Abstract
Approximately 95% of cervical cancer are caused by human papillomavirus (HPV) infection. Although it is estimated that HPV-associated cervical cancer will decrease with the widespread use of HPV vaccine, it may take time for HPV-associated cervical cancer to be eliminated. For the appropriate management of HPV-associated cervical cancer, it is important to understand the detailed mechanisms of cervical cancer development. First, the cellular origin of most cervical cancers is thought to be cells in the squamocolumnar junction (SCJ) of the uterine cervix. Therefore, it is important to understand the characteristics of SCJ for cervical cancer screening and treatment. Second, cervical cancer is caused by high risk HPV (HR-HPV) infection, however, the manner of progression to cervical cancer differs depending on the type of HR-HPV: HPV16 is characterized by a stepwise carcinogenesis, HPV18 is difficult to detect in precancerous lesions, and HPV52, 58 tends to remain in the state of cervical intraepithelial neoplasia (CIN). Third, in addition to the type of HPV, the involvement of the human immune response is also important in the progression and regression of cervical cancer. In this review, we demonstrate the carcinogenesis mechanism of HPV-associated cervical cancer, management of CIN, and the current treatment of CIN and cervical cancer.
Keywords: Human papillomavirus, Cervical cancer, Squamocolumnar junction
Epidemiology of cervical cancer and the introduction of HPV vaccination
Human papillomavirus (HPV) infection is the main cause of cervical cancer. Despite the recent introduction of an HPV vaccination, cervical cancer remains the fourth most commonly diagnosed cancer among women worldwide and the fourth leading cause of cancer-related death [1]. Approximately 10,000 patients were newly diagnosed with cervical cancer in 2019 in Japan, and the number is growing slightly [2]. Cervical cancer is classified into two major histologic types, squamous cell carcinoma (72.6%) and adenocarcinoma (21.8%) [3] and the 5-year survival rates by histologic type are 81.4% for squamous cell carcinoma and 75.8% for adenocarcinoma, with adenocarcinoma having a worse prognosis than squamous cell carcinoma [3]. More than 80% of cervical adenocarcinoma are HPV associated, but 10–15% of them, such as gastric-type adenocarcinoma and clear cell carcinoma, have been reported to be HPV independent [4]. However, squamous cell carcinomas, which comprise the majority of cervical cancers are mostly caused by HPV infection, and approximately 95% of cervical cancers are HPV-positive [5]. There are other rare histological types of HPV-associated cervical cancer, such as small cell carcinoma and glassy cell carcinoma [6, 7].
It is expected that HPV-associated cervical cancer will decrease with the widespread use of HPV prophylactic vaccines. In Sweden, where the HPV prophylactic vaccine was introduced at an early age, administration of the vaccine had a significant effect on cervical cancer prevention, with incidence ratios of 0.12 (95% CI 0.00–0.34) and 0.47 (95% CI 0.27–0.75) for women who received the HPV vaccine before age of 17 and between ages of 17 and 30, respectively [8]. In Australia, if current levels of vaccination and screening coverage are maintained the annual incidence of cervical cancer is estimated to fall below four cases per 100,000 women by 2028 (range 2021–35) [9].
In Japan, a publicly-funded HPV vaccination program for girls aged 13–16 began in 2010, and HPV vaccination was included in the national immunization program for 12–16 aged girls in April 2013. However, soon after the introduction of HPV vaccination, the Ministry of Health, Labor and Welfare (MHLW) in Japan decided to suspend proactive recommendation of the vaccine as there were repeated reports of diverse symptoms, including motor impairment and chronic pain, from girls who had been vaccinated. As a result, the prevalence of HPV prophylactic vaccines has fallen below 1% [10]. In January 2014, after investigating these adverse events, MHLW concluded that there was no evidence to suggest a causal relationship between the HPV vaccine and adverse events, however, MHLW did not resume its proactive recommendation. Since then, the Nagoya Study has also reported no association between the HPV vaccine and reported adverse events [11]. Considering these results, the Japanese government decided to resume the proactive recommendation of the HPV vaccine in April 2022.
To prevent cervical cancer, in addition to primary prevention by HPV vaccine, secondary prevention with early detection through screening is also important. However, the low examination rate for cervical cancer screening in Japan has also been an issue. The ideal screening rate for cervical cancer elimination is 70% [12], however, in Japan it is only around 40% [13]. As a screening method for cervical cancer, a combination of cytological diagnosis by Pap smear and HPV testing is becoming popular worldwide. In general, cytology alone has low sensitivity and a high false-negative rate [14] but combining it with HPV testing increases sensitivity [15] and reduces costs by extending the interval between examinations and completing the examinations earlier. Although the 2012 guidelines of the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology recommended a combination of cytology and HPV testing (co-testing) every 5 years between the ages of 30 and 65 [16], the latest American Cancer Society guidelines for 2020 recommend FDA-approved HPV testing every 5 years after age 25 [17]. Consequently, HPV testing is likely to become the main screening method for cervical cancer in the future. While the introduction of the HPV vaccine in the U.S. might have led to a decrease in false-positive results for precancerous lesions in HPV testing, the usefulness of cytological-based screening is still supported in Japan where the introduction of the vaccine has been delayed. According to the Japanese guideline for cervical cancer screening at National Cancer Center [18], both cytology-based screening and HPV testing alone are recommended.
