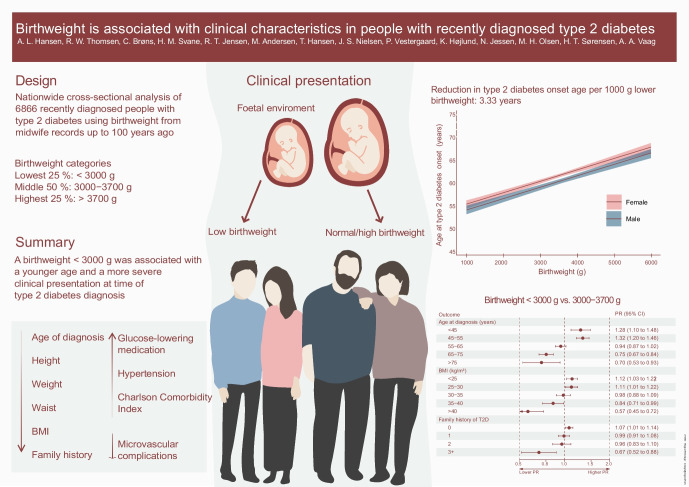
Abstract
Aims/hypothesis
Low birthweight is a risk factor for type 2 diabetes but it is unknown whether low birthweight is associated with distinct clinical characteristics at disease onset. We examined whether a lower or higher birthweight in type 2 diabetes is associated with clinically relevant characteristics at disease onset.
Methods
Midwife records were traced for 6866 individuals with type 2 diabetes in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort. Using a cross-sectional design, we assessed age at diagnosis, anthropomorphic measures, comorbidities, medications, metabolic variables and family history of type 2 diabetes in individuals with the lowest 25% of birthweight (<3000 g) and highest 25% of birthweight (>3700 g), compared with a birthweight of 3000–3700 g as reference, using log-binomial and Poisson regression. Continuous relationships across the entire birthweight spectrum were assessed with linear and restricted cubic spline regression. Weighted polygenic scores (PS) for type 2 diabetes and birthweight were calculated to assess the impact of genetic predispositions.
Results
Each 1000 g decrease in birthweight was associated with a 3.3 year (95% CI 2.9, 3.8) younger age of diabetes onset, 1.5 kg/m2 (95% CI 1.2, 1.7) lower BMI and 3.9 cm (95% CI 3.3, 4.5) smaller waist circumference. Compared with the reference birthweight, a birthweight of <3000 g was associated with more overall comorbidity (prevalence ratio [PR] for Charlson Comorbidity Index Score ≥3 was 1.36 [95% CI 1.07, 1.73]), having a systolic BP ≥155 mmHg (PR 1.26 [95% CI 0.99, 1.59]), lower prevalence of diabetes-associated neurological disease, less likelihood of family history of type 2 diabetes, use of three or more glucose-lowering drugs (PR 1.33 [95% CI 1.06, 1.65]) and use of three or more antihypertensive drugs (PR 1.09 [95% CI 0.99, 1.20]). Clinically defined low birthweight (<2500 g) yielded stronger associations. Most associations between birthweight and clinical characteristics appeared linear, and a higher birthweight was associated with characteristics mirroring lower birthweight in opposite directions. Results were robust to adjustments for PS representing weighted genetic predisposition for type 2 diabetes and birthweight.
Conclusion/interpretation
Despite younger age at diagnosis, and fewer individuals with obesity and family history of type 2 diabetes, a birthweight <3000 g was associated with more comorbidities, including a higher systolic BP, as well as with greater use of glucose-lowering and antihypertensive medications, in individuals with recently diagnosed type 2 diabetes.
Graphical Abstract
Supplementary Information
The online version contains peer-reviewed but unedited supplementary material available at 10.1007/s00125-023-05936-1.
Keywords: Age at diagnosis, Birthweight, Epidemiology, Fetal programming, Polygenic risk score, Type 2 diabetes
Introduction
Type 2 diabetes is a multifactorial heterogeneous disease associated with a range of complications [1]. There is increasing evidence that both low and high birthweight are associated with increased risk of type 2 diabetes in adult life [2–6]. Furthermore, low birthweight is a known risk factor for hypertension, dyslipidaemia, cardiovascular disease and neurocognitive dysfunction, even among people without type 2 diabetes [2, 7, 8]. In precision medicine, there is a strong focus on understanding clinically relevant disease segmentation and identifying individuals with the highest need for care. With low birthweight thus being a risk factor for not only type 2 diabetes but also for many of the most important type 2 diabetes comorbidities, one may speculate that having a birthweight outside the normal range among individuals with type 2 diabetes is associated with a differential clinical presentation at onset, with more comorbidities.
The thrifty phenotype hypothesis proposes that exposure to an adverse fetal environment may lead to metabolic adaptations that help prepare the low-birthweight child for survival in a sparse environment [9, 10]. However, the same adaptive changes may subsequently become detrimental when the individual is exposed to an affluent lifestyle, increasing the risk of developing type 2 diabetes and associated complications [10]. The theoretical framework of developmental programming involving impaired development of multiple organs, including but not restricted to those involved in glucose homeostasis [2, 9–11], raises the question of whether a lower birthweight in type 2 diabetes may be characterised by a more severe clinical presentation that potentially includes more comorbidities.
We conducted a cross-sectional analysis examining the relationship between lower birthweight and clinical characteristics reflecting disease severity, such as age at diabetes diagnosis, anthropomorphic measures, glucose and lipid metabolism, medications, family history of type 2 diabetes, and comorbidities. Due to reports of a potential U- or J-shaped association between birthweight and risk of type 2 diabetes [2, 8–10], we also examined the impact of high compared with normal birthweight on type 2 diabetes characteristics. Further acknowledging the potential impact of genetics on the associations between birthweight and type 2 diabetes [2, 9, 11], we also used polygenic scores (PS) to assess the quantitative genetic impacts of birthweight and type 2 diabetes on associations between birthweight and clinical characteristics. Finally, using a case–control design, we reaffirmed the association of low birthweight with the risk of type 2 diabetes in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort.
Methods
DD2 cohort
Since November 2010, individuals have been continuously enrolled into the nationwide DD2 cohort by general practitioners (GPs) and hospital outpatient clinics. The enrolment process, implementation, logistics, biobank and characteristics of the cohort have been described previously [12]. In brief, clinicians identify new type 2 diabetes patients and complete an online questionnaire. Urine and fasting blood samples are collected for storage in a biobank. From the beginning, the DD2 cohort aimed to enrol participants with newly diagnosed type 2 diabetes. In practice, however, the referral to DD2 has not always happened at the first identification of diabetes, when other clinical activity and therapy may have been more pertinent. Thus, the average time from the first-recorded glucose-lowering drug initiation after diabetes diagnosis to the enrolment date in the DD2 cohort is approximately 1 year [12]. The unique civil personal registration number assigned to all Danish residents can be used to link DD2 participants to different Danish health registries. Variables collected for the DD2 cohort have been described in previous publications [12, 13] and are presented at www.dd2.dk (accessed 8 March 2023). We do not have detailed information on ethnicity, but all individuals were born in Denmark between 1920 and 1988.
