Abstract
Purpose
To investigate the effect of intraoperative remimazolam sedation on postoperative sleep quality in elderly patients after total joint arthroplasty.
Methods
Between May 15, 2021 and March 26, 2022, 108 elderly patients (age ≥ 65 years) who received total joint arthroplasty under neuraxial anesthesia were randomized into remimazolam group (a loading dose of 0.025–0.1 mg/kg and followed by an infusion rate of 0.1–1.0 mg/kg/h till end of surgery) or routine group (sedation was given on patient’s requirement by dexmedetomidine 0.2–0.7 μg/kg/h). Primary outcome was the subjective sleep quality at surgery night which was evaluated by Richards Campbell Sleep Questionnaire (RCSQ). Secondary outcomes included RCSQ scores at postoperative first and second nights and numeric rating scale pain intensity within first 3 days after surgery.
Results
RCSQ score at surgery night was 59 (28, 75) in remimazolam group which was comparable with 53 (28, 67) in routine group (median difference 6, 95% CI − 6 to 16, P = 0.315). After adjustment of confounders, preoperative high Pittsburg sleep quality index was associated worse RCSQ score (P = 0.032), but not remimazolam (P = 0.754). RCSQ score at postoperative first night [69 (56, 85) vs. 70 (54, 80), P = 0.472] and second night [80 (68, 87) vs. 76 (64, 84), P = 0.066] were equivalent between two groups. Safety outcomes were comparable between the two groups.
Conclusions
Intraoperative remimazolam did not significantly improve postoperative sleep quality in elderly patients undergoing total joint arthroplasty. But it is proved to be effective and safe for moderate sedation in these patients.
Clinical trial number and registry URL
ChiCTR2000041286 (www.chictr.org.cn).
Supplementary Information
The online version contains supplementary material available at 10.1007/s00540-023-03193-5.
Keywords: Bispectral index, Remimazolam, Sedation, Sleep, Total joint arthroplasty
Introduction
Sleep disturbance frequently occurs in elderly patients after surgery due to perioperative anxiety, stress and postoperative pain [1]. In patients undergoing total joint arthroplasty, the reported incidence of sleep disturbance reaches up to 60% [2, 3]. Many studies have demonstrated that postoperative sleep disturbances are associated with worse clinical outcomes including increased risk of postoperative complications, prolonged duration of in-hospital stay, and poor quality of recovery [4].
Neuraxial anesthesia is commonly used for patients undergoing total joint arthroplasty [5]. These patients usually suffer from moderate to severe anxiety and pain which are considered as major risk factors of perioperative sleep disturbance [2, 3, 6]. Sedation during neuraxial anesthesia is an effective approach to alleviate intraoperative anxiety. Intraoperative sedatives, such as dexmedetomidine [7] and midazolam [8], are also reported to improve postoperative sleep quality. Furthermore, one randomized trial showed that intraoperative midazolam with bispectral index (BIS) around 77 provides better sleep quality than dexmedetomidine in patients after elective transurethral prostatic resection [8]. Yet, deficits of midazolam include a relative long-acting time for two to three hours and the potential risk of delirium [9]. Remimazolam is an ultra-short-acting benzodiazepine which takes significant advantage in shorter elimination half-life of several minutes and better recovery of cognitive function from procedure sedation in comparison with midazolam [10]. Up to now, there is little data to elucidate the relationship between intraoperative infusion of remimazolam and postoperative sleep quality.
Present study was designed to investigate the effect of intraoperative remimazolam for sedation on sleep quality at surgery night in elderly patients after total joint arthroplasty. We hypothesized that intraoperative sedation with remimazolam could improve sleep quality at surgery night compared with routine care group.
Methods
Participants
This randomized trial was approved by Biomedical Research Ethics Committee of Peking University First Hospital (No. 2020-350, Chairperson Prof. Yanyan Yu on December 15, 2020) and registered at Chinese Clinical Trial Registry (www.chictr.org.cn; No. ChiCTR2000041286; December 23, 2020). The study was conducted in Peking University First Hospital and Cangzhou People's Hospital. Written informed consents were obtained from all participants.
Elderly patients, aged 65 years old or above, who were scheduled for elective total joint arthroplasty under neuraxial anesthesia were enrolled. Patients were excluded if they met any of the following criteria: (1) allergy to remimazolam or dexmedetomidine; (2) sleep disorder requiring medical interventions (i.e., hypnotics) within recent 1 month; (3) Severe arrythmia including sick sinus syndrome, severe bradycardia (heart rate < 50 beats per minute), or atrioventricular block of second degree or above without pacemaker; (4) severe renal dysfunction (requiring renal replacement therapy); (5) Child–Pugh class C; and (6) American Society of Anesthesiologists physical status (ASA-PS) ≥ IV.
Randomization and group allocation
Patients were randomized into either remimazolam group or routine group at 1:1 ratio with a block size of 4 and stratified by study centers by an independent biostatistician (SAS 9.2. SAS Institute, Cary, NC). Random numbers were sealed in sequentially numbered opaque envelopes and stored at study centres until the end of the study. A researcher was designated to allocate random number 30 min before anesthesia and prepared trial drugs per protocol. This researcher was not involved in administration of intervention, follow-up, and data collection.
Anaesthesia and interventions
No premedication was given prior to surgery. All patients received standard monitoring including heart rate and rhythm, non-invasive blood pressure, arterial pulse saturation (SpO2), and Bispectral index (BIS). L2-3 or L3-4 was selected for neuraxial anaesthesia and 0.5% hyperbaric bupivacaine with a volume of 2–3 ml was given for spinal anaesthesia. Epidural catheterization was conducted if expected duration of surgery would be longer than 2 h. Routinely, methylprednisolone 40 mg was given before anaesthesia and tropisetron 5 mg was given at end of surgery.