The cellular origin of cervical cancer and HPV-associated carcinogenesis
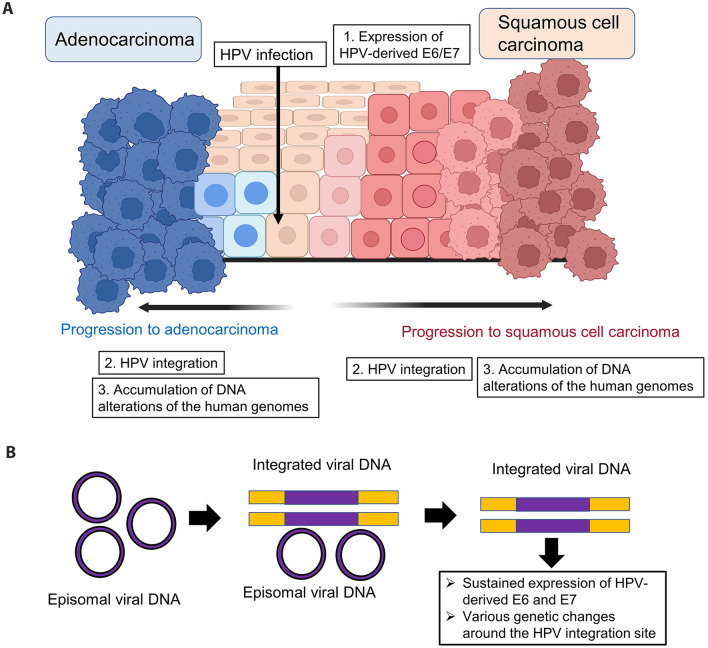
More than 200 genotypes of HPV have been reported [19]. Of these, only the mucosal type infects the uterine cervix. The International Agency for Research on Cancer (IARC) classifies HPV as Groups 1–4 based on the risk of carcinogenesis [20] and Group 1 or Group 2A with a high risk of carcinogenesis includes 13 types of HPV: HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. In particular, HPV types 16 and 18 are responsible for 70% of the HPV genotypes detectable in cervical cancer [21]. When HPV infects target cells, HPV-derived oncogenes E6 and E7 inactivate the tumor suppressor genes p53 and pRb, respectively, leading to apoptosis resistance and abnormal cell proliferation [22]. In approximately 90% of patients with HPV infection, the immune response spontaneously eliminates HPV-infected cells, but some patients develop a persistent infection, which is discussed in more detail in Chapters 3 and 4. A fraction of patients who develop persistent infections will progress to cervical cancer through the following steps. During persistent HPV infection, integration of the HPV genome into the human genome occurs, resulting in sustained expression of HPV-derived E6 and E7. HPV integration not only contributes to the persistent expression of HPV-derived E6/E7, but also induces various genetic alterations such as amplification of oncogenes, chromosomal rearrangements, and chromosomal instability around the HPV integration site [23, 24] (Fig. 1A, B). After accumulation of DNA alterations in the host (human) genomes, HPV-infected cells eventually progress to cervical cancer.
Fig. 1.
Carcinogenesis mechanism of cervical cancer. A Carcinogenesis mechanism of uterine squamous cell carcinoma and adenocarcinoma. Human papillomavirus (HPV) infects cells in the basal layer of the cervix and causes carcinogenesis over the years. Squamous cell carcinoma develops through a stepwise progression of its precancer lesions (cervical intraepithelial neoplasia, CIN). The steps to carcinogenesis include expression of HPV-derived oncogenes, E6/E7, HPV integration, and accumulation of human oncogene mutations. The figure was created with BioRender.com. B Changes in HPV genomic status. During persistent HPV infection, the integration of the HPV genome into the human genome occurs. HPV integration contributes to the sustained expression of HPV-derived E6/E7 as well as induces various genetic changes such as amplification of oncogenes, chromosomal rearrangements, and chromosomal instability around the HPV integration site
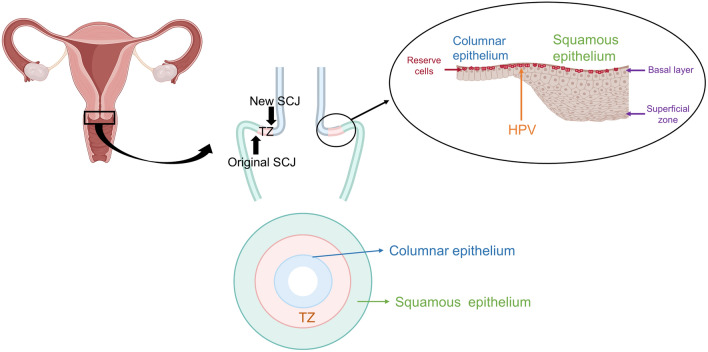
The uterine cervix consists of three distinct types of epithelium: the stratified squamous epithelium of the outer cervix, the monolayered columnar epithelium of the inner cervix, and the transformation zone (TZ) between the two (Fig. 2). The boundary between the squamous and columnar epithelium is called the squamocolumnar junction (SCJ) (Fig. 2).