Exposure: birthweight
The feasibility of extracting birthweight and associated variables for Danish residents using the Danish National Archive (DaNaAr) has been established [14, 15]. For all individuals in the DD2 cohort born in 1920–1988, and where data were potentially available in the DaNaAr, we extracted objectively ascertained midwife information on birthweight, non-singleton birth status and born-at-term status (see electronic supplementary material [ESM] Methods: birthweight data from original midwife records). Our main analysis focused on the lowest and highest quartiles of birthweight on the basis of considerations of statistical precision together with our assumption that associations would be observed in a dose–response relationship [2] across the entire birthweight spectrum. While our a priori hypothesis was that low birthweight would be associated with a more severe clinical presentation, we kept a parallel focus on high birthweight to avoid overlooking potential U- or J-shaped relationships. Birthweight was therefore divided into three categories: individuals below the lowest quartile (<25%, <3000 g); above the highest quartile (>75%, >3700 g); and between the lowest and highest quartile (25–75%, 3000–3700 g, as the reference group). In additional analyses, we reran our models, applying frequently used clinical definitions of low birthweight (often <2500 g) or high birthweight (often >4500 g) [16].
Population controls
We ascertained birth data for birth-date-matched controls for each DD2 cohort diabetes participant available in the DaNaAr. We identified control individuals by randomly choosing two births from the same page of the midwife paper records where the corresponding individual with diabetes was recorded (one page typically contains birth information on 6–8 consecutive deliveries). This allowed for matching of diabetes cases and controls to the closest available date of birth (within the same week), geographic location, hospital and individual midwife (ESM Methods: birthweight data from original midwife records).
Outcomes and covariates
Information on outcomes, covariates, definitions and codes is provided in ESM Table 1 [12]. This includes age at diagnosis, family history of type 2 diabetes, anthropomorphic measures (height, weight, BMI, waist circumference, waist/hip and waist/height ratios), BP, glucose and lipid metabolism, and comorbidities and diabetes-associated complications at time of disease onset. Variables were categorised based on prior publications [12]. If their distributions were substantially skewed, they were categorised based on their distributions. We defined outliers as values >5 SDs from the mean (ESM Table 1).
Statistical analyses
Cross-sectional analysis of characteristics associated with birthweight in the diabetes cohort
Descriptive data are provided as medians (IQR) for continuous variables and as counts (percentages) for categorical variables according to the three birthweight categories. To examine the associations between preselected clinical type 2 diabetes variables and birthweight, we performed log-binomial and robust (modified) Poisson regression [17] analyses to calculate prevalence ratios (PRs) with 95% CIs using birthweight <3000 g and birthweight >3700 g as exposures and birthweight 3000–3700 g as the reference category. PRs for all outcomes were adjusted for sex, family history of type 2 diabetes and age at DD2 enrolment (except when focusing on age at diagnosis). We refrained from additional adjustment in our main model because different metabolic and lifestyle factors may act as intermediates in the incompletely understood pathways between birthweight and type 2 diabetes. Further adjustments for BMI, physical activity, smoking and/or alcohol consumption were performed in extended exploratory analyses.
To investigate associations across the full birthweight spectrum, we performed linear regression analyses. To allow for potential non-linear relationships, we used two- and three-degree polynomials and restricted cubic spline regression analyses (ESM Methods: Exploring linear and non-linear relationships). All models were adjusted for sex, family history of type 2 diabetes and age at DD2 enrolment (except when focusing on age at diagnosis). Logarithmic transformations are presented as the percentage change in the outcome per 1000 g birthweight. To account for missing data when performing regression analysis, we employed multivariate imputations by chained equations (MICE) using the MICE package version 3.14.0 from R [18]. The percentage of missing values varied between 0% and 57% (blood lipids), with a mean percentage missing values of 13.75% across all variables. The distribution of missingness was similar across birthweight, sex and age at enrolment. Predictive mean matching was used for imputation of continuous variables, logistic regression for binary variables and polytomous regression for multilevel categorical variables. See ESM Methods (Multivariate imputations by chained equations [MICE] model specification) for further specification of the method and the missing data pattern.
Additional analyses
PS
Based on a DD2 subpopulation with available genotype data (n=2563), we calculated PS for developing type 2 diabetes and for higher birthweight [19, 20]. Genotyping was performed using the Global Screening Array-24 v2.0 (Illumina, San Diego, CA, USA). After removing individuals and variants with >5% missingness, the genotype data were imputed using the Haplotype Reference Consortium reference panel build GRCh37. PS was calculated using published weighted scores [19, 20]. We were able to include 94% of the variants in the type 2 diabetes PS (PGS000014) and 100% of the variants in the birthweight PS (PGS002105). We performed log-binomial regression analyses to calculate PRs with 95% CIs to investigate the associations between the PS for developing type 2 diabetes and the ascertained midwife record birthweight of the individuals with type 2 diabetes. All main analyses were then repeated in this subpopulation, further adjusting for the type 2 diabetes and birthweight PS. Self-reported information on family history of type 2 diabetes was validated against the PS for developing type 2 diabetes.
Case–control analysis of diabetes risk
Finally, in a case–control analysis of 6866 eligible individuals with type 2 diabetes (cases; see later) and the 17,780 birth-date-matched control individuals (ESM Methods: birthweight data from original midwife records), we performed logistic regression analysis to calculate ORs as a measure of relative risks of being diagnosed with type 2 diabetes, using <3000 g and >3700 g as exposures, and 3000–3700 g as the reference. We further re-analysed associations using conventional clinical cut-off points for low and high birthweight (<2500 g and >4500 g, respectively).
Sensitivity analyses
To investigate the potential impact of preterm birth on birthweight associations, we additionally adjusted our models for the variable ‘born-at-term’. We re-analysed the data using conventional clinical cut-off points for low and high birthweight (<2500 g and >4500 g). We further reran our analyses comparing individuals below the lowest and above the highest birthweight quartile with those between birthweight quartiles, while using observed quartiles specific to four subgroups: male and born-at-term (<25% [3200 g] and >75% [3800 g]); male and not born-at-term (<25% [2275 g] and >75% [2800 g]); female and born-at-term (<25% [3100 g] and >75% [3685 g]); and female and not born-at-term (<25% [2250 g] and >75% [2800 g]).