After confirmation of absolute anaesthesia level above T10, trial drugs were administrated by attending anaesthesiologists. In remimazolam group, a loading dose of remimazolam 0.025–0.1 mg/kg was initially given until the loss of consciousness, and then sedation depth was maintained at BIS 70–80 by continuous infusion at a rate of 0.1–1.0 mg/kg/h till end of surgery. Attending anaesthesiologist could adjust the infusion rate to maintain targeted sedation depth. Additional dosage of remimazolam 0.025–0.1 mg/kg could be given in necessary if the target BIS was not obtained at maximum infusion rate. In routine group, sedation was given at the discrete of patient’s demand with dexmedetomidine (i.e., 0.2–0.7 ug/kg/h) based on routine care. Since the BIS value was not routinely used for monitoring sedation in clinical setting, the readings of BIS value were blinded to attending anaesthesiologists in these patients.
To decrease potential bias, the anaesthesiologists did not take part in enrolment, randomization, postoperative follow-up and data collection. Any exchange of information above was not allowed during the study period. Blind method was also conducted to researchers, patients, and other related healthcare providers. Emergency unmasking of randomization could be taken in terms of severe adverse events which might result in prolonged in-hospital stay, increased medical expense, disability, and death.
Postoperative analgesia and follow-up
Multimodal analgesia was provided to keep the numeric rating pain score at rest (NRS, a 11-score scale, 0 indicating no pain and 10 for worst pain) less than 3. First, patient controlled intravenous analgesia (PCIA) pump was provided. It was programmed to deliver background infusion of sufentanil at a rate of 1 μg/h and a bolus of 2 μg sufentanil on demand with 8 min lockout time. Second, non-steroid anti-inflammatory drugs (NSAIDs, flurbiprofen 50 mg, parecoxib 40 mg or ketorolac 30 mg) was initially given at end of surgery and then at 12 h interval until postoperative 72 h. Third, ultrasound guided femoral nerve block for knee surgery or fascia iliaca compartment block for hip surgery was conducted with a single injection of 0.3% ropivacaine 20 ml.
Patients were visited twice daily (07:00–09:00 and 19:00–21:00, respectively) during postoperative first 3 days and then once daily during in-hospital stay. After discharge, patients were interviewed by telephone at postoperative 30 days.
Data collection and outcome assessment
Baseline variables such as demographic data, comorbidities, and major laboratory tests were collected. Sleep quality within recent 1 month was assessed by Pittsburgh Sleep Quality Index (PSQI, ranges from 0 to 21 with higher scores indicating worse sleep quality) [11]. Chronic pain was assessed by the Brief Pain Inventory (BPI, the mean score ranges from 0 to 10 with higher score indicating heavier intensity or worse pain-related function) [12]. Activity of daily living was assessed with the Barthel Index (ranges from 0 to 100 with higher score indicating better daily activity). Anxiety was assessed by Self-Rating Anxiety Scale (SAS, ranges from 20 to 80 with higher score for heavier anxiety) [13].
Primary outcome
Primary outcome was the subjective sleep quality at surgery night, assessed by the Chinese version Richards Campbell Sleep Questionnaire (RCSQ) at 07:00–09:00 on postoperative first morning [14, 15].
The RCSQ involves five domains including sleep depth, sleep latency, awakenings, returning to sleep, and overall sleep quality. Each domain is assessed by using a 100-mm visual analog scale with higher scores indicating better sleep. Overall RCSQ sleep score is defined as the mean value of above five domains and classified into four groups: very poor sleep with scores of 1–25, poor sleep with scores of 26–50, good sleep with scores of 51–75, and very good sleep with scores of 76–100. Although originally designed for critically ill patients in intensive care unit, the RCSQ has also been used among general surgical patients [16]. To improve the consistency of sleep assessment, the investigators who performed RCSQ was trained before study beginning and two times during study.
Secondary outcomes
RCSQ scores at postoperative first and second nights were assessed in line with primary outcome. NRS pain intensity and the incidence of postoperative nausea and vomiting (PONV) within postoperative first 3 days were recorded. Duration of postoperative in-hospital stay was defined as the interval between surgery day and discharge of hospital. Major complications requiring medical interventions (i.e., Clavien–Dindo classification 2 and above [17]) within postoperative 30 days were also collected.
Safety outcomes
Safety outcomes were monitored from administration of study drugs until 6 h after surgery including hypotension, hypertension, bradycardia, and desaturation. We also recorded the occurrence of intraoperative and early postoperative nausea and vomiting. Interventions of above adverse events were conducted according to routine practice.
Statistical analysis
Sample size calculation
In a pilot observation of 20 elderly patients, mean RCSQ score at surgery night was 50 with standard deviation (SD) of 15. We assumed that intraoperative remimazolam would increase the RCSQ score to 60 at surgery night. With statistical significance at 0.05 and power at 90%, the sample size required to detect the difference was 49 patients in each group (PASS 15.0, StataCorp. LP, College Station, TX, USA). Taking a dropout rate of 10%, we planned to enrol 54 patients in each group.
Data analysis
Continuous data with normal distribution were expressed as the mean (SD) and analyzed using independent samples t test, whereas data without normal distribution were expressed as median (interquartile range, IQR) and compared by Mann–Whitney U-test. Categorical data were presented as number (percentage) and compared by Chi-square test or Fisher’s exact test.
Primary outcome was analyzed in intention-to-treat and per protocol populations respectively. RCSQ score was presented as median (IQR) and compared by Mann–Whitney U-test. Median difference and 95% confidence interval (CI) were calculated with Hodges-Lehmann estimator. Multivariable generalized linear model was employed to investigate the association between interventions and RCSQ with adjustment of confounders including unbalanced variables between two groups and clinically important factors. A post-hoc analysis was administrated to compare the RCSQ between patients who received remimazolam and those treated with dexmedetomidine. Categorical outcomes were compared by Chi-square and estimated effect was presented as relative risk and 95% CI. Length of postoperative in-hospital stay was presented as median (IQR) and analyzed using the Kaplan–Meier survival analysis with log-rank test and hazard ratio (HR) calculated by Cox regression analysis.
BIS value was recorded at 1-min interval from initial administration of trial drug to end of surgery. Time-weighted average (TWA) BIS was calculated as the summation of BIS values multiplied by the referred time, and divided by the specified recording duration.