Fig. 2.
Structure of the uterine cervix. The uterine cervix consists of three distinct types of epithelia. The boundary between the squamous and columnar epithelium is referred to as the squamocolumnar junction (SCJ). Reserve cells are located in the basal layer of the cervical SCJ. SCJ squamocolumnar junction, TZ transformation zone. The figure was created with BioRender.com
In women of reproductive age, vaginal acid stimulation causes squamous transformation of the columnar epithelium of the uterine cervix. Stem cells (reserve cells) in the SCJ are thought to be involved in this transformation [25, 26]. The area between the original SCJ and the new SCJ is known as the transformation zone (TZ) [27]. There are various theories regarding the cellular origin of cervical cancer, including the theory that HPV infects SCJ cells as well as the theory that HPV infects reserve cells [26]. These two theories are not exclusive. The SCJ is a cuboidal cell that borders the squamous and columnar epithelium, and cells in this area are thought to have the ability to differentiate into squamous or columnar epithelium. SCJ cells are characterized by the expression of SCJ markers such as cytokeratin 7 (CK7), anterior gradient (AGR) 2, cluster differentiation (CD) 63, matrix metalloproteinase (MMP) 7 and guanine deaminase (GDA) [28]. SCJ was considered as the cellular origin of cervical cancer for the following two reasons. First, these SCJ markers are expressed in cervical cancer and its precursor lesion, cervical intraepithelial neoplasia (CIN) [28], and second, cervical cancer and CIN frequently originate from the SCJ region. Therefore, close monitoring of the SCJ region is extremely important in the examination of cervical lesions. In particular, the accuracy of colposcopy decreases with age because TZ often migrates into the cervical canal [29]. Therefore, understanding the pathogenesis of HPV-derived cervical cancer and the characteristics of TZ is essential during primary screening and close examination of HPV-infected cervical lesions.
Natural history of HPV-related cervical lesions according to HPV genotypes
HPV-associated cervical cancer includes squamous cell carcinoma, adenocarcinoma (including adenosquamous carcinoma), and small cell carcinoma. Squamous cell carcinoma, which accounts for approximately 70% of all cervical cancers, develops through CINs. CIN can be classified into three stages, CIN1, CIN2, and CIN3, or two stages as low-grade squamous intraepithelial lesions (LSIL, CIN1) and high-grade squamous intraepithelial lesions (HSIL, CIN2-3) [30]. HSIL is characterized by positive p16 due to HPV-induced cell cycle activation. In NCCN guideline 2022, HSIL is considered a precancerous lesion and HSIL is indicated for treatments such as cervical conization and laser vaporization [31]. The precancerous lesions of adenocarcinoma are adenocarcinoma in situ (AIS), which, unlike CIN, does not take the form of gradual progression. Unlike squamous cell carcinoma and adenocarcinoma with precancerous lesions, the precancerous lesions have not been identified in small cell carcinoma and the cell of origin is still unknown.
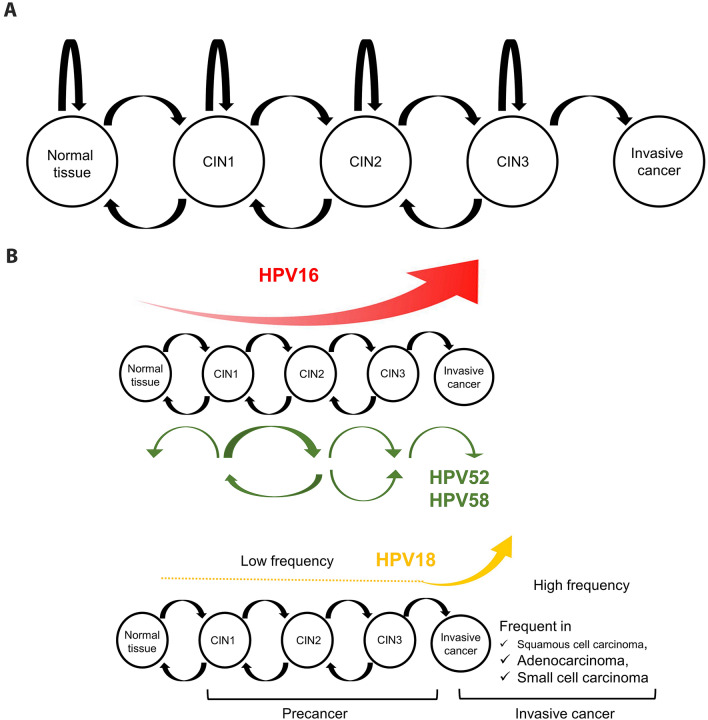
In general, HPV infection is temporary and about 90% of HPV infections are spontaneously eliminated within 2 years and do not develop into persistent infections [32]. However, a small proportion of these patients develop cervical cancer through persistent HPV infection. Infected HPV genotype is known to be involved in the progression of CIN. Among the 13 high-risk HPV types, 7 HPV types, HPV types 16, 18, 31, 33, 35, 52, and 58 have an approximately 20% rate of progression to CIN3 or higher within 5 years and are considered particularly high risk [33]. The progression of CIN is generally considered to be a gradual process from HPV infection through CIN1 to CIN2/3 to cervical cancer over the years. This change is not unidirectional but is characterized by repeated progression and regression and a bidirectional transition [34] (Fig. 3A). Recently, the Markov model, a statistical analysis method that takes into account the natural history of CIN, which is characterized by bidirectional transition, has been used to estimate the prognosis of CIN. Using the Markov model, it became clear that among these seven particularly high-risk types, the form of CIN progression differs depending on the HPV genotype; HPV16 is characterized by stepwise progression to CIN3 or higher, whereas HPV52 