Abstract
Loss of skeletal muscle mass is a primary feature of sarcopenia and cancer cachexia. In cancer patients, tumor-derived inflammatory factors promote muscle atrophy via tumor-to-muscle effects, which is closely associated with poor prognosis. During the past decade, skeletal muscle has been considered to function as an autocrine, paracrine, and endocrine organ by releasing numerous myokines. The circulating myokines can modulate pathophysiology in the other organs, as well as in the tumor microenvironment, suggesting myokines function as muscle-to-tumor signaling molecules. Here, we highlight the roles of myokines in tumorigenesis, particularly in terms of crosstalk between skeletal muscle and tumor. Better understanding of tumor-to-muscle and muscle-to-tumor effects will shed light on novel strategies for the diagnosis and treatment of cancer.
Keywords: Cancer cachexia, Myokine, Sarcopenia, Skeletal muscle, Tumor
INTRODUCTION
Skeletal muscle comprises approximately 40% of the whole body weight, and contains the largest body protein pool (1). The major functions of skeletal muscle are to contract to produce movement, maintain body temperature, store nutrients, and stabilize joints (1). In this regard, skeletal muscle wasting is closely associated with numerous human diseases (2). Sarcopenia is characterized by the decline in skeletal muscle mass and strength, emerging as a major health problem, particularly related to aging (3). Thus, maintaining skeletal muscle mass is crucial for human health and wellness.
During the past decade, skeletal muscle has been recognized as an endocrine organ (4). Skeletal muscle is composed of various types of cells, such as myocytes, fibroblasts, adipocytes, neurons, and connective tissues, which can release bioactive molecules referred to as ‘myokines’ (5). The myokines are peptides that are synthesized, expressed, and secreted by skeletal muscle fibers, including cytokines, interleukins (ILs), and neurotrophins (5). These myokines can regulate extracellular matrix organization, angiogenesis, and metabolism in both paracrine and endocrine manners (6).
Notably, most of the patients with advanced cancers represent sarcopenia with or without loss of fat mass, distinct from age-related sarcopenia or malnutrition, which is termed ‘cachexia’ (7). The cancer-associated cachexia is a multi-organ metabolic syndrome, which is not fully recovered by nutritional support, and accounts for 20% of cancer deaths (8). In this regard, skeletal muscle is suggested as a crucial player in carcinogenesis; however, its underlying mechanism is not fully understood. Herein, we focus on how skeletal muscle regulates cancer promotion and progression by affecting the tumor microenvironment (TME), particularly through myokines.
SKELETAL MUSCLE MASS AND CANCER: TUMOR-TO-MUSCLE EFFECTS
Cancer cachexia: tumor-to-muscle effects
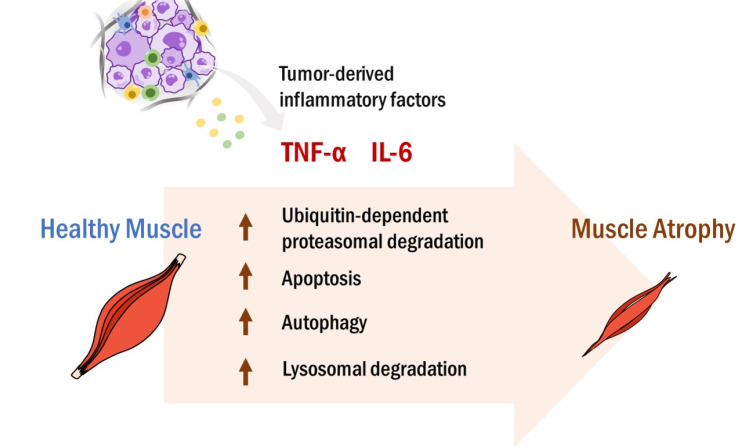
Cancer cachexia is a devastating syndrome of remarkable weight loss, muscle wasting, and anorexia, which occurs in about 30% of total cancer patients, and in 70-80% of advanced cancer patients (8). Both cancer cachexia and sarcopenia show loss of skeletal muscle mass, sharing a complex pathophysiology, while cachexia is involved with more inflammatory responses than sarcopenia (9). Cancer has long been considered as a chronic and systemic inflammatory status that creates TME more favorable to tumor promotion and progression, leading to metabolic alterations in multiple organs (10). Among the pro-inflammatory factors released from tumors, IL-6 and tumor necrosis factor α (TNF-α) are well known to trigger skeletal muscle wasting (11, 12). Notably, serum levels of IL-6 and TNF-α were dramatically increased in pancreatic cancer patients with weight loss, compared to those without weight change (13, 14). A prospective cohort study further demonstrated that the plasma IL-6 level was markedly higher in cachectic lung cancer patients than in non-cachectic patients (15). Higher IL-6 and TNF-α levels were associated with cachexia, as well as mortality in cancer patients (16, 17). In tumor-bearing murine models, the elevated serum levels of IL-6 and/or TNF-α were also correlated with skeletal muscle loss, further supporting the sarcopenic effects of cancer-derived inflammatory cytokines (18, 19). However, ablation of IL-6 attenuated skeletal muscle wasting in tumor-bearing mice (19, 20). Similarly, administration of an anti-TNF-α antibody improved cachectic conditions in tumor-bearing mice (21). As illustrated in Fig. 1, these data suggest that cancer-derived IL-6 and/or TNF-α promote skeletal muscle wasting via tumor-to-muscle effects.
Fig. 1.
Tumor-to-muscle effects. Tumor tissues constantly release various types of pro-inflammatory molecules into the bloodstream, maintaining the systemic and chronic inflammatory status. The circulating tumor-derived inflammatory factors, such as IL-6 and TNF-α, promote skeletal muscle atrophy through activating ubiquitin-dependent proteasomal degradation, apoptosis, autophagy, and/or lysosomal degradation. Loss of skeletal muscle is closely associated with poor survival in cancer patients.