All tests were two tailed and P < 0.05 was considered as statistically significant. Statistical analyses were performed with the SPSS 25.0 software (SPSS, Inc., Chicago, IL) and Python 3.7.0 software (Python Software Foundation, Beaverton, OR, USA).
Results
Participants
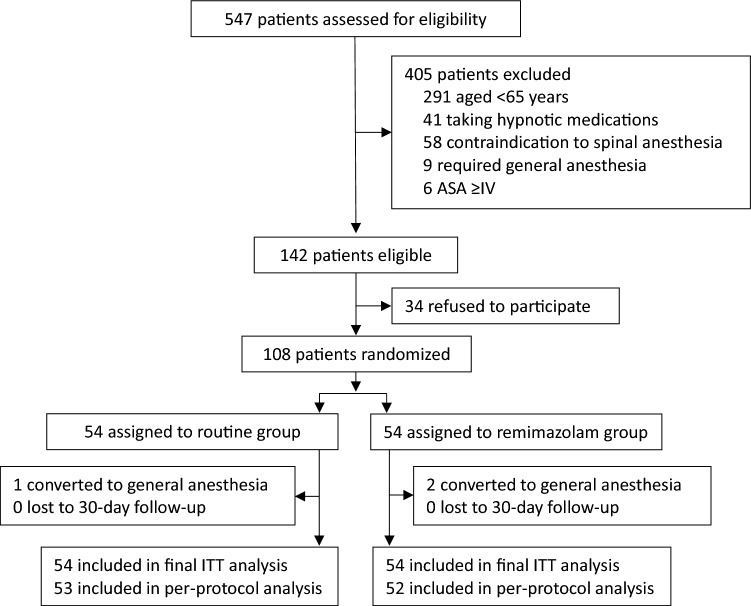
From May 15, 2021 to February 24, 2022, 547 patients were screened and 108 eligible patients were randomized (Fig. 1). The last follow-up was performed on March 26, 2022. Two patients in remimazolam group and one in routine group received general anaesthesia after randomization because of failure of spinal anaesthesia. In remimazolam group, 52 patients received remimazolam with a median dosage of 25.0 (20.0, 31.3) mg. In routine group, 83.3% (45/54) received dexmedetomidine with a median dosage of 35.0 (25.5, 50.0) μg. No patient was lost or died during 30-day follow-up.
Fig. 1.
Flowchart of the trial. ITT, intention-to-treat
Baseline variables including demographic data and comorbidities were listed in Table 1. Patients in remimazolam group had equivalent PSQI [6.5 (3.3) vs. 7.7 (3.5), P = 0.062], SAS [27 (25, 31) vs. 30 (26, 33), P = 0.061], and BPI severity scores [2.8 (1.2) vs. 3.1 (1.3), P = 0.252] in comparison with routine group. The incidence of hypertension was lower in remimazolam group than routine group (55.6% vs. 77.8%, P = 0.014). Perioperative variables were comparable between two groups, except higher percentage of total knee arthroplasty (90.7% vs. 75.9%, P = 0.039) and slightly lower TWA BIS [77 (7) vs. 85 (9), P < 0.001] in remimazolam group (Table 2). No additional narcotics or benzodiazepines were administered to any patient during surgery.
Table 1.
Baseline variables
| Remimazolam group (n = 54) | Routine group (n = 54) | P | |
|---|---|---|---|
| Age, year | 70.8 ± 4.4 | 71.8 ± 5.5 | 0.292 |
| Female, n | 39 (72.2%) | 40 (74.1%) | 0.828 |
| Body mass index, kg/m2 | 26.3 ± 3.6 | 26.2 ± 2.5 | 0.869 |
| Duration of education, year | 9 (3, 12) | 8 (3, 12) | 0.758 |
| Preoperative comorbidities, n | |||
| Hypertension | 30 (55.6%) | 42 (77.8%) | 0.014 |
| Coronary heart disease | 13 (24.1%) | 9 (16.7%) | 0.339 |
| Arrhythmiaa | 2 (3.7%) | 3 (5.6%) | > 0.99 |
| Stroke | 2 (3.7%) | 6 (11.1%) | 0.270 |
| Diabetes mellitus | 10 (18.5%) | 18 (33.3%) | 0.079 |
| Chronic obstructive pulmonary disease | 1 (1.9%) | 2 (3.7%) | > 0.99 |
| Alcoholismb | 2 (3.7%) | 3 (5.6%) | > 0.99 |
| ASA-PS classification, n | 0.430 | ||
| II | 31 (57.4%) | 35 (64.8%) | |
| III | 23 (42.6%) | 19 (35.2%) | |
| Pittsburgh sleep quality index, scorec | 6.5 ± 3.3 | 7.7 ± 3.5 | 0.062 |
| Self-rating anxiety scale, scored | 27 (25, 31) | 30 (26, 33) | 0.061 |
| Brief Pain Inventory, scoree | |||
| Severity score | 2.8 ± 1.2 | 3.1 ± 1.3 | 0.252 |
| Interference score | 3.6 ± 1.5 | 3.8 ± 1.4 | 0.553 |
| Barthel Index, scoref | 95 (90, 100) | 95 (85, 100) | 0.053 |
Data are presented as mean ± SD, median (interquartile range), or number (%)
ASA-PS, American Society of Anesthesiology physical status
aArrhythmia requiring medical therapy such as atrial fibrillation and atrioventricular block
bWeekly consumption of alcohol more than 150 mL or in equivalent dosage
cA self-rated questionnaire that assesses sleep quality within 1 month which ranges from 0 to 21 with higher score for worse sleep quality
dScore ranges from 20 to 80 with higher score indicating heavier anxiety
eIncludes a 4-item chronic pain severity scale and a 7-item pain-related-function interference scale. The mean scores range from 0 to 10 with higher score indicating heavier intensity or worse pain-related function
fAssessment of daily living activities. Score ranges from 0 to 100 with higher score for better activity
Table 2.