All analyses were performed using R statistical software version 4.1.2 [21]. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.
Ethics
This study was approved by the Danish Data Protection Agency (record number 2008–58–0035) and by the Regional Committees on Health Research Ethics for Southern Denmark (record number S-20100082). All cohort participants gave written informed consent.
Results
The DD2 cohort enrolled 8190 participants in the period 2010–2018. A total of 1030 participants were born after 1988, had an unknown birthplace or had incomplete birth data, leaving 7160 (87%) for whom we were able to trace complete midwife records. We excluded 294 individuals with non-singleton births, positive GAD (GAD) antibody (>30 kU/l) (to avoid potential misclassification of type 2 diabetes with autoimmune diabetes) or high-sensitivity C-reactive protein (hsCRP) >30 mg/l (indicating acute illness at time of enrolment). Our final study population included 6866 individuals with type 2 diabetes (Fig. 1), of whom n=1675 (24.4%) had a birthweight <3000 g, n=3525 (51.3%) had a birthweight of 3000–3700 g, and n=1666 (24.3%) had a birthweight >3700 g. Table 1 shows baseline characteristics overall and according to birthweight category. Median age at enrolment was 62 years; 8.4% were diagnosed with type 2 diabetes at <45 years of age and 8.0% at ≥75 years of age. The proportion of women was 41.2%, and 54.5% of the participants were enrolled by GPs. Further information on covariates according to birthweight categories can be found in ESM Tables 2–4.
Fig. 1.
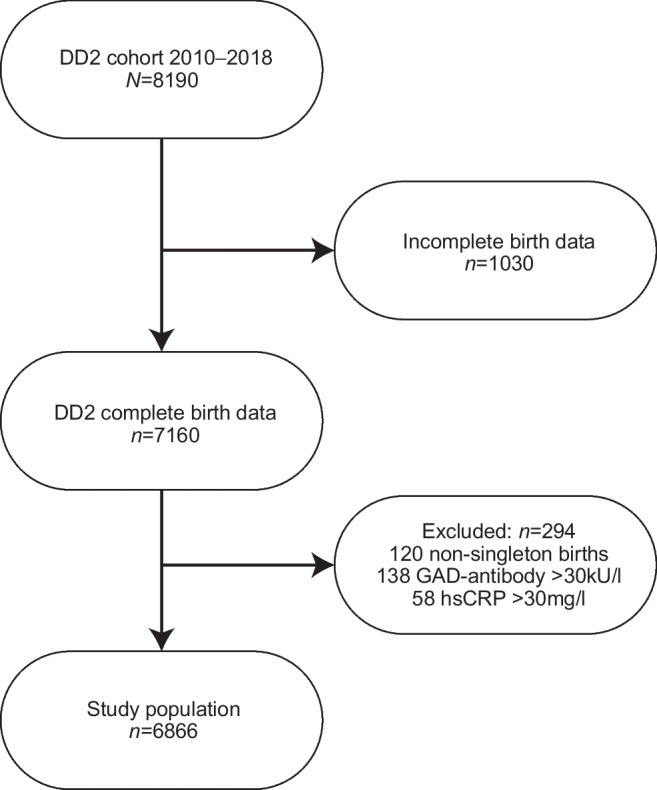
Flowchart of study population selection. Individuals may have fulfilled more than one of the exclusion criteria at DD2 enrolment
Table 1.
Baseline characteristics according to birthweight
| Enrolment characteristic | <3000 g (n=1675) | 3000–3700 g (n=3525) | >3700 g (n=1666) | Total (n=6866) |
|---|---|---|---|---|
| Sex | ||||
| Male | 810 (48.36) | 2099 (59.55) | 1128 (67.71) | 4037 (58.80) |
| Female | 865 (51.64) | 1426 (40.45) | 538 (32.29) | 2829 (41.20) |
| Age at enrolment, years | 59.3 (51.30–66.51) | 62.09 (53.46–68.39) | 64.53 (55.61–70.08) | 61.95 (53.18–68.52) |
| Age category at enrolment | ||||
| <45 years | 183 (10.93) | 296 (8.40) | 100 (6.00) | 579 (8.43) |
| 45–55 years | 438 (26.15) | 707 (20.06) | 298 (17.89) | 1443 (21.02) |
| 55–65 years | 549 (32.78) | 1153 (32.71) | 465 (27.91) | 2167 (31.56) |
| 65–75 years | 408 (24.36) | 1097 (31.12) | 620 (37.21) | 2125 (30.95) |
| ≥75 years | 97 (5.79) | 272 (7.72) | 183 (10.98) | 552 (8.04) |
| Age at diagnosis, years | 57.16 (49.35–64.50) | 59.83 (51.55–66.47) | 62.15 (53.47–68.07) | 59.8 (51.3–66.5) |
| Age category at diagnosis | ||||
| <45 years | 241 (14.39) | 410 (11.63) | 139 (8.34) | 790 (11.51) |
| 45–55 years | 483 (28.84) | 787 (22.33) | 330 (19.81) | 1600 (23.30) |
| 55–65 years | 559 (33.37) | 1249 (35.43) | 547 (32.83) | 2355 (34.30) |
| 65–75 years | 331 (19.76) | 903 (25.62) | 515 (30.91) | 1749 (25.47) |
| ≥75 years | 61 (3.64) | 176 (4.99) | 135 (8.10) | 372 (5.42) |
| Family history of type 2 diabetes | ||||
| n=0 | 816 (48.72) | 1715 (48.65) | 759 (45.56) | 3290 (47.92) |
| n=1 | 538 (32.12) | 1115 (31.63) | 560 (33.61) | 2213 (32.23) |
| n=2 | 250 (14.93) | 506 (14.35) | 249 (14.95) | 1005 (14.64) |
| n=3+ | 71 (4.24) | 189 (5.36) | 98 (5.88) | 358 (5.21) |
| Enrolment status | ||||
| GP clinic | 864 (51.58) | 1950 (55.32) | 928 (55.70) | 3742 (54.50) |
| Outpatient clinic | 811 (48.42) | 1575 (44.68) | 738 (44.30) | 3124 (45.50) |
| Weighta, kg | 87 (75–100) | 92 (81–106) | 97 (86.0–111.8) | 92 (80.7–106.0) |
| Weight categorya | ||||
| <80 kg | 452 (34.53) | 695 (24.69) | 218 (16.16) | 1365 (24.94) |
| 80–106 kg | 632 (48.28) | 1421 (50.48) | 696 (51.59) | 2749 (50.23) |
| ≥106 kg | 225 (17.19) | 699 (24.83) | 435 (32.25) | 1359 (24.83) |
| Heighta, cm | 170 (163–176) | 173 (166–179) | 176 (169–182) | 173 (166–180) |
| Height categorya | ||||
