Abstract
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are the most common healthcare-associated infections, with rates varying between countries. Antimicrobial resistance (AMR) among common HAP/VAP pathogens has been reported, and multidrug resistance (MDR) is of further concern across Middle Eastern countries. This narrative review summarizes the incidence and pathogens associated with HAP/VAP in hospitals across Gulf Cooperation Council (GCC) countries. A PubMed literature search was limited to available data on HAP or VAP in patients of any age published within the past 10 years. Reviews, non-English language articles, and studies not reporting HAP/VAP data specific to a GCC country were excluded. Overall, 41 articles, a majority of which focused on VAP, were selected for inclusion after full-text screening. Studies conducted over multiple years showed a general reduction in VAP rates over time, with Gram-negative bacteria the most commonly reported pathogens. Gram-negative isolates reported across GCC countries included Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae. Rates of AMR varied widely among studies, and MDR among A. baumannii, K. pneumoniae, Escherichia coli, P. aeruginosa, and Staphylococcus aureus isolates was commonly reported. In Saudi Arabia, between 2015 and 2019, rates of carbapenem resistance among Gram-negative bacteria were 19–25%; another study (2004–2009) reported antimicrobial resistance rates in Acinetobacter species (60–89%), P. aeruginosa (13–31%), and Klebsiella species (100% ampicillin, 0–13% other antimicrobials). Although limited genotype data were reported, OXA-48 was found in ≥ 68% of patients in Saudi Arabia with carbapenem-resistant Enterobacteriaceae infections. Ventilator utilization ratios varied across studies, with rates up to 0.9 reported in patients admitted to adult medical/surgical intensive care units in both Kuwait and Saudi Arabia. VAP remains a burden across GCC countries albeit with decreases in rates over time. Evaluation of prevention and treatment measures and implementation of a surveillance program could be useful for the management of HAP and VAP.
Keywords: Antimicrobial resistance, Critical care, Hospital-acquired pneumonia, Limited-resource countries, Mechanical ventilation, Surveillance, Ventilator-associated pneumonia
Key Summary Points
| Hospital-acquired and ventilator-associated pneumonia (HAP/VAP) infections represent potentially costly comorbidities, with rates of acquisition that vary widely between nations. |
| Articles described VAP rates in Gulf Cooperation Council member states that varied widely by nation and time, with multi-year studies suggesting a trend of decreasing VAP rates over the last 10–15 years. |
| The most commonly reported organisms associated with HAP/VAP infections were multidrug-resistant species in the genera Acinetobacter, Pseudomonas, and Klebsiella, although methicillin (meticillin)-resistant Staphylococcus aureus and Streptococcus pneumonia were also commonly identified. |
| The high frequency of treatment-resistant bacterial pathogens represents a challenge to further improvement in managing these infections. |
Introduction
Hospital-acquired infections (HAIs), also referred to by the broader term of healthcare-associated infections, are typically defined as infections that occur during care in a hospital facility that were not present or incubating at the time of admission [1]. HAIs usually manifest ≥ 48 h after hospital admission and include infections that present after hospital discharge. Hospital-acquired pneumonia (HAP; pneumonia occurring ≥ 48 h after hospital admission) and ventilator-associated pneumonia (VAP; pneumonia occurring ≥ 48 h after endotracheal intubation) are among the most frequent types of HAI both in high-income and low-/middle-income countries [1, 2]. Use of the term HAP varies across guidelines and studies [2]; in this review, HAP encompasses any pneumonia occurring ≥ 48 h after hospital admission and therefore includes VAP as a subset.
In general, there is wide variation in VAP rates reported across studies due to a combination of factors, including country, intensive care unit (ICU) type, patient case-mix, and differing definitions of VAP [3, 4]. Surveillance of device-associated HAIs during 2012–2017 in ICUs across 45 countries of the International Nosocomial Infection Control Consortium [INICC; predominantly countries with developing economies, including Bahrain, Kuwait, Saudi Arabia, and the United Arab Emirates (UAE)] determined pooled mean VAP rates for 11 ICU types [5]. VAP rates ranged from 7.4 (surgical cardiothoracic ICUs) up to 17.7 per 1000 ventilator-days (medical cardiac ICUs), which were considerably higher than those reported in the USA.
The pathogens most frequently associated with VAP include Pseudomonas aeruginosa, Acinetobacter baumannii, Gram-negative bacilli of the Enterobacterales family (particularly Escherichia coli and Klebsiella pneumoniae), and Staphylococcus aureus [2, 3]. Antimicrobial resistance rates, including multidrug resistance among common HAP/VAP pathogens, are substantial. International surveillance data report methicillin (meticillin) resistance in approximately one half of S. aureus isolates, high rates of cefepime and piperacillin-tazobactam resistance in P. aeruginosa (up to 30%), and carbapenem resistance in > 50% of A. baumannii isolates [2]. Of further concern, multidrug resistant (MDR) pathogens pose a serious problem in Middle Eastern countries due to multiple predisposing factors, including antibiotic misuse and overuse, continuous population movement, and inadequate infection prevention and control strategies [6]. Other inherently MDR organisms as a result of extensive carbapenem use (namely Stenotrophomonas maltophilia) are also encountered in critical care units. Furthermore, apart from bacteria, certain fungi (in particular Candida auris) can be challenging for hospitals and clinicians in view of MDR and their propensity to spread, persist in the hospital environment, and cause outbreaks in ICUs that are difficult to treat [7, 8].
Infection control and surveillance in Gulf Cooperation Council (GCC) countries is coordinated by the GCC Centre for Infection Control (GCC-CIC) [9]. In addition to providing evidence-based guidelines for infection control practices, the GCC-CIC conducts educational and training activities for personnel working in infection prevention and control [9]. Current standards for respiratory therapy procedures are contained in the GCC Infection Prevention and Control Manual, including detailed protocols for the setup and maintenance of mechanical ventilation circuits and artificial airways, specimen collection, personal protective equipment, and hand hygiene [10]. However, further initiatives and more extensive participation in training activities for infection surveillance and data management are needed, and there is not yet a comprehensive and integrated system in place for collecting and compiling data from hospitals throughout the region [9].
Although all GCC countries are classified as high-income, their economies are still developing, and their healthcare systems face many of the challenges common to other developing nations [9, 11]. Subsequently, this narrative review was undertaken to summarize available data related to the incidence and associated organisms of HAP and VAP in hospitals throughout GCC countries, with a view to identifying unmet medical needs and future challenges for the management of HAP/VAP in the region.
Methods
A PubMed database search was carried out on 13 November 2021 using the search terms: “pneumonia, ventilator associated”[MeSH Terms] OR (pneumonia AND “ventilator associated”) OR “ventilator-associated pneumonia” OR (ventilat* AND associated AND pneumonia) OR ((device OR catheter) AND associated AND pneumonia) OR “healthcare-associated pneumonia”[MeSH Terms] OR (“healthcare associated” AND pneumonia) OR “healthcare-associated pneumonia” OR (healthcare AND associated AND pneumonia) OR “hospital-acquired pneumonia” OR (“hospital acquired” AND pneumonia) OR (nosocom* AND pneumonia) AND (Gulf OR Bahrain OR Kuwait OR Oman OR Qatar OR “Saudi Arabia” OR “United Arab Emirates” OR UAE) NOT Review[publication type]. The search was additionally limited to English-language articles published in the preceding 10 years.
Search results were reviewed by the authors to identify articles reporting original data related to HAP or VAP in patients of any age; articles that did not report HAP or VAP data specific to a GCC country were excluded. Search results were initially screened for relevance on the basis of the title and abstract, then full copies of each identified article were further reviewed for relevance.
This article is based on previously conducted studies and does not contain any new studies with human participants performed by any of the authors.