and HPV58 are characterized by persistent CIN1-2 [35, 36] (Fig. 3B). It is also important to note that among high-risk HPVs, HPV18 has characteristics not found in other types: HPV18 has a detection rate of approximately 25% in cervical cancer among Japanese [21], which is the second most common following HPV16, but its probability of being found in precancerous lesions is lower than with other high-risk HPVs, only about 5% [37] (Fig. 3B). One of the reasons for this is related to the histological characteristics of HPV18, which is more common in adenocarcinomas and small cell carcinomas among cervical cancers, and it is more difficult to detect precancerous lesions in these histological types than in squamous cell carcinomas [38, 39]. However, the low detection rate of HPV18 in precancerous lesions cannot be explained by histological characteristics alone, and some unique mechanisms are thought to be involved in HPV18 carcinogenesis [37, 40]. A study analyzing the cellular origin of mixed carcinoma of the uterine cervix reported that maintenance of stem cell-like components is important for HPV18 carcinogenesis [40]. Although further studies are needed to elucidate HPV18-associated carcinogenesis, the low detection rate of precancerous lesions and the high incidence of invasive cancer suggest that HPV18-positive patients require more careful management than patients with other types of infection.
Fig. 3.
The natural history of HPV-associated cervical lesions. A Bidirectional transition of cervical intraepithelial neoplasia (CIN). The natural history of CIN shows a bidirectional transition between different states (normal, CIN1, CIN2, and CIN3). B HPV-genotype-specific characteristics of cervical cancer development. HPV16-related lesion is characterized by stepwise progression to CIN3 or higher, whereas HPV52 and HPV58-related lesions are characterized by their persistency between CIN1 and CIN2. HPV18 is frequently detected in rate in cervical cancer, but its detection rate in precancerous lesions is low. HPV human papillomavirus, CIN cervical intraepithelial neoplasia
Evasion of HPV-infected cells from human immunity
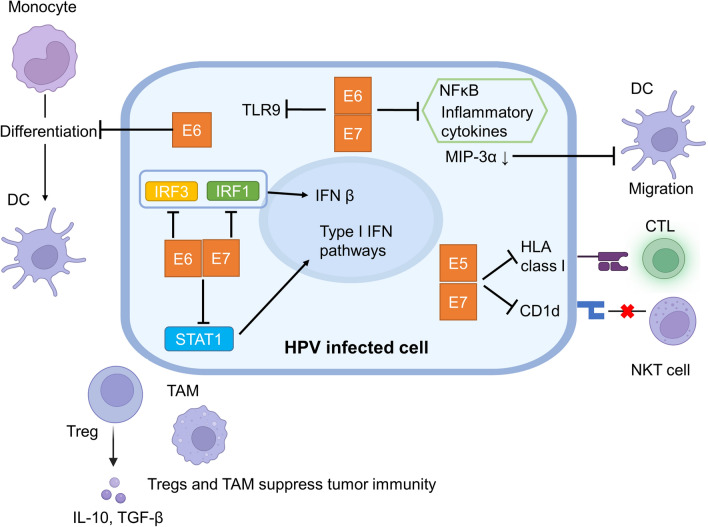
Evasion of human immune surveillance mechanisms is essential for persistent HPV infection to occur and its progression to cervical cancer. HPV infection of the cervix usually triggers a human immune response, and in most cases HPV-infected cells are eliminated. For example, keratinocytes respond to viral infection via pattern recognition receptors (PRRs) such as toll-like receptors (TLRs) and activate innate immunity through the expression of inflammatory cytokines. On the other hand, HPV-infected cells have mechanisms to evade the human immune response (Fig. 4). For example, HPV has been reported to suppress the expression of TLR9 in infected cells [41]. Furthermore, HR-HPV-derived E6 and E7 suppress nuclear factor-kappa B (NFκB) activity, resulting in decreased expression of inflammatory cytokines [42]. In addition, E6 and E7 also inhibit type I interferon (IFN) pathways by suppressing the activation of signal transducer and activator of transcription 1 (STAT1) [43]. Regarding the IFN pathway, E6 inhibits the activation of interferon regulatory factor (IRF) 3 and E7 inhibits IFN-β production by inhibiting IRF1 [44, 45]. HPV-derived E5 and E7 suppress the ability of host cells to present HPV antigens by downregulating the expression of Human leukocyte antigen (HLA) class I, which is necessary for antigen presentation [46–48]. HPV-infected cells are recognized by antigen-presenting cells such as tissue dendritic cells (Langerhans cells in the epithelium) and tissue macrophages, and cytotoxic T cells are activated via these antigen-presenting cells. On the other hand, HPV-infected keratinocytes inhibit Langerhans cell migration by reducing the expression of macrophage inflammatory protein (MIP)-3α [49], and HR-HPV-derived E6 inhibits the differentiation of monocytes into dendritic cells [50]. In CIN and cervical cancer, the distribution of Langerhans cells has been reported to be inversely correlated with E6 and E7 expression [51, 52]. Acting as an intermediate between innate and acquired immunity, natural killer T (NKT) cells are not restricted to MHC and recognize antigens presented on CD1d and produce IFN-γ to activate antiviral immunity [53]. HPV-derived E5 functions to suppress the CD1d-presenting ability of HPV-infected cells and evade recognition by NKT cells [53].