| <166 cm | 594 (37.98) | 828 (25.21) | 286 (18.31) | 1708 (26.64) |
| 166–180 cm | 784 (50.13) | 1785 (54.34) | 778 (49.81) | 3347 (52.21) |
| ≥180 cm | 186 (11.89) | 672 (20.46) | 498 (31.88) | 1356 (21.15) |
| BMIa, kg/m2 | 30.02 (26.67–33.98) | 30.64 (27.26–34.97) | 31.42 (28.08–35.50) | 30.76 (27.34–34.89) |
| BMI categorya | ||||
| <25 kg/m2 | 586 (34.99) | 1112 (31.55) | 478 (28.69) | 2176 (31.69) |
| 25–30 kg/m2 | 447 (26.69) | 911 (25.84) | 387 (23.23) | 1745 (25.42) |
| 30–35 kg/m2 | 378 (22.57) | 817 (23.18) | 433 (25.99) | 1628 (23.71) |
| 35–40 kg/m2 | 181 (10.81) | 423 (12.00) | 237 (14.23) | 841 (12.25) |
| ≥40 kg/m2 | 83 (4.96) | 262 (7.43) | 131 (7.86) | 476 (6.93) |
| Waist circumference, cm | 104 (95–114) | 107 (98–117) | 110 (100–120) | 107 (97–117) |
| Waist circumference category | ||||
| <94 cm male/<80 cm female | 202 (12.06) | 336 (9.53) | 117 (7.02) | 655 (9.54) |
| 94–102 cm male/80–88 cm female | 235 (14.03) | 485 (13.76) | 195 (11.70) | 915 (13.33) |
| ≥102 cm male/≥88 cm female | 1238 (73.91) | 2704 (76.71) | 1354 (81.27) | 5296 (77.13) |
| Waist/hip ratio | 0.97 (0.91–1.03) | 0.98 (0.92–1.04) | 1 (0.93–1.05) | 0.98 (0.92–1.04) |
| Waist/hip ratio category | ||||
| <0.92 | 491 (29.33) | 827 (23.54) | 341 (20.51) | 1659 (24.22) |
| 0.92–1.04 | 841 (50.24) | 1787 (50.87) | 868 (52.19) | 3496 (51.04) |
| ≥1.04 | 342 (20.43) | 899 (25.59) | 454 (27.30) | 1695 (24.74) |
| Waist/height ratioa | 0.61 (0.56–0.67) | 0.62 (0.56–0.68) | 0.62 (0.57–0.68) | 0.62 (0.56–0.68) |
| Waist/height ratio categorya | ||||
| <0.5 | 104 (6.64) | 201 (6.13) | 73 (4.67) | 378 (5.90) |
| 0.5–0.6 | 625 (39.91) | 1177 (35.87) | 551 (35.28) | 2353 (36.71) |
| ≥0.6 | 837 (53.45) | 1903 (58.00) | 938 (60.05) | 3678 (57.39) |
| Alcohol consumption | ||||
| ≤21/14 units per week male/female | 1592 (95.04) | 3266 (92.65) | 1557 (93.46) | 6415 (93.43) |
| >21/14 units per week male/female | 83 (4.96) | 259 (7.35) | 109 (6.54) | 451 (6.57) |
| Smoking statusa | ||||
| Never | 557 (48.48) | 1130 (46.08) | 496 (42.03) | 2183 (45.66) |
| Former | 352 (30.64) | 890 (36.30) | 448 (37.97) | 1690 (35.35) |
| Current | 240 (20.89) | 432 (17.62) | 236 (20.00) | 908 (18.99) |
| Physical activity | ||||
| 0 days per week | 239 (14.27) | 534 (15.15) | 245 (14.71) | 1018 (14.83) |
| 1–2 days per week | 351 (20.96) | 683 (19.38) | 329 (19.75) | 1363 (19.85) |
| 3–4 days per week | 390 (23.28) | 793 (22.50) | 389 (23.35) | 1572 (22.90) |
| 5–6 days per week | 254 (15.16) | 547 (15.52) | 222 (13.33) | 1023 (14.90) |
| 7 days per week | 441 (26.33) | 968 (27.46) | 481 (28.87) | 1890 (27.53) |
Data are shown as n (%) or median (IQR)
aContains missing data. The distribution of missing data can be found in ESM Methods: Multivariate imputations by chained equations (MICE) model specification
Age at diagnosis, family history of type 2 diabetes, and body composition
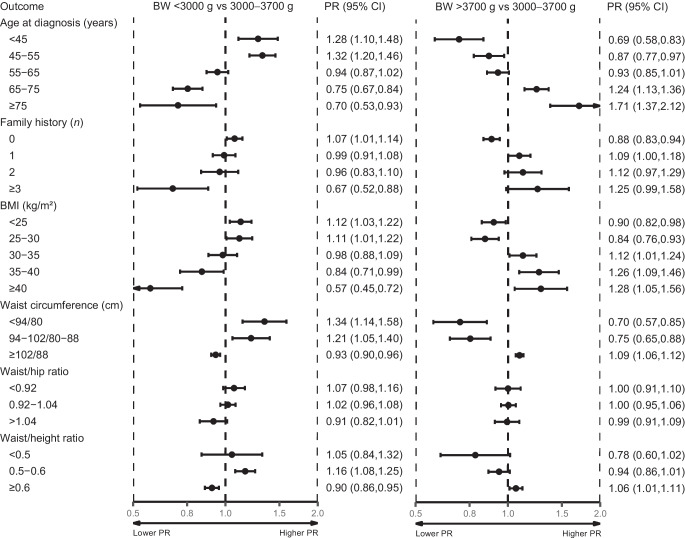
Compared with birthweight 3000–3700 g, birthweight <3000 g was associated with younger age at type 2 diabetes diagnosis (Fig. 2). The PR for age <45 years was 1.28 (95% CI 1.10, 1.48), and the PR for age ≥75 years was 0.70 (95% CI 0.53, 0.93). Birthweight <3000 g was associated with reporting fewer individuals with a family history of type 2 diabetes, with a PR of 1.07 (95% CI 1.01, 1.14) for reporting no type 2 diabetes-affected relatives and a PR of 0.67 (95% CI 0.52, 0.88) for reporting three or more relatives with type 2 diabetes (Fig. 2). Similarly, birthweight <3000 g was associated with a lower BMI, with a PR of 1.12 (95% CI 1.03, 1.22) for BMI <25 kg/m2, decreasing to a PR of 0.57 (95% CI 0.45, 0.72) for presence of severe obesity (≥40 kg/m2). Participants with birthweight <3000 g also had a smaller waist circumference, with a PR of 1.34 (95% CI 1.14, 1.58) for male participants with a waist circumference of <94 cm/female participants with waist circumference <80 cm and a PR for waist/height ratio of 0.90 (95% CI 0.86, 0.95) for a ratio ≥0.6 (Fig. 2).