Skeletal muscle mass as a cancer prognostic marker
Notably, low skeletal muscle mass is correlated with poor prognosis in various types of cancer, including pancreatic and oesophageal cancers (8, 22, 23). In contrast, increased skeletal muscle mass was associated with longer survival in pediatric patients with malignant solid cancers (24). Thus, skeletal muscle mass is considered a putative marker for predicting cancer prognosis.
Moreover, skeletal muscle mass can affect chemotherapy-induced toxicity in cancer patients. Cancer patients with loss of skeletal muscle mass presented susceptibility to chemotherapy-induced toxicity and poor prognosis, compared to patients with lean body mass (25, 26). A meta-analysis including 48 studies has demonstrated that low skeletal muscle mass is associated with dose-limiting toxicities in cancer patients under neoadjuvant/adjuvant chemotherapies or curative radio-chemotherapies (27). In cachectic tumor-bearing mice, 5-fluorouracil chemotherapy exacerbated skeletal muscle wasting (28). Likewise, sarcopenia has been reported as a poor prognostic marker in cancer patients treated with immune checkpoint inhibitors, implying that skeletal muscle mass is crucial for optimizing chemotherapeutic regimens (29, 30). Taken together, skeletal muscle mass can reflect the body status in cancer patients and skeletal muscle might prevent and/or block carcinogenic processes, possibly through its secretome, while tumors induce skeletal muscle atrophy via secretion of IL-6 and TNF-α.
MYOKINES: MUSCLE-TO-TUMOR EFFECTS
Myokines, exercise, and cancer
In addition to tumor-to-muscle effects, skeletal muscle can modulate TME through its secretome. These skeletal muscle-derived molecules, termed myokines, include more than 600 proteins, such as cytokines (IL-8; IL-15) and neurotrophins (brain-derived neurotrophic factor, BDNF) (5). Myokines exert biological functions in autocrine, paracrine, and endocrine fashions (6). In particular, exercise can continuously stimulate skeletal muscle contraction, resulting in the secretion of a large amount of myokines (31). These myokines enhance skeletal muscle hypertrophy in an autocrine manner (31). Once released into the systemic circulation, myokines facilitate communication between muscle and the other organs (6).
It is well established that exercise is inversely correlated to cancer risk (32, 33). Exercise and/or regular physical activities can inhibit tumor promotion and progression in animal models, as well as cancer patients (34-36). The secreted myokines during the physical activities can regulate metabolic pathways, immune system, and inflammation in TME (37). Thus, myokines play a key role in muscle-to-tumor effects, which mediates the beneficial effects of exercise in cancer patients. In the following sections, we discuss how skeletal muscle-derived myokines modulate cancer promotion and progression by affecting TME. Among various myokines, we focus on myokines that are known to be released by exercise from skeletal muscle and to function in TME in both direct and/or indirect manners.
Effects of myokines on TME
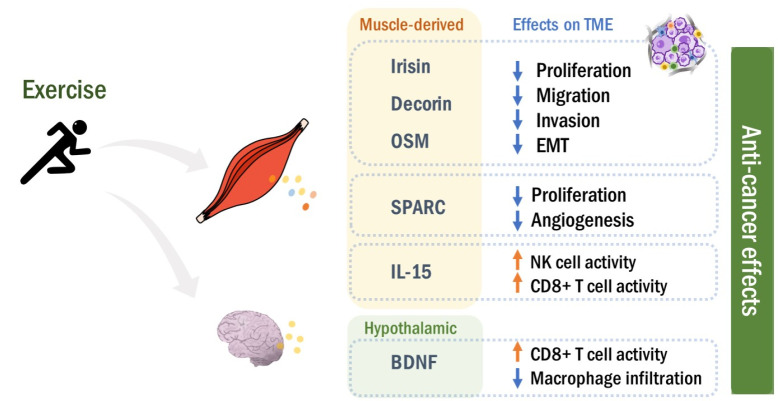
Irisin: Irisin, a recently discovered myokine, is a cleaved form of fibronectin type III domain-containing 5 (FNDC5) (38). During exercise, muscle contraction can upregulate the plasma levels of FNDC5 and its proteolytic product irisin (38, 39). Notably, it has been reported that the serum level of irisin was significantly reduced in patients with breast and liver cancers, compared to healthy individuals, implying the tumor-suppressive role of irisin (40, 41). In line with this notion, irisin treatment inhibited cell proliferation in breast and lung cancer cell lines (42, 43). Moreover, irisin prevented epithelial-to-mesenchymal transition (EMT), a major driver of cancer metastasis (43, 44). Exposure to irisin or overexpression of FNDC5/irisin diminished migration and invasion in different types of human cancer cell lines (43, 45). In ovarian cancer cell lines, irisin blocked hypoxia-inducible factor 1α signaling pathways responsible for the expression of metastasis markers, supporting its anti-metastatic activities (46). In the xenograft glioblastoma mouse model, administration of irisin dramatically repressed tumor growth (47). Furthermore, bladder cancer patients with lower serum irisin levels showed higher mortality (48). Taken together, these data suggest that irisin is a tumor-suppressive myokine.
Decorin: Decorin (DCN) is a member of the small leucine-rich repeat proteoglycan family that is ubiquitously expressed in connective tissues (49). During exercise, muscle contraction can upregulate DCN expression in human tendon and muscle, and also induce its secretion into the systemic circulation (50, 51). DCN has been reported to act as a soluble inhibitor of pan-receptor tyrosine kinases, such as Met receptor and epidermal growth factor receptor (EGFR), pro-survival signals for tumor cells (52). DCN treatment or overexpression reduced cell proliferation, migration, and EMT marker expression in various types of cancer cells, which can be mediated by the inhibition of Met/EGFR pathways (53-56). In consistent manner, systemic injection or overexpression of DCN suppressed tumor growth through blocking Met/EGFR pathways in the orthotopic xenograft models (54, 57). DCN significantly reduced angiogenesis and pulmonary metastasis in the tumor xenograft models (58, 59). In contrast, DCN ablation showed more and larger tumors in mice subcutaneously injected with murine colon cancer cells (53). Moreover, it has been reported that DCN is downregulated in tumors from patients with invasive breast and gastric cancers, which is associated with poor prognosis (60, 61). Overall, these data suggest that muscle-derived DCN can exert anti-carcinogenic activities in an endocrine manner, particularly in patients with DCN-low tumors.