Fig. 4.
Immune escape mechanisms of HPV-infected cells. TLR toll-like receptors, DC Dendritic cell, TAM tumor-associated macrophage, CTL cytotoxic T lymphocyte, Treg regulatory T cell, IFN interferon, IRF interferon regulatory factor, NKT natural killer T cell, IL-10 interleukin-10, TGF tumor growth factor, NFκB nuclear factor-kappa B, MIP macrophage inflammatory protein, HLA human leukocyte antigen, STAT1 signal transducer and activator of transcription 1. The figure was created with BioRender.com
We have described how the immune response is evaded by HPV-derived products, however, host characteristics are also associated with the immune response to HPV. In particular, the relationship between persistent HPV infection and HLA class I/II has been well studied, and the HLA class I/II polymorphisms have been reported to be associated with CIN progression [54–56]. Furthermore, loss of heterozygosity of HLA and HLA-A/B mutations are known to occur frequently in cervical cancer [5, 24]. In addition, immune cells that "suppress" tumor immunity, including regulatory T cells (Treg) and tumor-associated macrophages (TAM), are involved in CIN progression. Tregs suppress effector T cells via the production of interleukin (IL)-10 and tumor growth factor (TGF)-β [57]. Tregs are associated with the prognosis of CIN patients, with patients with high Treg counts causing persistent HPV infection and CIN [58, 59]. In addition, the presence of TAM has been reported to correlate with the prognosis of HPV-associated tumors [60, 61]. Likewise, HPV-derived products not only suppress immune responses but are also attenuated antigen-presenting capacity of HPV-infected cells. In addition to the immune escape by HPV-derived products, host characteristics including HLA polymorphisms and suppressive immune responses in the tumor microenvironment are associated with persistent HPV infection and the progression of CIN.
Therapeutics of HPV-associated cervical neoplasia
Treatment of cervical cancer includes surgery (± adjuvant therapy), concurrent chemoradiation or chemotherapy, depending on the clinical stage of the disease. Radiation-based therapy and chemotherapy are the primary treatment strategies for advanced or recurrent cervical cancer. Although chemotherapies are not as effective for cervical cancer, the addition of bevacizumab to chemotherapies improved overall survival from 13.3 to 16.8 months [62]. Furthermore, combination therapies with immune checkpoint inhibitors (ICIs) have recently been attracting attention as a treatment option for advanced or recurrent cervical cancer, with median overall survival extended from 16.5 to 24.4 months [63]. Thereafter, combination therapy of chemotherapies and pembrolizumab was covered by insurance in Japan. In addition, genome-based medicine based on cancer genome profiling tests (CGP) has become popular recently to provide personalized medicine. However, there are still some challenges in CGP-based personalized medicine. One of the main issues is that only a limited number of patients can be enrolled in clinical trials based on genomic information [64, 65] and further improvement is desired.
Treatment strategies based on the characteristics of carcinogenic mechanisms have been investigated in HPV-associated cervical tumors. Among these, therapeutic approaches that focus on the immune response against the HPV protein have been drawing attention (Table 1). We conducted a search for recent (completed after January 2015) or ongoing Phase 2–4 clinical trials using the search terms “HPV” and “immunotherapy” on two databases, ClinicalTrial.gov (https://clinicaltrials.gov/ct2/home) and jRCT (https://jrct.niph.go.jp/). Therapeutic vaccine therapy is one of these treatments whose efficacy is expected as a possible conservative treatment. Therapeutic vaccines currently being attempted are mainly against CIN2/3, including plasmid DNA vaccines consisting of two plasmids encoding the E6 and E7 genes [66, 67] and peptide vaccines, such as oral vaccines of HPV16 E7-expressing lactobacillus [68] and HPV16-E7 synthetic long peptide (E7LP) vaccination [69]. Another immunotherapy includes engineered T-cell therapies with TCR-engineered T cells targeting E7, which are highly effective in treatment-refractory HPV-related cancers, including cervical, vulvar, anal, and oropharyngeal cancers [70]. Another therapy focusing on anti-tumor immunity is tumor-infiltrating lymphocyte therapy (TIL therapy), a type of immunotherapy in which TILs are harvested from a patient's tumor tissue, cultured outside the body, and returned to the patient. It is expected to be effective in cervical cancer [71]. Besides TIL therapies, antibody-based therapeutics are also expected to activate anti-tumor immunity. For instance, tisotumab vedotin, an investigational antibody–drug conjugate (ADC) directed against tissue factor (TF), a protein highly prevalent in multiple solid tumors, has demonstrated antitumor activity with a manageable and tolerable safety profile in women with previously treated recurrent or metastatic cervical cancer in a phase II clinical trial (NCT03438396) [72]. Additionally, as cervical cancer is caused by viral carcinogenesis, there is hope for the development of therapeutics that can trigger immune responses against both HPV and tumors in the coming years.