Fig. 2.
Forest plot of age at type 2 diabetes diagnosis, family history of type 2 diabetes, and body composition. Adjusted PRs for age at diagnosis, anthropomorphic measures (BMI, waist circumference, waist/hip ratio and waist/height ratio), and family history of type 2 diabetes according to birthweight are shown. Age at diagnosis was only adjusted for sex and family history of type 2 diabetes. Family history of type 2 diabetes was adjusted for sex and age at enrolment. Body composition variables were adjusted for sex, age at enrolment and family history of type 2 diabetes. Waist circumference is shown for male/female participants Birthweight categories included a population of 1675 for <3000 g, 3525 for 3000–3700 g and 1666 for >3700 g
Compared with birthweight 3000–3700 g, birthweight >3700 g was associated with older age at type 2 diabetes diagnosis, with a PR for ≥75 years of 1.71 (95% CI 1.37, 2.12) (Fig. 2). Birthweight >3700 g was also associated with more family history of type 2 diabetes (PR for three or more affected relatives 1.25 [95% CI 0.99, 1.58]), higher BMI (PR for ≥ 40 kg/m2 was 1.28 [95% CI 1.05, 1.56]), larger waist circumference (PR for ≥102 cm [male]/88 cm [female] was 1.09 [95% CI 1.06, 1.12]) and higher waist/height ratio (PR for ≥0.6 was 1.06 [95% CI 1.01, 1.11]). No clear associations for birthweight <3000 g or >3700 g were observed for waist/hip ratio. All associations remained robust after further adjustment for BMI, alcohol, smoking status and physical activity (ESM Table 5).
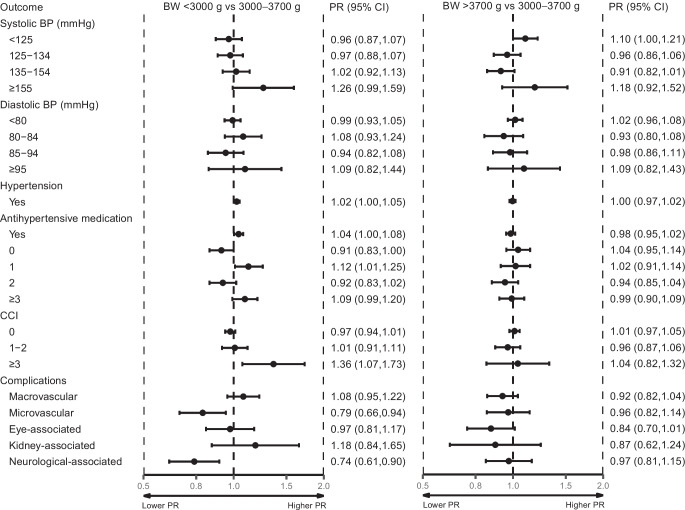
Hypertension and blood lipids
Compared with birthweight 3000–3700 g, birthweight <3000 g was associated with higher systolic BP (SBP) (PR for ≥155 mmHg was 1.26 [95% CI 0.99, 1.59)] and with use of three or more antihypertensive drugs (PR 1.09 [95% CI 0.99, 1.20]) (Fig. 3). After further adjustment for BMI, alcohol, smoking status and physical activity, these associations became stronger (ESM Table 5). Compared with birthweight 3000–3700 g, birthweight >3700 g was associated with lower SBP (PR for <125 mmHg was 1.10 [95% CI 1.00, 1.21]) (Fig. 3) and with lower total cholesterol (PR for <3.70 mmol/l was 1.16 [95% CI 1.03, 1.32]) (ESM Fig. 1). These associations remained after adjusting for BMI, alcohol, smoking and physical activity (ESM Table 5). No associations of birthweight <3000 g or >3700 g were observed for diastolic BP (DBP) (Fig. 3), triglycerides, HDL-cholesterol, LDL-cholesterol or use of lipid-lowering drugs (ESM Fig. 1). Total cholesterol was associated only with high birthweight (ESM Fig. 1, ESM Table 5).
Fig. 3.
Forest plot of BP, comorbidities and diabetes-associated complications. Adjusted PRs for BP variables, comorbidities and diabetes-associated complications according to birthweight are shown. Adjusted for sex, age at enrolment and family history of type 2 diabetes. Birthweight categories included a population of 1675 for <3000 g, 3525 for 3000–3700 g and 1666 for >3700 g
Metabolic variables
Compared with birthweight 3000–3700 g, birthweight <3000 g was associated with a greater use of glucose-lowering drugs (PR for use of three or more medications was 1.33 [95% CI, 1.06, 1.65]) (ESM Fig. 1). No clear associations were observed for measures of glucose homeostasis (blood glucose, HbA1c, C-peptide, HOMA2 insulin sensitivity (IS), HOMA2-B or hsCRP (ESM Fig. 1, ESM Table 5). Compared with birthweight 3000–3700 g, birthweight >3700 g was associated with higher C-peptide levels (PR for ≥1550 pmol/l was 1.14 [95% CI 1.02, 1.27]) and with a higher HOMA2-B (PR for >121 was 1.15 [95% CI 1.03, 1.28]) (ESM Fig. 1). After further adjusting for BMI, alcohol, smoking and physical activity, this association remained for C-peptide but not for HOMA2-Beta (ESM Table 5).
Comorbidities and diabetes complications
Compared with birthweight 3000–3700 g, birthweight <3000 g was associated with a greater burden of comorbidity, assessed by a Charlson Comorbidity Index (CCI) score of ≥3 (PR 1.36 [95% CI 1.07, 1.73]) (Fig. 3). With the qualification that PRs for individual diseases in the CCI would be too imprecise, we found that, in crude numbers, a greater proportion of participants with birthweight <3000 g, compared with birthweight 3000–3700 g, had myocardial infarctions, congestive heart failure, chronic pulmonary disease, and any malignant tumour at enrolment (ESM Table 4). Those with birthweight <3000 g were less likely to have been diagnosed with microvascular complications (PR 0.79 [95% CI 0.66, 0.94]) and specifically diabetes-associated neurological disease (PR 0.74 [95% CI, 0.61, 0.90]). No clear association was found for macrovascular complications or diabetes-associated eye or renal disease (Fig. 3). All associations remained after further adjustment for BMI, alcohol, smoking status and physical activity (ESM Table 5). For birthweight >3700 g compared with birthweight 3000–3700 g, no clear association was found for CCI or diabetes-associated complications (Fig. 3, ESM Table 5).
