Abstract
Introduction
The residual post-COVID maxillary mucormycosis defect (PCMMD) were extensive, due to unilateral or bilateral maxillectomies. The Goal of rehabilitation of PCMMD is to deliver a prosthetically driven reconstruction. FEA was to evaluate the biomechanical response of PSI struts (PSI 1), PSI Screw retained (PSI 2) and QZI to masticatory load on virtual simulation to improve accuracy and enhance the design.
Aim
To validate and compare the Biomechanical benefit of the PSI struts, PSI Screw retained, QZI in a case of rehabilitation of post-COVID maxillary mucormycosis defect (PCMMD) by FEA study.
Methodology
The result of stress to masticatory load on virtual simulation for (1) Maximum and minimum stress (Von Mises stress); (2) the Displacement (in three positions) and (3) the Deformation (Plastic strain) was compared on virtual simulation for PSI 1 and PSI 2 and QZI.
Conclusion
The FEA and comparative evaluation of PSI 1, PSI 2 and QZI showed a good resistance to displacement. The stress and strain values are low and acceptable. In comparison QZI shows more stress in the anterior region.
Keywords: Patient-specific implant, Maxillary reconstruction, Structural optimization, Biomechanical analysis, Finite element analysis, Additive manufacturing
Introduction
COVID-19 pandemic created a unique challenge to the mankind. The superinfection of mucor fungus was one such healthcare challenge. The treating surgeons worked hard to fight the epidemic of mucormycosis. As maxillofacial surgeon we treated cases of mucormycosis of jaws. Maxillary mucormycosis was much more prevalent and was treated by curettage and osteotomies for removal of affected bone of maxilla. The residual post-COVID maxillary mucormycosis defect (PCMMD) are extensive, unilateral/bilateral, almost complete maxilla (subtotal) is missing in few cases. The Goal of rehabilitation of PCMMD is to deliver a prosthetically Driven reconstruction. This is to restore facial contour and masticatory function. The traditional Maxillofacial prosthesis (Obturator) has been used previously [1]. There are challenges in using obturators for PCMMD.Customised Patient specific implants (PSI) prepared by virtual planning and CAD- CAM printing technology is recently used in literature for cases of maxillectomies [2]. In cases of bilateral maxillectomies, for lack of bony support quad zygomatic implants are used [3–5].
Thus, PSI and Quad Zygoma Implants (QZI) are options for the support of prosthesis in the severe maxillary defects of PCMMD. There is a need to study the biomechanical properties of PSI and QZI as its response to functional load of chewing. Finite element analysis has a role in preliminary evaluation of this biomechanical performance of these implants [6].
We designed two types of PSI depending on the design of dental implants for future prosthesis support: PSI with the struts (PSI 1) and PSI with Screw retained implants embedded in PSI (PSI 2). The purpose of the present FEA study was to Validate and compare the Biomechanical benefit of the PSI struts, PSI Screw retained, QZI in a case of rehabilitation of post-COVID maxillary mucormycosis defect (PCMMD) by FEA study.
The unique condition of PCMMD can be treated with similar principles of PSI or QZI. PSI was designed as wings, connectors and attachments either struts or screw retained implants. The ability of these implants to sustain the masticatory forces transmitted during function can be studied on virtual simulation.
Methodology
Finite element analysis (FEA) was used with objectives—To compare response of PSI struts, PSI Screw retained and QZI to masticatory load on virtual simulation for
Maximum and minimum stress (Von Mises stress)
the Displacement (in three positions)
the Deformation (Plastic strain)
FEA was to evaluate the biomechanical response of PSI struts, PSI Screw retained and QZI to masticatory load on virtual simulation to improve accuracy and enhance the design.