IL-15: IL-15, a cytokine that belongs to the four α-helix bundle family, is responsible for natural killer (NK) and T cell immunity (62). Besides the immune cells, IL-15 is abundantly expressed in skeletal muscle, acting as a myokine. Exercise increased IL-15 levels in skeletal muscle and circulation in human subjects (63-65). It is noteworthy that exercise-induced IL-15 expression was correlated with better prognosis in pan-cancer cohorts, suggesting IL-15 as an anti-tumorigenic myokine (66). Consistently, the serum IL-15 level was significantly increased by exercise in prostate cancer patients (35). The pooled sera isolated from the exercising patients reduced cell growth in human prostate cancer DU-145 cells (35). Its anti-cancer effects could be attributed to immune modulation in TME. In tumor-bearing mice, IL-15 was co-localized with CD8 T and NK cells in TME (67). Heterodimeric IL-15 injection suppressed metastatic burden through increasing intratumoral CD8 T and NK cells in several different mouse cancer models, implying the involvement of CD8 T and NK cells in immune modulation by IL-15 (68). Likewise, subcutaneous injection of recombinant human IL-15 induced the number of circulating CD8 T and NK cells, while decreasing the number of leukemic cells in the blood of patients with T-cell malignancies (69). Recently, Kurz et al. demonstrated that exercise enhanced CD8 T cell immunity via increasing IL-15, which suppressed tumor growth in a pancreatic cancer mouse model (70). Overall, these data support that skeletal muscle-derived IL-15 promotes anti-tumor immunity.
Secreted Protein Acidic and Rich in Cysteine: Secreted Protein Acidic and Rich in Cysteine (SPARC), also known as osteonectin, is a calcium-binding matricellular glycoprotein that is involved in development, wound repair, tissue remodeling, differentiation, and proliferation (71). It has been reported that exercise induced circulating SPARC levels in healthy adult men (72). Exercise increased skeletal muscle expression and serum level of SPARC in both human and mice, which suppressed colon tumorigenesis (34). In wild type (WT) mice, regular exercise significantly reduced the number of aberrant crypts driven by a chemical carcinogen azoxymethane (34). However, SPARC-null mice developed more colon tumors than WT mice, which was not attenuated by exercise (34). In a rat model bearing colon cancers, high-intensity swimming training enhanced serum SPARC levels, finally suppressing tumorigenesis (73). Likewise, exercise significantly stimulated SPARC release from the skeletal muscle in patients with metastatic castrate-resistant prostate cancer (35). The serum isolated from these patients suppressed cell growth in human prostate cancer cell lines (35). These data suggest that exercise-induced SPARC can act as an anti-carcinogenic muscle-to-tumor signaling molecule.
SPARC can suppress multiple stages of carcinogenesis. SPARC inhibited cell proliferation in different types of cancer cell lines (74-76). In consistent manner, SPARC-null mice showed accelerated growth of tumor grafts, compared with WT mice (75, 77). Moreover, overexpression of SPARC hampered the expression of EMT markers and migratory capability in gastric cancer cells (78). In athymic nude mice subcutaneously implanted with gastric cancer cells, SPARC overexpression diminished tumor growth and angiogenesis (78, 79). Said et al. demonstrated that SPACR silencing promoted tumor growth and invasiveness, while reducing stromal collagen in the transgenic prostate cancer mouse model (80). Furthermore, SPARC enhanced chemosensitivity in vivo (78, 81). Taken together, these data suggest SPARC as a tumor suppressive myokine.
In contrast, SPARC can act as a tumor promoter as well. SPARC is highly expressed in metastatic tumors, such as glioblastomas and melanoma (82). SPARC increased the expression of EMT markers, enhancing invasion and migration abilities in cancer cells (83, 84). Tumor-derived SPARC induced vascular permeability and lung metastasis in mice inoculated with melanoma cells via tail vein injection (85). Moreover, SPARC overexpression promoted cell proliferation in liver and pancreatic cancer cells, further supporting tumor-derived SPARC as a tumor promoter (86, 87). It has been reported that certain types of cancers display contradictory compartmentalized expression of SPARC in TME, which contributes to the complex functions of SPARC in tumorigenesis (88). In the case of pancreatic cancer, stromal expression of SPARC was associated with poor prognosis, while its tumoral expression showed no significance (89). Furthermore, Pan et al. suggested that the oncogenic roles of SPARC might be due to its autocrine secretion into TME (87). Therefore, the roles of SPARC on TME can vary depending on the site of secretion, either TME or skeletal muscle, as well as its localization in TME.
Oncostatin M: Oncostatin M (OSM) is a member of the IL-6 family cytokines that exerts biological functions through binding to its heterodimeric receptor complex comprising OSM receptor β (OSMRβ) and gp130 (90). Interestingly, exercise remarkably increased the serum level of OSM in healthy individuals and cancer patients (35, 91). OSM has been reported to suppress cell growth in breast cancer, lung adenocarcinoma, and glioblastoma cells, implying OSM as an anti-tumorigenic myokine (92-94). In the orthotopic mouse cancer model, OSM-expressing glioma cells were unable to generate tumor mass (95). In prostate cancer patients undertaking androgen deprivation therapy, the exercise program significantly enhanced the serum level of OSM, while the expression levels of the other myokines were not altered (96). Human prostate cancer DU145 cells exposed to the sera from these patients showed retarded cell growth, further supporting the anti-cancer effect of exercise-induced OSM (96). Similarly, the sera pooled from exercising mice contained abundant OSM levels, which inhibited the proliferation of human breast cancer MCF-7 cells (97). OSM-containing conditioned media reduced EMT phenotypes and cancer stemness in lung adenocarcinoma cells (98). Moreover, treatment with OSM suppressed migration and invasion in human lung adenocarcinoma cell lines, and also blocked tumor metastasis in vivo (92). These data suggest that skeletal muscle-secreted OSM during exercise can act as an anti-tumorigenic myokine.