Table 1.
Immune-stimulating therapies for HPV-associated cervical lesions in clinical trials
| Immune-stimulating therapies * | References |
|---|---|
| Therapeutic vaccine therapy | |
| Plasmid DNA vaccines | [66, 67] |
| Peptide vaccine | [68, 69] |
| Engineered T-cell therapies | [70] |
| TIL therapy | [71] |
| Antibody–drug conjugates | [72] |
*To note, immune checkpoint inhibitors alone are not included
The table summarizes representative immune-stimulating therapies for HPV-associated cervical cancer and precancer. These therapies were identified through searches of “ClinicalTrial.gov” and jRCT using keywords such as “Cervical cancer”, “HPV”, and “Immunotherapy” and filtered with “Phase II–IV studies” and “Interventional study”. Key terms for jRCT included “uterine cervix”, “cervical cancer”, or “HPV”
Abbreviations
- CIN
Cervical intraepithelial neoplasia
- CGP
Cancer genome profiling
- HLA
Human leukocyte antigen
- HR-HPV
High risk human papillomavirus
- HSIL
High-grade squamous intraepithelial lesion
- IFN
Interferon
- IRF
Interferon regulatory factor
- MHLW
The Ministry of Health, Labor and Welfare
- NKT
Natural killer T
- SCJ
Squamocolumnar junction
- TAM
Tumor-associated macrophages
- TIL
Tumor-infiltrating lymphocyte
- TLR
Toll-like receptors
- Treg
Regulatory T cells
- TZ
Transformation zone
Author contributions
Conception and design: MK, AT. Writing draft: MK, AT. Revision of the manuscript: KS, MM, YO.
Funding
Open access funding provided by Osaka University. This review was supported by a grant to A.T. by AMED [Grant Number: 22wm0325014h0003].
Declarations
Conflict of interest
No author has any conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Misako Kusakabe and Ayumi Taguchi contributed equally.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Center Japan Cancer registry and statistics (JP). Cancer Information Service. Available from https://ganjoho.jp/reg_stat/statistics/dl/index.html. Accessed Feb 2023.
- 3.Nagase S, Ohta T, Takahashi F, et al. Annual report of the committee on gynecologic oncology, the Japan society of obstetrics and gynecology: annual patient report for 2018 and annual treatment report for 2013. J Obstet Gynaecol Res. 2022;48:541–552. doi: 10.1111/jog.15134. [DOI] [PubMed] [Google Scholar]
- 4.Stolnicu S, Hoang L, Soslow RA. Recent advances in invasive adenocarcinoma of the cervix. Virchows Arch. 2019;475:537–549. doi: 10.1007/s00428-019-02601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network, Albert Einstein College of Medicine Analytical Biological Services et al. Integrated genomic and molecular characterization of cervical cancer. Nature. 2017;543:378–384. doi: 10.1038/nature21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Q, Hu Y, He Y, et al. Glassy cell carcinoma of cervix: an analysis for 20 cases and literatures review. Transl Cancer Res. 2020;9:2357–2362. doi: 10.21037/tcr.2020.03.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultheis AM, de Bruijn I, Selenica P, et al. Genomic characterization of small cell carcinomas of the uterine cervix. Mol Oncol. 2022;16:833–845. doi: 10.1002/1878-0261.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 9.Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:e19–e27. doi: 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- 10.Ueda Y, Yagi A, Ikeda S, et al. Beyond resumption of the Japanese government’s recommendation of the HPV vaccine. Lancet Oncol. 2018;19:1563–1564. doi: 10.1016/S1470-2045(18)30573-4. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Hosono A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res. 2018;5:96–103. doi: 10.1016/j.pvr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (2020) Global strategy to accelerate the elimination of cervical cancer as a public health problem. Available from https://www.who.int/publications/i/item/9789240014107 Accessed Feb 2023.
- 13.National Cancer Center Japan Cancer registry and statistics (JP). Cancer Information Service. Available from https://ganjoho.jp/reg_stat/statistics/stat/screening/screening.html Accessed Feb 2023.
- 14.Clavel C, Masure M, Bory JP, et al. Human papillomavirus testing in primary screening for the detection of high-grade cervical lesions: a study of 7932 women. Br J Cancer. 2001;84:1616–1623. doi: 10.1054/bjoc.2001.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 16.Saslow D, Solomon D, Lawson HW, et al. American cancer society, American society for colposcopy and cervical pathology, and American society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321–34621. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Center, Japan (2020) Updated cervical cancer screening guidelines. Available from http://canscreen.ncc.go.jp/shikyukeiguide2019.pdf Accessed Mar 2023.
