Abstract
Background
Research has shown that ingesting 0.3 g·kg−1 body mass sodium bicarbonate (NaHCO3) can improve time-to-exhaustion (TTE) cycling performance, but the influence of psychophysiological mechanisms on ergogenic effects is not yet understood.
Objective
This study retrospectively examined whether changes in TTE cycling performance are mediated by positive expectations of receiving NaHCO3 and/or the decline in blood bicarbonate during exercise.
Methods
In a randomised, crossover, counterbalanced, double-blind, placebo-controlled design, 12 recreationally trained cyclists (maximal oxygen consumption, 54.4 ± 5.7 mL·kg·min−1) performed four TTE cycling tests 90 min after consuming: (1) 0.3 g·kg−1 body mass NaHCO3 in 5 mL·kg−1 body mass solution, (2) 0.03 g·kg−1 body mass sodium chloride in solution (placebo), (3) 0.3 g·kg−1 body mass NaHCO3 in capsules and (4) cornflour in capsules (placebo). Prior to exercise, participants rated on 1–5 Likert type scales how much they expected the treatment they believe had been given would improve performance. Capillary blood samples were measured for acid-base balance at baseline, pre-exercise and post-exercise.
Results
Administering NaHCO3 in solution and capsules improved TTE compared with their respective placebos (solution: 27.0 ± 21.9 s, p = 0.001; capsules: 23.0 ± 28.1 s, p = 0.016). Compared to capsules, NaHCO3 administered via solution resulted in a higher expectancy about the benefits on TTE cycling performance (Median: 3.5 vs. 2.5, Z = 2.135, p = 0.033). Decline in blood bicarbonate during exercise was higher for NaHCO3 given in solution compared to capsules (2.7 ± 2.1 mmol·L−1, p = 0.001). Mediation analyses showed that improvements in TTE cycling were indirectly related to expectancy and decline in blood bicarbonate when NaHCO3 was administered in solution but not capsules.
Conclusions
Participants’ higher expectations when NaHCO3 is administered in solution could result in them exerting themselves harder during TTE cycling, which subsequently leads to a greater decline in blood bicarbonate and larger improvements in performance.
Key Points
Ingesting 0.3 g·kg−1 body mass sodium bicarbonate in solution and capsules improved time-to-exhaustion cycling performance
Positive expectancy about the benefits of sodium bicarbonate and decline in blood bicarbonate were higher when sodium bicarbonate was administered in solution compared with capsules
Improvements in time-to-exhaustion cycling performance for sodium bicarbonate administered in solution were related to expectancy and the enhanced extracellular buffering response
Keywords: Ergogenic aids, Placebo effect, Beliefs, Extracellular buffering, High-intensity exercise
Introduction
Sodium bicarbonate (NaHCO3) is suggested to be an effective ergogenic aid during high-intensity, short duration exercise [1, 2]. Ingesting 0.3 g·kg−1 body mass (BM) NaHCO3 60–90 min prior to exercise increases blood bicarbonate concentration by ~5 to 6 mmol·L−1 and in turn, elevates pH gradient between intracellular and extracellular compartments [3]. This allows for greater efflux of hydrogen cations (H+) from active musculature into circulation, reducing muscle pH during high-intensity exercise [4]. Although the deleterious effect of intramuscular acidosis on muscle fatigue is disputed [5, 6], declining muscle pH during intense exercise is believed to inhibit metabolic processes required for energy production from anaerobic pathways [7–9]. Enhanced extracellular buffering after NaHCO3 ingestion could therefore sustain anaerobic energy production and improve high-intensity exercise performance.
Whilst there is empirical support for the potential physiological mechanisms underlining the benefits of NaHCO3 on sports performance, it is clear that findings are equivocal [2]. Researchers have shown NaHCO3 administered in solution to improve time-to-exhaustion (TTE) cycling performance [10–12], whereas others have reported no improvements when NaHCO3 was given in capsules [13–15]. One reason for these inconsistencies between ingestion strategies could relate to participants’ expectancy, which is an anticipation of a future event that is thought to induce the placebo effect [16]. In short, placebo effects are positive outcomes arising from an individuals’ expected and/or learned responses to a treatment [17]. A plethora of research has identified that expectations about dietary supplements contributes to improvements in sporting performance [18]. That is, when an athlete ingests a supplement and expects it to be performance enhancing they are more likely to report improvements in performance than when they do not expect it to improve performance [18]. McClung & Collins [19] reported that when participants received NaHCO3 and expected to receive NaHCO3, their 1000-m running time trial improved more than when participants received NaHCO3 but expected a placebo. Furthermore, they found similar improvements in performance for participants receiving a placebo or NaHCO3 when they expected NaHCO3. Similar results have been reported elsewhere for NaHCO3 [20] and other dietary supplements such as caffeine and carbohydrates [21, 22]. These findings demonstrate that athletes’ expectations about ergogenic aids can influence whether positive outcomes are observed.
Since the placebo effect is a complex phenomenon that can be described from neurobiological and psychosocial perspectives [23], it is reasonable to suggest that any expectations about NaHCO3 may interact with physiological mechanisms underpinning pharmacological properties [18]. One example could be the potential interaction of expectation and decline in blood bicarbonate during exercise on performance. Irrespective of pre-exercise changes in blood bicarbonate after NaHCO3, it is possible that decline in blood bicarbonate during exercise directly infers the degree of extracellular buffering (i.e., amount of H+ removed from muscles) and thus the capacity for NaHCO3 to improve exercise performance [24]. Any relationship between expectancy and decline in blood bicarbonate might be explained by response expectancy theory [16]. In brief, this posits that predictions about responses can induce placebo effects, and in turn affect physiological processes [16, 25]. Theoretically, athletes that expect they have received NaHCO3, and that it will improve performance, might exert themselves to a greater extent during exercise due to psychologically manipulated expectations (i.e., reduced feelings of fatigue) [26] and thus, augment the extracellular buffering capacity of NaHCO3 (thus leading to greater decline in blood bicarbonate) [27]. However, no study has examined the interaction between expectations and decline in blood bicarbonate following NaHCO3 ingestion, therefore further work is warranted to determine its impact on sports performance.
Recently, we reported that participants are less likely to detect NaHCO3 administered in capsules than solution (67% vs. 17% ‘unsure’ ratings) partially due to the ‘poor’ taste of solution beverages [28]. Importantly, these differences in blinding between NaHCO3 ingestion strategies may influence participants’ expectations of positive outcomes (i.e., greater response expectancy after identifying NaHCO3) that mean researchers administering NaHCO3 in solution during randomised controlled trials overestimate its ‘true’ pharmacological effectiveness for improving exercise performance. Therefore, we retrospectively examined data from a previous study [28] to (1) determine the influence of expectation and decline in blood bicarbonate following NaHCO3 administered in solution and capsules on TTE cycling performance, and (2) examine if changes in performance are mediated by the expectation of receiving NaHCO3 and decline in blood bicarbonate.
Methods
Study Design
A randomised, crossover, double-blind, placebo-controlled, counterbalanced design was employed. Participants attended five laboratory visits to perform TTE cycling tests (1 x familiarisation, 4 x experimental trials). To control for order effects, participants were randomly assigned to receive one of four treatments (NaHCO3 and placebo administered in solution and capsules) at each visit in a balanced fashion using a Latin square sequence by a member of the research team not involved with data collection.
