Abstract
Introduction
Clostridioides difficile infection (CDI) is a globally recognized cause of morbidity and mortality with devastating effects on health-related quality of life (HRQoL). The objective of this study was to conduct the first systematic literature review (SLR) to assess the humanistic burden of CDI on patient experiences, including HRQoL and related constructs, and attitudes towards treatment alternatives.
Methods
An SLR was conducted to identify peer-reviewed articles that assessed CDI, including recurrent CDI (rCDI), and patient-reported outcomes or HRQoL. PubMed, Embase, and the Cochrane Collaboration abstracting services were used to conduct literature searches from 2010 to 2021 in the English language. This SLR was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria.
Results
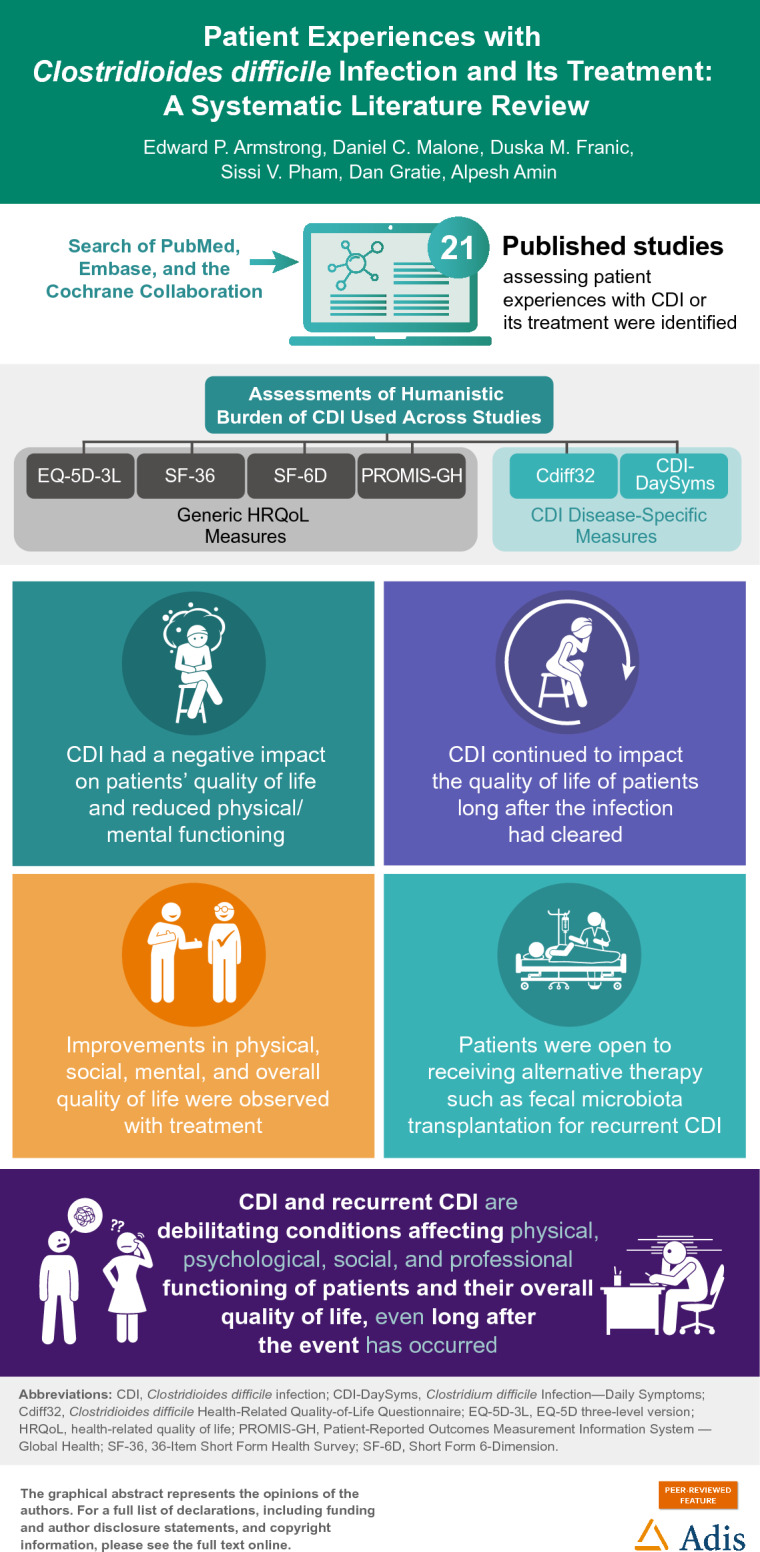
Of 511 identified articles, 21 met study inclusion criteria. The SLR showed CDI has a devastating impact on patients’ overall HRQoL that continues well beyond infection clearance. The impact of CDI on physical, emotional, social, and professional well-being rivaled abdominal symptoms of uncontrollable diarrhea, being worse for patients with rCDI. Patients with CDI feel isolated, depressed, lonely, and continue to be frightened of recurrences as well as being contagious to others. Most believe that they will never be free of CDI.
Conclusion
CDI and rCDI are debilitating conditions affecting physical, psychological, social, and professional functioning of patients’ HRQoL, even long after the event has occurred. The results of this SLR suggest that CDI is a devastating condition in need of better prevention strategies, improved psychological support, and treatments that address the microbiome disruption to break the cycle of recurrence. Additional safe and effective therapies are needed to address this unmet medical need.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00833-x.
Keywords: Clostridioides difficile, Infectious diarrhea, Microbiome, Patient engagement, Patient experience, Patient-reported outcomes, Quality of life, Survey
Plain Language Summary
Clostridioides difficile infection is a gut bacterial infection that can happen after a person has taken antibiotics to treat another infection. C. difficile infection can lead to other medical problems and death. This review of the literature aimed to understand how C. difficile infection (first, previous, and repeat occurrences), the severe diarrhea it causes, and available treatments (both old and new) for C. difficile infection can impact a person’s quality of life, daily self-care activities, and attitudes toward treatment. Results from this review of 21 studies showed that C. difficile infection has a negative impact on the quality of life of patients, affecting their physical, mental, and social health. C. difficile infection also disrupted the professional lives of patients and their ability to perform work activities. This negative effect continued over time, long after the infection had cleared because patients feared it would come back again. Treating C. difficile infection improved quality of life. Findings suggest that C. difficile infection is a devastating condition that needs better prevention strategies, improved psychological support, and treatments that stop the cycle of repeated gut infections by restoring good gut flora.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-023-00833-x.