The letter from IEC (IEC/06/03 Dated 26/09/2022) received for exemption from Ethical review. Selection of case of PCMMD of Bilateral defect in maxilla. This patient male, 62 year old gave history of COVID infection in March 2021. He was hospitalized for fifteen days and was on oxygen support. Two months after this he complained of mobility of teeth with pus discharge in upper jaw. He was diagnosed with Maxillary mucormycosis in June 2021. The extensive ablation of bilateral maxillary sinus debridement and maxillectomy for removal of necrosed maxillary bone was performed. High-resolution multi-slice CT scan was performed on the head and neck region by CT scanner. The voxel size was non-isotropic, with a width and height of 0.75 mm and a slice thickness of 1 mm.
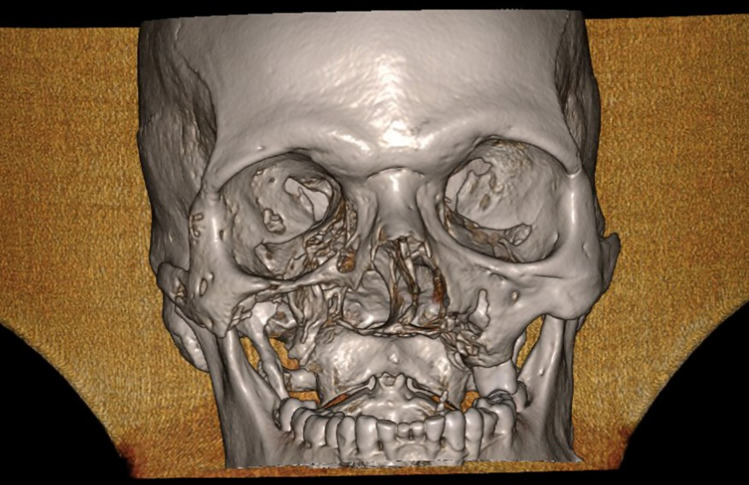
CT DICOM file was sent to Biomechanical engineer. All data in Digital Imaging and Communications in Medicine (DICOM) format were imported. Figure 1 is the CT image showing Bilateral defect of maxilla, loss of alveolar bone, palate, maxillary sinus walls, floor, nasal floor, part of lateral nasal walls. There was presence of complete, zygomatic bone, infraorbital rims, floor of orbit and nasal bone.
Fig. 1.
3-D CT image of a case for FEA
The CT image was converted into STL file format using DICOM to print format. This format was used afterwards using Geomagics free format. A 3-dimentonal reconstruction of images was produced with a surface triangulation technique. For this purpose, a redefinition of edges and surfaces from scanned images was conducted in order to build a simplified model covering most of volume of original geometry.
Throughout the process special care was taken to keep the surfaces of the implant in contact with the bone as faithful as possible to original model. Designing of the implant done by design tool present in the Geomagic free form software.
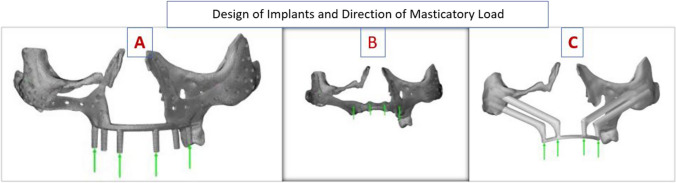
PSI implants were prepared by additive manufacturing with mirror finish outside and mat finish inside done by grinder by aluminum beat blasting. PSI was prepared from medical grade titanium TiAl6V4 with yield strength 116 MPa; Ultimate strength 1286 MPa and Fatigue strength 410 MPa. Thickness was 1.8 mm. PSI designed was single piece for bilateral defect. The parts were zygomatic wings, nasal wings connector bars and six points for dental attachments. Here in PSI struts(PSI 1) design six struts were incorporated in design (Fig. 2A) and in another design the screw retained dental Implants (ADIN Dental Implants) (PSI 2) were incorporated (Fig. 2B). The simulation of PSI 1 and 2 were used for FEA. The splinted four Zygomatic implants (Quad Zygoma Implant QZI) of Nobel BioCare were used for simulation (Fig. 2C). Virtual simulation and FEA performed on Dassault Simula software.