Participants1
Our sample size calculation conducted on G*Power (version 3.1.9.4) revealed that 12 participants were needed to achieve statistical power (β = 0.80; α = 0.05). This was based on using repeated measures analysis of variances (within-factors) to analyse differences in TTE cycling performance, with an expected medium effect size (partial eta squared, ηp2 = 0.06). Correlation between repeated measures was estimated from previous reliability data for a similar TTE cycling test [29]. To account for typical dropout rates, sixteen recreationally trained cyclists were recruited to participate in the study; however, two did not meet inclusion criteria, one withdrew because of injury and one withdrew due to side-effects after NaHCO3 ingestion. Therefore, 12 recreationally trained cyclists (9 men, 3 women; height, 176.3 ± 5.6 cm; body mass, 69.4 ± 8.1 kg; age, 29.3 ± 6.7 years; maximal oxygen consumption, 54.4 ± 5.7 mL·kg−1·min−1) completed study procedures. Inclusion criteria stipulated that participants were: i) aged between 18 and 40 years, ii) performing at least 3 h cycling per week, iii) unaware of the benefits of NaHCO3 on sport performance, iv) not using extra- or intracellular buffering aids during training (i.e., NaHCO3, beta-alanine), v) not intolerant to cornflour, and vi) not diagnosed with a medical condition that could impact high-intensity exercise. To control for any small confounding effect of menstrual cycle on anaerobic exercise performance [30], we asked female participants to record their menstrual cycle (using a calendar-based method) to ensure experimental trials occurred during the same phase (follicular: 1–14 d, or luteal: 14 d to start of next cycle). Ethical approval was gained from the lead authors’ Institutional Ethics Committee (ETH2021-0198). Research procedures were conducted in accordance with the revised Declaration of Helsinki (2013). All participants provided written informed consent prior to commencing study procedures.
Supplementation Protocol
Across four experimental trials, participants ingested: (1) 0.3 g·kg−1 BM NaHCO3 in 5 mL·kg−1 BM solution (SB-SOL), (2) 0.03 g·kg−1 BM sodium chloride in 5 mL·kg−1 BM solution (placebo; PL-SOL), (3) 0.3 g·kg−1 BM NaHCO3 within size 0 vegetarian capsules (SB-CAP), or (4) an equal number of capsules that contained cornflour (placebo; PL-CAP). Pilot testing revealed that 0.03 g·kg−1 BM sodium chloride provided the best taste-match with 0.3 g·kg−1 BM NaHCO3 in solution, whereas other commonly used doses (i.e., 0.07 g·kg−1 BM) were deemed too ‘salty’ [28]. Cornflour was chosen as the placebo for capsule trials as it is an inert substance that effectively blinds NaHCO3 supplementation [28]. Solution treatments were prepared in 4 mL·kg−1 BM double-strength sugar free orange squash (Sainsbury’s, UK) and 1 mL·kg−1 BM water, before being chilled (~12°C) for 1 h prior to consumption [28]. All capsules (Your Supplements, Stockport, UK) were manually filled using a capsule filling device (ALL-IN Capsule, USA) and each capsule contained approximately either 0.9 g NaHCO3 (Health Leads Ltd, UK) or 0.4 g cornflour (Sainsbury’s, UK). Capsule treatments were given to the nearest number of whole capsules (mean ± SD = 24 ± 3; range = 20–28) in an equal volume of squash/water consumed during sodium bicarbonate solution (SB-SOL) and placebo solution (PL-SOL) trials. Treatments were prepared by a member of the research team not involved with data collection and solution treatments were administered in opaque bottles to prevent participants visually distinguishing between them. Participants were given a 10-min period to consume treatments, alongside ingesting a carbohydrate-rich meal (1.5 g·kg−1 BM carbohydrates; toasted bread/jam, cereal bars) over a 30-min period to minimise the risk of gastrointestinal (GI) discomfort [31].
Blood Sampling
Finger prick capillary blood samples were taken to measure acid-base balance and lactate. To measure acid-base balance, 95 μL samples were collected into blood gas capillary tubes (Vitrex, Herlev, Denmark) and transferred into single-use i-STAT G3+ cartridges (Abbott, Illinois, USA). Samples were analysed for pH, bicarbonate and base excess using a portable blood gas analyser (i-STAT 1, Abbott, Illinois, USA), which has previously demonstrated excellent accuracy and precision for blood pH and bicarbonate (intraclass correlation coefficients, ICC = 0.88, 0.86) [32]. Additional 10 μL blood samples were collected into haemolysing cups (EKF Diagnostics, Cardiff, UK) and analysed for lactate using a Biosen C-Line (EKF Diagnostics; coefficient of variation < 1.5%, at a value of 12 mmol·L−1).
Questionnaires
Participants completed treatment assignment questionnaires that required them to indicate which treatment they thought had been administered (see Gurton et al. [28] for data) and rate on 1–5 Likert-type scales how much they expected this treatment would improve their performance (“1” = no expectations at all, “5” = extremely high expectations). This was adapted from a 44-item Likert-type scale previously used to assess expectancies in psychological research that has been suggested to show good reliability (coefficient alphas, 0.81–0.89) [33]. Participants also completed 100-mm visual analogue scales (0” = no symptom, “100” = severest symptom) to quantify aggregate Gastrointestinal (GI) discomfort for eight common side-effects [12].
Procedures
Participants attended the laboratory in a 3 h post-prandial state having avoided strenuous exercise and alcohol for 24 h. Caffeine consumption was also prohibited 12 h prior to testing. Nutritional intake was recorded via self-report food diaries for 24 h before the first trial and replicated prior to subsequent sessions (n = 11, one participant lost diary). Experimental trials were separated by 3–7 d to allow for appropriate washout of nutritional treatments. Testing was conducted at a similar time of day (± 2 h) to control for the confounding effects of circadian rhythms on exercise performance [34].
During the initial laboratory visit, anthropometric measures were recorded before participants performed a graded cycling test on an electronically braked SRM ergometer (Schoberer Rad Meßtechnik, Germany). Gaseous exchange was collected using a breath-by-breath metabolic analyser (Vyntus CPX, CareFusion GmbH, Germany). Participants completed a 5 min warm-up at 70 W and a self-selected cadence (60–90 rev·min−1). Power output during the first stage was prescribed according to participants’ fitness level, with increments of +5 W applied every 15 s such that volitional exhaustion occurred within ~8 to 12 min [35]. Average power output across the final 2 min (Wpeak) was used to calculate workload for TTE cycling tests [29]. Maximal oxygen consumption was recorded as the highest 30 s average for oxygen uptake [35]. Following 30 min recovery, participants were familiarised to the TTE cycling test. Workload was adapted from the 110% Wpeak chosen by Saunders et al. [29] to cause fatigue within ~5 min, as this duration has been shown to elicit the greatest ergogenic benefits for NaHCO3 ingestion [11]. Participants selected preferred bike dimensions (replicated during subsequent TTE tests) and completed a 5 min warm-up at 1.5 W·kg−1 BM. This intensity was chosen to prepare participants for TTE cycling, without diminishing extracellular buffering capacity [36]. Power output was then increased across 60 s (increments every 15 s) until desired workload was achieved, at which point TTE cycling commenced. Participants' cadence was visible, but time was concealed. Exercise was terminated when participants failed to maintain cadence > 60 rev·min−1 for 5 s despite verbal encouragement.