However, it is controversial as to whether OSM is anti-tumorigenic or pro-tumorigenic. In addition to muscle-to-tumor effects, OSM can be released within TME by tumor cells and/or stromal cells (99, 100). TME-derived OSM seems to exert pro-tumorigenic functions. Treatment with OSM induced proliferation in ovarian and prostate cancer cell lines (101, 102). OSM upregulated the expression of EMT markers and cancer stemness in pancreatic and cervical cancer cells (103, 104). In consistent manner, paracrine expression of OSM promoted invasiveness and angiogenesis of the grafts in vivo (105, 106). Of note, OSM suppressed or maintained the tumor sizes in xenograft models, while promoting metastatic features (104, 106). This might be explained by the lack of OSMR complex. OSM inhibited the growth of OSMR-intact cell growth, while cells with OSMR loss showed OSM resistance (102, 107). Thus, OSM might differentially affect tumorigenesis, depending on the cellular and molecular contexts.
BDNF: BDNF is a member of the nerve growth factor family that is mainly produced by neurons, regulating neuronal survival, differentiation, and apoptosis (108). In addition to neuron cells, skeletal muscle can synthesize BDNF (109). BDNF-null mice exhibited abnormal differentiation of myoblasts, decreased myotube size, and delayed muscle regeneration, suggesting that BDNF is a myokine responsible for skeletal muscle physiology (110). Of note, BDNF can be secreted from stromal cells in TME (111, 112). Higher expression of BDNF was associated with poor prognosis in various types of cancers (112, 113). Moreover, BDNF promoted cancer cell proliferation, migration, and invasion (112, 114). These data suggest that TME-derived BDNF can act as an oncogenic regulator.
In the case of skeletal-muscle derived BDNF, exercise can induce the skeletal muscle expression of BDNF in murine and human (109, 115). However, exercise was not able to increase plasma BDNF levels in human subjects, which means that muscle-derived BDNF might not be directly involved in muscle-to-tumor signaling (115). Rather, exercise seems to stimulate BDNF production in the brain (116). Hypothalamic BDNF expression inhibited tumor growth, while enhancing the anti-cancer CD8 T-cell immunity in C57BL/6 mice subcutaneously implanted with mouse melanoma cells (117, 118). Furthermore, brain infusion of BDNF reduced migration and intratumoral macrophage infiltration in the glioma mouse model (119). Although there is no strong evidence yet that skeletal muscle-derived BDNF directly affects tumorigenesis, exercise-induced hypothalamic BDNF expression can inhibit tumor promotion and progression. Taken together, these data imply that BDNF exerts tumor suppressive functions rather indirectly through the muscle-to-brain effects. Further investigation is required to elucidate a direct role of skeletal muscle-derived BDNF as a myokine.
CONCLUDING REMARKS
Currently, skeletal muscle is well recognized as a secretory organ (4). During exercise, skeletal muscle releases various types of myokines that control exercise adaptations in the autocrine and/or paracrine fashions (5). Moreover, myokines can travel through the circulation to the other organs, such as adipose tissues and brain, modulating pathophysiology (120, 121). Similarly, myokines play a crucial role in the interplay between skeletal muscle and tumor, which regulates tumor promotion and progression. While tumor-derived IL-6 and/or TNF-α contribute to skeletal muscle atrophy (Fig. 1), skeletal muscle-secreted myokines promote anti-tumor immunities and suppress tumor growth and metastatic abilities (Fig. 2). Thus, maintaining and/or recovery of skeletal muscle mass, a source of myokines, would be a novel and proactive approach to cancer treatment. Taken together, the integrative understanding of crosstalk between skeletal muscle and tumor will provide effective diagnosis/prognosis markers and novel chemotherapeutic targets.
Fig. 2.
Muscle-to-tumor effects. During exercise, muscle contraction stimulates the production of myokines. Muscle-derived myokines enter the circulation, and affect tumor promotion and progression by modulating TME in an endocrine fashion. In particular, BDNF seems to regulate TME rather indirectly through muscle-to-brain effects. Overall, exercise-derived myokines exert anti-tumorigenic functions in both direct and/or indirect manners.