Fig. 2.
Design of implants and direction of masticatory force for simulation. A PSI 1; B PSI 2; C QZI
The vertical force of 150 N on anterior component and 300 N posterior component were applied simulating load transfer from the prosthesis. It was considered that the force was uniformly distributed through the connector to the zygomatic component of the implants. The response was recorded as per the software settings for the maximum stress on the underlying remaining bone and on respective implants along with displacement and deformation.
FEA was done in four steps.
Import STL—in digitalized shape to surface software
Mesh of implant and Model is checked by the automation tool in the software
Application of loads, definition of material, tetrahedron mesh, check is done through structural model creation software
Mechanical scenarios—Static loading was done on implant in structural analysis
Then simulation is performed and results are given
in case of error, design and structure is slightly modified to dissolve error and obtain final FEA results.
Results
The data were collected as received from software. Table 1 shows the stress value in newton per meter square (N/m2) in PSI 1, PSI 2 and QZI for Von mises stress, Displacement at three components 1.1, 2.1 and 3.1.
Table 1.
Comparison of Von Mises stress and Displacement of PSI 1, PSI2 and QZI
| Sr no | Sensor name | PSI 1 | PSI 2 | QZI | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Min | Max | Min | Max | ||
| 1. | Von Mises stress overall (N_m2) | 1.37 | 1.63 | 2.28 | 1.92 | 4.35 | 1.92 |
| 2. | Von Mises stress on implant (N/m2) | 1.37 | 1.63 | 2.28 | 1.92 | 4.35 | 1.92 |
| 3. | Von Mises stress on bone (N/m2) | 0 | 1.37 | 0 | 1.35 | 0 | 2.28 |
| 4. | Displacement magnitude (mm) | 0 | 0.89 | 0 | 0.12 | 0 | 0.12 |
| 5. | Displacement component 1.1 (mm) | − 0.03 | 0.03 | − 0.02 | 0.04 | − 0.02 | 0.04 |
| 6. | Displacement component 2.1 (mm) | − 0.07 | 0.03 | − 0.001 | 0.06 | − 0.001 | 0.06 |
| 7. | Displacement component 3.1 (mm) | − 8.55 | 0.08 | − 0.001 | 0.10 | − 0.001 | 0.10 |
| 8. | Reaction force (N) | 0 | 24 | 0 | 193 | 0 | 193 |
PSI 1, patient specific implant with struts; PSI 2, patient specific implant with screw retained implants; QZI, quad zygomatic implant
The overall minimum Von mises stress developed were 1.37 (PSI 1); 2.28 (PSI 2) and 4.35 (QZI). The overall maximum Von mises stress developed were 1.63 (PSI 1); 1.92 (PSI 2) and 1.92 (QZI). The minimum Von mises stress developed on Implant were 1.37 (PSI 1); 2.28 (PSI 2) and 4.35 (QZI). The maximum Von mises stress developed on implant were 1.63 (PSI 1); 1.92 (PSI 2) and 1.92 (QZI). Whereas zero minimum Von mises stress developed on surrounding bone and the maximum Von mises stress developed were 1.37 (PSI 1); 1.35 (PSI 2) and 2.28 (QZI). The simulation of displacement magnitude (mm) for the anterior component 1.1, minimum was − 0.03(PSI 1); − 0.02 (PSI 2 and QZI), whereas maximum was 0.03(PSI 1); 0.04 (PSI 2 and QZI). The observation value of Displacement at middle component 2.1, minimum was − 0.07(PSI 1); − 0.001(PSI 2 and QZI), whereas maximum was 0.03(PSI 1); 0.06 (PSI 2 and QZI). The observation value of Displacement at posterior component 3.1, minimum was − 8.55(PSI 1); − 0.001(PSI 2 and QZI), whereas maximum was 0.08(PSI 1); 0.10 (PSI 2 and QZI).