Linear regression analyses
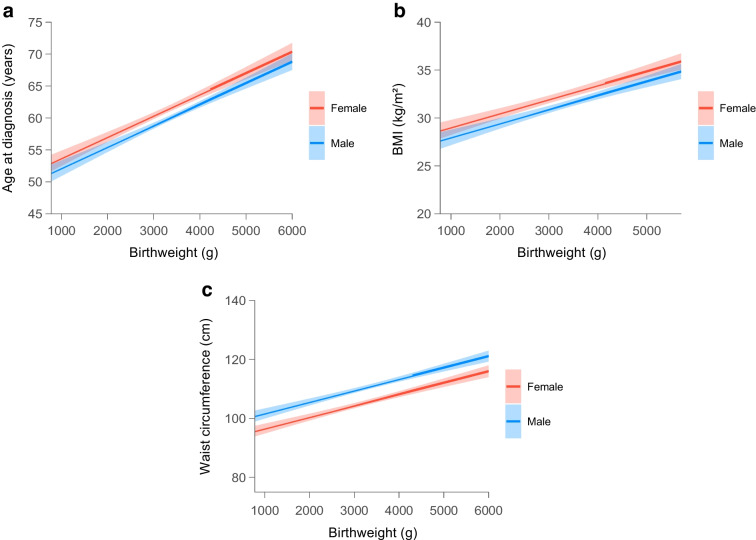
In a linear regression model, each 1000 g decrease in birthweight was associated with a 3.33 year (95% CI 2.86, 3.80) younger age at type 2 diabetes diagnosis, a 1.46 kg/m2 (95% CI 1.19, 1.73) lower BMI and a 3.90 cm (95% CI 3.26, 4.54) smaller waist circumference (Fig. 4a–c and ESM Table 6). These associations became stronger after further adjustment for BMI, alcohol, smoking status and physical activity (ESM Table 6). Finally, in linear regression models, no clear associations were found between birthweight and SBP, DBP, triglycerides, LDL-cholesterol, HDL-cholesterol, HbA1c, C-peptide, hsCRP, HOMA2-IS, or HOMA2-B (ESM Table 6). Sensitivity analyses using two- and three-degree polynomials, and restricted cubic spline regression models, were consistent with the linear regression results (ESM Methods: Exploring linear and non-linear relationship).
Fig. 4.
Linear regression plots of age at diagnosis (a), BMI (b) and waist circumference (c) according to birthweight stratified by sex and adjusted for age at enrolment and family history of type 2 diabetes. Estimate shows change in outcome per g change in birthweight with 95% CIs. Models were performed on the study population of 6866 individuals
Extended analyses
PS
Adjusting for type 2 diabetes and/or birthweight PS in the subpopulation with available genotypes did not attenuate any associations (ESM Table 7). No associations between type 2 diabetes PS and the three birthweight groups were observed, nor when using a linear regression model (ESM Fig. 2, ESM Tables 7 and 8). Type 2 diabetes PS was associated with reduced likelihood of reporting no family history of type 2 diabetes (PR 0.94 [95% CI 0.90, 0.98]), and with increased likelihood of reporting multiple relatives with type 2 diabetes (PR for three or more affected relatives was 1.48 [95% CI 1.24, 1.75]) (ESM Fig. 2).
Case–control analysis of diabetes risk
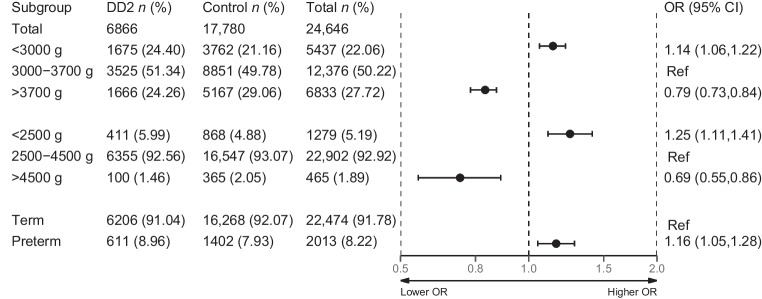
Compared with participants with birthweight 3000–3700 g, those with birthweight <3000 g had a sex- and year-of-birth-adjusted OR of 1.14 (95% CI 1.06, 1.22) of being diagnosed with type 2 diabetes (Fig. 5). Conversely, participants with birthweight >3700 g had an adjusted OR of 0.79 (95% CI 0.73, 0.84) of being diagnosed with type 2 diabetes. Using clinical definitions of low and high birthweight yielded stronger associations with diabetes risk (Fig. 5).
Fig. 5.
Forest plot of case–control analysis of diabetes risk. All DD2 participants included in the study are considered cases. All individuals in the matched control population are considered controls with the assumptions of being alive at enrolment of their matched DD2 participants and being free from type 2 diabetes. Logistic regressions were used to compute ORs for the association of birthweight with a type 2 diabetes diagnosis. Analysis was adjusted for sex and year at birth
Sensitivity analysis
Adjusting the main model further for born-at-term status did not attenuate the associations (ESM Table 9). Re-analysing our data using clinical definitions of low and high birthweight yielded similar or stronger associations compared with our main analyses (ESM Table 10). Further re-analysing our data with sex and born-at-term birthweight categories yielded similar associations (ESM Table 11).
Discussion
Compared with participants with type 2 diabetes falling within the 50% middle range of birthweight, participants with the lowest 25% birthweight were younger at disease onset, had a lower prevalence of obesity, and a greater burden of comorbidity including an SBP ≥155 mmHg. These participants also displayed greater use of glucose-lowering and antihypertensive medications and were less likely to have a diabetes-associated neurological disease at enrolment. Additionally, participants with birthweight <3000 g were less likely to report a family history of type 2 diabetes. Opposite relationships were generally observed for participants with type 2 diabetes who were in the 25% highest compared with lowest birthweight groups. Adjustment for currently known genetic predispositions to type 2 diabetes and birthweight, using PS, did not attenuate the associations. Finally, the case–control analysis reconfirmed the association between low birthweight and type 2 diabetes risk in the current study population.
The extent to which a lower birthweight in type 2 diabetes is associated with a distinct subphenotype has previously only been studied in 1509 individuals born in the limited time between 1952 and 1966 [22]. Our findings, that a lower birthweight is associated with younger age and less obesity at type 2 diabetes onset, are in accordance with Paulina et al’s results [22]. However, because the DD2 cohort is not restricted to any narrow age interval, we were able to document a more than threefold higher estimate of the impact of 1000 g birthweight on age at type 2 diabetes diagnosis, when compared with Paulina et al (i.e. 3 years vs 0.8 years).