Results
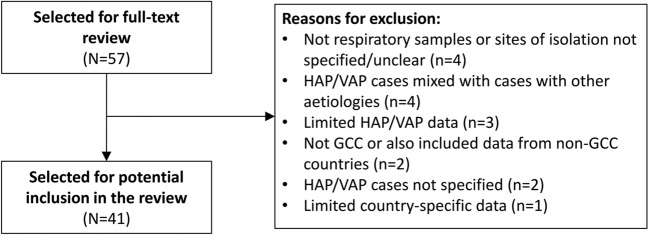
Based on title and abstract, 57 articles were identified for full-text review. Among the full-text articles assessed, 41 were ultimately selected for potential inclusion in the review (Fig. 1), including studies conducted in Saudi Arabia (n = 32), Kuwait (n = 4), Qatar (n = 2), Oman (n = 1), UAE (n = 1), and Bahrain, Oman, and Saudi Arabia (n = 1). The majority of identified studies focused on VAP, either exclusively or in the context of HAIs, with only 2 publications [12, 13] focusing on HAP; thus, the majority of the discussion in the current review focuses on VAP.
Fig. 1.
Selection process. GCC Gulf Cooperation Council, HAP hospital-associated pneumonia, VAP ventilator-associated pneumonia
VAP Rates
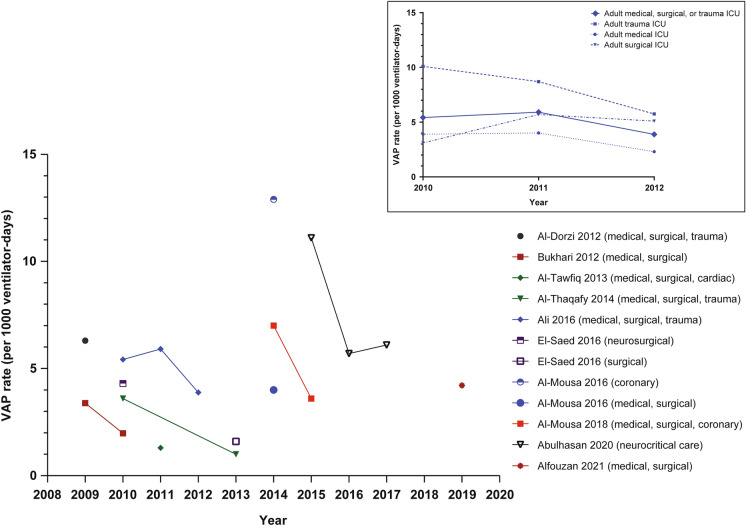
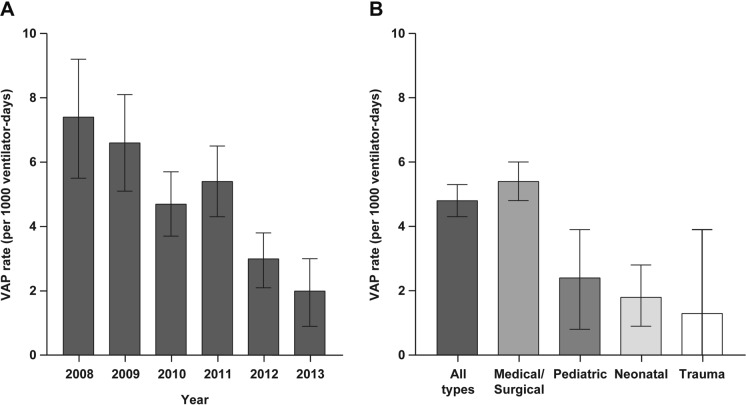
Annual VAP rates varied widely between studies and over time (Fig. 2). In the studies that reported data for multiple years, there was a general trend for reduced VAP rates over time. In support of this observation, a longitudinal study (2008–2013) conducted in Bahrain, Oman, and Saudi Arabia showed a general trend for reduced ICU-associated VAP rates over time (Fig. 3A) [9]. A number of time-series analyses reported reduced VAP rates with VAP prevention bundles [9, 14–19]. Studies identified a need for further study of prevention and intervention programs, evidence-based treatment guidelines, and increased VAP-related education activities for staff to ensure that protocols are properly implemented [20–22].
Fig. 2.
VAP rates in adult ICUs across GCC countries as reported by various studies included in this review [9, 14, 17–19, 23, 29, 32, 34]. The inset shows ICU type for data from work by Ali et al. (2016), which was the only included study describing rates in a single GCC nation, Qatar [23]. GCC Gulf Cooperation Council, ICU intensive care unit, VAP ventilator-associated pneumonia
Fig. 3.
VAP rates across ICUs in Bahrain, Oman, and Saudi Arabia, 2008–2013, as reported by El-Saed 2016 [9] A by year and B by ICU type. Note that, for panel B, the VAP rate for trauma ICUs is reported for the time period 2010–2013 (not 2008–2013). ICU intensive care unit, VAP ventilator-associated pneumonia
One study from Qatar from 2010 to 2012 that analyzed data by ICU type demonstrated higher VAP rates within trauma ICUs than within medical or surgical ICUs (Fig. 2, inset) [23]. In contrast, a study from Bahrain, Oman, and Saudi Arabia from 2008 to 2013 reported higher VAP rates within medical/surgical ICUs than trauma ICUs, although data from trauma ICUs were collected only from 2010 to 2013 (Fig. 3B) [9].
Infective Agents and Antimicrobial Resistance
Table 1 summarizes the reported associated organisms of HAP/VAP and antimicrobial resistance data. It is noteworthy that isolation of organisms from respiratory samples is not evidence that they are causative of the HAP/VAP unless they are isolated from both the blood culture and respiratory samples at the same time, which is uncommon. Thus, for the purposes of this review, we refer to organisms as being associated with VAP.
Table 1.