On arrival to the laboratory during experimental trials, baseline capillary blood samples were taken and GI discomfort questionnaires were completed. Participants then consumed nutritional treatments (SB-SOL, SB-CAP, PL-SOL or PL-CAP) and the carbohydrate-rich meal. Additional GI discomfort questionnaires were filled in at 30-min and 60-min post-consumption. Capillary blood samples were performed prior to exercise, which was 85 min post-consumption. At this point, participants repeated GI discomfort questionnaires and completed treatment assignment questionnaires. TTE cycling tests commenced 90 min after consuming supplements and were performed as described during the familiarisation visit. Final capillary blood samples and GI discomfort questionnaires were repeated post-exercise.
Statistical Analysis
Data were analysed using SPSS version 26.0 (IBM, New York, USA). Descriptive data are presented as mean ± SD (unless otherwise stated) and the α-level of statistical significance was set at p < 0.05. Normality was determined from Shapiro-Wilk tests and sphericity was assessed using Mauchly’s test, with violations corrected via Greenhouse-Geisser. Two-way repeated measures analysis of variance (ANOVA) were used to establish significant treatment * time interactions for blood analyses and least significant difference post-hoc pairwise comparisons were conducted to determine treatment differences at each time point [37]. One-way repeated measures ANOVA were used to determine whether there were any differences between treatments for TTE cycling performance and decline in blood bicarbonate. Effect sizes for ANOVA main effects and interactions are reported as partial eta squared (ηp2), with values of 0.1, 0.25 and 0.4 representing small, medium and large effects, respectively [38]. Between treatment effects sizes (g) were calculated by dividing mean difference by pooled SD and applying Hedges g bias correction to account for the small sample size [39]. These were interpreted as trivial (≤ 0.20), small (0.20–0.49), moderate (0.50–0.79) or large (≥ 0.80) [38]. Friedman tests were conducted on non-normally distributed data (i.e., expectancy, aggregate GI discomfort) with median and Z values reported for between treatment comparisons. Non-normally distributed effect sizes (r) were calculated from Z/√n, with 0.10, 0.24 and 0.37 considered small, medium, and large, respectively [40].
To examine whether changes in TTE were mediated by expectancy of NaHCO3 and decline in blood bicarbonate, we used Process v4.0 [41] SPSS macro (model 6), which simultaneously tests direct and indirect effects in a serial mediation model. Briefly, serial mediation was chosen as we hypothesised that expectations would affect decline in blood bicarbonate that in turn, would influence sport performance; as opposed to parallel mediation approaches that would not consider the influence of expectations on decline in blood bicarbonate [42]. Direct effects are the effects of the predictor variable (i.e., treatment) on an outcome variable (i.e., TTE) that occur separately to each mediator, while indirect effects are the effects of treatment on TTE via expectancy and decline in blood bicarbonate. Given that the predictor variable cannot be treated as an ordinal or interval measurement, we included this as a multi-categorical independent variable [43]. Indicator coding was used, which automatically generated k−1 dummy variables to represent the four treatments. By recoding multi-categorical variables into k−1 separate dummy variables, the mathematical equivalent of analysis of covariance is modelled and the linear mediation model can be estimated. The predictor variable is entered into the mediation model to quantify the indirect and direct effects of being in one treatment compared to a reference group.
To understand the indirect effect of treatment on TTE, we conducted three mediation analyses whereby one dummy variable was coded as the reference group, and the rest as comparisons. In the first, second and third analyses, SB-SOL, SB-CAP and PL-CAP were used as the reference group, respectively, which allowed us to compare the following: SB-SOL versus SB-CAP (D1), SB-SOL versus PL-SOL (D2), SB-SOL versus PL-CAP (D3), SB-CAP versus PL-SOL (D4), SB-CAP versus PL-CAP (D5) and PL-SOL versus PL-CAP (D6). Bootstrapping was set at 10,000 samples to control for Type I error [41] and bias corrected 95% CI were calculated. An effect was significant when the CI did not contain zero. The partially standardised indirect effect (PSIE) is reported as the effect size, with values of 0.01, 0.09 and 0.25 representing small, medium, and large effect sizes, respectively [44].
Results
Blood Metabolites
Significant two-way interaction effects (treatment * time) were observed for bicarbonate (F(6, 66) = 49.688, ηp2 = 0.819, p < 0.001), pH (F(6, 66) = 12.664, ηp2 = 0.535, p < 0.001), base excess (F(6, 66) = 40.463, ηp2 = 0.786, p < 0.001) and lactate (F(2.791, 30.702) = 9.975, ηp2 = 0.476, p < 0.001). Bicarbonate, pH and base excess were higher pre-exercise for SB-SOL and SB-CAP compared with PL-SOL and PL-CAP (p < 0.05; Table 1). There were no differences in pre-exercise pH or lactate between SB-SOL and SB-CAP (p > 0.05). However, bicarbonate (+2.2 mmol·L−1, p = 0.007, g = 1.04) and base excess (+1.7 mmol·L−1, p = 0.043, g = 0.82) were higher pre-exercise after SB-SOL compared with SB-CAP. Bicarbonate, pH, base excess and lactate were also higher post-exercise for SB-SOL and SB-CAP compared with PL-SOL and PL-CAP (p < 0.05; Table 1). There were no differences in post-exercise blood metabolites between SB-SOL and SB-CAP (p > 0.05).
Table 1.
Mean ± SD for blood metabolites at baseline, pre-exercise and post-exercise
| SB-SOL | SB-CAP | PL-SOL | PL-CAP | |
|---|---|---|---|---|
| pH | ||||
| Baseline | 7.445 ± 0.031 | 7.442 ± 0.027 | 7.443 ± 0.021 | 7.434 ± 0.026 |
| Pre-exercise | 7.521 ± 0.031 * | 7.521 ± 0.040 * | 7.438 ± 0.030 | 7.447 ± 0.032 |
| Post-exercise | 7.287 ± 0.057 * | 7.293 ± 0.073 * | 7.226 ± 0.064 | 7.237 ± 0.058 |
| Bicarbonate (mmol·L−1) | ||||
| Baseline | 26.8 ± 1.7 | 26.1 ± 1.5 | 26.5 ± 1.5 | 26.6 ± 1.5 |
| Pre-exercise | 34.4 ± 2.4 *# | 32.2 ± 1.6 * | 26.4 ± 1.1 | 27.1 ± 1.3 |
| Post-exercise | 15.5 ± 2.6 * | 15.9 ± 3.2 * | 12.6 ± 2.5 | 13.0 ± 1.9 |
| Base excess (mmol·L−1) | ||||
| Baseline | 2.8 ± 1.7 | 2.1 ± 1.6 | 2.4 ± 1.3 | 2.5 ± 1.1 |
| Pre-exercise | 10.6 ± 2.2 *# | 8.9 ± 1.8 * | 2.5 ± 1.2 | 3.2 ± 1.6 |
| Post-exercise | -9.8 ± 3.1 * | -9.3 ± 3.9 * | −13.4 ± 3.1 | −12.9 ± 2.6 |
| Lactate (mmol·L−1) | ||||
| Baseline | 1.34 ± 0.36 | 1.18 ± 0.37 | 1.23 ± 0.47 | 1.11 ± 0.33 |
| Pre-exercise | 1.50 ± 0.35 | 1.80 ± 0.62 | 1.74 ± 0.40 | 1.70 ± 0.53 |
| Post-exercise | 14.09 ± 3.15 * | 13.58 ± 2.95 * | 11.26 ± 2.17 | 11.73 ± 2.83 |
*p < 0.05 versus PL-SOL and PL-CAP, #p < 0.05 versus SB-CAP
TTE, Expectancy and Decline in Blood Bicarbonate
Significant main effects of treatment were observed for TTE (F(1.727, 18.998) = 6.147, ηp2 = 0.358, p = 0.011) and decline in blood bicarbonate (F(3, 33) = 34.923, ηp2 = 0.760, p < 0.001). TTE was greater for SB-SOL compared with PL-SOL (+27.0 s, 95% CI: 13.0, 40.9, p = 0.001, g = 0.34) and PL-CAP (+29.7 s, 95% CI: 12.9, 46.6, p = 0.003, g = 0.37), and for SB-CAP compared with PL-CAP (+23.0 s, 95% CI: 5.2, 41.0, p = 0.016, g = 0.32). There were no differences for TTE between SB-SOL and SB-CAP (p = 0.598) or between PL-SOL and PL-CAP (p = 0.561). Mean and individual responses for TTE cycling performance are show in Fig. 1. Decline in blood bicarbonate was higher for SB-SOL compared with PL-SOL (+5.1 mmol·L−1, p < 0.001, g = 1.64) and PL-CAP (+4.8 mmol·L−1, p < 0.001, g = 1.58), and for SB-CAP compared with PL-SOL (+2.4 mmol·L−1, p = 0.001, g = 0.84) and PL-CAP (+2.2 mmol·L−1; p = 0.001; g = 0.77). Decline in blood bicarbonate was also greater for SB-SOL compared with SB-CAP (+2.7 mmol·L−1, p = 0.001, g = 0.79). There were no differences for decline in blood bicarbonate between PL-SOL and PL-CAP (p = 0.598). Mean and individual responses for decline in blood bicarbonate during TTE cycling are shown in Table 2.