Despite younger age at diagnosis, participants with type 2 diabetes with birthweight <3000 g had an increased CCI score of ≥3 compared with those with birthweight 3000–3700 g. Several CCI diseases are known type 2 diabetes comorbidities as well as being diseases associated with low birthweight independent of type 2 diabetes [2, 8, 11, 15, 23–31]. Despite a greater use of antihypertensive medications, a birthweight <3000 g was also associated with an SBP ≥155 mmHg. Besides type 2 diabetes, hypertension is probably the disease most consistently associated with low birthweight [32, 33]. The pathophysiology of type 2 diabetes involves multiple organ dysfunctions [11], and the associations between low birthweight and several diseases [2, 7, 8] besides type 2 diabetes may likewise represent manifestations of aberrant organ development beyond those involved in glucose homeostasis.
A birthweight <3000 g was associated with a lower prevalence of diabetes-associated neurological disease, while it had no impact on the prevalence of diabetes-associated eye or renal disease. However, given their younger age at diagnosis, increased SBP, increased antihypertensive drug usage, and greater use of glucose-lowering therapies for similar glucose levels, participants with type 2 diabetes with lower birthweight (<3000 g) may be at increased risk of both micro- and macrovascular complications with increasing duration of diabetes [34].
Interestingly, a previous study of only 177 individuals with type 2 diabetes reported increased mortality rate among those with both lowest and highest birthweight [27]. While this supports the finding of an increased disease severity among individuals with the lowest birthweights, and to some extent also the reports of U- or J-shaped associations between birthweight and risk of developing type 2 diabetes, it does not support our findings of a relatively milder clinical disease presentation among those individuals with the highest birthweights. However, further data are needed to understand this and both type 2 diabetes and its comorbidities are in general more associated with lower as opposed to higher birthweight.
The fetal insulin hypothesis proposes that the association between low birthweight and type 2 diabetes could be confounded by genetic factors underlying both low birthweight and increased type 2 diabetes risk [8]. However, after adjustment for known genetic predispositions to type 2 diabetes and birthweight using PS, all associations remained unchanged or even strengthened (ESM Table 7). Moreover, the findings that the participants with type 2 diabetes with birthweight <3000 g less frequently reported a positive family history of type 2 diabetes and, conversely, that those with birthweight >3700 g more frequently reported a family history of type 2 diabetes, further support the conclusion that enrichment for genetic variants associated with type 2 diabetes is unlikely to explain the differential characteristics observed. Interestingly, recorded family history of type 2 diabetes was closely associated with type 2 diabetes PS (ESM Fig. 2), providing cross-validation of both measures as accurately reflecting the genetic predisposition to type 2 diabetes.
Strengths of this study include a large well-characterised cohort of individuals recently diagnosed with type 2 diabetes, with more than 50% recruited from Denmark’s primary healthcare sector. Birthweights were ascertained from digitised original midwife records. Adjustments for the currently known genetic predisposition to type 2 diabetes and birthweight (PS), as well as the finding of fewer individuals with a family history of type 2 diabetes among the lower-birthweight participants with type 2 diabetes, do not suggest that the associations are genetically determined. Results obtained using categorical birthweight groups were supported by continuous models.
Limitations include the cross-sectional study design, which has an inherent risk of sampling bias and precludes causal inferences and the exploration of temporal relationships. However, participant inclusion was nationwide and included only those with new-onset type 2 diabetes from both general practice and hospital clinics. Furthermore, birthweight registrations preceded determinations of clinical type 2 diabetes characteristics and our case–control analysis confirmed that low birthweight is associated with type 2 diabetes in this contemporary cohort. This result is noteworthy as the control group included individuals with unknown later type 2 diabetes disease status and (unlike previous studies reporting associations between low birthweight and type 2 diabetes prevalence) included live-born individuals who may have passed away in early childhood due to prematurity and/or low birthweight. Although the association between lower birthweights and younger age at diabetes diagnosis in theory could be explained by increased contact with and use of the healthcare system, this is unlikely to explain the large impact on type 2 diabetes onset age of 3.33 years per 1000 g birthweight. There were missing data for some covariates. However, characteristics with clear associations were missing relatively little data. Due to relevant treatment having already been started at enrolment, many participants had near normalised BP, lipids and/or glucose levels. Specifically, the increased use of glucose-lowering medication may have masked an association between low birthweight and elevated plasma glucose or HbA1c levels. However, increased medication use in its own right reflects disease severity. The finding that individuals with birthweight <3000 g were more likely to have an SBP ≥155 mmHg in the face of increased use of antihypertensive drugs underscores the strength of the association between low birthweight and hypertension burden in those with recently diagnosed type 2 diabetes. Although we did not have information on ethnicity, all patients were born in Denmark, and our study population is therefore likely to be relatively homogeneous. Further studies in other ethnicities are needed to validate our findings. Finally, we lacked information on education, income and potential maternal/paternal factors related to birthweight and metabolic health.
Conclusion
Birthweight across the entire spectrum was associated with distinct and clinically relevant type 2 diabetes characteristics. Specifically, a birthweight <3000 g was associated with younger age at diagnosis, lower prevalence of obesity, fewer individuals with a family history of type 2 diabetes, and greater use of glucose-lowering medications, as well as a larger burden of comorbidity including hypertension, in individuals recently diagnosed with type 2 diabetes. Further prospective studies are needed to elucidate the impact of birthweight on disease trajectories, comorbidities, complications and mortality in individuals with type 2 diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- CCI
Charlson comorbidity index score
- DaNaAr
Danish National Archive
- DD2
Danish Centre for Strategic Research in Type 2 Diabetes
- DBP
Diastolic BP
- GP
General practitioner
- hsCRP
High-sensitivity C-reactive protein
- IS
Insulin sensitivity
- MICE
Multivariate imputations using chained equations
- PR
Prevalence ratio
- PS
Polygenic score
- SBP
Systolic BP
Acknowledgements
The authors are grateful to all participants in the DD2 study and to the healthcare personnel who recruited the participants at the general practices and outpatient clinics. The authors sincerely thank the DD2 management and the DD2 data managers. An abstract version of this study was presented at the EASD 2022 annual meeting in Stockholm, 22 September 2022.
Data availability
Danish data protection legislation does not allow sharing of the individual-level personal data used for this study. However, a data dictionary for variables used in the study and analysis code is in preparation and will be shared and made publicly available on the DD2 website, www.dd2.dk (expected in late 2023). Requests to access the Danish health registries used in this study can be sent from researchers at authorised research institutions to the Danish Health Data Authority by e-mail to forskerservice@sundhedsdata.dk. Requests to use the primary collected DD2 data can be made at https://dd2.dk/forskning/ansoeg-om-data.