- 19.Araldi RP, Assaf SMR, Carvalho RF, et al. Papillomaviruses: a systematic review. Genet Mol Biol. 2017;40:1–21. doi: 10.1590/1678-4685-GMB-2016-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100b:1–441. [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma Y, Kusumoto-Matsuo R, Takeuchi F, et al. Human papillomavirus genotype distribution in cervical intraepithelial neoplasia grade 2/3 and invasive cervical cancer in Japanese women. Jpn J Clin Oncol. 2014;44:910–917. doi: 10.1093/jjco/hyu112. [DOI] [PubMed] [Google Scholar]
- 22.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: roles of E6 and E7 proteins. Cancer Sci. 2007;98:1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu Z, Zhu D, Wang W, et al. Genome-wide profiling of HPV integration in cervical cancer identifies clustered genomic hot spots and a potential microhomology-mediated integration mechanism. Nat Genet. 2015;47:158–163. doi: 10.1038/ng.3178. [DOI] [PubMed] [Google Scholar]
- 24.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506:371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delvenne P, Herman L, Kholod N, et al. Role of hormone cofactors in the human papillomavirus-induced carcinogenesis of the uterine cervix. Mol Cell Endocrinol. 2007;264:1–5. doi: 10.1016/j.mce.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 26.López J, Ruíz G, Organista-Nava J, et al. Human papillomavirus infections and cancer stem cells of tumors from the uterine cervix. Open Virol J. 2012;6:232–240. doi: 10.2174/1874357901206010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manga SM, Kincaid KD, Boitano TKL, et al. Misoprostol and estradiol to enhance visualization of the transformation zone during cervical cancer screening: an integrative review. Eur J Obstet Gynecol Reprod Biol. 2022;269:16–23. doi: 10.1016/j.ejogrb.2021.11.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren H, Jia M, Zhao S, et al. Factors correlated with the accuracy of colposcopy-directed biopsy: a systematic review and meta-analysis. J Invest Surg. 2022;35:284–292. doi: 10.1080/08941939.2020.1850944. [DOI] [PubMed] [Google Scholar]
- 30.WHO Classification of Tumours Editorial Board (2020) Female genital tumours, 4, 5th ed.. World Health Organization classification of tumours
- 31.National Comprehensive Cancer Network (2023) NCCN guidelines cervical cancer, version 1.2023. Available from https://www.nccn.org/login?ReturnURL=https://www. http://nccn.org/professionals/physician_gls/pdf/cervical.pdf Accessed Feb 2023
- 32.Ho GY, Bierman R, Beardsley L, et al. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–428. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto K, Oki A, Furuta R, et al. Predicting the progression of cervical precursor lesions by human papillomavirus genotyping: a prospective cohort study. Int J Cancer. 2011;128:2898–2910. doi: 10.1002/ijc.25630. [DOI] [PubMed] [Google Scholar]
- 34.Loopik DL, Bentley HA, Eijgenraam MN, et al. The natural history of cervical intraepithelial neoplasia Grades 1, 2, and 3: A systematic review and meta-analysis. J Low Genit Tract Dis. 2021;25:221–231. doi: 10.1097/LGT.0000000000000604. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi A, Hara K, Tomio J, et al. Multistate markov model to predict the prognosis of high-risk human papillomavirus-related cervical lesions. Cancers (Basel) 2020;12(2):270. doi: 10.3390/cancers12020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikesu R, Taguchi A, Hara K, et al. Prognosis of high-risk human papillomavirus-related cervical lesions: A hidden Markov model analysis of a single-center cohort in Japan. Cancer Med. 2022;11:664–675. doi: 10.1002/cam4.4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulk S, Berkhof J, Rozendaal L, et al. The contribution of HPV18 to cervical cancer is underestimated using high-grade CIN as a measure of screening efficiency. Br J Cancer. 2007;96:1234–1236. doi: 10.1038/sj.bjc.6603693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 39.Stoler MH, Mills SE, Gersell DJ, et al. Small-cell neuroendocrine carcinoma of the cervix. A human papillomavirus type 18-associated cancer. Am J Surg Pathol. 1991;15:28–32. doi: 10.1097/00000478-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Kusakabe M, Taguchi A, Tanikawa M, et al. Cells with stem-like properties are associated with the development of HPV18-positive cervical cancer. Cancer Sci. 2023;114:885–895. doi: 10.1111/cas.15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasan UA, Zannetti C, Parroche P, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the toll-like receptor 9 promoter. J Exp Med. 2013;210:1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards KH, Wasson CW, Watherston O, et al. The human papillomavirus (HPV) E7 protein antagonises an imiquimod-induced inflammatory pathway in primary human keratinocytes. Sci Rep. 2015;5:12922. doi: 10.1038/srep12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan EL, Macdonald A. Manipulation of JAK/STAT signalling by high-risk HPVs: potential therapeutic targets for HPV-associated malignancies. Viruses. 2020;12(9):977. doi: 10.3390/v12090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JS, Kim EJ, Kwon HJ, et al. Inactivation of interferon regulatory factor-1 tumor suppressor protein by HPV E7 oncoprotein. Implication for the E7-mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem. 2000;275:6764–6769. doi: 10.1074/jbc.275.10.6764. [DOI] [PubMed] [Google Scholar]