Fig. 1.
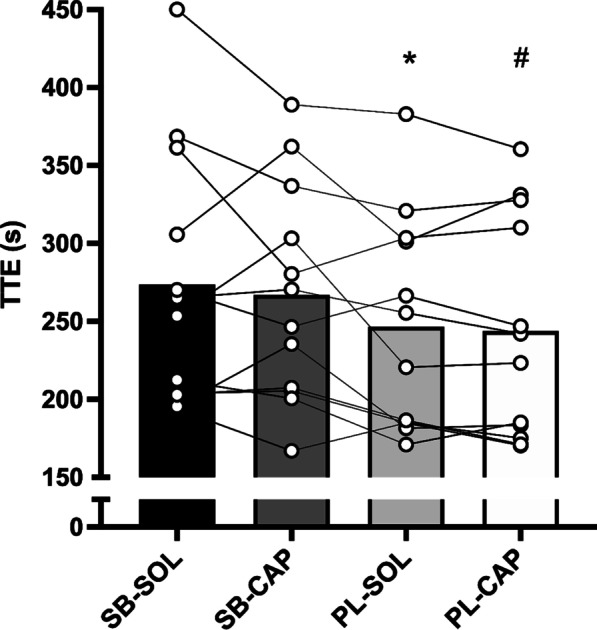
TTE cycling performance for each treatment. Data presented as mean (bar) and individual responses; * p < 0.05 versus SB-SOL, # p < 0.05 versus SB-SOL & SB-CAP
Table 2.
Mean and individual responses for decline in blood bicarbonate
| Participant | Decline in blood bicarbonate during TTE cycling (mmol·L−1) | |||
|---|---|---|---|---|
| SB-SOL | SB-CAP | PL-SOL | PL-CAP | |
| 1 | − 12.8* | − 8.9 | − 9.7 | − 8.2 |
| 2 | − 21.6* | − 15.5 | − 14.5 | − 14.3 |
| 3 | − 18.5 | − 19.6* | − 16.6 | − 14.4 |
| 4 | − 14.9 | − 15.7* | − 13.8 | − 13.8 |
| 5 | − 24.2* | − 20.6 | − 17.5 | − 17.2 |
| 6 | − 17.7* | − 15.1 | − 15.7 | − 14.6 |
| 7 | − 21.4* | − 16.9 | − 13.3 | − 15.4 |
| 8 | − 18.3* | − 16.0 | − 14.0 | − 14.8 |
| 9 | − 24.0* | − 20.1 | − 16.0 | − 17.6 |
| 10 | − 19.9* | − 17.3 | − 11.6 | − 13.6 |
| 11 | − 16.6* | − 15.5 | − 11.1 | − 13.7 |
| 12 | − 17.5* | − 14.4 | − 12.6 | − 12.1 |
| Mean | − 19.0 | − 16.3 | − 13.9 | − 14.1 |
| SD | 3.5 | 3.1 | 2.4 | 2.4 |
*Largest decline in bicarbonate for each participant
A significant effect of treatment was observed for expectancy (χ2(3) = 15.370, p = 0.002). Expectancy was highest for SB-SOL (Median: 3.5) compared with SB-CAP (Z = 2.135, p = 0.033, r = 0.61), PL-SOL (Z = 3.004, p = 0.003, r = 0.87) and PL-CAP (Z = 2.451, p = 0.014, r = 0.71). There were no differences in expectancy for SB-CAP (Median: 2.5) compared with PL-SOL (Z = 0.870, p = 0.385) and PL-CAP (Z = 0.316, p = 0.752), or between PL-SOL and PL-CAP (Median: 2.0 vs. 2.0, Z = −0.553, p = 0.580).
Indirect Effects of Expectancy and Decline in Blood Bicarbonate
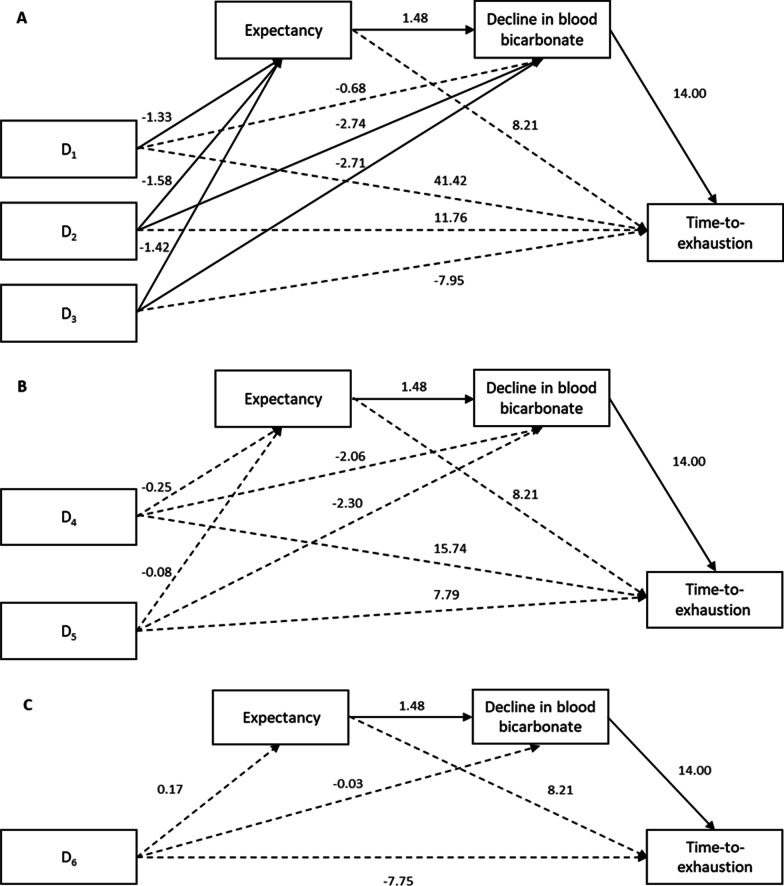
Results of the mediation analyses are shown in Table 3 and Fig. 2A–C. In all analyses, no direct effects of treatment on TTE were shown (Fig. 2A–C). Instead, when compared to SB-CAP, PL-SOL and PL-CAP, SB-SOL had a significant indirect effect on TTE via expectations and decline in blood bicarbonate (Table 3). Similarly, when compared to PL-SOL and PL-CAP, SB-CAP was indirectly related to TTE via decline in blood bicarbonate (Table 3). No indirect effects were shown for PL-CAP or PL-SOL via expectation or decline in blood bicarbonate on TTE (Table 3). In short, participants reported higher expectations about the effectiveness of SB-SOL, which resulted in a higher decline in blood bicarbonate, and in turn, improved TTE cycling performance, whereas improvements for SB-CAP were the result of decline in blood bicarbonate. For PL-SOL and PL-CAP, expectation and decline in blood bicarbonate were not directly or indirectly related to TTE.