Discussion
FEA gives biomechanical insight into structural behavior of the particular designs by simulation. This virtual simulation provides a structural analysis of how a particular product or design would react under stress in real world.
PCMMD was a three-dimensional defect in midface. The defects were extensive and rarely follow any classification pattern used as a basis for reconstruction of the maxillary defects. The PCMMD was due to subtotal maxillectomy. Autogenous grafts cannot replicate these anatomical structures. PSI has been recently used for the maxillary defects. These were prepared by additive technology for the customized 3D titanium PSI [2, 7–9]. Quad zygoma implant supported prosthesis has been used for the defect of maxilla. It was considered as safe, predictable, and cost-effective treatment modality [3–5, 10].
The references cited above for use of PSI and QZI for support of maxillofacial prosthesis are case reports or series with successful results.
The FEA studies for use of two zygomatic implants along with anterior dental implants had been shown to have maximum stress distribution to zygoma and less to anterior implants [11–13]. The thickness and cortication of zygomatic bone is sufficient to provide the anchorage and support to bear the masticatory load [11].
The clinical situation of PCMMD was result of subtotal maxillectomy defect. It was extensive and there was no anterior support. The use of four (Quad) zygoma increased the bony anchorage and distribution of stress. However, absence of anterior implants, and support of lateral surface of maxilla increased the unsupported length of zygomatic implant. This warrants to study the stress distribution by FEA study.
The Biomechanical properties of the Indigenously designed PSI and the zygomatic implants available was performed. We considered need to study the biomechanical properties of these implants. The normal biting forces in the range of 150–300 N were the forces applied on the implants. The site of force application was at the attachment of prosthesis to attachment bars.
In an article on maxillofacial prosthodontics of mucormycosis defects, use of obturators as maxillofacial prosthesis was prevalent. The need to use of remote implant support by use of anchorage from pterygoid, zygomatic bone for improvement in prosthesis retention and support. The need of reduction of weight of Maxillofacial prosthesis was discussed. The reduction of weight of prosthesis was critically important when the prosthesis is suspended without much remnant bone and tooth support [1, 14].
The PSI or QZI supported the prosthesis. These will transfer a lot of forces on the struts, connectors and bars. The present study has studied the biomechanical properties of these implants on virtual simulation. FEA bears a principal role in virtual evaluation of the designed PSI and QZI for PCMMD. The stress, strain and displacement distribution were simulated for occlusal loading. The FEA would give preliminary validation of structural analysis. This had been used for validation of most promising designs [6, 15, 16].
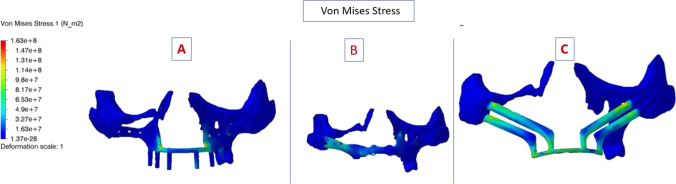
Table 1 shows all the values of interpretation. The maximum Von mises stress on implant for PSI 1, for PSI 2 and QZI was 1.63, 1.92 and 1.92 N/m2, respectively. Value of maximum Von Mises stress on Implant was higher in QZI followed by PSI 2and PSI 1. The maximum Von Mises stress on bone for PSI 1, PSI 2 and QZI was 1.37, 1.34, and 2.28 N/m2, respectively. This was higher in QZI and almost equal in PSI1 and PSI 2. Figure 3 shows the Von Mises stress in colour coding in three implants. The higher stress is seen on the zygomatic implant and its splinted bar. The highest von Mises stresses are shown in red, while the lowest are shown in dark blue. There are more blue areas on PSI 1 and PSI 2.
Fig. 3.