Perioperative data
| Remimazolam group (n = 54) | Routine group (n = 54) | P | |
|---|---|---|---|
| Study center, n | > 0.999 | ||
| Site 1 | 39 | 39 | |
| Site 2 | 15 | 15 | |
| Type of surgery, n | 0.039 | ||
| Total knee arthroplasty | 49 (90.7%) | 41 (75.9%) | |
| Total hip arthroplasty | 5 (9.3%) | 13 (24.1%) | |
| Intraoperative drugs | |||
| Dexmedetomidine, μga | – | 35.0 (25.5, 50.0) (n = 45) | – |
| Bupivacaine, mgb | 12.6 ± 2.3 (n = 52) | 12.3 ± 2.2 (n = 53) | 0.560 |
| Epidural lidocaine, n | 15 (28.8%) (n = 52) | 19 (35.8%) (n = 53) | 0.443 |
| Epidural ropivacaine, n | 5 (9.6%) (n = 52) | 10 (18.9%) (n = 53) | 0.176 |
| Use of tropisetron, n | 46 (85.2%) | 49 (90.7%) | 0.375 |
| Use of vasopressors, nc | 8 (14.8%) | 7 (13.0%) | 0.781 |
| Duration of anesthesia, min | 180 ± 35 | 173 ± 41 | 0.326 |
| Duration of surgery, min) | 118 ± 36 | 116 ± 36 | 0.825 |
| TWA BIS from incision to the end of surgeryd | 77 ± 7 | 85 ± 9 | < 0.001 |
| Cumulative time of BIS, mine | |||
| ≥ 81 | 11 (0, 26) | 98 (69, 113) | < 0.001 |
| 70–80 | 72 (59, 97) | 9 (1, 18) | < 0.001 |
| ≤ 69 | 11 (2, 28) | 1 (0, 8) | < 0.001 |
| Intraoperative fluid balance | |||
| Total intraoperative infusion, ml | 1500 (1275, 1800) | 1500 (1300, 1800) | 0.477 |
| Allogenic blood transfusion, nf | 7/54 (13%) | 12/54 (22%) | 0.206 |
| Estimated blood loss, ml | 100 (50, 150) | 100 (50, 200) | 0.311 |
| Intraoperative urine output, ml | 400 (200, 763) | 400 (250, 525) | 0.810 |
| Ultrasound-guided nerve block, n | 0.064 | ||
| Femoral nerve block | 49 (90.7%) | 42 (77.8%) | |
| Fascia iliaca compartment block | 5 (9.3%) | 12 (22.2%) | |
| Types of NSAIDS, ng | 0.691 | ||
| Flurbiprofen axetil | 41 (76%) | 40 (74%) | |
| Parecoxib | 2 (3.7%) | 4 (7.4%) | |
| Ketorolac tromethamine | 11 (20.4%) | 10 (18.5%) | |
| Postoperative use of oral opioids, nh | |||
| Surgery day | 21 (38.9%) | 28 (51.9%) | 0.176 |
| First day | 26 (48.1%) | 29 (53.7%) | 0.564 |
| Second day | 31 (57.4%) | 29 (53.7%) | 0.699 |
| Postoperative use of sedatives, ni | |||
| Night at surgery | 2 (3.7%) | 6 (11.1%) | 0.270 |
| First night | 10 (18.5%) | 6 (11.1%) | 0.279 |
| Second night | 5 (9.3%) | 5 (9.3%) | > 0.99 |
| Dosage of sufentanil by PCIA, μg | |||
| Surgery day | 26 (20, 35) | 26 (20, 38) | 0.692 |
| First day | 61 (48, 78) | 59 (49, 74) | 0.614 |
| Second day | 100 (74, 111) | 97 (81, 100) | 0.454 |
P values in bold indicate < 0.05
Data are presented as mean ± SD, median (interquartile range), or number (%)
TWA, time-weighted average; BIS, bispectral index; PCIA, patient-controlled intravenous analgesia
aUsed in routine care group
bUsed for spinal anesthesia
cIncluding ephedrine, metaraminol, and phenylephrine
dBIS value was recorded at 1 min interval from initial administration of trial drug to end of surgery. TWA BIS was calculated as the summation of BIS values multiplied by the referred duration, and divided by the whole recording duration
eCalculated as the accumulated time of BIS values from incision to the end of surgery within predefined references
fIncluding packaged red blood cell and fresh frozen plasm
gNSAIDs was given at end of surgery and then at 12 h interval until postoperative 72 h including flurbiprofen, parecoxib, and ketorolac
hIncluding oral oxycodone acetaminophen, pentazocine, and tramadol
iIncluding estazolam, oxazepam, and zolpidem
Primary outcome
The RCSQ score at surgery night was 59 (28, 75) in remimazolam group which was comparable with 53 (28, 67) in routine care group (median difference 6, 95% CI − 6 to 16, P = 0.315, Table 3 and Supplemental Fig. S1). This result was also verified in per protocol analysis [62 (27, 77) vs. 52 (28, 66), MD = 6 (− 4 to 16), P = 0.211]. In post-hoc analysis, patients in remimazolam group had similar RSCQ in comparison with those treated with dexmedetomidine [62 (27, 77) vs. 54 (28, 70), MD = 4 (− 8 to 16), P = 0.494, Table 3 and Supplemental Table S1].
Table 3.