Key Summary Points
| This is the first systematic literature review to assess the humanistic burden of Clostridioides difficile infection (CDI) on patient experiences and attitudes toward treatment alternatives. |
| The burden of CDI on physical and mental functioning was similar between patients who had active CDI and those who had cleared infection, indicating that CDI has a lasting impact on health-related quality of life (HRQoL) even after successful treatment. |
| Increased CDI disease severity and recurrence were associated with lower overall and specific HRQoL scores. |
| CDI and its associated symptoms have a debilitating impact on physical and mental health; the impact on physical, mental, social, and work/professional functioning during and after CDI clearance is profound. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.23268626.
Introduction
Clostridioides difficile infection (CDI) is a globally recognized cause of morbidity and mortality [1, 2]. Over the past decade, CDI cases have not only risen but increased in severity across the European Union (EU) and the United States (USA) [2, 3]. CDI is one of the most common, healthcare-associated bacterial infections that accounts for 15–39% of antibiotic-induced diarrhea in the USA, with adults recently completing a course of antibiotics and those older than 65 years of age being at greatest risk [3–6]. Although healthcare-associated infections have decreased, community-onset associated cases have risen over recent years in children and adults younger than 65 years of age [5, 7, 8]. Approximately 20–27% of all CDI cases are community associated with an incidence rate of 20–30 per 100,000 population across the USA, Europe, and Canada [2]. Patients with recurrent CDI (rCDI) face dire clinical sequelae, including poorer prognosis and higher complication rates [8–13]. CDI is rated as an urgent antibiotic threat by the US Centers for Disease Control and Prevention [3].
Although patient-reported outcomes (PROs) are frequently measured in noncommunicable chronic conditions, these are limited to communicable diseases. The first study conducted that assessed the lived experience of CDI on health-related quality of life (HRQoL) showed an overwhelming impact not only on physical functioning but also revealed the devastating sequalae on psychological and social domains of health [14]. CDI resulted in physical suffering, uncontrollable and explosive diarrhea, exhaustion, and shame. Patients also reported that they lacked an understanding of CDI and would benefit from better healthcare provider communication.
Antibiotics are necessary for immediate symptom relief of CDI [6, 15, 16]; however, recurrence after antibiotic use occurs in 15–30% of cases depending on the antibiotic used [15]. This is further compounded by those experiencing recurrences being at continued risk and the emergence of resistant strains [17]. Over the past few years, experimental fecal microbiota transplantation (FMT) has been shown to be a potential option to prevent further instances of rCDI treatment [14, 15, 18]. However, there are safety risks associated with its use, including transmission of host pathogens and its invasive administration mode requiring colonoscopy or enema [19]. FMT post antibiotic therapy, while resulting in wide efficacy rates ranging from 68% to 83% and 90% for open-label and real-world studies, respectively, did provide proof of concept that microbiome recovery improved clinical outcomes [15, 18]. As a result, new oral microbiome restoration products are currently undergoing clinical trials to meet US Food and Drug Administration (FDA) standards of efficacy and safety.
Despite advances in CDI treatment, no systematic literature review (SLR) has been conducted to capture patient experience data (PED) in CDI. PED encompasses the impact of the patient’s lived experiences that cannot be informed by objective numerical clinical measures, consistent with a patient-centric care model [20]. PED provides information about the humanistic burden of the condition, its impact on their HRQoL, related constructs, and treatment preferences. PED can be collected directly from patients using PROs or HRQoL measures. PED can also be sourced more broadly by the patient’s family members or caregivers, and those that treat patients. Therefore, PED encompasses information not typically collected in clinical trials, but critical for including the patient’s voice required for patient-centric care, especially for conditions considered life-threatening [20]. Furthermore, PED has gained increased traction globally with a greater importance being placed on meaningful patient outcomes [20, 21]. The purpose of this study was to conduct the first SLR to determine the humanistic burden of CDI, including the impact of rCDI, to capture patient experiences with the condition and treatments as assessed by PROs or HRQoL measures and related constructs, and attitudes towards treatment alternatives.
Methods
An SLR was conducted to identify peer-reviewed publications that assessed CDI broadly or rCDI and the attributes of PROs and HRQoL, including citations in reference lists. PubMed, Embase, and the Cochrane Collaboration abstracting services were used to conduct the SLR from 2010 to 2021, restricted to human studies and conducted in the English language. This limitation was imposed as only one disease-specific HRQoL instrument for CDI was identified since 2016 [22] by ePROVIDE™ using the broad therapeutic area search terms “CDI” and “Clostridium Difficile,” as individual searches (see Supplemental file 1 for the full list of search terms). Therefore, this SLR was anticipated to capture the full spectrum of PRO and HRQoL articles in CDI. ePROVIDE™ is a search engine that provides descriptive information on published clinical outcome assessments by condition, including PRO and HRQoL measures [23]. Reviewers (EA and DM) independently conducted literature searches and reviewed article titles and abstracts for possible inclusion in the analysis with any differences resolved by reaching consensus after collaboratively reviewing articles. This SLR was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria [24]. See Supplemental file 2 for the completed PRISMA checklist. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Papers were eligible for inclusion if they met the following criteria: empirical studies assessing the impact of CDI on HRQoL or PROs more generally to fully capture the patient’s experience. For the purposes of this review, the term CDI encompasses primary CDI (pCDI), rCDI, or current history of CDI. HRQoL data differentiated by CDI recurrence have been presented where available, see for example Han et al. [25] and Garey et al. [22]; however, data were frequently pooled without differentiating by CDI recurrence (see for example, Wilcox et al. [26], Talbot et al. [27], Kleinman et al. [28]) or CDI status (current, prior, or no CDI) (see Heinrich et al. [29] and Paul et al. [30]). Excluded were papers solely measuring treatment efficacy, economic outcomes, clinician-reported outcomes, reviews, or commentaries.