Table 3.
Indirect effects of expectancy and decline in blood bicarbonate on TTE
| Pathway | b (SE) | 95% CI | PSIE (SE) | 95% CI |
|---|---|---|---|---|
| Indirect effects on TTE via expectancy | ||||
| D1 | − 10.95 (14.3) | − 40.50 to 16.58 | − 0.15 (0.20) | − 0.57 to 0.23 |
| D2 | − 13.00 (17.25) | − 49.83 to 17.65 | − 0.18 (0.24) | − 0.71 to 0.24 |
| D3 | − 11.63 (15.55) | − 45.35 to 16.16 | − 0.16 (0.22) | − 0.64 to 0.22 |
| D4 | − 2.05 (15.49) | − 18.16 to 5.67 | − 0.03 (0.08) | − 0.25 to 0.08 |
| D5 | − 0.68 (5.37) | − 14.48 to 8.34 | − 0.01 (0.08) | − 0.20 to 0.12 |
| D6 | 1.37 (4.96) | − 7.1 to 14.62 | 0.02 (0.07) | − 0.10 to 0.20 |
| Indirect effects on TTE via decline in blood bicarbonate | ||||
| D1 | − 9.45 (18.29) | − 46.51 to 26.73 | − 0.13 (0.25) | − 0.64 to 0.38 |
| D2 | − 38.33 (20.39) | − 81.49 to − 2.15 | − 0.53 (0.28) | − 1.12 to − 0.03 |
| D3 | − 37.93 (18.87) | − 76.18 to − 2.61 | − 0.53 (0.26) | − 1.04 to − 0.04 |
| D4 | − 28.88 (15.49) | − 63.25 to 1.75 | − 0.40 (0.21) | − 0.88 to 0.02 |
| D5 | − 28.48 (15.12) | − 61.49 to − 0.73 | − 0.39 (0.21) | − 0.84 to − 0.01 |
| D6 | 0.39 (12.29) | − 23.04 to 25.96 | 0.01 (0.17) | − 0.31 to 0.37 |
| Indirect effects on TTE via expectancy and decline in blood bicarbonate | ||||
| D1 | − 27.64 (15.08) | − 63.68 to − 5.63 | − 0.38 (0.20) | − 0.84 to − 0.09 |
| D2 | − 32.83 (16.10) | − 71.32 to − 8.33 | − 0.45 (0.20) | − 0.92 to − 0.13 |
| D3 | − 29.37 (15.05) | − 65.97 to − 6.75 | − 0.41 (0.19) | − 0.86 to − 0.10 |
| D4 | − 5.18 (8.47) | − 23.18 to 10.98 | −0.07 (0.11) | − 0.31 to 0.15 |
| D5 | − 1.73 (8.72) | − 19.48 to 16.59 | − 0.02 (0.12) | − 0.26 to 0.23 |
| D6 | 3.46 (8.12) | − 12.50 to 21.95 | 0.05 (0.11) | − 0.17 to 0.30 |
Unstandardised coefficients are shown
Bold indicates difference (p < 0.05)
SE standard error, PSIE partially standardized indirect effect, TTE time-to-exhaustion
D1 = SB-SOL versus SB-CAP, D2 = SB-SOL versus PL-SOL, D3 = SB-SOL versus PL-CAP, D4 = SB-CAP versus PL-SOL, D5 = SB-CAP versus PL-CAP, D6 = PL-SOL versus PL-CAP
Fig. 2.
A–C The effects of treatment on TTE and the mediating role of expectancy and decline in blood bicarbonate. Note: Values presented are the unstandardised regression coefficients. A solid line represents a significant relationship. A Reference group = SB-SOL. D1 = SB-SOL versus SB-CAP, D2 = SB-SOL versus PL-SOL, D3 = SB-SOL versus PL-CAP. B Reference group = SB-CAP. D4 = SB-CAP versus PL-SOL, D5 = SB-CAP versus PL-CAP. C Reference group = PL-SOL. D6 = PL-SOL versus PL-CAP
Gastrointestinal Discomfort
All participants reported GI discomfort following SB-SOL and SB-CAP, with ‘gut fullness’ the most common side-effect. There were no differences in aggregate GI discomfort between treatments at 30 min (χ2(3) = 3.051, p = 0.351), 60 min (χ2(3) = 5.250, p = 0.154), pre-exercise (χ2(3)=5.797, p = 0.122) and post-exercise (χ2(3) = 2.971, p = 0.396).
Discussion
The aims of our retrospective study were (1) to determine the influence of expectancy and decline in blood bicarbonate following NaHCO3 administered in solution and capsules on TTE cycling performance, and (2) examine if changes in performance are mediated by the expectation of receiving NaHCO3 and decline in blood bicarbonate. Administering NaHCO3 in solution and capsules were indirectly related to TTE cycling performance via decline in blood bicarbonate, while giving NaHCO3 in solution resulted in greater expectations that it would improve performance. Our mediation analyses also revealed an indirect relationship between expectancy and decline in blood bicarbonate on TTE cycling performance when NaHCO3 was administered in solution, but not for the other treatments. These findings suggest that participants’ reporting greater expectancy after consuming NaHCO3 in solution may subconsciously exert themselves harder during an exercise task due to psychologically manipulated expectations (i.e., reduced feelings of fatigue). In turn, this is likely to lead to a greater decline in blood bicarbonate that may indicate an enhanced extracellular buffering response that contributes to improvement in performance.