Efficacy outcomes
| Remimazolam group group (n = 54) | Routine group (n = 54) | Estimated effect size (95% CI) | Pa | |
|---|---|---|---|---|
| Primary outcome | ||||
| RCSQ at surgery night, scoreb | ||||
| Intention-to-treat | 59 (28, 75) | 53 (28, 67) | MD = 6 (− 6, 16) | 0.315 |
| Per protocol | 62 (27, 77) (n = 52) | 52 (28, 66) (n = 53) | MD = 6 (− 4, 16) | 0.211 |
| Secondary outcomes | ||||
| RCSQ after surgery, scoreb | ||||
| First night | 69 (56, 85) | 70 (54, 80) | MD = 2 (− 4, 10) | 0.472 |
| Second night | 80 (68, 87) | 76 (64, 84) | MD = 4 (0, 10) | 0.066 |
| NRS pain intensity at rest, scorec | ||||
| First day | 1 (0, 3) | 0 (0, 2) | MD = 0 (0, 1) | 0.114 |
| Second day | 1 (0, 3) | 1 (0, 2) | MD = 0 (0, 1) | 0.206 |
| Third day | 1 (0, 3) | 0 (0, 2) | MD = 0 (0, 0) | 0.313 |
| Postoperative nausea and vomiting, n | 10 (18.5%) | 7 (13.0%) | RR = 1.43 (0.59, 3.48) | 0.428 |
| Major complications, nd | 1 (1.9%) | 3 (5.6%) | RR = 0.33 (0.04, 3.11) | 0.618 |
| Length of in-hospital stay after surgery, day | 7 (6, 10) | 7 (7, 11) | HR = 1.16 (0.79, 1.70) | 0.449 |
Data are presented as median (interquartile range) or number (%)
MD, median difference or mean difference; HR, hazard ratio; RR, relative risk; RCSQ, Richards Campbell Sleep Questionnaire; NRS, Numeric Rating Scale
aCalculated as BIS-guided group versus or minus routine group
bThe RCSQ involves five domains including sleep depth, sleep latency, awakenings, returning to sleep, and sleep quality. Overall RCSQ sleep score is defined as the mean value of above five domains and it ranges from 0 to 100 with higher score for better sleep
cPain intensity at rest was assessed by numeric rating scale (11-point scale, 0 for no pain and 10 for the worst pain)
dComplications requiring medical interventions (i.e., Clavien–Dindo classification 2 and above) within postoperative 30 days. One patient in BIS-guided group suffered lower limbs venous thrombosis. Three patients in routine group suffered complications including cardiac injury, new-onset atrial fibrillation, and urinary tract infection
Unbalanced variables between two groups (history of hypertension and total hip replacement surgery) and factors with clinical significance (age, female, preoperative PSQI, SAS, BPI and intraoperative TWA BIS) were considered as confounders. After adjustment, multivariable generalized linear regression showed that remimazolam was not associated with RCSQ score at surgery night (β 1.80, 95% CI − 9.46 to 13.06, P = 0.754, Supplemental Table S2). Higher preoperative PSQI score (indicating poorer sleep quality) was associated with decrement of RCSQ score (β − 1.67, 95% CI − 3.19 to − 0.14, P = 0.032).
Secondary outcomes
RCSQ score at postoperative first night [69 (56, 85) vs. 70 (54, 80), P = 0.472] and second night [80 (68, 87) vs. 76 (64, 84), P = 0.066] were comparable between two groups (Table 3). At second night, patients in remimazolam group had slightly higher scores in domains of return to sleep and sleep quality (P = 0.040 and 0.046, respectively, Supplemental Table S3). Pain intensity was comparable between two groups during postoperative 3 days (Table 3). The incidences of PONV and major complications did not differ statistically between study groups. Patients in two groups had similar duration of postoperative in-hospital stay (Table 3).
Exploratory outcomes
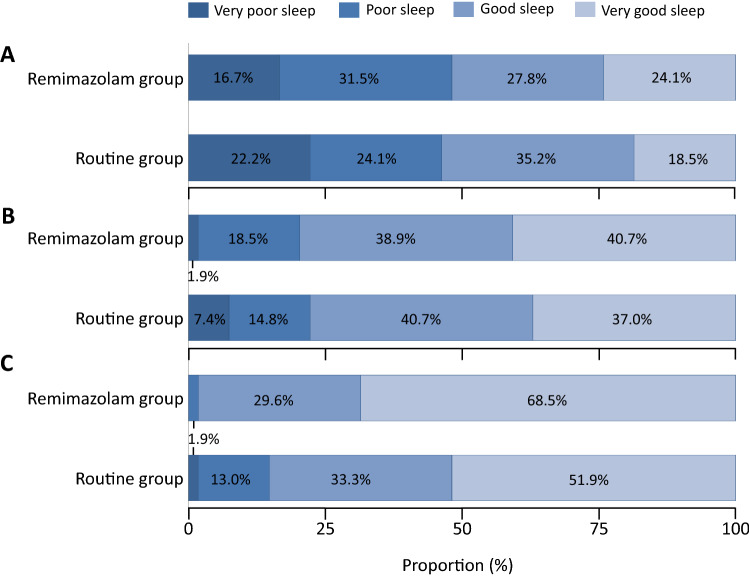
Classifications of RCSQ quality at each night were presented in Fig. 2. Constituent ratio of sleep quality was comparable at surgery night and postoperative first night. At postoperative second night, patients in remimazolam group had higher proportion of good sleep than routine group (98.1% vs. 85.2%, P = 0.015, Fig. 2). Subgroup analysis showed no significant differences of RCSQ scores between high dose and low dose of drug both in remimazolam group and dexmedetomidine group (Table S4).
Fig. 2.
Richards Campbell Sleep Questionnaire scores distribution between two groups of the first 3 nights after surgery. Horizontal stacked bar graphs show Richards Campbell Sleep Questionnaire score distribution between BIS-guided sedation group and routine care group of A night of the surgery, B first night after surgery and C second night after surgery. Bars are labelled with proportions
Safety outcomes
Patients in routine group had slightly higher incidence of intraoperative hypotension but without statistical significance (16.7% vs. 14.8%, P > 0.99). There was also no statistical significance in other adverse events including hypertension, bradycardia, tachycardia, respiratory depression, nausea and vomiting (Table 4).
Table 4.