HAP-/VAP-associated organisms and antimicrobial resistance
| Article | Study period | Study population and setting | ICU type | Isolated organisms | Antimicrobial resistance |
|---|---|---|---|---|---|
| Saudi Arabia | |||||
| Almangour et al., 2021 [45] | May 2015–May 2019 | Adults ≥ 18 years treated at two hospitals in Riyadh who developed VAP due to MDR Gram-negative pathogens and who were treated with aerosolized plus IV colistin (n = 65) or IV colistin alone (n = 70) | NOS |
MDR Gram-negative MDR P. aeruginosa (51%) MDR A. baumannii (35%) MDR K. pneumoniae (12%) |
No colistin-resistant isolates were identified |
| Mahmoud et al., 2021 [25] | Jul 2016–Sep 2019 | Adults > 18 years classified as “comfort care” or “support care” patients and required an infection-related admission to KAMC hospital (Riyadh) during their first year | NA |
Gram-negative Pseudomonas spp., n = 58 (17%) Acinetobacter spp., n = 28 (8%) |
MDR, 92 of 342 (27%); Carbapenem resistance, 65 of 342 (19%) |
|
Gram-positive S. aureus, n = 45 (13%) | |||||
| Osman et al., 2020 [31] | Jan 2015–Mar 2018 (pre-bundle, Jan 2015–Feb 2017; post-bundle, Mar 2017–Mar 2018) | Patients aged 1 month to 14 years admitted to pediatric ICU of KAMC hospital, Jeddah, and requiring MV for > 48 h (n = 131) | Pediatric |
Gram-negative (pre-bundle; post-bundle) P. aeruginosa (36%; 27%) K. pneumoniae (12%; 36%) S. maltophilia (15%; 9%) A. baumannii (12%; 9%) E. cloacae (9%; 9%) E. coli (6%; 9%) H. influenzae (6%; 0%) Moraxella catarrhalis (3%; 0%) Serratia marcescens (3%; 0%) Gram-positive S. aureus (9%; 0%) S. pneumoniae (6%; 0%) |
Carbapenem-resistant Gram-negative, 11 of 44 (25%) Pre-bundle group (26%) Post-bundle group (18%) |
| Alsulami et al., 2020 [46] | Nov 2014–Jun 2019 | Patients aged 14–84 years who underwent open heart surgery with cardiopulmonary bypass in the surgery department of a single hospital, Jeddah (n = 200) | Surgical |
Gram-negative H. influenzae, n = 3 |
NR |
| Alraddadi et al., 2019 [13] | Jan–Nov 2017; Dec 2017–Aug 2018 (date ranges were compared since CAZ-AVI was not available until Dec 2017) |
Patients > 18 years at a single hospital in Jeddah who had CRE infections: Treated with CAZ-AVI for ≥ 24 h (total n = 10; HAP n = 5) Treated with other agents (total n = 28; HAP n = 14) |
NA |
Gram-negative, CAZ-AVI; comparator K. pneumoniae, 7 of 10 (70%); 23 of 28 (82%) E. coli, 3 of 10 (30%); 5 of 28 (18%) |
Carbapenem resistance (100%) |
| Alshamrani et al., 2019 [47] | May 2017 | All inpatients of all ages at six hospitals in five cities | NA |
Gram-negative Pseudomonas spp. (19%) Klebsiella spp. (19%) E. coli (13%) Acinetobacter spp. (7%) |
NR |
|
Gram-positive S. aureus (7%) | |||||
| Alyami et al., 2020 [48] | Jun 2013–May 2019 | Patients of all ages infected or colonized by Chryseobacterium/Elizabethkingia spp. at Prince Sultan Military Medical City, Riyadh | NOS |
Gram-negative E. meningoseptica, 22 of 27 (81%) C. indologenes, 4 of 27 (15%) Other Chryseobacterium/Elizabethkingia spp., 1 of 27 (4%) |
NR |
| Balkhy et al., 2020 [26] | 2008–2016 | Patients of all ages diagnosed with DA-HAIs in ICUs of Ministry of National Guard Health Affairs (MNGHA) hospitals in Riyadh, Jeddah, Alhassa, and Dammam | NOS |
Gram-negative Pseudomonas spp., 20 of 76 (26%) Acinetobacter spp., 18 of 76 (24%) Klebsiella spp., 10 of 76 (13%) E. coli, 6 of 76 (8%) S. maltophilia, 4 of 76 (5%) Enterobacter spp., 3 of 76 (4%) Serratia spp., 2 of 76 (3%) Other, 6 of 76 (8%) |
MRSA (50%) MDR Acinetobacter spp. (89%) Pseudomonas spp. (25%) Klebsiella spp. (11%) Enterobacter spp. (33%) E. coli (17%) Serratia spp. (0%) Stenotrophomonas spp. (0%) |
|
Gram-positive S. aureus, 2 of 76 (3%) | |||||
|
Fungi Candida spp., 4 of 76 (5%) Non-candidal yeast, 1 of 76 (1%) | |||||
| Bosaeed et al., 2020 [28] | Jan 2017–Dec 2018 | Patients ≥ 18 years diagnosed with MDR P. aeruginosa infection and treated with ceftolozane-tazobactam for ≥ 72 h at the KAMC, Riyadh | NOS |
MDR Gram-negative P. aeruginosa, n = 19 |
MDR P. aeruginosa (HAP/VAP cases): Ceftazidime and ciprofloxacin resistant, 1 of 3 (33%) Amikacin resistant, 1 of 3 (33%) |
| Hala et al., 2019 [49] | Nov 2017 | Adult male patient (mid-60s) admitted with trauma-associated airway obstruction who underwent a percutaneous tracheostomy and developed infection 1 week after surgery | NA |
Gram-negative Klebsiella quasipneumoniae subspecies similipneumoniae |
CRE |
| Abdallah et al., 2018 [12] | Feb–Mar 2017 | Male aged 31 years admitted to ICU post-exploratory laparotomy/thoracotomy with associated chest and laparotomy infections (and further complications of septic shock, acute kidney injury, and internal fixation of broken elbow) | NA |
Gram-negative P. stuartii K. pneumoniae A. baumannii |
CRE P. stuartii K. pneumoniae MDR A. baumannii |
| Al-Abdely et al., 2018 [15] | Sep 2013–Feb 2017 | 37 adult ICUs in 22 hospitals across 14 cities in Saudi Arabia | NOS |
Gram-negative, baseline; intervention A. baumannii, 46 of 110 (42%); 169 of 405 (42%) K. pneumoniae, 14 of 110 (13%); 51 of 405 (13%) P. aeruginosa, 13 of 110 (12%); 61 of 405 (15%) Acinetobacter spp., 8 of 110 (7%); 21 of 405 (5%) E. coli, 3 of 110 (3%); 14 of 405 (3%) S. maltophilia, 0 of 110 (0%); 9 of 405 (2%) |
NR |
|
Gram-positive, baseline; intervention S. aureus, 11 of 110 (10%); 28 of 405 (7%) | |||||
|
Fungi, baseline; intervention C. albicans, 6 of 110 (5%); 4 of 405 (1%) Candida spp., 1 of 110 (1%); 7 of 405 (2%) | |||||
| Alessa et al., 2018 [50] | NR | Male African-American nursing home resident aged 79 years (with multiple comorbidities, including end-stage renal disease, receiving intermittent hemodialysis and with history of P. aeruginosa airway colonization) who presented to hospital with shortness of breath and potential nasogastric tube misplacement/suspected aspiration pneumonia | NA |
Gram-negative P. aeruginosa |
MDR P. aeruginosa |
| Khan et al., 2016 [51] | Aug 2003–Dec 2010 | All patients on MV for > 48 h in the KAMC tertiary care hospital adult ICU, Riyadh (patients with burns, brain death, do-not-resuscitate orders, or transferred from other hospitals were excluded) | Adult |
Gram-negative (EO-VAP; LO-VAP) H. influenzae (25%; 2%) P. aeruginosa (5%; 16%) A. baumannii (13%; 18%) K. pneumoniae (10%; 5%) Enterobacter spp. (6%; 6%) |
NR |
|
Gram-positive (EO-VAP; LO-VAP) MSSA (11%; 7%) MRSA (5%; 4%) S. pneumoniae (8%; 2%) | |||||
| Al-Obeid et al., 2015 [33] | Jan–Dec 2012 |
A. baumannii isolates collected from patients at the Security Forces Hospital During 2012, 12 XDR- A. baumannii strains were isolated from tracheal samples of ICU patients with serious VAP (mean age 59.2 years) |
NOS |
Gram-negative A. baumannii (100%) |
XDR- A. baumannii (> 90%) |
| Shaath et al., 2014 [52] | Mar–Sep 2010 | Postoperative cardiac patients aged < 14 years in the pediatric cardiac ICU of KAMC hospital, Riyadh | Pediatric | Gram-negative (33%) | NR |