In agreement with the proposed ergogenic effect of acute NaHCO3 ingestion on TTE cycling performance [10–12], SB-SOL and SB-CAP significantly improved TTE cycling performance compared to placebo treatments in our cohort of male and female recreationally trained cyclists (Fig. 1). Recent evidence suggests that the effect of NaHCO3 on high-intensity exercise performance could be gender-specific [45], however given our small sample size of female participants (n = 3) it was not possible to further explore this argument. Whilst this is the first study to compare the benefits of NaHCO3 given in solution and capsules on TTE cycling performance, it seems that researchers using solution ingestion approaches are more likely to report improvements than those opting for capsules [13–15]. We recently demonstrated that participants perceive solution NaHCO3 beverages to have a ‘worse’ taste than NaHCO3 administered in capsules, which sometimes results in them successfully identifying what treatment has been given [28]. Other researchers have suggested that taste alone can be ergogenic, owing to centrally acting mechanisms that alter participants’ perception of effort [46, 47]. Since perception of effort is a primary determinant of exercise performance in motivated individuals [48, 49], it is likely that participants’ expectations that they have received NaHCO3 influences ergogenic benefits. We argue that participants able to detect NaHCO3 experience higher response expectancies (i.e., prediction that exercise will be easier) that in turn, increase their belief that they can exert more effort during an exercise task [26]. Our results highlight that the administration of NaHCO3 in solution may therefore overestimate its ‘true’ efficacy during randomised controlled trials whereby psychological components could also contribute to benefits [13–15]. It is important that researchers adequately blind participants from the treatment given to provide a more accurate inference to the pharmacological effects of NaHCO3 on sports performance outcomes.
As expected, participants’ expectancy was highest for SB-SOL compared to the other treatments, likely due to the ‘poor’ taste of solution NaHCO3 beverages and GI side-effects weakening blinding efficacy [28], which subsequently increased participants’ expectation of positive outcomes. Participants’ expectancy is believed to induce placebo effects [16], but as we did not directly deceive participants about which treatment had been received, instead evaluating whether differences in acute NaHCO3 ingestion strategy altered expectancy, it is possible that some participants were unable to identify NaHCO3 in either solution or capsules (see Gurton et al. [28] for data). This may have limited participants’ expectation about the effect of NaHCO3 and thus, reduced any potential influence on TTE cycling performance. Since response expectancies are one of the psychological components believed to contribute towards placebo effects [25], it is crucial researchers measure participants’ expectation when evaluating the efficacy of acute NaHCO3 ingestion for improving sports performance.
Administering NaHCO3 in solution and capsules elevated acid-base balance pre- and post-exercise compared to placebo treatments (Table 1). Achieving an >5 mmol·L−1 increase in blood bicarbonate is considered crucial for maximising performance benefits after acute ingestion of 0.3 g·kg−1 BM NaHCO3 [1], but improvements have still been shown for alternative supplementation approaches (i.e., chronic and smaller dosing strategies) despite changes in blood bicarbonate failing to reach this ‘ergogenic’ threshold [50, 51]. In these circumstances, it is possible that decline in blood bicarbonate during exercise is more important, as this directly infers whether enhanced buffering response for NaHCO3 was fully used throughout an exercise task [24]. Decline in blood bicarbonate during TTE cycling was greater for SB-SOL and SB-CAP than their respective placebo treatments, with highest decline reported following SB-SOL in 83% of participants and SB-CAP in 17% of participants (Table 2). Importantly, we also found that decline in blood bicarbonate was greater for NaHCO3 administered in solution than capsules. According to arguments made by da Silva et al. [52], if total blood volume is ~5 L, then as decline in blood bicarbonate was ~3.0 mmol·L−1 higher for SB-SOL compared with SB-CAP, we can assume that that an extra ~15 mmoles of H+ could have been neutralised (based on the 1:1 stoichiometry of bicarbonate buffering system). Whilst post-exercise blood lactate was elevated for both NaHCO3 treatments compared with placebos, there were no differences between SB-SOL and SB-CAP. Despite enhanced extracellular buffering potential for SB-SOL, these results seem to suggest that lactate-H+ cotransport via the monocarboxylate transporter 1/4 was not further augmented by giving NaHCO3 in solution [8]. Our findings reinforce arguments by Higgins et al. [11] that enhanced TTE cycling performance after NaHCO3 is not solely contributed to augmented metabolic flux, however further research is needed to understand the influence of decline in blood bicarbonate on the performance enhancing effects of acute and chronic NaHCO3 supplementation.
This was the first study to examine the mediating role of expectancy and decline in blood bicarbonate on the ergogenic benefits of NaHCO3 (Fig. 2A–C). Firstly, whilst expectancy was increased for SB-SOL, it was not directly related to TTE performance (Table 3). Previous studies that demonstrated positive expectancy effects of NaHCO3 on exercise performance employed standardized scripts that deceived participants about which treatment had been given and outlined the ‘proven’ ergogenicity of NaHCO3 [19, 20]. These methodological differences likely increased expectancy compared to our study [26], as some participants would have been unaware of which treatment had been given, or the potential benefits for NaHCO3. Secondly, both SB-SOL and SB-CAP were indirectly related to TTE cycling performance via decline in blood bicarbonate, suggesting this could predict the ergogenic effects of NaHCO3. It is logical to theorize that participants who report greatest decline in blood bicarbonate would be more likely to see improvements in performance, as this indicates whether enhanced extracellular buffering for NaHCO3 was utilised [24]. Notably, there was also an indirect relationship between expectancy and decline in blood bicarbonate on TTE performance for SB-SOL, but not SB-CAP (Table 3). In other words, participants with the highest expectation (i.e., prediction that exercise will be ‘easier’) for SB-SOL may have exerted themselves harder during TTE cycling, which augmented physiological response resulting in a greater decline in blood bicarbonate. As such, we argue that these participants were able to capitalise on both the psychological and physiological effects of NaHCO3 and in turn, improve TTE cycling performance to a larger extent than when one is absent [27]. Improvements during TTE cycling for SB-SOL were therefore influenced by participants’ expectations [16, 25], whereas improvements for SB-CAP can be attributed to NaHCO3 pharmacological properties augmenting blood bicarbonate buffering capacity. These findings from our mediation analyses reinforce that administering NaHCO3 in solution may overestimate its ‘true’ efficacy during randomised controlled trials because of participants’ expectancy that the treatment would improve their performance [17, 18]. Our study bridges the gap between placebo effect and sports performance research by offering an alternative explanation for the equivocal effect of NaHCO3 on TTE cycling performance. Considering these findings, we recommend that researchers administer NaHCO3 in capsules when examining sports performance outcomes, allowing them to attribute ergogenic benefits to treatment efficacy instead of placebo effects such as response expectancy.
There are methodological limitations that need to be considered when interpreting results and should be addressed in the future. We did not include a control group (i.e., no treatment given) during the present study, which would have helped better understand the effects of expectations on TTE cycling performance [53]. We also chose to administer treatments 90 min pre-exercise, as this time-frame is believed to elicit an almost certain increase in blood bicarbonate >5 mmol·L−1 (~97% probability) [54]. Despite this, we found that pre-exercise blood bicarbonate was ~2.0 mmol·L−1 higher for SB-SOL versus SB-CAP. Considering that peak changes in blood bicarbonate following 0.3 g·kg−1 BM NaHCO3 typically occur later when administered in capsules [54], it is possible differences for decline in blood bicarbonate between NaHCO3 treatments were due to participants achieving a greater alkalotic state for SB-SOL pre-exercise, as well as greater effort owing to greater expectations from identifying treatments. Future work examining decline in blood bicarbonate between different NaHCO3 ingestion strategies needs to adopt a time-to-peak approach to ensure pre-exercise blood bicarbonate is similar between treatments [36, 50].