Funding
Open access funding provided by Lund University. The DD2 study was supported by the Danish Agency for Science (grant no. 09–067009 and 09–075724), the Danish Health and Medicines Authority, the Danish Diabetes Association, the Region of Southern Denmark, and the Novo Nordisk Foundation (grant no. NNF17SA0030962–2, NNF20O0063292 and NNF17SA0030364). Project partners are listed on the www.DD2.nu website. The study funders were not involved in the design of the study, the collection, analysis and interpretation of data, or writing the report. They did not impose any restrictions regarding publication of the report. The Department of Clinical Epidemiology, Aarhus University, receives funding for other studies in the form of institutional research grants to (and administered by) Aarhus University. None of these studies has any relation to the present study.
Authors’ relationships and activities
CB owns stock in Novo Nordisk. MHO has received payment or honoraria for lectures, presentations or educational events from AstraZeneca and Boehringer Ingelheim, and has unpaid positions as chairperson of the Danish Hypertension Society and a Nucleus member of the Working Group for Prevention and Rehabilitation, Danish Society of Cardiology. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
AAV conceived and designed the study and is the guarantor of the study. JSN was the principal manager of the Danish Centre for Strategic Research in Type 2 Diabetes (DD2). ALH performed the statistical analysis, prepared the first draft of the manuscript, and revised the draft. All authors contributed to the interpretation of data and critically revised the content of the draft. AAV, RWT and HTS supervised the study. All authors read and approved the manuscript, and gave final approval of the version to be published. As such, they had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aleksander L. Hansen, Email: aleksander.hansen@regionh.dk
Allan A. Vaag, Email: allan.vaag@med.lu.se
References
- 1.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knop MR, Geng TT, Gorny AW, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7(23):e008870. doi: 10.1161/jaha.118.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 4.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130(4 Pt 1):278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 6.Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm Metab Res. 2014;46(13):911–920. doi: 10.1055/s-0034-1395561. [DOI] [PubMed] [Google Scholar]
- 7.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease. Hypertension. 2006;47(3):502–508. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62–67. doi: 10.1007/bf00399095. [DOI] [PubMed] [Google Scholar]
- 9.Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol. 2008;32(3):213–218. doi: 10.1053/j.semperi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71(5 Suppl):1344s–1352s. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 11.Vaag AA, Grunnet LG, Arora GP, Brøns C. The thrifty phenotype hypothesis revisited. Diabetologia. 2012;55(8):2085–2088. doi: 10.1007/s00125-012-2589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen DH, Nicolaisen SK, Berencsi K, et al. Danish centre for strategic research in type 2 diabetes (DD2) project cohort of newly diagnosed patients with type 2 diabetes: a cohort profile. BMJ Open. 2018;8(4):e017273. doi: 10.1136/bmjopen-2017-017273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gedebjerg A, Bjerre M, Kjaergaard AD, et al. Mannose-binding lectin and risk of cardiovascular events and mortality in type 2 diabetes: a Danish cohort study. Diabetes Care. 2020;43(9):2190–2198. doi: 10.2337/dc20-0345. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen P, Vaag AA, Kyvik KO, Møller Jensen D, Beck-Nielsen H. Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia. 1997;40(4):439–446. doi: 10.1007/s001250050698. [DOI] [PubMed] [Google Scholar]
- 15.Mellemkjaer L, Olsen ML, Sørensen HT, Thulstrup AM, Olsen J, Olsen JH. Birth weight and risk of early-onset breast cancer (Denmark) Cancer Causes Control. 2003;14(1):61–64. doi: 10.1023/a:1022570305704. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . ICD-10: international statistical classification of diseases and related health problems / World Health Organization: tenth revision. 2. Geneva: World Health Organization; 2004. [Google Scholar]
- 17.Talbot D, Mésidor M, Chiu Y, Simard M, Sirois C. An alternative perspective on the robust poisson method for estimating risk or prevalence ratios. Epidemiology. 2023;34(1):1–7. doi: 10.1097/ede.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 18.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 19.Khera AV, Chaffin M, Aragam KG, et al. Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat Genet. 2018;50(9):1219–1224. doi: 10.1038/s41588-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Privé F, Aschard H, Carmi S, et al. Portability of 245 polygenic scores when derived from the UK Biobank and applied to 9 ancestry groups from the same cohort. Am J Hum Genet. 2022;109(1):12–23. doi: 10.1016/j.ajhg.2021.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Development Core Team (2022) R: a language and environment for statistical computing. In. R Foundation for statistical computing, Vienna, Austria
- 22.Paulina C, Donnelly LA, Pearson ER. The impact of birthweight on subsequent phenotype of type 2 diabetes in later life. Diabetic Med. 2022;39(7):e14792. doi: 10.1111/dme.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Rooij SR, Painter RC, Phillips DI, et al. Hypothalamic-pituitary-adrenal axis activity in adults who were prenatally exposed to the Dutch famine. Eur J Endocrinol. 2006;155(1):153–160. doi: 10.1530/eje.1.02193. [DOI] [PubMed] [Google Scholar]
- 26.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Leibson CL, Burke JP, Ransom JE, et al. Relative risk of mortality associated with diabetes as a function of birth weight. Diabetes Care. 2005;28(12):2839–2843. doi: 10.2337/diacare.28.12.2839. [DOI] [PubMed] [Google Scholar]
- 28.Eriksson JG, Salonen MK, Kajantie E, Osmond C. Prenatal growth and CKD in older adults: longitudinal findings from the Helsinki birth cohort study, 1924–1944. Am J Kidney Dis. 2018;71(1):20–26. doi: 10.1053/j.ajkd.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 29.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427(3):333–347. doi: 10.1042/bj20091861. [DOI] [PubMed] [Google Scholar]
- 31.Brøns C, Thuesen ACB, Elingaard-Larsen LO, et al. Increased liver fat associates with severe metabolic perturbations in low birth weight men. Eur J Endocrinol. 2022;186(5):511–521. doi: 10.1530/eje-21-1221. [DOI] [PubMed] [Google Scholar]
- 32.Mu M, Wang SF, Sheng J, et al. Birth weight and subsequent blood pressure: a meta-analysis. Arch Cardiovasc Dis. 2012;105(2):99–113. doi: 10.1016/j.acvd.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996;94(12):3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- 34.Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–2474. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Danish data protection legislation does not allow sharing of the individual-level personal data used for this study. However, a data dictionary for variables used in the study and analysis code is in preparation and will be shared and made publicly available on the DD2 website, www.dd2.dk (expected in late 2023). Requests to access the Danish health registries used in this study can be sent from researchers at authorised research institutions to the Danish Health Data Authority by e-mail to forskerservice@sundhedsdata.dk. Requests to use the primary collected DD2 data can be made at https://dd2.dk/forskning/ansoeg-om-data.