Analysis
Quantitative
First, identified PED articles were grouped into two categories: studies assessing the impact of CDI burden and interventional studies. Impact of CDI burden included PRO or HRQoL standalone studies. PROs identified were categorized as generic or condition-specific. Generic measures enable HRQoL to be measured across different conditions, severities, interventions, and age groups. However, this breadth comes at a cost—generic measures lack the sensitivity to measure changes over time within the condition as a result of a lack of content validity and item relevance. In contrast, condition-specific measures have greater content validity, ask questions considered more relevant to patients, and are better able to measure meaningful HRQoL changes, but are not generalizable to other conditions [31, 32]. Interventional studies included all PED studies with any CDI intervention: new or standard-of-care. Second, within the two categories, patient experiences with CDI (impact of CDI burden) and patient experiences with CDI interventions (attitudes towards treatment) were also assessed. Categorizations were not mutually exclusive and therefore a study could be grouped into more than one category. Frequency counts were conducted for the number of articles within each of the categories, in addition to a description of the PED findings. This SLR focused solely on PED, including qualitative studies, and excluded studies that only measured efficacy, such as randomized controlled trials. Therefore, risk of bias tools, such as the Risk of Bias (Rob 2) guidance from the Cochrane Collaboration, were not applicable [33]. Also, with the lack of a primary outcome of interest in studies on patient experiences with CDI, it was neither possible to calculate a fail-safe N, funnel plot to evaluate the results for publication bias, other measures of reporting bias, nor conduct a meta-analysis [34].
Qualitative
A thematic analysis was used to assess patient experiences with CDI and its treatment using an inductive approach. A data-driven approach was used given the exploratory nature of the present study [35] and was based on author consensus (DM, DF, and EA). Thematic analyses have been recognized for their added value for data synthesis that cannot be achieved using alternative approaches in SLRs [36].
Results
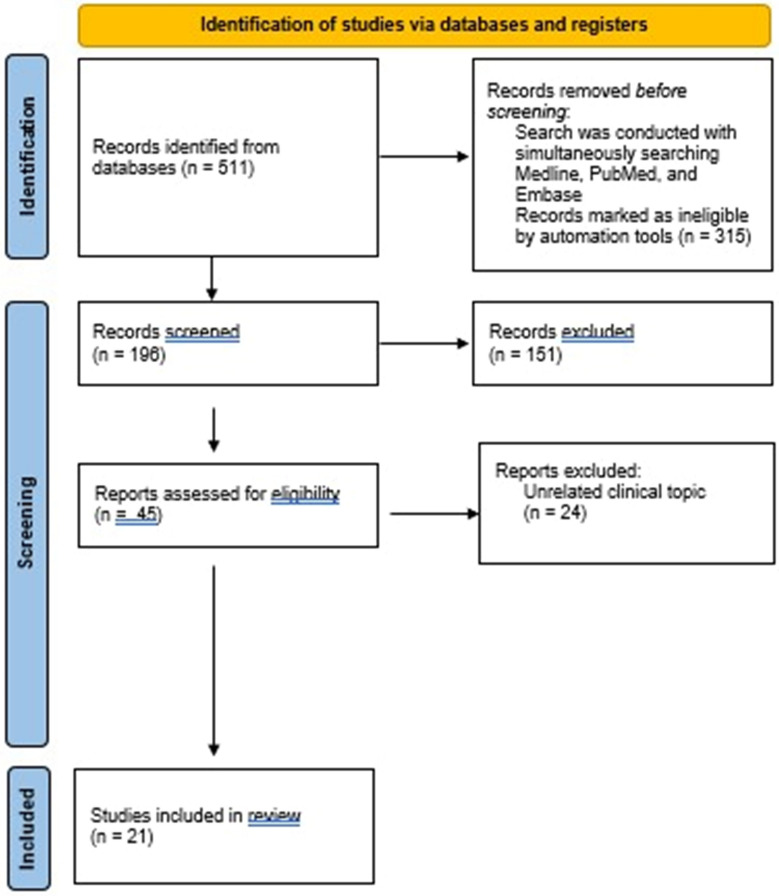
Twenty-one published articles assessing patient experiences with CDI or its treatment were identified. Figure 1 summarizes the PRISMA article selection process. Eighty percent of identified studies (17 of 21) were published after 2015. Across the 21 studies, humanistic CDI burden was assessed using four generic and two CDI disease-specific PRO measures designed for use in clinical trials. The generic HRQoL measures were EuroQol EQ-5D-3L (index and visual analogue scale [EQ VAS]) [25, 26, 30, 37], 36-Item Short Form Health Survey (SF-36)/RAND-36 [22, 29, 38–40], Short Form 6-Dimension (SF-6D) [29], and National Health Institute’s Patient-Reported Outcomes Measurement Information System—Global Health (PROMIS-GH) [25, 27, 28, 41]. The CDI disease-specific PROs included the C. difficile Health-Related Quality-of-Life Questionnaire (Cdiff32) [16, 22, 25] and the Clostridium difficile Infection–Daily Symptoms (CDI-DaySyms) [27, 28]. Seven studies identified used a qualitative approach to capture HRQoL and patient experiences or preferences with CDI [14, 41–46]. Table 1 summarizes the identified articles on patient experiences with CDI included in this review by study category.
Fig. 1.
Flow diagram for systematic review processes
Table 1.