| Balkhy et al., 2014 [27] | Oct 2004–Jun 2009 | Adult ICU of KAMC tertiary care hospital, Riyadh | Adult |
Gram-negative Acinetobacter (35%) P. aeruginosa (25%) Klebsiella spp. (6%) Enterobacter spp. (4%) Haemophilus spp. (4%) S. maltophilia (3%) E. coli (2%) |
MDR Acinetobacter spp. (60–89%) 3-class (86%) 4-class (69%) P. aeruginosa (13–31%) 3-class (13%) 4-class (10%) S. aureus (oxacillin 42%) Coagulase-neg staphylococci (VRE 17%; oxacillin 100%) |
|
Gram-positive S. aureus (including MRSA; 17%) Coagulase-neg staphylococci (3%) | |||||
| El-Saed et al., 2013 [53] | Aug 2003–Jun 2009 | Adult general ICU of KAMC tertiary care hospital, Riyadh | Adult/general |
Gram-negative Acinetobacter spp. (27%) P. aeruginosa (22%) Klebsiella spp. (7%) Haemophilus spp. (6%) Enterobacter spp. (5%) |
NR |
|
Gram-positive S. aureus (15%; 37 of 70 were MRSA) | |||||
| Bukhari et al., 2012 [19] | Jan–Dec 2010 | Patients admitted to the adult medical/surgical ICU at Hera General Hospital, Makkah | Adult |
Gram-negative P. aeruginosa (31%) A. baumannii (28%) K. pneumoniae (14%) E. coli (8%) |
MDR A. baumannii (100%) P. aeruginosa (93%) K. pneumoniae (83%) MRSA (15%) |
|
Gram-positive MRSA (15%) S. aureus (2%) | |||||
| Al-Dorzi et al., 2012 [14] | Aug 2003–Jun 2009 | Patients admitted to the adult ICU of KAMC hospital in Riyadh who required MV (burn patients and brain death patients were excluded) | General | Gram-negative, 251 of 327 (77%) | NR |
| Gram-positive, 60 of 327 (20%) | |||||
| Kuwait | |||||
| Alfouzan et al., 2021 [29] | 2018, 2019 | Patients ≥ 18 years admitted to a surgical/medical ICU at Farwania Hospital, a government secondary-care hospital in Kuwait | General |
Gram-negative A. baumannii, 9 of 30 (30%) K. pneumoniae, 7 of 30 (23%) P. aeruginosa, 3 of 30 (10%) E. coli, 2 of 30 (7%) Enterobacter cloacae, 2 of 30 (7%) Morganella morganii, 1 of 30 (3%) Citrobacter koseri, 1 of 30 (3%) |
NR (not specified in patients with HAP/VAP) |
|
Gram-positive Methicillin-susceptible S. aureus, 1 of 30 (3%) | |||||
| Abulhasan et al., 2020 [32] | Jan 2015–Dec 2017 | Patients admitted to the Neurocritical Care Unit of Ibn Sina Hospital, a tertiary care teaching hospital in Kuwait (97% of patients included in the study were aged ≥ 18 years) | Neurocritical |
Gram-negative Klebsiella spp., 11 of 33 (33%) E. coli, 4 of 33 (12%) P. aeruginosa, 4 of 33 (12%) Enterobacter, 5 of 33 (15%) A. baumannii, 1 of 33 (3%) S. maltophilia, 1 of 33 (3%) Other Enterobacteriaceae, 1 of 33 (3%) |
NR (not specified for HAP patients) |
|
Gram-positive S. aureus, 5 of 33 (15%) MRSA, 1 of 33 (3%) | |||||
| Al-Mousa et al., 2018 [16] | Jan 2014–Mar 2015 (baseline period, Jan–Mar 2014; intervention period, Apr 2014–Mar 2015) | Patients admitted to three adult ICUs in two hospitals in Kuwait City | General |
Gram-negative (baseline; intervention) A. baumannii (58%; 59%) P. aeruginosa (8%; 13%) Stenotrophomonas spp. (8%; 6%) K. pneumoniae (8%; 0%) Serratia marcescens (8%; 0%) Enterobacter spp. (0%; 6%) E. coli (0%; 3%) Morganella morganii (0%; 3%) |
NR |
|
Gram-positive S. aureus (8%; 0%) | |||||
|
Fungi Candida spp. (0%, 9%) | |||||
| Oman, Qatar, or UAE | |||||
| Sannathimmappa et al., 2021 [30] | Jan 2017–Aug 2019 | All bacterial isolates from ET aspirates of ventilated patients (> 95% of sampled patients were aged ≥ 21 years) at a tertiary care ministry hospital in the North-Batinah region, Oman | NOS |
Gram-negative A. baumannii (31%) K. pneumoniae (24%) P. aeruginosa (23%) E. coli (3%) Other Enterobacteriaceae (5%) S. maltophilia (2%) |
MDR A. baumannii (86%) K. pneumoniae (73%) E. coli (67%) P. aeruginosa (24%) S. aureus (53%) Enterococcus spp. (50%) ESBL E. coli, 3 of 4 (75%) K. pneumoniae, 6 of 35 (17%) Carbapenem resistance K. pneumoniae, 23 of 35 (66%) |
|
Gram-positive S. aureus (4%) MRSA (5%) Streptococcus spp. (2%) Enterococcus spp. (1%) | |||||
| Ali et al., 2016 [23] | Jan 2010–Dec 2012 | All patients ≥ 15 years clinically diagnosed with VAP in three adult ICUs (medical, surgical, and trauma) of Hamad General Hospital, Qatar | General |
Gram-negative Pseudomonas spp., 39 of 106 (37%) Klebsiella spp., 25 of 106 (24%) Enterobacter spp., 24 of 106 (23%) Acinetobacter spp., 23 of 106 (22%) Haemophilus spp., 16 of 106 (15%) E. coli, 5 of 106 (5%) Stenotrophomonas spp., 4 of 106 (4%) |
MDR, 43 of 106 (41%) |
|
Gram-positive Staphylococcus spp., 21 of 106 (20%) Streptococcus spp., 5 of 106 (5%) | |||||
| Arumugam et al., 2018 [54] | Jan 2010–Jan 2013 | Adult trauma patients admitted to the Trauma Centre of Hamad General Hospital who required intubation (either before hospitalization or in the trauma room) and ventilation (patients with burns, drowning, death, or discharged or those who were transferred to other facilities < 48 h after admission were excluded) | Trauma |
Gram-negative (All VAP cases; VAP in PHI; VAP in TRI) K. pneumoniae (36%; 22%; 38%) H. influenzae (30%; 30%; 29%) E. cloacae (12%; 9%; 15%) P. aeruginosa (12%; 9%; 15%) A. baumanii (7%; 4%; 9%) Moraxella catarrhalis (5%; 4%; 6%) K. oxytoca (4%; 4%; 3%) K. ozonae (5%; 13%; 0%) |
MDR A. baumannii, 1 of 23 (4%) patients intubated before hospitalization A. baumannii and S. aureus, 2 of 34 (6%) patients intubated on arrival in the trauma room |
|
Gram-positive S. aureus (28%; 35%; 24%) S. pneumoniae (16%; 13%; 18%) | |||||
| Sonnevend et al., 2013 [55] | 2009–2011 | Clinically relevant NDM-producing carbapenem-resistant Enterobacteriaceae isolated from major hospitals in the UAE | NA |
Gram-negative K. pneumoniae, 3 of 5 (60%) E. coli, 2 of 5 (40%) |
Carbapenem resistance K. pneumoniae (100%) E. coli (100%) |
CAZ-AVI ceftazidime-avibactam, CRE carbapenem-resistant Enterobacteriaceae, DA device-associated, ESBL extended-spectrum β-lactamase, EO early onset, ET endotracheal, HAI hospital-acquired infection, HAP hospital-acquired pneumonia, ICU intensive care unit, IV intravenous, KAMC King Abdulaziz Medical City, LO late onset, MDR multidrug-resistant, MIC minimum inhibitory concentration, MRSA methicillin-resistant S. aureus, MSSA methicillin-sensitive S. aureus, MV mechanical ventilation, NA not applicable, NDM New Delhi metallo-beta-lactamase, NOS not otherwise specified, NR not reported, PHI prehospital intubation, TRI trauma room intubation, UAE United Arab Emirates, VAP ventilator-associated pneumonia, XDR extensively drug-resistant
Gram-negative bacteria were the most commonly reported organisms overall, including Acinetobacter species (particularly A. baumannii), Pseudomonas species (particularly P. aeruginosa), and Klebsiella species (particularly K. pneumoniae). Gram-positive bacteria included methicillin-resistant S. aureus and Streptococcus pneumoniae. Other pathogens of note were S. maltophilia, which is an important nosocomial pathogen in children in Saudi Arabia [24], and carbapenem-resistant Providencia stuartii [12] (Table 1).
