0 (0.0) | NA |
| Stopping MPA or CNI, n (%) | 18 (56.3) | 7 (50.0) | 0.471 |
CKD: chronic kidney disease, BMI: body mass index, RT-PCR: reverse transcriptase-polymerase chain reaction, MPA: mycophenolic acid derivatives, CNI: calcineurin inhibitor.
Calculated using the CKD-EPI formula.
Values are presented as mean ± SD or as median and IQR.
As an additional analysis, we looked at the correlation between different laboratory parameters (peak CRP, peak ferritin, fibrinogen, peak D-dimer, peak procalcitonin, e-GFR) and the level of SARS-Cov-2 IgG antibodies. There was no significant correlation between those parameters (data not shown).
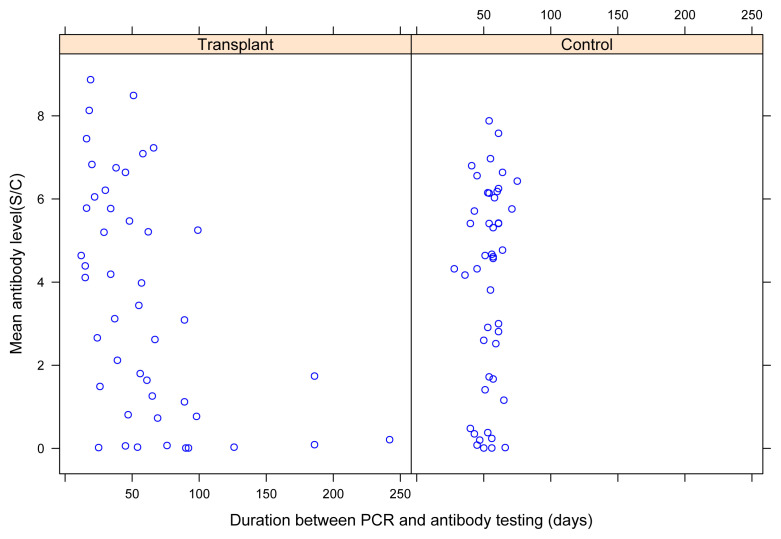
Finally, we analyzed the correlation between the SARS-Cov-2 IgG antibody levels and the duration between RT-PCR and antibody testing. The antibody level in kidney transplant recipients and controls based on the duration following RT-PCR testing is shown in Figure. Visual examination revealed that kidney transplant recipients who had a longer duration between RT-PCR and antibody testing had lower antibody levels. There was a significant correlation between the antibody levels and the duration between RT-PCR and antibody testing in transplant recipients (r = −0.532, p < 0.001), whereas no statistically significant correlation was found between the two parameters in the controls (r = 0.198, p = 0.186). Additionally, we constructed a multivariate regression model where we used antibody levels as the dependent variable and the study group along with the duration following RT-PCR testing as the independent variables. This analysis showed that the study group was not an independent determinant of antibody levels (data not shown).
Figure.
SARS-CoV-2 IgG antibody levels among transplant recipients and controls, according to the duration between RT-PCR and IgG testing.
4. Discussion
We found that kidney transplant recipients developed an antibody response following COVID-19; 69.6% of the patients with COVID-19 history had IgG antibodies and the mean antibody level and the seropositivity rate were similar to that of the control group. To the best of our knowledge, the largest report about antibody response in kidney transplant recipients is from Azzi et al. [9]. In this report, the researchers examined 69 kidney transplant recipients who had an RT-PCR-confirmed COVID-19 diagnosis, and 55 (80.0%) of them had a positive antibody response. The authors used the same test as ours to measure the antibody levels after a median of 44 days following RT-PCR positivity. Hartzell et al. examined anti-SARS-Cov2 IgG antibodies in 16 kidney transplant recipients following a mean of 16.1 days of RT-PCR testing and reported the antibody positivity rate as 60.0% and 63.6%, depending on immunosuppressive drug use [17]. Burack et al. examined 39 kidney transplant recipients and found an antibody positivity rate of 41.0% [7].