Conclusions
In this retrospective study, we found that 0.3 g·kg−1 BM NaHCO3 given in both solution and capsules improved TTE cycling performance. There was an indirect relationship between expectancy and decline in blood bicarbonate on TTE cycling for NaHCO3 administered in solution, but not capsules. These findings suggest that improvements in TTE cycling performance for NaHCO3 administered in solution were likely the result of greater response expectancies (i.e., predictions that exercise would be easier) and extracellular buffering response, whereas benefits for NaHCO3 given in capsules were due to pharmacological properties augmenting blood bicarbonate buffering capacity. Participants able to detect that NaHCO3 has been given may exert themselves harder during exercise, which in turn allows them to capitalise on both the psychological and physiological effects of NaHCO3, subsequently leading to increased performance benefits compared with when only one is present. In light of our findings, we recommend researchers examining potential ergogenic benefits for NaHCO3 adopt capsule ingestion strategies to ensure positive effects can be attributed to treatment efficacy instead of participants’ expectations.
Acknowledgements
We gratefully acknowledge the commitment of all participants.
Author Contributions
Conceptualisation, WHG, LAG and PH; Methodology, WHG, LAG and PH; Formal Analysis, WHG, GGM, and PH; Investigation, WHG and GGM; Resources, WHG, DGK and PH; Writing—Original Draft, MKR, WHG and PH; Writing—Review & Editing, MKR, WHG, GGM, LAG, DGK and PH. Project Administration, WHG. All authors have read and approved the manuscript.
Funding
No external funding was received.
Availability of Data and Materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval and Consent to Participate
Ethical approval was gained from the Canterbury Christ Church University Ethics Committee (ETH2021-0198). Research procedures were conducted in accordance with the revised Declaration of Helsinki (2013). All participants provided written informed consent prior to commencing study procedures.
Consent for Publication
All participants gave consent to publish the data presented in this manuscript.
Competing Interests
WHG, GGM, LAG, MKR, DGK and PH can confirm that there are no competing interests related to the study outcome or the supplement investigated.
Footnotes
Participants included in a previous study by Gurton et al. [28] titled “efficacy of sodium bicarbonate ingestion strategies for protecting blinding”. This study had separate aims, which were to compare the blinding efficacy of various sodium bicarbonate ingestion strategies. Published within European Journal of Applied Physiology (September 2022).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carr AJ, Hopkins WG, Gore CJ. Effects of acute alkalosis and acidosis on performance. Sports Med. 2011;41:801–14. doi: 10.2165/11591440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.de Oliveira LF, Dolan E, Swinton PA, Durkalec-Michalski K, Artioli GG, McNaughton LR, et al. Extracellular buffering supplements to improve exercise capacity and performance: a comprehensive systematic review and meta-analysis. Sports Med. 2022;52:505–26. doi: 10.1007/s40279-021-01575-x. [DOI] [PubMed] [Google Scholar]
- 3.Bishop D, Edge J, Davis C, Goodman C. Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc. 2004;36:807–13. doi: 10.1249/01.MSS.0000126392.20025.17. [DOI] [PubMed] [Google Scholar]
- 4.Hollidge-Horvat MG, Parolin ML, Wong D, Jones NL, Heigenhauser GJF. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Am J Physiol-Endocrinol Metab. 2000;278:E316–29. doi: 10.1152/ajpendo.2000.278.2.E316. [DOI] [PubMed] [Google Scholar]
- 5.Westerblad H. Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc. 2016;48:2339–42. doi: 10.1249/MSS.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 6.Fitts RH. The role of acidosis in fatigue: pro perspective. Med Sci Sports Exerc. 2016;48:2335–8. doi: 10.1249/MSS.0000000000001043. [DOI] [PubMed] [Google Scholar]
- 7.Spriet LL, Lindinger MI, McKelvie RS, Heigenhauser GJ, Jones NL. Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J Appl Physiol. 1989;66:8–13. doi: 10.1152/jappl.1989.66.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Messonnier L, Kristensen M, Juel C, Denis C. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J Appl Physiol. 2007;102:1936–44. doi: 10.1152/japplphysiol.00691.2006. [DOI] [PubMed] [Google Scholar]
- 9.Debold EP, Beck SE, Warshaw DM. Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol-Cell Physiol. 2008;295:C173–9. doi: 10.1152/ajpcell.00172.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenzie DC, Coutts KD, Stirling DR, Hoeben HH, Kuzara G. Maximal work production following two levels of artificially induced metabolic alkalosis. J Sports Sci. 1986;4:35–8. doi: 10.1080/02640418608732096. [DOI] [PubMed] [Google Scholar]
- 11.Higgins MF, James RS, Price MJ. The effects of sodium bicarbonate (NaHCO3) ingestion on high intensity cycling capacity. J Sports Sci. 2013;31:972–81. doi: 10.1080/02640414.2012.758868. [DOI] [PubMed] [Google Scholar]
- 12.Gurton WH, Gough LA, Sparks SA, Faghy MA, Reed KE. Sodium bicarbonate ingestion improves time-to-exhaustion cycling performance and alters estimated energy system contribution: a dose-response investigation. Front Nutr. 2020;7:154. doi: 10.3389/fnut.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sale C, Saunders B, Hudson S, Wise JA, Harris RC, Sunderland CD. Effect of β-alanine plus sodium bicarbonate on high-intensity cycling capacity. Med Sci Sports Exerc. 2011;43:1972–8. doi: 10.1249/MSS.0b013e3182188501. [DOI] [PubMed] [Google Scholar]
- 14.Saunders B, Sale C, Harris RC, Sunderland C. Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform. 2014;9:627–32. doi: 10.1123/ijspp.2013-0295. [DOI] [PubMed] [Google Scholar]
- 15.de Araujo Dias GF, da Eira Silva V, de Salles Painelli V, Sale C, Giannini Artioli G, Gualano B, Saunders B. (In) consistencies in responses to sodium bicarbonate supplementation: a randomised, repeated measures counterbalanced and double-blind study. PLoS ONE. 2015;10:14. doi: 10.1371/journal.pone.0143086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch I. Response expectancy as a determinant of experience and behavior. Am Psychol. 1985;40:1189–202. doi: 10.1037/0003-066X.40.11.1189. [DOI] [Google Scholar]
- 17.Beedie CJ, Foad AJ. The placebo effect in sports performance. Sports Med. 2009;39:313–29. doi: 10.2165/00007256-200939040-00004. [DOI] [PubMed] [Google Scholar]
- 18.Hurst P, Schipof-Godart L, Szabo A, Raglin J, Hettinga F, Roelands B, et al. The Placebo and Nocebo effect on sports performance: a systematic review. Eur J Sport Sci. 2020;20:279–92. doi: 10.1080/17461391.2019.1655098. [DOI] [PubMed] [Google Scholar]
- 19.McClung M, Collins D. “Because I know It will!”: placebo effects of an ergogenic aid on athletic performance. J Sport Exerc Psychol. 2007;29:382–94. doi: 10.1123/jsep.29.3.382. [DOI] [PubMed] [Google Scholar]
- 20.Higgins MF, Shabir A. Expectancy of ergogenicity from sodium bicarbonate ingestion increases high-intensity cycling capacity. Appl Physiol Nutr Metab. 2016;41:405–10. doi: 10.1139/apnm-2015-0523. [DOI] [PubMed] [Google Scholar]