Among studies that reported antimicrobial resistance, rates of resistance varied widely. Multidrug resistance (i.e., resistance to ≥ 1 agent in ≥ 3 antimicrobial classes) was frequently reported among A. baumannii, K. pneumoniae, E. coli, P. aeruginosa, and S. aureus isolates [23, 25–30]; vancomycin-resistant Enterococcus [26] and MDR Enterococcus species [26, 30] were rarely reported. A study in Saudi Arabia conducted from 2015 to 2018 reported carbapenem-resistant Gram-negative organisms in 25% of all VAP cases [31], while another study from Saudi Arabia from 2016 to 2019 reported 19% [25]. Two studies in Kuwait, one in Qatar, and one in Oman reported extended-spectrum beta-lactamase K. pneumoniae (17–32%), E. coli (75%), or Enterobacterales isolates (24%) [23, 29, 30, 32]. From 2004 to 2009, a tertiary care hospital in Riyadh reported 60–89% resistance to all tested antimicrobials for Acinetobacter species and 13–31% for P. aeruginosa; Klebsiella species were fully resistant to ampicillin and 0–13% were resistant to other tested antimicrobials [27].
Genotype data were sparse in the reviewed studies. One Saudi Arabian study that analyzed 12 extensively drug-resistant (XDR; defined as resistant to at least four classes of antimicrobials, including carbapenems) A. baumannii isolates from patients with VAP reported that none of the 12 isolates harbored a class B carbapenemase gene; class A carbapenemase blaGES was detected in 2 out of 12 isolates. Insertion sequence ISAba1 was detected in 5 out of 12 isolates, all isolates harbored Acinetobacter-derived cephalosporinases, and all isolates were carbapenem resistance–associated OM protein negative (i.e., had no evidence of efflux-mediated carbapenemase resistance) [33]. In another study in Saudi Arabia in patients with carbapenem-resistant infections, OXA-48 was the predominant carbapenemase in ≥ 68% of patients [13].
Ventilator Utilization Ratios
Table 2 shows reported VAP rates and ventilator utilization ratios, which varied widely over time between ICU type and between studies. A study in Kuwaiti patients from 2013 to 2015 reported a utilization rate of 0.9 for adult medical/surgical ICUs and 0.1 for adult coronary ICUs [34]. Another study in Saudi Arabia reported utilization rates of 0.7, 0.6, and 0.2 for adult medical, surgical, and cardiac ICUs, respectively [17], while a study from 2008 to 2013 conducted in Bahrain, Oman, and Saudi Arabia reported rates of 0.5–0.6 across adult ICUs [9]. There was no discernable trend over time in that study, with reported utilization ratios of 0.5 in 2008, 0.6 in 2012, and 0.5 in 2013 [9]. Another study in Saudi Arabia reported a decrease in utilization rates from 0.7 in 2003 to 0.5 in 2009 [14], while a study in Kuwait reported a rate increase from 0.7 to 0.8 in 2014 to 2015 [16].
Table 2.
Reported VAP rates and ventilator utilization ratios—all ICU types
| Source | City/country | ICU type | Year(s) | VAP definition used | VAP ratea | Ventilator utilization ratiob |
|---|---|---|---|---|---|---|
| Al-Dorzi et al., 2012 [14] | Riyadh, Saudi Arabia | Adult medical, surgical, trauma | 2003 | US CDC criteria | 19.1 | 0.7 |
| 2009 | 6.3 | 0.5 | ||||
| 2003–2009 | 15.9 | 0.6 | ||||
| Bukhari et al., 2012 [19] | Makkah, Saudi Arabia | Adult medical, surgical | 2009 | Pneumonia in a patient intubated and ventilated at the time of, or ≤ 48 h before the onset of the event | 3.4 | NR |
| 2010 | 2.0 | NR | ||||
| Al-Tawfiq et al., 2013 [17] | Dhahran, Saudi Arabia | Adult medical, surgical, cardiac | 2004 | MV with chest X-ray showing new or progressive infiltrate, consolidation, cavitation, or pleural effusion | 9.8 | NR |
| 2011 | 1.3 | NR | ||||
| 2004–2011 | 4.5 | NR | ||||
| Adult medical | 2004–2011 | 5.0 (0.4–9.4) | 0.7 (0.6–0.7) | |||
| Adult surgical | 2004–2011 | 4.9 (2.1–8.4) | 0.6 (0.5–0.7) | |||
| Adult cardiac | 2004–2011 | 1.9 (0–3.7) | 0.2 (0.2–0.3) | |||
| Al-Thaqafy et al., 2014 [18] | Riyadh, Saudi Arabia | Adult medical, surgical, trauma | 2010 | US CDC/NHSN criteria | 3.6 | 0.7 |
| 2013 | 1.0 | 0.6 | ||||
| Shaath et al., 2014 [52] | Riyadh, Saudi Arabia | Pediatric cardiac (postcardiac surgery patients) | 2010 | US CDC criteria | 29.0 | 0.3 |
| Ali et al., 2016 [23] | Doha, Qatar | Adult medical, surgical, trauma | 2010 | US CDC criteria | 5.4 | NR |
| 2011 | 5.9 | NR | ||||
| 2012 | 3.9 | NR | ||||
| Adult medical | 2010 | 3.9 | NR | |||
| 2011 | 4.0 | NR | ||||
| 2012 | 2.3 | NR | ||||
| Adult surgical | 2010 | 3.1 | NR | |||
| 2011 | 5.7 | NR | ||||
| 2012 | 5.1 | NR | ||||
| Adult trauma | 2010 | 10.1 | NR | |||
| 2011 | 8.7 | NR | ||||
| 2012 | 5.8 | NR | ||||
| Al-Mousa et al., 2016 [34] | Kuwait City, Kuwait | Adult medical, surgical | Nov 2013–Mar 2015 | US CDC/NHSN criteria | 4.0 (2.9–5.3) | 0.9 (0.9–0.9) |
| Adult coronary | Nov 2013–Mar 2015 | 12.9 | 0.1 (0.1–0.1) | |||
| Pediatric | Nov 2013–Mar 2015 | 0.3 (0.2–4.6) | 0.7 (0.7–0.7) | |||
| Neonatal | Nov 2013–Mar 2015 | 1.0 | 0.5 (0.4–0.5) | |||
| El-Saed et al., 2016 [9] | Bahrain, Oman, and Saudi Arabia | Medical/surgical, neurosurgical, surgical, trauma, pediatric, pediatric cardiothoracic, and neonatal | 2008–2013 | NHSN criteria | 4.8 (4.3–5.3) | 0.6 (0.6–0.6) |
| 2008 | 7.4 (5.5–9.2) | 0.5 (0.5–0.5) | ||||
| 2009 | 6.6 (5.1–8.1) | 0.6 (0.6–0.6) | ||||
| 2010 | 4.7 (3.7–5.7) | 0.6 (0.6–0.6) | ||||
| 2011 | 5.4 (4.3–6.5) | 0.6 (0.6–0.6) | ||||
| 2012 | 3.0 (2.1–3.8) | 0.6 (0.6–0.6) | ||||
| 2013 | 2.0 (0.9–3.0) | 0.5 (0.5–0.5) | ||||
| Medical/surgical | 2008–2013 | 5.4 (4.8–6.0) | 0.6 (0.6–0.6) | |||
| Pediatric | 2008–2013 | 2.4 (0.8–3.9) | 0.5 (0.5–0.5) | |||
| Neonatal | 2008–2013 | 1.8 (0.9–2.8) | 0.3 (0.3–0.3) | |||
| Trauma | 2010–2013 | 1.3 (0.0–3.9) | 0.6 (0.6–0.6) | |||
| Neurosurgical | 2010 | 4.3 (0.1–8.5) | 0.6 (0.6–0.6) | |||
| Pediatric cardiothoracic | 2010 | 3.5 (0.1–7.0) | 0.6 (0.6–0.6) | |||
| Surgical | 2013 | 1.6 (0.0–4.8) | 0.5 (0.4–0.5) | |||
| Banjar et al., 2017 [20] | Makkah region, Saudi Arabia | Adult, surgical, coronary care, pediatric, and neonatal | 2013 | NR | 6.9c (IQR, 3.6–10.3) | NR |
| Makkah, Makkah, Saudi Arabia | Not specified | 2013 | 6.4c (IQR, 3.2–8.2) | NR | ||
| Jeddah, Makkah, Saudi Arabia | Not specified | 2013 | 8.8c (IQR, 4.6–20.2) | NR | ||
| Taif, Makkah, Saudi Arabia | Not specified | 2013 | 6.1c (IQR, 3.6–11.6) | NR | ||
| Qunfudah, Makkah, Saudi Arabia | Not specified | 2013 | 4.4c (IQR, 0.6–6.3) | NR | ||
| Al-Mousa et al., 2018 [16] | Kuwait City, Kuwait | Adult medical/surgical, coronary | Jan–Mar 2014 | US CDC/NHSN criteria | 7.0 (3.8–11.8) | 0.7 |
| Apr 2014–Mar 2015 | 3.6 (2.5–5.0) | 0.8 | ||||
| Gaid et al., 2018 [56] | Provinces of Asser, Jeddah, Riyadh, and Qassim, Saudi Arabia | Adult medical/surgical | May 2015–Feb 2016 | NR | 18.1–26.6 | 0.5 (0.5–0.6) |
| Provinces of Asser and Taif, Saudi Arabia | Adult medical/surgical | Sep 2013–Mar 2015 | 9.3–20.7 | 0.3 (0.3–0.3) | ||