Patient experiences with CDI by study category, number of studies, and source
| Study category | No. of studies (n = 21)a | Sources |
|---|---|---|
| Impact of CDI burden | ||
| Generic HRQoL measures | ||
| EQ-5D | 4 | Barbut et al. [37], Paul et al. [30], Wilcox et al. [26], Han et al. [25] |
| SF-36/RAND-36/SF-6D | 5 | Heinrich et al. [29], Lee et al. [39], Kao et al. [40], Misra et al. [38], Garey et al. [22] |
| PROMIS-GH | 4 | Weaver et al. [41], Han et al. [25], Talbot et al. [27], Kleinman et al. [28] |
| CDI disease-specific PROs | ||
| Cdiff32 | 3 | Han et al. [25], Garey et al. [22], Kao et al. [16] |
| CDI-DaySyms | 2 | Kleinman et al. [28], Talbot et al. [27] |
| Qualitative studies: patient experiences with CDI | 7 | Madeo et al. [14], Guillemin et al. [43], Vent-Schmidt et al. [44], Pakyz et al. [45], Lurienne et al. [46], Weaver et al. [41], Zellmer et al. [42] |
| Interventional studies | ||
| Clinical trials: FMT | 4 | Kao et al. [40], Kao et al. [16], Misra et al. [38], Lee et al. [39] |
| Clinical trials: antibiotics | 1 | Paul et al. [30] |
| Qualitative studies: patient and provider attitudes toward CDI treatment | 4 | Gill et al. [47], Zellmer et al. [42], Pakyz et al. [45], Zipursky et al. [48] |
CDI, Clostridioides difficile infection; CDI-DaySyms, Clostridium difficile Infection–Daily Symptoms; Cdiff32, Clostridioides difficile Health-Related Quality-of-Life Questionnaire; EQ-5D, EuroQol EQ-5D questionnaire; FMT, fecal microbiota transplantation; HRQoL, health-related quality of life; PRO, patient-reported outcome; PROMIS-GH, Patient-Reported Outcomes Measurement Information System—Global Health; SF-6D, Short Form 6-Dimension; SF-36, 36-Item Short Form Health Survey
aStudies can be grouped into more than one category
Nine PRO studies were identified with a primary objective to assess patient perceptions and experiences with CDI treatment. Most (eight of nine) evaluated the impact of different FMT formulations on HRQoL [16, 38–40, 42, 45, 47, 48], and one article also included overall physician perceptions toward experimental FMT treatment [47]. Finally, one additional study assessed the impact of antibiotic treatment on HRQoL in patients with CDI [30]. The 21 reviewed studies on patient experiences with CDI are described in Table 2 which highlights the PED findings of PROs, HRQoL, and patient experiences with CDI and attitudes towards treatment (Results column).
Table 2.
Summary of studies on patient experiences with CDI reviewed by year
| Sourcea | Year published | No. of patients | Country | Measurement tool(s) | Patient population | Clinical trial | Results |
|---|---|---|---|---|---|---|---|
| Kao et al. [16] | 2021 | 19 | Canada | Cdiff32 | Adults (56–67 years of age) with mild–moderate rCDI | FMT via oral capsule vs colonoscopy | No CDI recurrences were reported at day 40 (primary endpoint) in 79% (15/19) of patients and improved to 95% (18/19) after re-treatment. Patients showed improvements on all 3 Cdiff32 domains: physical, social, and mental and the global score at 40 days (primary endpoint) and 130 days |
| Han et al. [25] | 2021 | 100 | USA | Cdiff32, EQ-5D (index), PROMIS-GH | Adults hospitalized with pCDI or rCDI | PROMIS-GH GPH and GMH summary scores and EQ-5D index scores for mean score (pooled pCDI and rCDI) were significantly lower than the reference population. Severe CDI, rCDI, and number of stools were associated with lower Cdiff32 scores | |
| Lurienne et al. [46] | 2020 | 350 | USA | Qualitative and survey study to understand the burden of CDI on HRQoL | Adults with current (active CDI) or past CDI (56% pCDI: 44% 1 or more recurrences) | Impact of CDI on HRQoL is long-lasting, affecting physical and mental domains, work, and finances. Priority needs to be placed on prevention and treatment. Almost all respondents (active or history of CDI) feared recurrence | |
| Gill et al. [47] | 2020 | 54 | Australia | Survey developed to measure patient and physician perceptions of FMT treatment for rCDI or refractory CDI | Patients who had received FMT for rCDI | 69% of patients reported having no concerns regarding FMT prior to the procedure; 30% of patients had aesthetic concerns (“yuck factor”); 96% of patients would recommend FMT to others; and 94% were satisfied with treatment outcome | |
| Vent-Schmidt et al. [44] | 2020 | 167 | Canada | Survey developed to assess patient experiences with CDI. A qualitative component assessed patient priorities and physician perceptions of FMT | Patients with a history of a primary or recurrent CDI. Patients or caregivers (in lieu of patients) were surveyed | 62% of patients with CDI did not experience symptom resolution within 7 days of initiating treatment. There was a reduction in self-assessed quality of life from prior to post CDI, demonstrating a lasting effect after resolution of CDI | |
| Barbut et al. [37] | 2019 | 80 | France | EQ-5D-3L (index and EQ VAS) | Patients from 7 hospitals (acute care settings) with current CDI | Acute CDI episode resulted in a loss of utility from 0.54 to 0.05 (where 0 = death and 1 = perfect health). The low baseline score suggests significant illness and poor HRQoL prior to admission. CDI episode utility scores had a mean adjusted decrement of 0.492 (SD 0.398) from baseline. The decrement increased significantly with CDI severity, worse for women, and those > 65 years of age. The QALY loss for CDI episode, not integrating excess mortality, was 0.028 (SD 0.053) | |
| Paul et al. [30] | 2019 | 69 | USA | ED-5D-3L | Current CDI | Antibiotics: ridinilazole vs vancomycin | Patients receiving ridinilazole had significant improvement in EQ-5D-3L mean change from baseline in index and EQ VAS scores as early as day 5, but no significant improvements with vancomycin (mean scores not reported) |
| Talbot et al. [27] | 2019 | 168 | USA, Canada, Australia, Korea, and Europe | CDI-DaySyms ADL, GSRS, PROMIS global items | Patients with mild to moderate or severe CDI: pCDI or first recurrence | Psychometric validation of the newly developed CDI-DaySyms, a 10-item, 3-domain, CDI disease-specific PRO assessing abdominal symptoms, pain, and systemic/other symptoms | |
| Heinrich et al. [29] | 2018 | 2411 | USA, EU (UK, France, Germany, Italy, and Spain), China, and Brazil | SF-36, SF-6D, WPAI | ≥ 18 years old either currently being treated (n = 299), previously treated (n = 2111), or never diagnosed with CDI (n = 350,370) (NHWS data) | Current CDI and prior CDI had significantly lower HRQoL relative to never having CDI for SF-36 PCS and MCS and SF-6D (utility). Current CDI and prior CDI missed more work and greater impairment while working and more activity impairment than never having CDI. Current CDI and prior CDI had significantly greater percentage of work missed, degree of impairment while working, degree of overall work impairment, and degree of activity impairment due to health than never CDI | |
| Kleinman et al. [28] | 2018 | 34 | USA, Switzerland, Sweden | CDI-DaySyms, PROMIS-GH item | Patients with active CDI (pCDI or rCDI) | Development of the CDI-DaySyms: 13-item, CDI-specific PRO designed to measure symptoms relevant to the general CDI population | |
| Kao et al. [40] | 2017 | 116 | Canada |