We did not identify any specific risk factors for lack of seroconversion following COVID-19. However, we noted a trend toward lower antibody levels in patients who had a longer postinfection duration. A similar observation was reported by Benotmane et al. [18] as they examined 29 kidney transplant recipients hospitalized for COVID-19 and measured antibody levels up to six months after COVID-19. During the follow-up, 20.7% of the patients became seronegative. A considerable IgG reduction was observed in patients treated with calcineurin inhibitors and steroids. No statistically significant difference was found regarding disease severity.
In kidney transplant recipients who did not have positive RT-PCR testing, we found a similar SARS-CoV-2 IgG antibody positivity rate to that in our controls. In line with our results, a recent study reported that the seroprevalence rate was 6.6% in asymptomatic people [19]. In another study, SARS-CoV-2 seroprevalence in healthy blood donors was reported as 3% [20].
Several studies have reported that kidney transplant recipients with COVID-19 have a high mortality rate as high as 40% [1–6]. It has been shown that increasing age and the presence of comorbid diseases are associated with an increased risk of mortality [21,22]. In our study, all kidney transplant recipients recovered except one. All the patients followed the infectious control measures rigorously, while most of the infected patients were younger, had low creatinine levels, and did not have many comorbidities. We closely followed up those patients and changed the immunosuppressive therapy as soon as we were aware of the COVID-19 infection. These factors might be responsible for the low mortality in our study.
Our study had some limitations. We did not formally screen all patients; we might have overlooked some patients who had severe COVID-19 and died. However, it is unlikely that we missed mild cases because most of the patients were in close telephone contact with transplant coordinators during this period. Because of the pandemic, transplantation activity was stopped. Therefore, our cohort consists of long-term transplanted patients. The absence of the patients in the first six months after transplantation when immunosuppression is the strongest might have influenced the results. Another limitation of this study was that the date of infection was defined as the date of PCR positivity, as opposed to the date of symptom onset. Since transplant recipients may exhibit prolonged shedding of the virus, the date of PCR positivity may not always be an accurate estimate of the infection onset. In addition, kidney transplant recipients and controls had different distribution characteristics regarding the duration between COVID-19 and antibody testing. All the controls were tested for antibodies at least four weeks, while no controls were tested beyond 12 weeks postinfection. However, the distribution of the transplant recipients between different IgG testing durations was homogenous. Finally, we did not examine the parameters related to cellular immunity.
In conclusion, kidney transplant recipients seem to have an antibody response similar to that of the general population at the early post-COVID-19 period. However, similar to the general population, there is a tendency toward lower antibody levels with increasing postinfection duration. Therefore, we suggest a caution for humoral immunity in kidney transplant recipients following COVID-19; at least for three months postinfection. The follow-up of antibody levels and booster vaccination might also be warranted.
Acknowledgment/conflict of interest
This present work was supported by the İstanbul branch of Turkish Society of Nephrology (grant number: 2711_2020).
The authors declare no conflicts of interest.
Funding Statement
This present work was supported by the İstanbul branch of Turkish Society of Nephrology (grant number: 2711_2020).
Footnotes
Informed consent
The study protocol was approved by the Clinical Research Ethics Committee of İstanbul University-Cerrahpaşa (approval no: 2021-2921) and the Ministry of Health’s Scientific Committee (approval no: 2020-11-30T14_57_30). The study was conducted in accordance with the 1975 Declaration of Helsinki, as revised in 2013. Informed consent was obtained from all individual participants included in the study.