- 21.Hurst P, Schipof-Godart L, Hettinga F, Roelands B, Beedie C. Improved 1000-m running performance and pacing strategy with caffeine and placebo: a balanced placebo design study. Int J Sports Physiol Perform. 2019;15:483–8. doi: 10.1123/ijspp.2019-0230. [DOI] [PubMed] [Google Scholar]
- 22.Clark VR, Hopkins WG, Hawley JA, Burke LM. Placebo effect of carbohydrate feedings during a 40-km cycling time trial. Med Sci Sports Exerc. 2000;32:1642–7. doi: 10.1097/00005768-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Peiris N, Blasini M, Wright T, Colloca L. The placebo phenomenon: a narrow focus on psychological models. Perspect Biol Med. 2018;61:388–400. doi: 10.1353/pbm.2018.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindh A, Peyrebrune M, Ingham S, Bailey D, Folland J. Sodium bicarbonate improves swimming performance. Int J Sports Med. 2008;29:519–23. doi: 10.1055/s-2007-989228. [DOI] [PubMed] [Google Scholar]
- 25.Raglin J, Szabo A, Lindheimer JB, Beedie C. Understanding placebo and nocebo effects in the context of sport: a psychological perspective. Eur J Sport Sci. 2020;20:293–301. doi: 10.1080/17461391.2020.1727021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindheimer JB, Szabo A, Raglin JS, Beedie C. Advancing the understanding of placebo effects in psychological outcomes of exercise: lessons learned and future directions. Eur J Sport Sci. 2020;20:326–37. doi: 10.1080/17461391.2019.1632937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beedie C, Foad A, Hurst P. Capitalizing on the placebo component of treatments. Curr Sports Med Rep. 2015;14:284. doi: 10.1249/JSR.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 28.Gurton WH, Matta GG, Gough LA, Hurst P. Efficacy of sodium bicarbonate ingestion strategies for protecting blinding. Eur J Appl Physiol. 2022;122:2555–63. doi: 10.1007/s00421-022-05031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders B, Sale C, Harris RC, Morris JG, Sunderland C. Reliability of a high-intensity cycling capacity test. J Sci Med Sport. 2013;16:286–9. doi: 10.1016/j.jsams.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael MA, Thomson RL, Moran LJ, Wycherley TP. The impact of menstrual cycle phase on athletes’ performance: a narrative review. Int J Environ Res Public Health. 2021;18:1667. doi: 10.3390/ijerph18041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr A, Slater G, Gore C, Dawson B, Burke L. Effect of sodium bicarbonate on [HCO3-], pH, and gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2011;21:189–94. doi: 10.1123/ijsnem.21.3.189. [DOI] [PubMed] [Google Scholar]
- 32.Dascombe BJ, Reaburn PRJ, Sirotic AC, Coutts AJ. The reliability of the i-STAT clinical portable analyser. J Sci Med Sport. 2007;10:135–40. doi: 10.1016/j.jsams.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Kushner MG, Sher KJ, Wood MD, Wood PK. Anxiety and drinking behavior: moderating effects of tension-reduction alcohol outcome expectancies. Alcohol Clin Exp Res. 1994;18:852–60. doi: 10.1111/j.1530-0277.1994.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 34.Reilly T. Human circadian rhythms and exercise. Crit Rev Biomed Eng. 1990;18:165–80. [PubMed] [Google Scholar]
- 35.Yoon B-K, Kravitz L, Robergs R. VO2max, protocol duration, and the VO2 plateau. Med Sci Sports Exerc. 2007;39:1186–92. doi: 10.1249/mss.0b13e318054e304. [DOI] [PubMed] [Google Scholar]
- 36.Gurton WH, Faulkner SH, James RM. Effect of warm-up and sodium bicarbonate ingestion on 4-km cycling time-trial performance. Int J Sports Physiol Perform. 2021;16:1573–9. doi: 10.1123/ijspp.2020-0743. [DOI] [PubMed] [Google Scholar]
- 37.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen J. Statistical power analysis for the behavioral sciences. 2. New York: Routledge; 1988. [Google Scholar]
- 39.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivarsson A, Andersen MB, Johnson U, Lindwall M. To adjust or not adjust: Nonparametric effect sizes, confidence intervals, and real-world meaning. Psychol Sport Exerc. 2013;14:97–102. doi: 10.1016/j.psychsport.2012.07.007. [DOI] [Google Scholar]
- 41.Hayes AF. Introduction to mediation, moderation, and conditional process analysis, second edition: a regression-based approach. New York: Guilford Publications; 2017. [Google Scholar]
- 42.Charalambous A, Giannakopoulou M, Bozas E, Paikousis L. Parallel and serial mediation analysis between pain, anxiety, depression, fatigue and nausea, vomiting and retching within a randomised controlled trial in patients with breast and prostate cancer. BMJ Open. 2019;9:e026809. doi: 10.1136/bmjopen-2018-026809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67:451–70. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 44.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16:93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- 45.Durkalec-Michalski K, Zawieja EE, Zawieja BE, Michałowska P, Podgórski T. The gender dependent influence of sodium bicarbonate supplementation on anaerobic power and specific performance in female and male wrestlers. Sci Rep. 2020;10:1878. doi: 10.1038/s41598-020-57590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gam S, Guelfi KJ, Fournier PA. New insights into enhancing maximal exercise performance through the use of a bitter tastant. Sports Med. 2016;46:1385–90. doi: 10.1007/s40279-016-0522-0. [DOI] [PubMed] [Google Scholar]
- 47.Best R, McDonald K, Hurst P, Pickering C. Can taste be ergogenic? Eur J Nutr. 2021;60:45–54. doi: 10.1007/s00394-020-02274-5. [DOI] [PubMed] [Google Scholar]
- 48.Marcora SM, Staiano W, Manning V. Mental fatigue impairs physical performance in humans. J Appl Physiol. 2009;106:857–64. doi: 10.1152/japplphysiol.91324.2008. [DOI] [PubMed] [Google Scholar]
- 49.Marcora SM, Staiano W. The limit to exercise tolerance in humans: mind over muscle? Eur J Appl Physiol. 2010;109:763–70. doi: 10.1007/s00421-010-1418-6. [DOI] [PubMed] [Google Scholar]
- 50.Gough LA, Williams JJ, Newbury JW, Gurton WH. The effects of sodium bicarbonate supplementation at individual time-to-peak blood bicarbonate on 4-km cycling time trial performance in the heat. Eur J Sport Sci. 2021;22:1856–64. doi: 10.1080/17461391.2021.1998644. [DOI] [PubMed] [Google Scholar]
- 51.Durkalec-Michalski K, Nowaczyk PM, Adrian J, Kamińska J, Podgórski T. The influence of progressive-chronic and acute sodium bicarbonate supplementation on anaerobic power and specific performance in team sports: a randomized, double-blind, placebo-controlled crossover study. Nutr Metab. 2020;17:38. doi: 10.1186/s12986-020-00457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva R, Oliveira L, Saunders B, Kratz C, Painelli V, Silva V, et al. Effects of β-alanine and sodium bicarbonate supplementation on the estimated energy system contribution during high-intensity intermittent exercise. Amino Acids. 2019;51:83–96. doi: 10.1007/s00726-018-2643-2. [DOI] [PubMed] [Google Scholar]
- 53.Beedie C, Benedetti F, Barbiani D, Camerone E, Cohen E, Coleman D, et al. Consensus statement on placebo effects in sports and exercise: the need for conceptual clarity, methodological rigour, and the elucidation of neurobiological mechanisms. Eur J Sport Sci. 2018;18:1383–9. doi: 10.1080/17461391.2018.1496144. [DOI] [PubMed] [Google Scholar]
- 54.Farias De Oliveira L, Saunders B, Yamaguchi G, Swinton P, Giannini Artioli G. Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Med Sci Sports Exerc. 2020;52:1801–8. doi: 10.1249/MSS.0000000000002313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.