Adverse events
| Remimazolam group (n = 54) | Routine group (n = 54) | P | |
|---|---|---|---|
| Intraoperative period | |||
| Hypotension, na | 8 (14.8%) | 9 (16.7%) | > 0.99 |
| Hypertension, nb | 2 (3.7%) | 4 (7.4%) | 0.678 |
| Bradycardia, nc | 1 (1.9%) | 2 (3.7%) | > 0.99 |
| Tachycardia, nd | 2 (3.7%) | 0 (0%) | 0.495 |
| Desaturation, ne | 1 (1.9%) | 1 (1.9%) | > 0.99 |
| Nausea, n | 0 (0%) | 2 (3.7%) | 0.495 |
| Vomiting, n | 0 (0%) | 0 (0%) | – |
| Postoperative period | |||
| Desaturation, ne | 0 (0%) | 0 (0%) | – |
| Nausea, n | 2 (3.7%) | 3 (5.6%) | > 0.99 |
| Vomiting, n | 0 (0%) | 0 (0%) | – |
Data are presented as number (%)
aDefined as systolic blood pressure < 90 mmHg or a decrease of > 20% from baseline
bDefined as systolic blood pressure > 160 mmHg or an increase of > 20% from baseline
cDefined as heart rate < 50 beat per minute or a decrease of > 20% from baseline
dDefined as heart rate > 100 bpm or an increase of > 20% from baseline
eDefined as pulse oxygen saturation < 90% or an absolute decrease of > 5% from baseline
Discussion
The present study demonstrated that intraoperative remimazolam sedation (kept at BIS 70–80), compared with those mainly treated with dexmedetomidine, did not significantly improve postoperative sleep quality in elderly patients receiving total joint arthroplasty under neuraxial anaesthesia. However, remimazolam is proved to be effective and safe for moderate sedation in elderly during reginal anaesthesia.
Nowadays, sleep disturbance after surgery has become a major concern especially in elderly patients. In this study, very poor or poor sleep quality happened in about half of patients at surgery night and its severity gradually alleviated during the following two nights, which was in line other studies [3, 18]. We employed PSQI for baseline sleep quality assessment and RCSQ for postoperative nightly assessment in the study. Both of the two instruments had been validated in Chinese patients [11, 14, 15]. PSQI is considered as a reliable scale to assess chronic sleep quality within 1 month [11]. For acute sleep quality assessment, numeric rating sleep scale was commonly used, but this method is merely a rough report of subjective sleep quality. Compared with PSQI and NRS scale, RCSQ reflects the acute change of sleep quality and includes five dimensional assessments of patient’s sleep [14, 15]. Recent studies have validated the effectiveness and reliability of RCSQ in general surgery patients [16].
In the present study, remimazolam was titrated to maintain sedation depth at BIS 70–80. This is based on the result of previous study in which intraoperative midazolam with BIS of 75–80 improved postoperative sleep quality [8]. Benzodiazepines is the mainstream for sedation in orthopaedic patients and account for 80% of sedatives in patients undergoing total joint arthroplasty [19], although with concerns on increased risks of delirium, apnoea and hypoxia [9, 20]. These adverse effects may be partially attributed to its relative long-acting time from several hours to days [19]. Remimazolam is a new agent of benzodiazepine family with ultra-short half-life time [21]. Thus, it preserves significant advantage in better recovery of consciousness and psychomotor performance including task execution and memory after procedure sedation [22].
The effect of remimazolam on sleep quality might be underestimated by the relatively small sample size of our study. Beyond anxiety, sleep disturbance arises from multiple etiologies including pain intensity, environmental noise, and procedures of medical therapy [2, 3, 23]. On the other hand, the ultra-short half-time of remimazolam may also limit its therapeutic effect. Thus, for sample size calculation, we conservatively proposed an assumption of a 20% increment in RCSQ score by remimazolam. Although no statistical significance was observed between two groups, median RCSQ score at surgery night was about 59 in remimazolam group and 53 in routine group which was close to our assumption (i.e., 60 vs. 50). This result indicated that intraoperative remimazolam may be used to improve sleep quality. However, further studies with large sample size are needed to validate this assumption, especially if postoperative remimazolam during surgery night will have better performance. For example, a randomized controlled study reported that patients who received zolpidem for 2 weeks after total knee arthroplasty had better sleep quality and lower pain scores [24].
Sedation strategy in routine group is based on daily clinical practice in two participating centres because of the following concerns. First, sedation at the discrete of patient’s demand and anaesthesiologist’s advice is the most common approach in most centres. The data originated from routine group are close to real-world practice and this facilitates the generalization of our result. Second, most sedatives were given in terms of clinical experience but not BIS-guided sedation in daily practice. Thus, BIS readings in routine group were masked to attending anaesthesiologists, which helps to alleviate the influence of BIS monitoring on daily practice. Third, previous evidence reported that intraoperative dexmedetomidine at 0.2–0.4 μg/kg/h might decrease the incidence of severe sleep disturbance at surgery night [25], and low dose dexmedetomidine was commonly used for sedation in our routine practice. In the present study, the median dosage of dexmedetomidine in routine group was 0.3 μg/kg/h, possibly optimizing postoperative sleep quality to some extent. However, we noticed that about half of these patients still complained sleep disturbance. This result is partially inconsistent with previous studies which reviewed the protective effect of dexmedetomidine on sleep [7]. As we discussed above, sleep disturbance is multifactorial. Further studies are needed to verify multidiscipline interventions on sleep quality, not only by sedatives.
We noticed that patients in routine group have lighter sedation depth in comparison with remimazolam group. We took two steps to evaluate the effect of BIS value on outcome. Multivariable linear regression was firstly administrated to adjust TWA BIS and other confounders. Second, a post-hoc analysis was employed to compare outcomes in patients with remimazolam and dexmedetomidine after exclusion of 9 patients without dexmedetomidine in routine group. The result was consistent with intention-to-treat analysis. Based on clinical experience, anaesthesiologists are prone to maintain lighter sedation in avoidance of adverse effects such hypotension and bradycardia by dexmedetomidine. In this study, we also observed slightly higher incidence of hypotension in routine care group but without statistical significance. However, the effect of sedation depth on patient’s prognosis is uncertain till now. In patients undergoing hip surgery, BIS-guided propofol light sedation (BIS > 80 versus deep BIS < 50) was associated with 50% reduction in postoperative delirium and 1 year mortality [26, 27]. But these results were not supported by the STRIDE trials which employed modified Observer's Assessment of Alertness/Sedation score (MOAA/S) as target of depth (lighter sedation 3–5 vs. heavier sedation 0–2) [28, 29].