| Provinces of Najran and Tabuk, Saudi Arabia | Adult medical/surgical | Sep 2013–Feb 2016 | 0.9–16.4 | 0.7 (0.6–0.7) | ||
| Provinces of Taif, Hail, and Madina, Saudi Arabia | Adult medical/surgical | Sep 2015–Mar 2016 | 10.1–51.6 | 0.9 (0.9–1.0) | ||
| Province of Riyadh, Saudi Arabia | Adult medical/surgical | Jan 2015–Feb 2016 | 186.5 | 0.1 (0.04–0.1) | ||
| Abulhasan et al., 2020 [32] | Kuwait | Neurocritical care (97% adult patients) | 2015 | US CDC/NHSN criteria | 11.1 | 0.4 |
| 2016 | 5.7 | 0.2 | ||||
| 2017 | 6.1 | 0.4 | ||||
| Ahmed et al., 2021 [57] | Riyadh, Saudi Arabia | Not specified (60-bed hospital) | 2019 | US CDC criteria | 2.1 | NR |
| Alfouzan et al., 2021 [29] | Kuwait | Adult medical/surgical | 2018, 2019 | Pneumonia in a patient on MV for > 2 days on the date of event | 4.2 | 0.7 |
CI confidence interval, ICU intensive care unit, IQR interquartile range, MV mechanical ventilation, NHSN National Healthcare Safety Network, NR not reported, US CDC US Centers of Disease Control and Prevention, VAP ventilator-associated pneumonia
aPer 1000 ventilator-days (where provided, error is 95% CI, unless otherwise indicated)
bVentilator utilization ratio is the number of ventilator days/number of patient days (where provided, error is 95% CI)
cMedian value
Discussion
This narrative review was undertaken to summarize available data related to the incidence and causative organisms of HAP and VAP in hospitals throughout GCC countries. Data from multi-year studies suggest that VAP rates have generally decreased over time in GCC countries [16, 18, 19, 23, 32]. Although time-series analyses suggest that VAP prevention bundles reduce VAP rates [9, 14–19], the contribution of individual components is somewhat unclear. A recent review found no high-level evidence to support an association between ventilator bundle implementation and reduced ventilator-associated event (VAE) risk [3]. Indeed, findings suggest that chlorhexidine and stress ulcer prophylaxis may actually increase VAE risk, while reduced mechanical ventilation duration will likely reduce VAE risk [3]. In controlled studies, a significant impact of probiotics on VAP rate has not been observed. These studies include a randomized controlled study carried out in ICUs across North America and Saudi Arabia to assess the effect of the probiotic Lactobacillus rhamnosus GG on VAP development in critically ill patients. Results of the study showed no significant difference in VAP incidence between the probiotic and placebo groups, or for any of the other prespecified outcomes [35].
Additionally, our literature review suggests that the most common organisms associated with VAP in GCC countries were Gram-negative bacteria, particularly Acinetobacter species, Pseudomonas species, and Klebsiella species, as well as Enterococcus species, with MDR reported in many studies. The types of organisms associated with HAP/VAP and the proportions of positive isolates were similar between the countries studied (Saudi Arabia, Kuwait, and Oman; Qatar; or UAE). E. coli and P. aeruginosa were present in up to approximately 40% of isolates, and A. baumannii and K. pneumoniae were isolated from > 50% of isolates in at least one of the included studies.
Our analysis also found a wide range of ventilator utilization ratios, with higher ratios increasing the likelihood of developing VAP. Thus, ventilator utilization ratio may be a useful outcome measure in VAP prevention studies [18]. However, ventilator utilization ratios are necessarily higher in certain patient populations (e.g., very-low-birth-weight infants, patients with chronic obstructive pulmonary disease); therefore, it may be misleading to compare ratios across different hospitals/facilities due to differences in patient populations [36].
Infection prevention programs commonly incorporate multimodal horizontal strategies that aim to reduce risk from all nosocomial pathogens, such as hand hygiene protocols, environmental disinfection, and prevention bundles [37, 38]. Internationally, many organizations have developed VAP prevention guidelines and tools, and VAP bundles have been widely implemented in hospitals [39–41]. While infection control and surveillance in GCC countries is coordinated by the GCC-CIC and evidence-based guidelines for infection control practices have been provided by the organization [9], local differences in antimicrobial policies and antimicrobial stewardship programs may exist [42]. Substantial variability has been noted among the VAP bundles adopted by different hospitals in other countries, although utilized components frequently include head-of-bed elevation by 30–45°, daily interruption of sedation and assessment of readiness to extubate, use of endotracheal tubes with subglottic secretion drainage ports, and avoidance of ventilator circuit changes unless visible soiling is present [40, 41]. The INICC Multidimensional Approach for VAP rate reduction incorporates multiple strategies: a VAP prevention bundle; education; outcome and process surveillance; and provision of feedback related to VAP rates, VAP consequences, and performance [34]. VAP data also constitute part of the performance dashboard of hospital infection prevention and control committees.
A limitation of this analysis is that the data were compiled from different and diverse healthcare organizations and laboratories, which have variations in definitions and diagnostic limitations, as well as differing methodologies and technologies used in the detection, speciation, and susceptibility testing of isolated organisms from patients with VAP [3]. An illustration of this variation is the implementation of the VAE model by the US National Healthcare Safety Network (NHSN) in 2013 [43], which resulted in a large difference in reported VAP incidences between the USA and Europe [3]. Further evidence of these variations and the limitations they place on these data analyses was found in the surveillance of device-associated HAIs in ICUs across the INICC, which showed that pooled mean VAP rates ranged from 7.4 to 17.7 per 1000 ventilator-days depending on ICU type. These were considerably higher than 2012/2013 US NHSN pooled means (0.7–3.6 per 1000 ventilator-days) [5]. Accordingly, comparison of national VAP rates with the USA has been cautioned against due to varying classifications. Also, studies evaluating the effect of prevention bundles on VAP rates may be difficult to generalize to the overall population. This paper is a review of different retrospective studies, and thus, generalization of the impact of prevention bundles is difficult due to missing data since, generally, most papers were not studying prevention bundles and a few studies correlated bundle implementation with VAP rates. Another limitation of the current review is that all of the included studies were conducted before the onset of the coronavirus disease 2019 (COVID-19) pandemic and, therefore, do not take into consideration the likely increases in VAP rates [44].