Funding Statement
ACKNOWLEDGEMENTS This research was supported by National Research Foundation of Korea (NRF) Grants funded by the Korean Government (grant numbers NRF-2020R1C1C1003338 and NRF-2022M3 A9F3016364 to NYS), and by the Yonsei Signature Research Cluster Program (2023-22-0011).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 3.Prokopidis K, Giannos P, Reginster JY, et al. Sarcopenia is associated with a greater risk of polypharmacy and number of medications: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:671–683. doi: 10.1002/jcsm.13190.0506ad5eae3c47a2bec01b728d449f27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iizuka K, Machida T, Hirafuji M. Skeletal muscle is an endocrine organ. J Pharmacol Sci. 2014;125:125–131. doi: 10.1254/jphs.14R02CP. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 6.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41:594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–106. doi: 10.1016/j.coph.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. doi: 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 9.Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review. Gerontology. 2014;60:294–305. doi: 10.1159/000356760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narsale AA, Carson JA. Role of interleukin-6 in cachexia: therapeutic implications. Curr Opin Support Palliat Care. 2014;8:321–327. doi: 10.1097/SPC.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel HJ, Patel BM. TNF-alpha and cancer cachexia: molecular insights and clinical implications. Life Sci. 2017;170:56–63. doi: 10.1016/j.lfs.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355–1358. [PubMed] [Google Scholar]
- 14.Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12–15. doi: 10.1093/jjco/28.1.12. [DOI] [PubMed] [Google Scholar]
- 15.dic D, Sr, Plestina S, Sverko-Peternac A, Nikolac N, Simundic AM, Samarzija M. Cancer cachexia, sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value. Support Care Cancer. 2016;24:4495–4502. doi: 10.1007/s00520-016-3287-y. [DOI] [PubMed] [Google Scholar]
- 16.Bilir C, Engin H, Can M, Temi YB, Demirtas D. The prognostic role of inflammation and hormones in patients with metastatic cancer with cachexia. Med Oncol. 2015;32:56. doi: 10.1007/s12032-015-0497-y. [DOI] [PubMed] [Google Scholar]
- 17.Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle. 2012;3:245–251. doi: 10.1007/s13539-012-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuyama T, Ishikawa T, Okayama T, et al. Tumor inoculation site affects the development of cancer cachexia and muscle wasting. Int J Cancer. 2015;137:2558–2565. doi: 10.1002/ijc.29620. [DOI] [PubMed] [Google Scholar]
- 19.Rupert JE, Narasimhan A, Jengelley DHA, et al. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J Exp Med. 2021;218:e20190450. doi: 10.1084/jem.20190450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pototschnig I, Feiler U, Diwoky C, et al. Interleukin-6 initiates muscle- and adipose tissue wasting in a novel C57BL/6 model of cancer-associated cachexia. J Cachexia Sarcopenia Muscle. 2023;14:93–107. doi: 10.1002/jcsm.13109.562eec85d9b4414fbcf6d13bb3bc9bf1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang EA, Park JM, Jin W, Tchahc H, Kwon KA, Hahm KB. Amelioration of cancer cachexia with preemptive administration of tumor necrosis factor-alpha blocker. J Clin Biochem Nutr. 2022;70:117–128. doi: 10.3164/jcbn.21-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi MH, Yoon SB. Sarcopenia in pancreatic cancer: effect on patient outcomes. World J Gastrointest Oncol. 2022;14:2302–2312. doi: 10.4251/wjgo.v14.i12.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang P, Zhou J, Xiao X, et al. The prognostic value of sarcopenia in oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:3–16. doi: 10.1002/jcsm.13126.1a88ce9a40bb4a85b21f796b31ddd0e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omori A, Kawakubo N, Takemoto J, et al. Effects of changes in skeletal muscle mass on the prognosis of pediatric malignant solid tumors. Pediatr Surg Int. 2022;38:1829–1838. doi: 10.1007/s00383-022-05225-9. [DOI] [PubMed] [Google Scholar]
- 25.Becker JN, Hermann R, Wichmann J, Sonnhoff M, Christiansen H, Bruns F. Low skeletal muscle mass is predictive of dose-limiting toxicities in head and neck cancer patients undergoing low-dose weekly cisplatin chemoradiotherapy. PLoS One. 2023;18:e0282015. doi: 10.1371/journal.pone.0282015.b3e76d5f6a8f407db2d1dac85605d329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jong C, Chargi N, Herder GJM, et al. The association between skeletal muscle measures and chemotherapy-induced toxicity in non-small cell lung cancer patients. J Cachexia Sarcopenia Muscle. 2022;13:1554–1564. doi: 10.1002/jcsm.12967.f4b04b35132f42edbe3b991ffdca1e2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surov A, Pech M, Gessner D, et al. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis. Clin Nutr. 2021;40:5298–5310. doi: 10.1016/j.clnu.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KT, Swiderski K, Ryall JG, et al. Mechanisms of chemotherapy-induced muscle wasting in mice with cancer cachexia. JCSM Rapid Communications. 2021;5:102–116. doi: 10.1002/rco2.50.4b80acbe02104dee8ebddccaedd7c9ba [DOI] [Google Scholar]
- 29.Cortellini A, Bozzetti F, Palumbo P, et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep. 2020;10:1456. doi: 10.1038/s41598-020-58498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S, Liu Z, Ren Y, et al. Sarcopenia was a poor prognostic predictor for patients with advanced lung cancer treated with immune checkpoint inhibitors. Front Nutr. 2022;9:900823. doi: 10.3389/fnut.2022.900823.8b1a087c5d5147ee821f9cebe18be2cc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann C, Weigert C. Skeletal muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb Perspect Med. 2017;7:a029793. doi: 10.1101/cshperspect.a029793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51:1252–1261. doi: 10.1249/MSS.0000000000001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore SC, Lee IM, Weiderpass E, et al. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoi W, Naito Y, Takagi T, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–889. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Taaffe DR, Galvao DA, et al. Acute effect of high-intensity interval aerobic exercise on serum myokine levels and resulting tumour-suppressive effect in trained patients with advanced prostate cancer. Prostate Cancer Prostatic Dis. 2022 doi: 10.1038/s41391-022-00624-4. doi: 10.1038/s41391-022-00624-4. [DOI] [PubMed] [Google Scholar]