SF-36 Survey developed to assess patient perceptions of FMT treatment |
Adults (18–90 years of age) with rCDI at 3 academic medical centers | FMT via oral capsule vs colonoscopy | FMT administered orally was not inferior to colonoscopy in preventing rCDI at 12 weeks. Prevention of rCDI after a single FMT treatment was 96.2% in both groups. Both modes of treatment improved SF-36 8 subscale scores in 4 weeks. There was no significant difference in HRQoL improvement by route of administration. Significantly greater proportion receiving capsules rated experience as “not at all unpleasant” |
| Lee et al. [39] | 2017 | 219 | Canada | RAND SF-36 | rCDI and refractory CDI | FMT fresh or frozen | Improvement in SF-36 8 subscale scores from baseline in 5 weeks (P < 0.05). In 7 (excluding pain) of the 8 SF-36 subscales, significant improvement was achieved as early as day 10 posttreatment |
| Weaver et al. [41] | 2017 | 119 | USA | Structured survey including selected PROMIS items/scales (GH items and isolation), emotional distress, and attitudes towards hygiene/infection and prevention | Patients with rCDI within 15 to 56 days | 58.5% rated their diarrhea as severe, 30.7% reported severe exhaustion, and 29.1% reported having severe abdominal pain. Median number of days patients were unable to participate in normal activities was 12. Respondents with severe ratings of diarrhea had 3.08 times higher odds of more days of inactivity compared to respondents with moderate/low diarrhea severity. Severe diarrhea had higher odds of being hospitalized compared to low to moderate severity. 55.6% of respondents worried about getting sick again and about being contagious (37.3%) | |
| Wilcox et al. [26] | 2017 | 30 | UK | EQ-5D-3L (index and EQ VAS) | Patients admitted with CDI (rCDI and pCDI). HRQoL was assessed comparing 30 patients hospitalized with mild/moderate and severe CDI vs healthy UK population | Mean EQ-5D-3L index values were 46% lower in patients with CDI vs UK population norms (0.42 vs 0.78). EQ VAS scores were 38% lower in CDI vs UK population norms (48 vs 77) | |
| Garey et al. [22] | 2016 | 98 | USA |
Cdiff32 RAND SF-36 |
Hospitalized patients with acute CDI (pCDI and rCDI) and ambulatory patients with multiple CDI episodes (rCDI) | Psychometric validation of the newly developed Cdiff32: 32 items related to the physical, mental, and social health of all patients with CDI | |
| Pakyz et al. [45] | 2016 | 17 | USA | Qualitative interview study | rCDI | FMT via nasogastric tube in an ambulatory setting | Before receiving FMT, findings were continuous diarrhea and weight loss, mental changes (depression, wanting to die, fear), quality of life by being unable to perform normal activities, and medication cost concerns. Respondents reached a “tipping point,” experiencing feelings of hopelessness which caused them to pursue FMT. During FMT noted a lack of “ick factor.” After FMT had symptom relief, but residual fears of recurrence. Patient activation through seeking information and empowerment was present |
| Zellmer et al. [42] | 2016 | 17 | USA | Survey developed addressing (1) patient perspectives on FMT treatment and (2) impact of CDI on HRQL (physical, social, emotional, and functioning) | Patients with CDI (2 active and 13 recovered, 2 unsure) who had previously received FMT | Patients reported that CDI affected their HRQoL, including physical, social, and emotional functioning. The impact on social functioning was the greatest with most reporting unemployment and one-third being socially inactive | |
| Guillemin et al. [43] | 2014 | 24 | France and USA | Qualitative study to determine the patient experience of CDI on their lives from admission, during, and after discharge | Patients admitted to hospital with CDI | Patients reported continuous, watery, and uncontrollable diarrhea that impacted daily lives. Diarrhea prevented usual daily activities and caused “collapse” of social lives. Humiliation and embarrassment were noted. Distress worsened once hospitalized. Feelings of loneliness and worry when placed in isolation. Improvement in psychological and physical parameters after discharge. Most patients reported persistent worry and fear of recurrent episodes, and they were more careful about their diet and hygiene | |
| Misra et al. [38] | 2014 | 34 | USA | SF-36 | Patients with rCDI | FMT (live microbiota suspension) via enema | After treatment, mean SF-36 MCS score increased from 43.6 (SD 13.0) at baseline to 51.3 (SD 11.2) at day 60, and mean PCS score increased from 44.9 (SD 10.3) at baseline to 46.0 (SD 11.1) at day 60 |
| Zipursky et al. [48] | 2012 | 192 | USA | Survey developed to assess attitudes towards FMT treatment for rCDI | Hypothetical scenarios of rCDI in lieu of patients | When patients were provided with FMT efficacy data only, 85% preferred FMT, while 15% preferred antibiotic treatment. Once patients were informed of the fecal source FMT, the preference for FMT decreased from 85% to 81% (P = not significant). FMT preference increased significantly if offered as a pill (90%) or if recommended by their physician (94%). Although FMT was rated as unappealing, women more so than men and older less so than younger, were willing to consider this alternative, with 77% willing to pay out-of-pocket for FMT | |
| Madeo et al. [14] | 2010 | 15 | USA | Qualitative study: interviews conducted to elicit patient experiences with CDI | Convenience sample of patients hospitalized with CDI | Thematic analysis revealed 4 key themes of CDI: physical suffering, lack of control over bowel function, lack of understanding of CDI, and loss of privacy and dignity |
ADL, activities of daily living; CDI, Clostridioides difficile infection; CDI-DaySyms, Clostridium difficile Infection–Daily Symptoms; Cdiff32, Clostridioides difficile Health-Related Quality-of-Life Questionnaire; EU, European Union; EQ-5D, EuroQol EQ-5D questionnaire; FMT, fecal microbiota transplantation; GMH, global mental health; GPH, global physical health; GSRS, Gastrointestinal Symptom Rating Scale; HRQoL, health-related quality of life; MCS, mental component summary; NHWS, National Health and Wellness Survey; pCDI, first or primary CDI occurrence; PCS, physical component summary; PRO, patient-reported outcome; PROMIS-GH, Patient-Reported Outcomes Measurement Information System—Global Health; QALY, quality-adjusted life year; rCDI, recurrent CDI; SD, standard deviation; SF-36, 36-Item Short Form Health Survey; SF-6D, Short Form 6-Dimension; UK, United Kingdom; USA, United States; VAS, visual analog scale; WPAI, Work Productivity and Activity Impairment
aAll 21 studies cited were peer-reviewed articles with 3 being abstracts [30, 38, 39]
Impact of CDI Burden: Generic HRQoL Measures
EQ-5D
Developed by the EuroQoL Group, EQ-5D-3L consists of two components: the self-classifier (index) and EQ VAS [49]. The EQ-5D-3L index is a 5-item measure assessing mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, and can be scored to provide utilities (preferences or HRQoL) where 0 equals death and 1.0 equals full health. The EQ VAS assesses HRQoL on a thermometer scale anchored from 0 (worst possible health) to 100 (best imaginable health) [50].