Conclusions
Although VAP rates have generally decreased over time in GCC countries, VAP and associated MDR organisms remain a burden. Overall, there is a need for further research, including prospective, randomized, multicenter studies carried out in the Gulf Region, to assess the diagnosis, prevention, and treatment of VAP. Research should also include details of bundles used to reduce VAP incidence, protocols of care, and toolkits used to carry out root-cause analysis on VAP infections. Furthermore, it would be useful to have an overarching multi-Gulf center surveillance program to continually monitor the incidence and prevalence of VAP and HAP and, indeed, other HAIs across the GCC countries.
Acknowledgements
Funding
This study was sponsored by Pfizer Inc, who also funded the journal’s Rapid Service Fee.
Medical Writing/Editorial Assistance
Editorial/medical writing support was provided by Philippa Jack, PhD, and Sheena Hunt, PhD, of ICON (Blue Bell, PA) and was funded by Pfizer Inc.
Author Contributions
Jehad S. Abdalla, May Albarrak, Almunther Alhasawi, Tariq Al Musawi, Basem M. Alraddadi, Walid Al Wali, Ashraf Elhoufi, Ashraf Hassanien, Nervana Habashy, and Ayman Kurdi contributed to the study conception and design. All authors commented on previous versions of the manuscript and approved the final manuscript.
Disclosures
Nervana Habashy, Ashraf Hassanien, and Ayman Kurdi are current or former employees of Pfizer Inc. and may hold stock or stock options. Jehad S. Abdalla, May Albarrak, Almunther Alhasawi, Tariq Al Musawi, Basem M. Alraddadi, Walid Al Wali, and Ashraf Elhoufi have nothing to disclose.
Compliance with Ethics Guidelines
This review article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf. Accessed 2 Nov 2022.
- 2.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uçkay I, Ahmed QA, Sax H, Pittet D. Ventilator-associated pneumonia as a quality indicator for patient safety? Clin Infect Dis. 2008;46(4):557–563. doi: 10.1086/526534. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012–2017: device-associated module. Am J Infect Control. 2020;48(4):423–432. doi: 10.1016/j.ajic.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 6.Dandachi I, Chaddad A, Hanna J, Matta J, Daoud Z. Understanding the epidemiology of multi-drug resistant gram-negative bacilli in the Middle East using a one health approach. Front Microbiol. 2019;10:1941. doi: 10.3389/fmicb.2019.01941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salah H, Sundararaju S, Dalil L, et al. Genomic epidemiology of Candida auris in Qatar reveals hospital transmission dynamics and a South Asian origin. J Fungi (Basel). 2021;7(3):240. doi: 10.3390/jof7030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaukat A, Al Ansari N, Al Wali W, et al. Experience of treating Candida auris cases at a general hospital in the state of Qatar. IDCases. 2021;23:e01007. doi: 10.1016/j.idcr.2020.e01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Saed A, Al-Jardani A, Althaqafi A, et al. Ventilator-associated pneumonia rates in critical care units in 3 Arabian Gulf countries: a 6-year surveillance study. Am J Infect Control. 2016;44(7):794–798. doi: 10.1016/j.ajic.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 10.GCC Centre for Infection Control. The GCC Infection Prevention and Control Manual. 3rd ed. Riyadh, Kingdom of Saudi Arabia: Ministry of National Guard Health Affairs; 2018, https://www.moh.gov.om/documents/236878/4737288/GCC+IPC-Manual+2018+3rd+edition.pdf/.
- 11.United Nations. World Economic Situation and Prospects—Statistical Annex. New York; 2020.
- 12.Abdallah M, Alhababi R, Alqudah N, Aldyyat B, Alharthy A. First report of carbapenem-resistant Providencia stuartii in Saudi Arabia. New Microbes New Infect. 2018;26:107–109. doi: 10.1016/j.nmni.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alraddadi BM, Saeedi M, Qutub M, Alshukairi A, Hassanien A, Wali G. Efficacy of ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. BMC Infect Dis. 2019;19(1):772. doi: 10.1186/s12879-019-4409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Dorzi HM, El-Saed A, Rishu AH, Balkhy HH, Memish ZA, Arabi YM. The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control. 2012;40(9):794–799. doi: 10.1016/j.ajic.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Al-Abdely HM, Khidir Mohammed Y, Rosenthal VD, et al. Impact of the International Nosocomial Infection Control Consortium (INICC)'s multidimensional approach on rates of ventilator-associated pneumonia in intensive care units in 22 hospitals of 14 cities of the Kingdom of Saudi Arabia. J Infect Public Health. 2018;11(5):677–684. doi: 10.1016/j.jiph.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mousa HH, Omar AA, Rosenthal VD, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional approach on rates of ventilator-associated pneumonia in intensive care units of two hospitals in Kuwait. J Infect Prev. 2018;19(4):168–176. doi: 10.1177/1757177418759745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Tawfiq JA, Amalraj A, Memish ZA. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004–2011. Int J Infect Dis. 2013;17(12):e1207–e1211. doi: 10.1016/j.ijid.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Al-Thaqafy MS, El-Saed A, Arabi YM, Balkhy HH. Association of compliance of ventilator bundle with incidence of ventilator-associated pneumonia and ventilator utilization among critical patients over 4 years. Ann Thorac Med. 2014;9(4):221–226. doi: 10.4103/1817-1737.140132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bukhari SZ, Hussain WM, Banjar AA, Fatani MI, Karima TM, Ashshi AM. Application of ventilator care bundle and its impact on ventilator associated pneumonia incidence rate in the adult intensive care unit. Saudi Med J. 2012;33(3):278–283. [PubMed] [Google Scholar]
- 20.Banjar A, Felemban M, Dhafar K, et al. Surveillance of preventive measures for ventilator associated pneumonia (VAP) and its rate in Makkah Region hospitals, Saudi Arabia. Turk J Med Sci. 2017;47(1):211–216. doi: 10.3906/sag-1510-105. [DOI] [PubMed] [Google Scholar]
- 21.Alshahwan SI, Alsowailmi G, Alsahli A, et al. The prevalence of complications of pneumonia among adults admitted to a tertiary care center in Riyadh from 2010–2017. Ann Saudi Med. 2019;39(1):29–36. doi: 10.5144/0256-4947.2019.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutiérrez JM, Millare PA, Al-Shenqiti YA, Enaya AA. Exposure to reprocessed single-use tracheal suction catheter and ventilator-associated pneumonia risk: a preliminary, single unit-based, matched case-control study. J Crit Care. 2016;32:145–151. doi: 10.1016/j.jcrc.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Ali HS, Khan FY, George S, Shaikh N, Al-Ajmi J. Epidemiology and outcome of ventilator-associated pneumonia in a heterogeneous ICU population in Qatar. Biomed Res Int. 2016;2016:8231787. doi: 10.1155/2016/8231787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alqahtani JM. Emergence of Stenotrophomonas maltophilia nosocomial isolates in a Saudi children's hospital. Risk factors and clinical characteristics. Saudi Med J. 2017;38(5):521–527. doi: 10.15537/smj.2017.5.16375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahmoud E, Abanamy R, Binawad E, et al. Infections and patterns of antibiotic utilization in support and comfort care patients: a tertiary care center experience. J Infect Public Health. 2021;14(7):839–844. doi: 10.1016/j.jiph.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Balkhy HH, El-Saed A, Alshamrani MM, et al. High burden of resistant gram negative pathogens causing device-associated healthcare infections in a tertiary care setting in Saudi Arabia, 2008–2016. J Glob Antimicrob Resist. 2020;23:26–32. doi: 10.1016/j.jgar.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Balkhy HH, El-Saed A, Maghraby R, et al. Drug-resistant ventilator associated pneumonia in a tertiary care hospital in Saudi Arabia. Ann Thorac Med. 2014;9(2):104–111. doi: 10.4103/1817-1737.128858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosaeed M, Ahmad A, Alali A, et al. Experience with ceftolozane-tazobactam for the treatment of serious Pseudomonas aeruginosa infections in Saudi tertiary care center. Infect Dis (Auckl). 2020;13:1178633720905977. doi: 10.1177/1178633720905977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alfouzan W, Dhar R, Abdo NM, Alali WQ, Rabaan AA. Epidemiology and microbiological profile of common healthcare associated infections among patients in the intensive care unit of a general hospital in Kuwait: a retrospective observational study. J Epidemiol Glob Health. 2021;11(3):302–309. doi: 10.2991/jegh.k.210524.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sannathimmappa MB, Nambiar V, Aravindakshan R, Al-Kasaby NM. Profile and antibiotic-resistance pattern of bacteria isolated from endotracheal secretions of mechanically ventilated patients at a tertiary care hospital. J Educ Health Promot. 2021;10(1):195. doi: 10.4103/jehp.jehp_1517_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osman S, Al Talhi YM, AlDabbagh M, Baksh M, Osman M, Azzam M. The incidence of ventilator-associated pneumonia (VAP) in a tertiary-care center: comparison between pre- and post-VAP prevention bundle. J Infect Public Health. 2020;13(4):552–557. doi: 10.1016/j.jiph.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Abulhasan YB, Abdullah AA, Shetty SA, Ramadan MA, Yousef W, Mokaddas EM. Health care-associated infections in a neurocritical care unit of a developing country. Neurocrit Care. 2020;32(3):836–846. doi: 10.1007/s12028-019-00856-8. [DOI] [PubMed] [Google Scholar]
- 33.Al-Obeid S, Jabri L, Al-Agamy M, Al-Omari A, Shibl A. Epidemiology of extensive drug resistant Acinetobacter baumannii (XDRAB) at Security Forces Hospital (SFH) in Kingdom of Saudi Arabia (KSA) J Chemother. 2015;27(3):156–162. doi: 10.1179/1973947815y.0000000019. [DOI] [PubMed] [Google Scholar]
- 34.Al-Mousa HH, Omar AA, Rosenthal VD, et al. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am J Infect Control. 2016;44(4):444–449. doi: 10.1016/j.ajic.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone J, Meade M, Lauzier F, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA. 2021;326(11):1024–1033. doi: 10.1001/jama.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. The NHSN Standardized Utilization Ratio (SUR). April 2022. https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sur-guide-508.pdf. Accessed 2 Nov 2022.