- 36.Kim J-S, Galvão DA, Newton RU, Gray E, Taaffe DR. Exercise-induced myokines and their effect on prostate cancer. Nat Rev Urol. 2021;18:519–542. doi: 10.1038/s41585-021-00476-y. [DOI] [PubMed] [Google Scholar]
- 37.Huang Q, Wu M, Wu X, Zhang Y, Xia Y. Muscle-to-tumor crosstalk: the effect of exercise-induced myokine on cancer progression. Biochim Biophys Acta Rev Cancer. 2022;1877:188761. doi: 10.1016/j.bbcan.2022.188761. [DOI] [PubMed] [Google Scholar]
- 38.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huh JY, Panagiotou G, Mougios V, et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Provatopoulou X, Georgiou GP, Kalogera E, et al. Serum irisin levels are lower in patients with breast cancer: association with disease diagnosis and tumor characteristics. BMC Cancer. 2015;15:898. doi: 10.1186/s12885-015-1898-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pazgan-Simon M, Zuwala-Jagiello J, Kukla M, Grzebyk E, Simon K. Serum concentrations of selected adipokines in virus-related liver cirrhosis and hepatocellular carcinoma. Clin Exp Hepatol. 2020;6:235–242. doi: 10.5114/ceh.2020.99517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gannon NP, Vaughan RA, Garcia-Smith R, Bisoffi M, Trujillo KA. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int J Cancer. 2015;136:197–202. doi: 10.1002/ijc.29142. [DOI] [PubMed] [Google Scholar]
- 43.Shao L, Li H, Chen J, et al. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem Biophys Res Commun. 2017;485:598–605. doi: 10.1016/j.bbrc.2016.12.084. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361–373. doi: 10.1007/s11684-018-0656-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu L, Ye Y, Sun Y, et al. Low FNDC5/Irisin expression is associated with aggressive phenotypes in gastric cancer. Front Pharmacol. 2022;13:981201. doi: 10.3389/fphar.2022.981201.b36e6933a9d24bd3b77541f5b5aee339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alizadeh Zarei M, Seyed Hosseini E, Haddad Kashani H, Ahmad E, Nikzad H. Effects of the exercise-inducible myokine irisin on proliferation and malignant properties of ovarian cancer cells through the HIF-1 alpha signaling pathway. Sci Rep. 2023;13:170. doi: 10.1038/s41598-022-26700-2.0c60f91b32d34226bec99356b763f046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CW, Chang YH, Lee HH, et al. Irisin, an exercise myokine, potently suppresses tumor proliferation, invasion, and growth in glioma. FASEB J. 2020;34:9678–9693. doi: 10.1096/fj.202000573RR. [DOI] [PubMed] [Google Scholar]
- 48.Esawy MM, Abdel-Samd KM. The diagnostic and prognostic roles of serum irisin in bladder cancer. Curr Probl Cancer. 2020;44:100529. doi: 10.1016/j.currproblcancer.2019.100529. [DOI] [PubMed] [Google Scholar]
- 49.Sofeu Feugaing DD, Gotte M, Viola M. More than matrix: the multifaceted role of decorin in cancer. Eur J Cell Biol. 2013;92:1–11. doi: 10.1016/j.ejcb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Heinemeier KM, Bjerrum SS, Schjerling P, Kjaer M. Expression of extracellular matrix components and related growth factors in human tendon and muscle after acute exercise. Scand J Med Sci Sports. 2013;23:150–161. doi: 10.1111/j.1600-0838.2011.01414.x. [DOI] [PubMed] [Google Scholar]
- 51.Kanzleiter T, Rath M, Gorgens SW, et al. The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun. 2014;450:1089–1094. doi: 10.1016/j.bbrc.2014.06.123. [DOI] [PubMed] [Google Scholar]
- 52.Neill T, Schaefer L, Iozzo RV. Decoding the matrix: instructive roles of proteoglycan receptors. Biochemistry. 2015;54:4583–4598. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bi X, Pohl NM, Qian Z, et al. Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 2012;33:326–330. doi: 10.1093/carcin/bgr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X, Villodre ES, Larson R, et al. Decorin-mediated suppression of tumorigenesis, invasion, and metastasis in inflammatory breast cancer. Commun Biol. 2021;4:72. doi: 10.1038/s42003-020-01590-0.a35e725f4a31444ab59fa8714d1e455f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen H, Wang Z, Yang N, Zhang J, Liang Z. Decorin inhibits proliferation and metastasis in human bladder cancer cells by upregulating P21. Medicine (Baltimore) 2022;101:e29760. doi: 10.1097/MD.0000000000029760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia Y, Feng Q, Tang B, et al. Decorin suppresses invasion and EMT phenotype of glioma by inducing autophagy via c-Met/Akt/mTOR axis. Front Oncol. 2021;11:659353. doi: 10.3389/fonc.2021.659353.8f6d1e0b05504463a1117266a4faeb86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buraschi S, Pal N, Tyler-Rubinstein N, Owens RT, Neill T, Iozzo RV. Decorin antagonizes Met receptor activity and down-regulates beta-catenin and Myc levels. J Biol Chem. 2010;285:42075–42085. doi: 10.1074/jbc.M110.172841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed CC, Waterhouse A, Kirby S, et al. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 59.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 60.Basak D, Jamal Z, Ghosh A, et al. Reciprocal interplay between asporin and decorin: implications in gastric cancer prognosis. PLoS One. 2021;16:e0255915. doi: 10.1371/journal.pone.0255915.d056a9ab7d624000a3e8501c6df573c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Troup S, Njue C, Kliewer EV, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 62.Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011;22:19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Tamura Y, Watanabe K, Kantani T, Hayashi J, Ishida N, Kaneki M. Upregulation of circulating IL-15 by treadmill running in healthy individuals: is IL-15 an endocrine mediator of the beneficial effects of endurance exercise? Endocr J. 2011;58:211–215. doi: 10.1507/endocrj.K10E-400. [DOI] [PubMed] [Google Scholar]