CDI showed a substantial negative impact on patients’ HRQoL in all EQ-5D studies reviewed across the USA, UK, and EU. CDI significantly reduced mean utility (HRQoL) by a range of 0.4 to 0.5 during a CDI episode from baseline in the EU and UK [26, 37]. In the UK, EQ-5D index values (EQ VAS scores) were 46% (38%) significantly lower in patients with CDI compared with the UK population norms, 0.4 versus 0.8 (48 vs 77), respectively [26]. Similarly, EQ-5D-3L index values dropped substantially by a mean of 0.5 from baseline during an acute CDI episode to a mean of 0.05 and significantly worsened with age (> 65 years), positive utility at baseline (i.e., EQ-5D-3L index value > 0), and CDI disease severity. Of note, the majority (53%) of patients with CDI rated an acute CDI episode as worse than death, mean EQ-5D-3L index value = − 0.3 [37]. Similarly, in a cross-sectional study, mean values with the EQ-5D-3L index were lower for rCDI (0.5) than pCDI (0.6) (P = 0.13) [25].
SF-36
SF-36 is the most widely used generic HRQoL measure included in clinical studies globally. Two versions of SF-36 were included in the reviewed CDI studies: Medical Outcomes Study (MOS) SF-36 and RAND-36. Both consist of the same set of 36 items from the MOS but use different scoring algorithms [50]. SF-36 consists of a physical component summary (PCS) score (including subscales of general health, physical functioning, role-physical, and bodily pain) and a mental component summary (MCS) score (social functioning, vitality, mental health, and role-emotional). MOS SF-36 uses norm-based scores (mean = 50 and standard deviation [SD] = 10), where a higher score is preferable.
Overall, SF-36 summary scores and almost all eight subscale scores showed CDI significantly reduced both physical and mental functioning [29, 39]. PCS and MCS scores were lower for those with current or past CDI versus no CDI [29]. SF-36 mean MCS score was worse for those with rCDI than pCDI (P < 0.05) [22]. Almost all SF-36 subscale scores were reported as ≤ 40, except for general health [29, 39] and vitality [29], with the greatest negative impact being on role-physical, role-emotional, and social functioning (mental health subscales) in patients with current CDI [29] or rCDI [39].
The largest PRO study identified utilized data from the National Health and Wellness Survey, an international self-report survey (Table 2). Patients with current and prior CDI had significantly lower MCS (39, 43 vs 46) and PCS (39, 41 vs 46) scores than those who have never had CDI adjusting for covariates, that is, 7 points and 3 to 5 points, respectively (P < 0.05) [29]. All differences were considered clinically meaningful (at least 2 points) [25].
RAND SF-36 domain scores for patients (n = 219) with rCDI or refractory CDI at a Canadian medical center showed decreased HRQoL (worse HRQoL) for all eight subscales (mean): physical functioning (32.2), role limitations (physical, 12.9), role limitations (emotional, 30.0), energy or fatigue (26.5), emotional well-being (55.0), social functioning (28.9), pain (40.4), and general health (40.8) at baseline [39]. Similarly, Kao et al. [40] reported lower baseline MOS SF-36 subscale scores for patients with rCDI (mean randomized to oral vs FMT at baseline): physical functioning (45 vs 35), role-physical (0 vs 0), role-emotional (0 vs 0), vitality (30 vs 25), mental health (60 vs 60), social functioning (25 vs 25), bodily pain (45 vs 45); and general health (50 vs 55) at baseline (n = 116). Adjusted utility as measured by SF-6D (where 0 = death and 1.0 = full health) was significantly lower with current or previous CDI than for those that never experienced CDI, where utility equals 0.58, 0.64 versus 0.71, respectively (P < 0.05) [29].
PROMIS-GH
PROMIS-GH is a 10-item generic HRQoL measure assessing physical, mental, and social health. PROMIS-GH provides two summary scores for global physical health (GPH) and global mental health (GMH) standardized with mean = 50 and SD = 10 in the reference population. Higher scores indicate better health [25]. In other words, a score of 40 can be interpreted as 1 SD below the reference population mean [51]. US GMH thresholds were defined as poor (< 29), fair (29–39), good (40–47), very good (49–55), and excellent (≥ 56) health. US GPH thresholds were defined as poor (< 35), fair (35–41), good (42–49), very good (50–57), and excellent (≥ 58) health [52].
Patients hospitalized with CDI (n = 100) showed fair to poor GPH and GMH summary scores for pCDI (38 and 44) and rCDI (33 and 39), respectively. The pooled GMH scores were significantly lower than the US general population (P < 0.02) [25].
Impact of CDI Burden: CDI Disease-Specific PRO Measures
Prior to 2016, there were no CDI disease-specific PRO measures despite the long-term symptoms and poor HRQoL experienced by patients with CDI. Therefore, two instruments, Cdiff32 and CDI-DaySyms, were tailored to capture concepts important to patients with CDI. The relevance of the two disease-specific PROs for patients with CDI were confirmed by thematic analyses from qualitative studies in CDI [14, 41, 43, 45, 46].
Cdiff32 is a 32-item multidimensional HRQoL measure assessing physical, mental, and social domains in patients with acute and rCDI. Cdiff32 domain and overall scores range from 0 (worst) to 100 (best) [22]. Cdiff32 was included in three PRO studies [16, 22, 25], with two comparing Cdiff32 data for pCDI versus rCDI [16, 22]. Cross-sectional data showed Cdiff32 overall (40.7 vs 50.0, P = 0.04) and mental domain (33.4 vs 46.2, P = 0.004) scores were significantly lower (worse) for rCDI than pCDI (P < 0.001). CDI disease severity and recurrence were associated with lower Cdiff32 overall HRQoL (n = 100, pCDI and rCDI) [25]. Cdiff32 overall HRQoL and all domain scores (physical, social, and mental) were significantly worse for rCDI than pCDI hospitalized patients [22]. Kao et al. [16] showed Cdiff32 overall and all domain scores significantly improved in patients with rCDI after successful treatment (P < 0.002).