To date, few studies have evaluated the effectiveness and safety of remimazolam infusion for sedation in elderly patients during spinal anaesthesia. Our study reported that a loading dose followed by continuous infusion of remimazolam could be safely and effectively used for intraoperative targeted sedation without increasing risks of adverse outcomes such as hypotension and respiratory depression.
Preoperative sleep disturbance is associated with postoperative sleep and pain intensity [30, 31]. Our study also found that poor preoperative sleep quality (higher PSQI scores) was associated with postoperative sleep disturbance. This indicates that improvement of preoperative sleep quality may alleviate the risk of postoperative sleep disturbance.
Our study had several limitations. First, architecture of sleep was monitored by RCSQ but not polysomnography. It is true that polysomnography represents a gold-standard tool for evaluation of sleep disorders [32], yet it was impractical in perioperative settings and may interfere with patient’s medical care, even sleep quality. Previous study showed that RCSQ had high consistence with polysomnography [33]. Second, the sample size was underpowered. Sample size calculation was based on a SD of 15 in pilot observation. But the actual SD was about 26. Further studies with larger sample size should be considered. Third, sedation depth was monitored by BIS. Previous studies showed that BIS may be inappropriate for monitoring dexmedetomidine sedation [34]. Objective scales such as MOAA/S may be better. However, frequent assessments will interrupt sedation continuity. This is not friendly to patient’s experiences. Another question is the effect of sedation depth on sleep quality. We employed multivariable linear regression and post hoc analysis to explore the association. Fourth, our study only focused on elderly patients undergoing orthopaedic surgery. Further studies are needed to identify the generalizability in other populations.
In conclusion, for elderly patients undergoing total joint arthroplasty under neuraxial anaesthesia, intraoperative remimazolam can be safely and effectively used for sedation, but its effect on postoperative sleep quality is still uncertain.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1: Richards Campbell Sleep Questionnaire scores of the first 3 nights after surgery. The box and whiskers plots show medians, interquartile ranges and outer ranges, and individual points mean mild outliers (o, which are outside 1.5 times of interquartile range). (PDF 201 KB)
Table S1: Primary and secondary Outcomes among patients receiving remimazolam and dexmedetomidine. Table S2: Factors associated with score of Richards Campbell Sleep Questionnaire at surgery night. Table S3: The scores in each domain of Richards Campbell Sleep Questionnaire. Table S4: The differences of Richards Campbell Sleep Questionnaire scores between high dose or low dose of dexmedetomidine and remimazolam. (DOCX 26 KB)
Acknowledgements
The authors would like to thank Ms Mo Li and Na-Ping Chen (Department of Anesthesiology and Critical Care Medicine, Peking University First Hospital, Beijing, China) for the help in data collection and statistical consultation.
Author contributions
CMD and JY helped conduct the study, analyze the data, and write the manuscript and contributed equally to the study. CJZ helped conduct the study. ML, HYL and YXW helped design the study and conduct the study. ZTM and DLM helped design the study and revise the manuscript. DLM is responsible for the overall content and acted as the principal investigator of this study. All authors participated in revision, approved for publication and took accountability for the report.
Funding
The present study was supported by National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital, No. 2022CR74, Dong-Liang Mu) and National Key R&D Program of China (2018YFC2001800, Dong-Liang Mu). The sponsors had no role in the design or conduct of the trial; data collection, trial management, data analysis, interpretation of the results, or preparation and approval of the manuscript.
Data availability
The dataset analysed in the current study is available from the corresponding author on reasonable request.
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chun-Mei Deng and Zhao-Ting Meng have contributed equally to the study.
References
- 1.Su X, Wang DX. Improve postoperative sleep: what can we do. Curr Opin Anaesthesiol. 2018;31:83–88. doi: 10.1097/ACO.0000000000000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning BT, Kearns SM, Bohl DD, Edmiston T, Sporer SM, Levine BR. Prospective assessment of sleep quality before and after primary total joint replacement. Orthopedics. 2017;40:e636–636e640. [DOI] [PubMed]
- 3.Wang Y, Liu Y, Li X, Lv Q, Xia Q, Wang X, Shao Y. Prospective assessment and risk factors of sleep disturbances in total hip and knee arthroplasty based on an Enhanced Recovery After Surgery concept. Sleep Breath. 2021;25:1231–1237. doi: 10.1007/s11325-020-02213-y. [DOI] [PubMed] [Google Scholar]
- 4.Sipilä RM, Kalso EA. Sleep well and recover faster with less pain—a narrative review on sleep in the perioperative period. J Clin Med. 2021;10:2000. doi: 10.3390/jcm10092000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memtsoudis SG, Cozowicz C, Bekeris J, Bekere D, Liu J, Soffin EM, Mariano ER, Johnson RL, Hargett MJ, Lee BH, Wendel P. Anaesthetic care of patients undergoing primary hip and knee arthroplasty: consensus recommendations from the International Consensus on Anaesthesia-Related Outcomes after Surgery group (ICAROS) based on a systematic review and meta-analysis. Br J Anaesth. 2019;123:269–287. doi: 10.1016/j.bja.2019.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10:321–326. doi: 10.5664/jcsm.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Lin D, Sun Y, Wu A, Wei C. Effect of dexmedetomidine on postoperative sleep quality: a systematic review. Drug Des Devel Ther. 2021;15:2161–2170. doi: 10.2147/DDDT.S304162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan WF, Miao EY, Jin F, Ma H, Lu HW. Changes in first postoperative night bispectral index after daytime sedation induced by dexmedetomidine or midazolam under regional anesthesia: a randomized controlled trial. Reg Anesth Pain Med. 2016;41:380–386. doi: 10.1097/AAP.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 9.Memtsoudis S, Cozowicz C, Zubizarreta N, Weinstein SM, Liu J, Kim DH, Poultsides L, Berger MM, Mazumdar M, Poeran J. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Reg Anesth Pain Med. 2019;2019:1. [DOI] [PubMed]