- 37.Bearman G, Doll M, Cooper K, Stevens MP. Hospital infection prevention: how much can we prevent and how hard should we try? Curr Infect Dis Rep. 2019;21(1):2. doi: 10.1007/s11908-019-0660-2. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14:S3–S5. doi: 10.1016/j.ijid.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Institute for Healthcare Improvement. How-to Guide: Prevent Ventilator-Associated Pneumonia. February 2012. http://www.ihi.org/resources/Pages/Tools/HowtoGuidePreventVAP.aspx. Accessed Nov 2022.
- 40.Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(suppl 2):S133–S154. doi: 10.1017/s0899823x00193894. [DOI] [PubMed] [Google Scholar]
- 41.Hellyer TP, Ewan V, Wilson P, Simpson AJ. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc. 2016;17(3):238–243. doi: 10.1177/1751143716644461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al Salman J, Al Dabal L, Bassetti M, et al. Promoting cross-regional collaboration in antimicrobial stewardship: findings of an infectious diseases working group survey in Arab countries of the Middle East. J Infect Public Health. 2021;14(7):978–984. doi: 10.1016/j.jiph.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Fan Y, Gao F, Wu Y, Zhang J, Zhu M, Xiong L. Does ventilator-associated event surveillance detect ventilator-associated pneumonia in intensive care units? A systematic review and meta-analysis. Crit Care. 2016;20(1):338. doi: 10.1186/s13054-016-1506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner-Lastinger LM, Pattabiraman V, Konnor RY, et al. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: a summary of data reported to the National Healthcare Safety Network—ADDENDUM. Infect Control Hosp Epidemiol. 2022;43(1):137. doi: 10.1017/ice.2022.10. [DOI] [PubMed] [Google Scholar]
- 45.Almangour TA, Alruwaili A, Almutairi R, et al. Aerosolized plus intravenous colistin vs intravenous colistin alone for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria: a retrospective cohort study. Int J Infect Dis. 2021;108:406–412. doi: 10.1016/j.ijid.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Alsulami OA, Konkar AE, Alalyani AA, et al. Postoperative pneumonia following open heart surgery. Cureus. 2020;12(9):e10320. doi: 10.7759/cureus.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alshamrani MM, El-Saed A, Alsaedi A, et al. Burden of healthcare-associated infections at six tertiary-care hospitals in Saudi Arabia: a point prevalence survey. Infect Control Hosp Epidemiol. 2019;40(3):355–357. doi: 10.1017/ice.2018.338. [DOI] [PubMed] [Google Scholar]
- 48.Alyami AM, Kaabia NM, AlQasim MA, et al. Chryseobacterium/Elizabethkingia species infections in Saudi Arabia. Saudi Med J. 2020;41(3):309–313. doi: 10.15537/smj.2020.3.24985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hala S, Antony CP, Alshehri M, et al. First report of Klebsiella quasipneumoniae harboring blaKPC-2 in Saudi Arabia. Antimicrob Resist Infect Control. 2019;8:203. doi: 10.1186/s13756-019-0653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alessa MA, Almangour TA, Alhossan A, Alkholief MA, Alhokail M, Tabb DE. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa pneumonia in a patient receiving intermittent hemodialysis. Am J Health Syst Pharm. 2018;75(9):e184–e188. doi: 10.2146/ajhp170056. [DOI] [PubMed] [Google Scholar]
- 51.Khan R, Al-Dorzi HM, Tamim HM, et al. The impact of onset time on the isolated pathogens and outcomes in ventilator associated pneumonia. J Infect Public Health. 2016;9(2):161–171. doi: 10.1016/j.jiph.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Shaath GA, Jijeh A, Faruqui F, Bullard L, Mehmood A, Kabbani MS. Ventilator-associated pneumonia in children after cardiac surgery. Pediatr Cardiol. 2014;35(4):627–631. doi: 10.1007/s00246-013-0830-1. [DOI] [PubMed] [Google Scholar]
- 53.El-Saed A, Balkhy HH, Al-Dorzi HM, Khan R, Rishu AH, Arabi YM. Acinetobacter is the most common pathogen associated with late-onset and recurrent ventilator-associated pneumonia in an adult intensive care unit in Saudi Arabia. Int J Infect Dis. 2013;17(9):e696–701. doi: 10.1016/j.ijid.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Arumugam SK, Mudali I, Strandvik G, El-Menyar A, Al-Hassani A, Al-Thani H. Risk factors for ventilator-associated pneumonia in trauma patients: a descriptive analysis. World J Emerg Med. 2018;9(3):203–210. doi: 10.5847/wjem.j.1920-8642.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sonnevend A, Al Baloushi A, Ghazawi A, et al. Emergence and spread of NDM-1 producer Enterobacteriaceae with contribution of IncX3 plasmids in the United Arab Emirates. J Med Microbiol. 2013;62(Pt 7):1044–1050. doi: 10.1099/jmm.0.059014-0. [DOI] [PubMed] [Google Scholar]
- 56.Gaid E, Assiri A, McNabb S, Banjar W. Device-associated nosocomial infection in general hospitals, Kingdom of Saudi Arabia, 2013–2016. J Epidemiol Glob Health. 2018;7(suppl 1):S35–s40. doi: 10.1016/j.jegh.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed N, Abbasi MS, Bin-Shuwaish MS, et al. Knowledge attitude and practice of safety measures for corona virus disease-19 (COVID-19) among general population. Niger J Clin Pract. 2021;24(7):1037–1043. doi: 10.4103/njcp.njcp_394_20. [DOI] [PubMed] [Google Scholar]