Von Mises stress image showing the deformation scale in colour coding the minimum is dark blue. A PSI 1; B PSI 2; C QZI
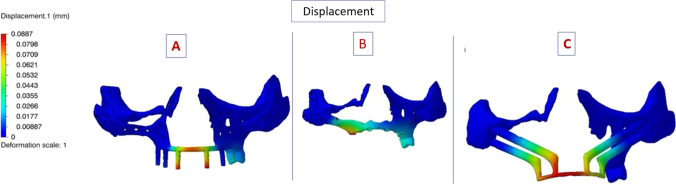
The minimum displacement magnitude of all was zero. The maximum displacement magnitude of PSI 1 was 0.89 mm, that of PSI 2 and QZI was 0.12 mm. PSI 1 displacement value was higher than PSI 2 and QZI, which are equal. The displacement magnitude at the individual component minimum is in negative value and maximum value were similar in anterior and posterior component of three implants. For anterior component (1.1) the displacement value of PSI 1 was 0.03 mm and for PSI 2 as well as QZI was 0.04 mm. For component 2.1 the displacement value of PSI 1 was 0.03 mm and for PSI 2 as well as QZI was 0.06 mm. For component 3.1 the displacement value of PSI 1 was 0.08 mm and for PSI 2 as well as QZI was 0.10 mm. Figure 4 shows that the displacement (red colour) was in anterior region.
Fig. 4.
Displacement seen on deformation scale with colour coding, the minimum—dark blue and maximum—red. A PSI 1; B PSI 2; C QZI
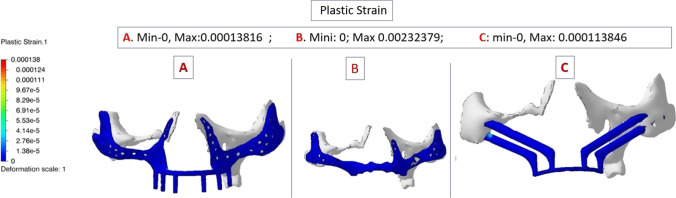
Plastic strain of deformation is shown in Fig. 5, it was equal for all three implants. The lower values are in dark Blue indicate low values of plastic strain.
Fig. 5.
Plastic strain seen on deformation scale with colour coding,the minimum—dark blue. A PSI 1; B PSI 2; C QZI
All three implants PSI 1, PSI 2 and QZI show minimum displacement, good resistance to displacement and deformation. However, among the three the QZI shows more stress in the medial implant and connecting bars.
In a FEA study of a PSI design on the severely atrophic maxilla by Mommaerts MY, this displacement constraints were chosen to be a maximum vertical displacement of 0.1 mm. They accepted this after the experimentation of FEA on bone [17].
The displacement values of the present FEA study of all three implants at component 1.1 and 2.1 were below 0.1. Only values at component 3.1 were 0.1 for PSI 2 and QZI. Thus, the displacement values are acceptable for all three designs.
The total maximum masticatory force of around 150 N in upright direction for the dental implant and masticatory force studies were assumed by Kaman et al., Demeko et al., and Liu et al. [18–20].
The vertical masticatory force value of 300N was used by other researchers (Saini, Miyamoto) [21, 22]
In the present study vertical load of 150 N in anterior and 300 N in posterior loading points estimated the reaction force of 24 N in PSI 1 and 195 N each in PSI 2 as well as QZI (Table 1). These forces can show the estimated response of the implants to the average daily use of the prosthesis.
The 3D finite element analysis is a virtual stress analysis widely used for studying the stress and strain biomechanical effects. The new designs of PSI 1 and PSI 2 would be fixed to the zygoma with micro-screws through individual titanium network. The six dental implant like connectors would support the future prosthesis. In case of QZI, the connecting bar produces splinting of the four implants inserted in zygoma. The future abutments connected to these four zygoma implants for virtual simulation. These abutments would support the future prosthesis. These three designs were biocompatible structure for future maxillofacial prosthesis. The prosthesis would support the lips, cheeks and will give function of chewing. This is a graft-less solution, possibility of immediate provisional prosthesis. A huge defect of PCMMD can be restored with the maxillofacial prosthesis with lighter weight and better retention using PSI or QZI. The optimum satisfaction of patient and quality of life improvement are expected after fulfilling the biomechanical requirement of PSI and QZI [23].