- 10.Jhuang BJ, Yeh BH, Huang YT, Lai PC. Efficacy and safety of remimazolam for procedural sedation: a meta-analysis of randomized controlled trials with trial sequential analysis. Front Med (Lausanne) 2021;8:641866. doi: 10.3389/fmed.2021.641866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 12.Wang XS, Mendoza TR, Gao SZ, Cleeland CS. The Chinese version of the brief pain inventory (BPI-C): its development and use in a study of cancer pain. Pain. 1996;67:407–416. doi: 10.1016/0304-3959(96)03147-8. [DOI] [PubMed] [Google Scholar]
- 13.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 14.Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8:131–144. doi: 10.1891/1061-3749.8.2.131. [DOI] [PubMed] [Google Scholar]
- 15.Chen LX, Ji DH, Zhang F, Li JH, Cui L, Bai CJ, Liu H, Liang Y. Richards–Campbell sleep questionnaire: psychometric properties of Chinese critically ill patients. Nurs Crit Care. 2019;24:362–368. doi: 10.1111/nicc.12357. [DOI] [PubMed] [Google Scholar]
- 16.Allen RW, Burney CP, Davis A, Henkin J, Kelly J, Judd BG, Ivatury SJ. Deep sleep and beeps: sleep quality improvement project in general surgery patients. J Am Coll Surg. 2021;232:882–888. doi: 10.1016/j.jamcollsurg.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, De Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 18.Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Br J Anaesth. 2012;109:769–775. doi: 10.1093/bja/aes252. [DOI] [PubMed] [Google Scholar]
- 19.Cozowicz C, Zhong H, Illescas A, Athanassoglou V, Poeran J, Reichel JF, Poultsides LA, Liu J, Memtsoudis SG. The perioperative use of benzodiazepines for major orthopedic surgery in the United States. Anesth Analg. 2022;134:486–495. doi: 10.1213/ANE.0000000000005854. [DOI] [PubMed] [Google Scholar]
- 20.Athanassoglou V, Cozowicz C, Zhong H, Illescas A, Poeran J, Liu J, Poultsides L, Memtsoudis SG. Association of perioperative midazolam use and complications: a population-based analysis. Reg Anesth Pain Med. 2022;47:228–233. doi: 10.1136/rapm-2021-102989. [DOI] [PubMed] [Google Scholar]
- 21.Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–651. doi: 10.1097/ALN.0000000000003103. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Sang N, Song K, Zhong W, Wang H, Jiang J, Huang Y, Hu P. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–624. doi: 10.1016/j.clinthera.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Lin D, Huang X, Sun Y, Wei C, Wu A. Perioperative sleep disorder: a review. Front Med (Lausanne) 2021;8:640416. doi: 10.3389/fmed.2021.640416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong L, Wang Z, Fan D. Sleep quality effects recovery after total knee arthroplasty (TKA)—a randomized, double-blind, controlled study. J Arthroplasty. 2015;30:1897–1901. doi: 10.1016/j.arth.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Duan G, Wang K, Peng T, Wu Z, Li H. The effects of intraoperative dexmedetomidine use and its different dose on postoperative sleep disturbance in patients who have undergone non-cardiac major surgery: a real-world cohort study. Nat Sci Sleep. 2020;12:209–219. doi: 10.2147/NSS.S239706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, Mears SC. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown CH, Azman AS, Gottschalk A, Mears SC, Sieber FE. Sedation depth during spinal anesthesia and survival in elderly patients undergoing hip fracture repair. Anesth Analg. 2014;118:977–980. doi: 10.1213/ANE.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieber FE, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JPP, Jaberi M, Hasenboehler EA. Effect of depth of sedation in older patients undergoing hip fracture repair on postoperative delirium: the STRIDE randomized clinical trial. JAMA Surg. 2018;153:987–995. doi: 10.1001/jamasurg.2018.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieber F, Neufeld KJ, Gottschalk A, Bigelow GE, Oh ES, Rosenberg PB, Mears SC, Stewart KJ, Ouanes JPP, Jaberi M, Hasenboehler EA. Depth of sedation as an interventional target to reduce postoperative delirium: mortality and functional outcomes of the strategy to reduce the incidence of postoperative delirium in elderly patients randomised clinical trial. Br J Anaesth. 2019;122:480–489. doi: 10.1016/j.bja.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Y, Hou H, Zhang J, Wang X, Wang L, Wu Y, Deng L. The effect of preoperative sleep quality on the target plasma concentration of propofol and postoperative sleep in patients undergoing painless gastroscopy. BMC Anesthesiol. 2023;23:9. doi: 10.1186/s12871-022-01957-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjurström MF, Irwin MR, Bodelsson M, Smith MT, Mattsson-Carlgren N. Preoperative sleep quality and adverse pain outcomes after total hip arthroplasty. Eur J Pain. 2021;25:1482–1492. doi: 10.1002/ejp.1761. [DOI] [PubMed] [Google Scholar]
- 32.Markun LC, Sampat A. Clinician-focused overview and developments in polysomnography. Curr Sleep Med Rep. 2020;6:309–321. doi: 10.1007/s40675-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagatomo K, Masuyama T, Iizuka Y, Makino J, Shiotsuka J, Sanui M. Validity of an under-mattress sensor for objective sleep measurement in critically ill patients: a prospective observational study. J Intens Care. 2020;8:16. doi: 10.1186/s40560-020-0433-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–1815. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1: Richards Campbell Sleep Questionnaire scores of the first 3 nights after surgery. The box and whiskers plots show medians, interquartile ranges and outer ranges, and individual points mean mild outliers (o, which are outside 1.5 times of interquartile range). (PDF 201 KB)
Table S1: Primary and secondary Outcomes among patients receiving remimazolam and dexmedetomidine. Table S2: Factors associated with score of Richards Campbell Sleep Questionnaire at surgery night. Table S3: The scores in each domain of Richards Campbell Sleep Questionnaire. Table S4: The differences of Richards Campbell Sleep Questionnaire scores between high dose or low dose of dexmedetomidine and remimazolam. (DOCX 26 KB)
Data Availability Statement
The dataset analysed in the current study is available from the corresponding author on reasonable request.