- 64.Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol (1985) 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen AR, Mounier R, Plomgaard P, et al. Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol. 2007;584:305–312. doi: 10.1113/jphysiol.2007.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo Z, He Z, Qin H, et al. Exercise-induced IL-15 acted as a positive prognostic implication and tumor-suppressed role in pan-cancer. Front Pharmacol. 2022;13:1053137. doi: 10.3389/fphar.2022.1053137.19ca331f40e74c398f381e855cc39aef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reyes AF, Goldusky J, Bhimalli P, Marzo AL, Schneider JR. Tracking fluorescently labeled IL-15 and anti-PD-1 in the tumor microenvironment and draining lymph nodes. J Immunol Methods. 2022;505:113253. doi: 10.1016/j.jim.2022.113253. [DOI] [PubMed] [Google Scholar]
- 68.Stravokefalou V, Stellas D, Karaliota S, et al. Heterodimeric IL-15 (hetIL-15) reduces circulating tumor cells and metastasis formation improving chemotherapy and surgery in 4T1 mouse model of TNBC. Front Immunol. 2022;13:1014802. doi: 10.3389/fimmu.2022.1014802.2b9cb05ca9884ddbbe501fcf04cf4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miljkovic MD, Dubois SP, Muller JR, et al. Interleukin-15 augments NK cell-mediated ADCC of alemtuzumab in patients with CD52+ T-cell malignancies. Blood Adv. 2023;7:384–394. doi: 10.1182/bloodadvances.2021006440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurz E, Hirsch CA, Dalton T, et al. Exercise-induced engagement of the IL-15/IL-15Ralpha axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. 2022;40:720–737. doi: 10.1016/j.ccell.2022.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyamoto T, Shimizu Y, Matsuo Y, et al. Effects of exercise intensity and duration on a myokine, secreted protein acidic and rich in cysteine. Eur J Sport Sci. 2022;22:1401–1410. doi: 10.1080/17461391.2021.1953152. [DOI] [PubMed] [Google Scholar]
- 73.Matsuo K, Sato K, Suemoto K, et al. A mechanism underlying preventive effect of high-intensity training on colon cancer. Med Sci Sports Exerc. 2017;49:1805–1816. doi: 10.1249/MSS.0000000000001312. [DOI] [PubMed] [Google Scholar]
- 74.Yiu GK, Chan WY, Ng SW, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. doi: 10.1007/springerreference_176868. [DOI] [PubMed] [Google Scholar]
- 77.Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. doi: 10.1158/1541-7786.215.2.4. [DOI] [PubMed] [Google Scholar]
- 78.Ma J, Ma Y, Chen S, et al. SPARC enhances 5-FU chemosensitivity in gastric cancer by modulating epithelial-mesenchymal transition and apoptosis. Biochem Biophys Res Commun. 2021;558:134–140. doi: 10.1016/j.bbrc.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Zhang JL, Chen GW, Liu YC, et al. Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One. 2012;7:e44618. doi: 10.1371/journal.pone.0044618.678149d436954b0bbfaca91329a2b786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Said N, Frierson H, Chernauskas D, Conaway M, Motamed K, Theodorescu D. The role of SPARC in the TRAMP model of prostate carcinogenesis and progression. Oncogene. 2009;28:3487–3498. doi: 10.1038/onc.2009.205. [DOI] [PubMed] [Google Scholar]
- 81.Rahman M, Chan AP, Tang M, Tai IT. A peptide of SPARC interferes with the interaction between caspase8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One. 2011;6:e26390. doi: 10.1371/journal.pone.0026390.c40c2c6686a244a9a4a8c452d7363355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng J, Tang L. SPARC in tumor pathophysiology and as a potential therapeutic target. Curr Pharm Des. 2014;20:6182–6190. doi: 10.2174/1381612820666140619123255. [DOI] [PubMed] [Google Scholar]
- 83.Grant JL, Fishbein MC, Hong LS, et al. A novel molecular pathway for snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev Res (Phila) 2014;7:150–160. doi: 10.1158/1940-6207.CAPR-13-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez-Moncada F, Torres MJ, Lavanderos B, Cerda O, Castellon EA, Contreras HR. SPARC induces E-cadherin repression and enhances cell migration through integrin alphavbeta3 and the transcription factor ZEB1 in prostate cancer cells. Int J Mol Sci. 2022;23:5874. doi: 10.3390/ijms23115874.6cc32a5b306440a3a03364f095c09d6b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tichet M, Prod'Homme V, Fenouille N, et al. Tumour-derived SPARC drives vascular permeability and extravasation through endothelial VCAM1 signalling to promote metastasis. Nat Commun. 2015;6:6993. doi: 10.1038/ncomms7993. [DOI] [PubMed] [Google Scholar]
- 86.Gao ZW, Liu C, Yang L, et al. SPARC Overexpression promotes liver cancer cell proliferation and tumor growth. Front Mol Biosci. 2021;8:775743. doi: 10.3389/fmolb.2021.775743.ddf2a948dbc34a91bbab4874626dd155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pan K, Huang X, Jia X. SPARC promotes pancreatic cancer cell proliferation and migration through autocrine secretion into the extracellular milieu. Oncol Lett. 2021;21:485. doi: 10.3892/ol.2021.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009;3:255–273. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neuzillet C, Tijeras-Raballand A, Cros J, Faivre S, Hammel P, Raymond E. Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32:585–602. doi: 10.1007/s10555-013-9439-3. [DOI] [PubMed] [Google Scholar]
- 90.Richards CD. The enigmatic cytokine oncostatin m and roles in disease. ISRN Inflamm. 2013;2013:512103. doi: 10.1155/2013/512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hwang JH, McGovern J, Minett GM, et al. Mobilizing serum factors and immune cells through exercise to counteract age-related changes in cancer risk. Exerc Immunol Rev. 2020;26:80–99. [PubMed] [Google Scholar]
- 92.Pan CM, Wang ML, Chiou SH, Chen HY, Wu CW. Oncostatin M suppresses metastasis of lung adenocarcinoma by inhibiting SLUG expression through coordination of STATs and PIASs signalings. Oncotarget. 2016;7:60395. doi: 10.18632/oncotarget.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grant SL, Hammacher A, Douglas AM, et al. An unexpected biochemical and functional interaction between gp130 and the EGF receptor family in breast cancer cells. Oncogene. 2002;21:460–474. doi: 10.1038/sj.onc.1205100. [DOI] [PubMed] [Google Scholar]
- 94.Halfter H, Friedrich M, Resch A, et al. Oncostatin M induces growth arrest by inhibition of Skp2, Cks1, and cyclin A expression and induced p21 expression. Cancer Res. 2006;66:6530–6539. doi: 10.1158/0008-5472.CAN-04-3734. [DOI] [PubMed] [Google Scholar]