Limitation of the study: The CT image is used for simulation had only the bone tissue. The soft tissue component could not be studied. FEA was studied on models and not on Real structures. In FEA study all the results of stress, strain or displacement are approximated cannot obtain the difference between the obtained results and the real one. The statistical tests cannot be applied on the data obtained.
Conclusion
The FEA and comparative evaluation of PSI 1, PSI 2 and QZI showed a good resistance to displacement. The stress and strain values are low and acceptable. In comparison QZI shows more stress in the anterior region.
Abbreviations
- PSI
Patient specific implants
- QZI
Quad zygoma implant
- PCMMD
Post-COVID maxillary mucormycosis defect
- FEA
Finite element analysis
Declarations
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Varsha Sunil Manekar, Email: varsha_manekar@yahoo.co.in.
Abhay N. Datarkar, Email: abhaydatarkar@yahoo.com
Ashlesha Ghormode, Email: ashlesha.ghormade@gmail.com.
Surendra Daware, Email: surendra.daware@gmail.com.
Prashant Pandilwar, Email: prashant.pandilwar@rediffmail.com.
Pranav Sapkal, Email: pranav@lucidimplants.com.
References
- 1.Ali IE, Chugh A, Cheewin T, Hattori M, Sumita YI. The rising challenge of mucormycosis for maxillofacial prosthodontists in the covid-19 pandemic: a literature review. J Prosthodont Res. 2022 doi: 10.2186/jpr.JPR_D_21_00264. [DOI] [PubMed] [Google Scholar]
- 2.Rattan V, Rai S, Jolly SS, Meena VK. Maxillofacial reconstruction with Patient-specific Implants. J Postgrad Med Educ Res. 2019;53(1):34–37. doi: 10.5005/jp-journals-10028-1309. [DOI] [Google Scholar]
- 3.Soltanzadeh P, Su JM, Habibabadi SR, Kattadiyil MT. Obturator fabrication incorporating computer-aided design and 3-dimensional printing technology: a clinical report. J Prosthet Dent. 2019;121(4):694–697. doi: 10.1016/j.prosdent.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 4.Vega LG, Gielincki W, Fernandes RP. Zygoma implants reconstruction of acquired maxillary bony defects. Oral Maxillofac Surg Clin North Am. 2013;25(2):223–239. doi: 10.1016/j.coms.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Rathee M, Singh S, Malik S, Divakar S, Alam M. Reconstruction and rehabilitation of maxillary defects secondary to mucormycosis. Saudi J Oral Dent Res. 2022;7(1):1–7. doi: 10.36348/sjodr.2022.v07i01.001. [DOI] [Google Scholar]
- 6.Zhong S, Shi Q, Van Dessel J, Gu Y, Sun Y, Yang S, Politis C. Biomechanical validation of structural optimized patient-specific mandibular reconstruction plate orienting additive manufacturing. Comput Methods Programs Biomed. 2022;1(224):107023. doi: 10.1016/j.cmpb.2022.107023. [DOI] [PubMed] [Google Scholar]
- 7.Rotaru H, Schumacher R, Kim SG, Dinu C. Selective laser melted titanium implants: a new technique for the reconstruction of extensive zygomatic complex defects. Maxillofac Plast Recon Surg. 2015;37:1–4. doi: 10.1186/s40902-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melville JC, Manis CS, Shum JW, Alsuwied D. Single-unit 3D-printed titanium reconstruction plate for maxillary reconstruction: the evolution of surgical reconstruction for maxillary defects—a case report and review of current techniques. J Oral Maxillofac Surg. 2019;77(4):874–e1. doi: 10.1016/j.joms.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Glas HH, Vosselman N, de Visscher SA. The use of 3D virtual surgical planning and computer aided design in reconstruction of maxillary surgical defects. Curr Opin Otolaryngol Head Neck Surg. 2020;28(2):122–8. doi: 10.1097/MOO.0000000000000618. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt BL, Pogrel MA, Young CW, Sharma A. Reconstruction of extensive maxillary defects using zygomaticus implants. J Oral Maxillofac Surg. 2004;62:82–89. doi: 10.1016/j.joms.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Romeed SA, Malik R, Dunne SM. Zygomatic implants: the impact of zygoma bone support on biomechanics. J Oral Implantol. 2014;40(3):231–7. doi: 10.1563/AAID-JOI-D-11-00245. [DOI] [PubMed] [Google Scholar]