- 45.Ronco LV, Karpova AY, Vidal M, et al. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campo MS, Graham SV, Cortese MS, et al. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology. 2010;407:137–142. doi: 10.1016/j.virol.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 47.Cortese MS, Ashrafi GH, Campo MS. All 4 di-leucine motifs in the first hydrophobic domain of the E5 oncoprotein of human papillomavirus type 16 are essential for surface MHC class I downregulation activity and E5 endomembrane localization. Int J Cancer. 2010;126:1675–1682. doi: 10.1002/ijc.25004. [DOI] [PubMed] [Google Scholar]
- 48.Li W, Deng XM, Wang CX, et al. Down-regulation of HLA class I antigen in human papillomavirus type 16 E7 expressing HaCaT cells: correlate with TAP-1 expression. Int J Gynecol Cancer. 2010;20:227–232. doi: 10.1111/IGC.0b013e3181cceec5. [DOI] [PubMed] [Google Scholar]
- 49.Guess JC, McCance DJ. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J Virol. 2005;79:14852–14862. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iijima N, Goodwin EC, Dimaio D, et al. High-risk human papillomavirus E6 inhibits monocyte differentiation to Langerhans cells. Virology. 2013;444:257–262. doi: 10.1016/j.virol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jimenez-Flores R, Mendez-Cruz R, Ojeda-Ortiz J, et al. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans’ cells in the human female genital tract. Immunology. 2006;117:220–228. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang B, Xue M. Correlation of E6 and E7 levels in high-risk HPV16 type cervical lesions with CCL20 and Langerhans cells. Genet Mol Res. 2015;14:10473–10481. doi: 10.4238/2015.September.8.8. [DOI] [PubMed] [Google Scholar]
- 53.Miura S, Kawana K, Schust DJ, et al. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol. 2010;84:11614–11623. doi: 10.1128/JVI.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto K, Maeda H, Oki A, et al. HLA class II DRB1*1302 allele protects against progression to cervical intraepithelial neoplasia grade 3: a multicenter prospective cohort study. Int J Gynecol Cancer. 2012;22:471–478. doi: 10.1097/IGC.0b013e3182439500. [DOI] [PubMed] [Google Scholar]
- 55.Paaso A, Jaakola A, Syrjänen S, et al. From HPV infection to lesion progression: the role of HLA alleles and host immunity. Acta Cytol. 2019;63:148–158. doi: 10.1159/000494985. [DOI] [PubMed] [Google Scholar]
- 56.Chan PK, Cheung JL, Cheung TH, et al. HLA-B alleles, high-risk HPV infection and risk for cervical neoplasia in southern Chinese women. Int J Cancer. 2006;118:1430–1435. doi: 10.1002/ijc.21528. [DOI] [PubMed] [Google Scholar]
- 57.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kojima S, Kawana K, Tomio K, et al. The prevalence of cervical regulatory T cells in HPV-related cervical intraepithelial neoplasia (CIN) correlates inversely with spontaneous regression of CIN. Am J Reprod Immunol. 2013;69:134–141. doi: 10.1111/aji.12030. [DOI] [PubMed] [Google Scholar]
- 59.Molling JW, de Gruijl TD, Glim J, et al. CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121:1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 60.Kawachi A, Yoshida H, Kitano S, et al. Tumor-associated CD204+ M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018;109:863–870. doi: 10.1111/cas.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen XJ, Han LF, Wu XG, et al. Clinical significance of CD163+ and CD68+ tumor-associated macrophages in high-risk HPV-related cervical cancer. J Cancer. 2017;8:3868–3875. doi: 10.7150/jca.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (gynecologic oncology group 240) Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 64.Sunami K, Ichikawa H, Kubo T, et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: a hospital-based study. Cancer Sci. 2019;110:1480–1490. doi: 10.1111/cas.13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386:2078–2088. doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hillemanns P, Denecke A, Woelber L, et al. A therapeutic antigen-presenting cell-targeting DNA vaccine VB10.16 in HPV16-positive high-grade cervical intraepithelial neoplasia: Results from a phase I/IIa trial. Clin Cancer Res. 2022;28:4885–4892. doi: 10.1158/1078-0432.CCR-22-1927. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda Y, Adachi K, Tomio K, et al. A placebo-controlled, double-blind randomized (phase IIB) trial of oral administration with HPV16 E7-expressing Lactobacillus, GLBL101c, for the treatment of cervical intraepithelial neoplasia Grade 2 (CIN2) Vaccines (Basel) 2021;9:329. doi: 10.3390/vaccines9040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Domingos-Pereira S, Galliverti G, Hanahan D, et al. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer. 2019;7:122. doi: 10.1186/s40425-019-0593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nagarsheth NB, Norberg SM, Sinkoe AL, et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat Med. 2021;27:419–425. doi: 10.1038/s41591-020-01225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stevanović S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coleman RL, Lorusso D, Gennigens C, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]