- 95.Friedrich M, Hoss N, Stogbauer F, et al. Complete inhibition of in vivo glioma growth by oncostatin M. J Neurochem. 2001;76:1589–1592. doi: 10.1046/j.1471-4159.2001.00202.x. [DOI] [PubMed] [Google Scholar]
- 96.Kim JS, Wilson RL, Taaffe DR, Galvão DA, Gray E, Newton RU. Myokine expression and tumor-suppressive effect of serum after 12 wk of exercise in prostate cancer patients on ADT. Med Sci Sports Exerc. 2022;54:197. doi: 10.1249/MSS.0000000000002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hojman P, Dethlefsen C, Brandt C, Hansen J, Pedersen L, Pedersen BK. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am J Physiol Endocrinol Metab. 2011;301:E504–E510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 98.Wang ML, Pan CM, Chiou SH, et al. Oncostatin m modulates the mesenchymal-epithelial transition of lung adenocarcinoma cells by a mesenchymal stem cell-mediated paracrine effect. Cancer Res. 2012;72:6051–6064. doi: 10.1158/0008-5472.CAN-12-1568. [DOI] [PubMed] [Google Scholar]
- 99.Tripathi C, Tewari BN, Kanchan RK, et al. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget. 2014;5:5350–5368. doi: 10.18632/oncotarget.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Araujo AM, Abaurrea A, Azcoaga P, et al. Stromal oncostatin M cytokine promotes breast cancer progression by reprogramming the tumor microenvironment. J Clin Invest. 2022;132:148667. doi: 10.1172/JCI148667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Q, Zhu J, Sun F, Liu L, Liu X, Yue Y. Oncostatin M promotes proliferation of ovarian cancer cells through signal transducer and activator of transcription 3. Int J Mol Med. 2011;28:101–108. doi: 10.3892/ijmm.2011.647. [DOI] [PubMed] [Google Scholar]
- 102.Mori S, Murakami-Mori K, Bonavida B. Oncostatin M (OM) promotes the growth of DU 145 human prostate cancer cells, but not PC-3 or LNCaP, through the signaling of the OM specific receptor. Anticancer Res. 1999;19:1011–1015. [PubMed] [Google Scholar]
- 103.Smigiel JM, Parameswaran N, Jackson MW. Potent EMT and CSC phenotypes are induced by oncostatin-M in pancreatic cancer. Mol Cancer Res. 2017;15:478–488. doi: 10.1158/1541-7786.MCR-16-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kucia-Tran JA, Tulkki V, Smith S, et al. Overexpression of the oncostatin-M receptor in cervical squamous cell carcinoma is associated with epithelial-mesenchymal transition and poor overall survival. Br J Cancer. 2016;115:212–222. doi: 10.1038/bjc.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith DA, Kiba A, Zong Y, Witte ON. Interleukin-6 and oncostatin-M synergize with the PI3K/AKT pathway to promote aggressive prostate malignancy in mouse and human tissues. Mol Cancer Res. 2013;11:1159–1165. doi: 10.1158/1541-7786.MCR-13-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lapeire L, Hendrix A, Lambein K, et al. Cancer-associated adipose tissue promotes breast cancer progression by paracrine oncostatin M and Jak/STAT3 signaling. Cancer Res. 2014;74:6806–6819. doi: 10.1158/0008-5472.CAN-14-0160. [DOI] [PubMed] [Google Scholar]
- 107.Lacreusette A, Nguyen JM, Pandolfino MC, et al. Loss of oncostatin M receptor beta in metastatic melanoma cells. Oncogene. 2007;26:881–892. doi: 10.1038/sj.onc.1209844. [DOI] [PubMed] [Google Scholar]
- 108.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci. 2015;11:1164–1178. doi: 10.5114/aoms.2015.56342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 110.Clow C, Jasmin BJ. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol Biol Cell. 2010;21:2182–2190. doi: 10.1091/mbc.e10-02-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miknyoczki SJ, Lang D, Huang L, Klein-Szanto AJ, Dionne CA, Ruggeri BA. Neurotrophins and Trk receptors in human pancreatic ductal adenocarcinoma: expression patterns and effects on in vitro invasive behavior. Int J Cancer. 1999;81:417–427. doi: 10.1002/(SICI)1097-0215(19990505)81:3<417::AID-IJC16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 112.Sinkevicius KW, Kriegel C, Bellaria KJ, et al. Neurotrophin receptor TrkB promotes lung adenocarcinoma metastasis. Proc Natl Acad Sci U S A. 2014;111:10299–10304. doi: 10.1073/pnas.1404399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shen T, Cheng X, Xia C, et al. Erlotinib inhibits colon cancer metastasis through inactivation of TrkB-dependent ERK signaling pathway. J Cell Biochem. 2019;120:11248–11255. doi: 10.1002/jcb.28400. [DOI] [PubMed] [Google Scholar]
- 114.Zhang SY, Hui LP, Li CY, Gao J, Cui ZS, Qiu XS. More expression of BDNF associates with lung squamous cell carcinoma and is critical to the proliferation and invasion of lung cancer cells. BMC Cancer. 2016;16:171. doi: 10.1186/s12885-016-2218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Matthews VB, Astrom MB, Chan MH, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–1418. doi: 10.1007/s00125-009-1364-1. [DOI] [PubMed] [Google Scholar]
- 116.Ibeas K, Herrero L, Mera P, Serra D. Hypothalamus-skeletal muscle crosstalk during exercise and its role in metabolism modulation. Biochem Pharmacol. 2021;190:114640. doi: 10.1016/j.bcp.2021.114640. [DOI] [PubMed] [Google Scholar]
- 117.Xiao R, Bergin SM, Huang W, et al. Environmental and genetic activation of hypothalamic BDNF modulates T-cell immunity to exert an anticancer phenotype. Cancer Immunol Res. 2016;4:488–497. doi: 10.1158/2326-6066.CIR-15-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cao L, Liu X, Lin EJ, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. doi: 10.1016/j.cell.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garofalo S, D'Alessandro G, Chece G, et al. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623. doi: 10.1038/ncomms7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fang P, She Y, Yu M, Min W, Shang W, Zhang Z. Adipose-muscle crosstalk in age-related metabolic disorders: the emerging roles of adipo-myokines. Ageing Res Rev. 2023;84:101829. doi: 10.1016/j.arr.2022.101829. [DOI] [PubMed] [Google Scholar]
- 121.Jena BP, Larsson L, Gatti DL, Ghiran I, Cho WJ. Understanding brain-skeletal muscle crosstalk impacting metabolism and movement. Discoveries (Craiova) 2022;10:e144. doi: 10.15190/d.2022.3. [DOI] [PMC free article] [PubMed] [Google Scholar]