References
- 1. Elias M, Pievani D, Randoux C, Louis K, Denis B, et al. COVID-19 infection in kidney transplant recipients: disease incidence and clinical outcomes. Journal of the American Society of Nephrology. 2020;31(10):2413–2423. doi: 10.1681/ASN.2020050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cravedi P, Mothi SS, Azzi Y, Haverly M, Farouk SS, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. American Journal of Transplantation. 2020;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abolghasemi S, Mardani M, Sali S, Honarvar N, Baziboroun M. COVID-19 and kidney transplant recipients. Transplant Infectious Disease. 2020;22(6):e13413. doi: 10.1111/tid.13413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sran K, Olsburgh J, Kasimatis T, Clark K, Gökmen R, et al. COVID-19 in Kidney Transplant Patients from a Large UK Transplant Centre: Exploring Risk Factors for Disease Severity. Transplantation Proceedings. 2021;53(4):1160–1168. doi: 10.1016/j.transproceed.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hilbrands LB, Duivenvoorden R, Vart P, Franssen C, Hemmelder MH, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrology, Dialysis, Transplantation. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Meester J, De Bacquer D, Naesens M, Meijers B, Couttenye MM, et al. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. Journal of the American Society of Nephrology. 2021;32(2):385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burack D, Pereira MR, Tsapepas DS, Harren P, Farr MA, et al. Prevalence and Predictors of SARS-CoV-2 Antibodies among Solid Organ Transplant Recipients with Confirmed Infection. American Journal of Transplantation. 2021;21(6):2254–2261. doi: 10.1111/ajt.16541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nature Medicine. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 9. Azzi Y, Parides M, Alani O, Loarte-Campos P, Bartash R, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney International. 2020;98(6):1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alkurt G, Murt A, Aydin Z, Tatli O, Agaoglu NB, et al. Seroprevalence of coronavirus disease 2019 (COVID-19) among health care workers from three pandemic hospitals of Turkey. PloS One. 2021;16(3):e0247865. doi: 10.1371/journal.pone.0247865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prendecki M, Clarke C, McKinnon T, Lightstone L, Pickering MC, et al. SARS-CoV-2 Antibody Point-of-Care Testing in Dialysis and Kidney Transplant Patients With COVID-19. Kidney Medicine. 2021;3(1):54–59e1. doi: 10.1016/j.xkme.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo L, Ren L, Yang S, Xiao M, Chang D, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clinical Infectious Disease. 2020;71(15):778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Long Q-X, Tang X-J, Shi Q-L, Li Q, Deng H-J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 14. Zhao J, Yuan Q, Wang H, Liu W, Liao X, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clinical Infectious Disease. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gandhi R, Lynch J, Del Rio C. Mild or moderate COVID-19. The New England Journal of Medicine. 2020;383(18):1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 16. Trabulus S, Karaca C, Balkan II, Dincer MT, Murt A, et al. Kidney function on admission predicts in-hospital mortality in COVID-19. Plos One. 2020;15(9):e0238680. doi: 10.1371/journal.pone.0238680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hartzell S, Bin S, Benedetti C, Haverly M, Gallon L, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. American Journal of Transplantation. 2020;20(11):3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benotmane I, Gautier Vargas G, Velay A, Wendling M-J, Perrin P, et al. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. American Journal of Transplantation. 2021;21(6):2307–2310. doi: 10.1111/ajt.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stout RL, Rigatti SJ. Seroprevalence of SARS-CoV-2 Antibodies in the US Adult Asymptomatic Population as of September 30, 2020. JAMA Network Open. 2021;4(3):e211552. doi: 10.1001/jamanetworkopen.2021.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grzelak L, Temmam S, Planchais C, Demeret C, Tondeur L, et al. A comparison of four serological assays for detecting anti–SARS-CoV-2 antibodies in human serum samples from different populations. Science Translational Medicine. 2020;12(559):eabc3103. doi: 10.1126/scitranslmed.abc3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebinger JE, Achamallah N, Ji H, Claggett BL, Sun N, et al. Pre-existing traits associated with Covid-19 illness severity. PloS One. 2020;15(7):e0236240. doi: 10.1371/journal.pone.0236240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Navari Y, Bagheri AB, Akhavan Rezayat A, Seyed Alinaghi S, Najafi S, et al. Mortality risk factors in kidney-transplanted patients with COVID-19: A systematic review and regression analysis. Health Science Reports. 2021;4(4):e427. doi: 10.1002/hsr2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]