CDI-DaySyms is a 10-item multidimensional PRO designed to measure symptoms relevant to patients with mild to moderate or severe CDI. CDI-DaySyms measures three symptom domains: diarrhea, abdominal, and systemic/other symptoms. CDI-DaySyms was not included in any clinical trials [27].
Impact of CDI Burden: Qualitative Studies
In addition to validated measures, qualitative studies further supported the concepts that CDI disease-specific PROs identified as important to patients [14, 41–46]. Namely, the symptoms associated with CDI on HRQoL were profound and affected physical, mental (psychological), social (impacts of daily activities), and role (work or professional activities) functioning during, and long after, CDI clearance [14, 22, 27, 29, 41, 43–46]. These multifactorial and debilitating consequences of CDI on HRQoL worsened with longer duration of illness and even a single recurrence. Alarmingly, the impact of CDI on physical and mental functioning was similar for those with active CDI when compared with those with cleared infection [22, 27, 46]. The protracted nature of CDI on role functioning also had significant financial implications for patients with CDI and continued after clearance of the infection. For those that had ceased work, unemployment continued for a mean of 118 days (during the infection) and 310 days (after clearance of infection) [46]. The HRQoL domains identified as important to patients with CDI based on the qualitative study and CDI-specific PRO measures, Cdiff32 and CDI-DaySyms, are compiled in the next section.
Physical Functioning
The CDI abdominal symptoms of continuous, watery, uncontrollable diarrhea were experienced by 60% of patients and occurred for days. These symptoms were described as debilitating, exhausting, and affected an individual’s ability to perform his/her usual activities. Specific physical symptoms attributed to CDI included disturbed sleep, fatigue, lack of energy, lightheadedness, weight loss, loss of appetite, flatulence, abdominal pain, cramping and bloating, frequent and urgent bathroom use, and atrocious smell [14, 22, 41, 43, 45, 46]. CDI was reported as physical suffering, “I am too tired to do anything!” (patient with CDI [46, p. 9]) and “I was completely drained, I could barely make it to the bathroom” (patient with rCDI [45, p. 556]).
Mental Functioning
The mental impact of CDI was high and lingering, including feelings of anxiousness, isolation, loneliness, depression, stress, irritation, anger, humiliation, shame, and embarrassment with loss of bowel control [22, 41, 43, 45, 46]. Patients with rCDI stated feeling so isolated and depressed that they “would rather die” [45, p. 556]. Additionally, most patients continued to fear worsening of CDI (92%) and future CDI recurrences (87%), with many believing that they will never be rid of it (41%), independent of recurrence history or symptom improvement [46]. Patients reported overwhelming fear, “[Feeling] scared. Very scared and thinking I can die from this…” [43, p. 100]. They continued to worry that certain foods would make it worse or that a course of antibiotics would precipitate a recurrence. Some have also reported cognitive impairment. CDI causes emotional distress and was reported as shocking and scary: “CDI is the worst of everything that I have ever had” [43, p. 97]. “I have documented PTSD” [46, p. 9].
Social Functioning
CDI affected social or leisure activities, including inability to meet with friends and family, being worried about being a burden, being frightened of being contagious, and feeling ostracized as friends and family feared contracting CDI. As one patient with rCDI described that “The hardest part was the imprisonment” (patient with rCDI [43, p. 101]). There was stigma associated with the disease. Although it has not been associated with impacting the risk for rCDI episodes, frequent handwashing was reported in almost half of all respondents [22, 41, 43, 45, 46]. The stigma associated with CDI resulted in patients feeling like an outcast and the disintegration of their social life, “I feel like everyone is afraid of me” [46, p. 9].
Role Functioning
CDI also has a negative impact on a patient’s ability to work effectively or on role functioning (professional lives) with approximately half having to stop work, and “I can’t work at all” [46, p. 9]. The impact of abdominal symptoms on role functioning was anticipated; however, CDI continued to be detrimental for almost half of the patients with CDI long after the infection had cleared. Current and prior history of CDI negatively affected role functioning because of the severe diarrheal symptoms, unpredictable need for immediate bathroom access, abdominal pain, and exhaustion resulting in an inability to work, use of sick leave, and fear of dismissal due to extended absences. The fatigue, even after hospital discharge, was so severe and continued for an extended period that some had difficulty walking and needed support for housework [14, 29, 41, 43, 45, 46].
Interventional Studies
Clinical Trials
Four clinical trials assessed the impact of unapproved FMT on HRQoL in patients with rCDI [16, 38–40]. Three were single-arm studies conducted in patients with rCDI [16, 38, 39], and one compared different FMT routes of administration (Table 2) [40]. One study compared the impact of antibiotic treatment (ridinilazole vs vancomycin) on HRQoL in patients with CDI [30]. Of the five clinical trials, one showed an 8-point improvement in SF-36 MCS score at 8 weeks in patients with rCDI, but not PCS score [38]. For the remaining four studies, HRQoL improved significantly from baseline, independent of the PRO measure used (EQ-5D index, SF-36, and Cdiff32) or active treatment (antibiotic or FMT), in 10 days for most or all domains [16, 39], in all domains at 4–5 weeks with FMT [16, 39], and at 12 weeks [16, 39, 40]. Ridinilazole-treated patients also showed significant improvements in HRQoL in all five EQ-5D domains at 5 days, although vancomycin treatment took longer to show HRQoL improvement (Table 2) [30]. For SF-36, seven of eight subscale scores improved at day 10 after FMT with the pain domain lagging behind in one study (P < 0.05) [39], and all eight subscales improved at 4–5 weeks after CDI resolution (P < 0.05) [39, 40]. The symptom of diarrhea improved within days with successful treatment of FMT in a single-arm study [39]. Similarly, for Cdiff32, significant improvements in physical, social, mental, and overall HRQoL were observed with successful treatment by day 40 in patients with rCDI [16] (Table 2).