- 12.Korkmaz FM, Korkmaz YT, Yaluğ S, Korkmaz T. Impact of dental and zygomatic implants on stress distribution in maxillary defects: a 3-dimensional finite element analysis study. J Oral Implantol. 2012;38(5):557–67. doi: 10.1563/AAID-JOI-D-10-00111. [DOI] [PubMed] [Google Scholar]
- 13.Wen H, Guo W, Liang R, Xiang L, Long G, Wang T, Deng M, Tian W. Finite element analysis of three zygomatic implant techniques for the severely atrophic edentulous maxilla. J Prosthet Dent. 2014;111(3):203–15. doi: 10.1016/j.prosdent.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Gaur V, Patel K, Palka L. An implant-supported prosthetic rehabilitation of a patient with a bilateral subtotal maxillectomy defect secondary to rhino-orbital-cerebral mucormycosis: a clinical report of a graftless approach. J Prosthet Dent. 2021 doi: 10.1016/j.prosdent.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Freytag M, Shapiro V, Tsukanov I. Finite element analysis in situ. Finite Elem Anal Des. 2011;47(9):957–972. doi: 10.1016/j.finel.2011.03.001. [DOI] [Google Scholar]
- 16.Biegler M, Marko A, Graf B, Rethmeier M. Finite element analysis of in-situ distortion and bulging for an arbitrarily curved additive manufacturing directed energy deposition geometry. Addit Manuf. 2018;24:264–272. [Google Scholar]
- 17.Mommaerts MY. Evolutionary steps in the design and biofunctionalization of the additively manufactured sub-periosteal jaw implant ‘AMSJI’ for the maxilla. Int J Oral Maxillofac Surg. 2018 doi: 10.1016/j.ijom.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Kaman S, et al. Stress analysis of zygomatic implants on the augmented maxillary sinus: is it necessary to graft? Implant Dent. 2017;26:1–8. doi: 10.1097/ID.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 19.Demenko V, Linetskiy I, Linetska L, Yefremov O. Load-carrying capacity of short implants in edentulous posterior maxilla: a finite element study. Med Eng Phys. 2019;71:30–37. doi: 10.1016/j.medengphy.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, et al. Effects of different positions and angles of implants in maxillary edentulous jaw on surrounding bone stress under dynamic loading: a three-dimensional finite element analysis. Comput Math Methods Med. 2019;23:56. doi: 10.1155/2019/8074096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saini H, Ackland DC, Gong L, Cheng LK, Röhrle O. Occlusal load modelling significantly impacts the predicted tooth stress response during biting: a simulation study. Comput Methods Biomech Biomed Eng. 2020;23(7):261–270. doi: 10.1080/10255842.2020.1711886. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto S, Ujigawa K, Kizu Y, Tonogi M, Yamane GY. Biomechanical threedimensional finite-element analysis of maxillary prostheses with implants. Design of number and position of implants for maxillary prostheses after hemimaxillectomy. Int J Oral Maxillofac Surg. 2010;39:1120–1126. doi: 10.1016/j.ijom.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Carnicero A, Peláez A, Restoy-Lozano A, Jacquott I, Perera R. Improvement of an additively manufactured subperiosteal implant structure design by finite elements based topological optimization. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-94980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]