Qualitative Studies: Patient and Provider Attitudes Toward CDI Treatment
Overall, the PED with CDI treatment highlighted patient and provider preferences for new therapies that had become available since 2012, experimental FMT, over or in comparison to standard-of-care therapies (Table 1) [42, 45, 47, 48]. FMT treatment acceptability increased significantly if offered as an odorless, colorless pill (81% vs 90%, P = 0.002), and if recommended by their physician in any administration route including pill, nasogastric tube, enema, or colonoscopy (81% vs 94%, P < 0.001). FMT was rated as unappealing, by women more so than men, in terms of the need to handle the stool, odor, and receiving FMT by nasogastric tube at 28% vs 53%, 45% vs 73%, and 52% vs 68%, respectively (P < 0.05). Similarly, FMT was rated as unappealing, by older more so than younger respondents, in terms of the need to handle the stool, odor, and color at 54% vs 28%, 71% vs 47%, and 72% vs 52%, respectively (P < 0.05). Overall, respondents acknowledged the unappealing nature of FMT but would still consider this experimental treatment given the lack of treatment options for rCDI and the trust they have in their physicians [48].
These early findings were echoed by others with FMT in two studies who considered it unappealing but necessary given the lack of options to eradicate infection [42, 45]. Prior to receiving FMT, patients had reached a “tipping point,” experiencing feelings of hopelessness which led them to pursue the experimental treatment [45]. Following FMT, respondents experienced symptom relief but had residual fears of disease recurrence [45]. Overall, patients with rCDI experiences toward FMT were positive after treatment. They were grateful to their physicians for providing an alternate treatment option but wished it was provided earlier during the acute rCDI episode and recommended further provider education regarding alternate rCDI treatments [45].
In an Australian survey focused on patient and physician barriers to FMT use, 50 physicians, although aware of FMT availability, perceived that 90% of patients would consider the treatment but 10% would not [47]. Practitioners were reluctant because they thought patients may not consider it acceptable (aesthetic concerns) and feared FMT could lead to pathogen transmission. In contrast, of the 54 patients who were treated with FMT for refractory or rCDI, 94% by colonoscopy, almost all (96%) stated that they would recommend FMT to others and 51 (94%) were satisfied with their treatment outcome. Patient perceptions prior to receiving FMT therapy were more reserved with 17 (31%) patients reporting being initially hesitant when first offered FMT. This was attributed to aesthetic concerns, the “yuck” [47, p. 951] or “gross factor” [47, p. 953], with six of 54 concerned about infection risk. When comparing different FMT routes of administration, patients preferred oral (66%) over colonoscopy-delivered (44%) treatment (P = 0.01). Most patients reported FMT was innovative (63%) and natural (41%) but “unpleasant, gross, or disgusting” (30%) [40, p. 1989] (individual items so percentages do not add up to 100). Overall, if required, 97% stated that they would consider alternative therapy again for rCDI [40] and it was physician perceptions, rather than patient aesthetic concerns, that were considered a barrier to FMT therapy in rCDI [47].
Discussion
This is the first SLR to assess the humanistic burden of CDI on patient experiences and attitudes toward treatment alternatives. HRQoL studies showed the debilitating impact of CDI, beyond physical functioning, was far greater and more protracted than originally perceived [38, 46]. The ongoing and uncontrollable diarrhea had a profound mental health impact resulting in patients feeling depressed. In addition, the impact of this multifactorial condition on physical, mental, social, and role domains failed to wane after CDI clearance [46] and was worse for those with a longer infection or even a single recurrence. Repeated recurrences were expected to worsen CDI sequalae and HRQoL [22, 25, 46].
The profound effect of CDI on HRQoL can be more readily interpreted when compared with the HRQoL scores of other gastrointestinal conditions, in addition to those of population norms. The decrement in utility for chronic inflammatory bowel disease (IBD) as measured by EQ-5D-3L (EQ VAS) was 0.1 (− 14.4) [53] with a mean EQ-5D preference of 0.81. IBD impacts all eight SF-36 subscales with the lowest means being for vitality (52–57) [54]. IBD has been described as debilitating and may lead to life-threatening complications. The decrement in mean utility for CDI was far worse at 0.4–0.5 with a mean EQ-5D-3L index value of 0.5–0.6 (although lower for rCDI than pCDI; P > 0.05) and teen values for SF-36 subscales indicating potential floor effects. The HRQoL patient experience data showed CDI has a more devastating effect on HRQoL than Crohn’s disease or ulcerative colitis [53, 54]. Most patients with an acute CDI episode reported that this was a fate worse than death with an EQ-5D-3L index value less than zero [37], which explains the quote that they “would rather die” (patient with rCDI [45, p. 556]). This demonstrates the devastation CDI has on HRQoL and supports that “the impact of CDI on HRQoL is different from other diarrheal diseases” [29, p. 2869].
Few studies provided data comparing the impact of rCDI versus pCDI on HRQoL. Generic measures (PROMIS-GH and SF-36) showed poorer HRQoL for mental health (GMH and MCS), but not for physical health (GPH or PCS) [22, 25]. Conversely, Cdiff32 showed poorer HRQoL across all domains (physical, mental, and social) and overall HRQoL for rCDI versus pCDI [22]. This discrepancy can be attributed to the lack of sensitivity of generic PROs, while the CDI-specific measures are designed to capture subtle and important changes to patients with the condition. Additionally, given the very low HRQoL scores of patients with CDI, there is most likely a floor effect that prohibits measurement of HRQoL worsening independent of their existence. This would also explain why for EQ-5D, recurrence was only a predictor for poorer HRQoL when the EQ-5D-3L index value was greater than zero and can be explained by most patients having negative utilities during an acute event [37]. Furthermore, as the utilities were most likely skewed and close to zero, some have found natural log transformations useful [55, 56].
The recent burgeoning number of studies on patient experiences with CDI shows the importance placed on the patients’ voice with these being integrated as study endpoints. As a result, use of a patient-centric approach by including patient preferences and goals for treatment and discharge plans is anticipated to optimize outcomes for patients with CDI.
Treatments associated with reducing recurrent disease episodes would be highly valued by patients. Of the limited treatment options available, several small open-label studies suggest that unapproved FMT has efficacy in reducing rCDI but carries inherent risk of transmission of undetected and emerging infectious agents [15, 57]. Improvement in HRQoL with FMT was seen in all HRQoL domains observed. PRO studies identified in this review consistently showed that although patients acknowledged that FMT was unappealing, almost all reported they were satisfied with treatment and would recommend it to others [42, 47, 48]. It is likely that patient acceptance of alternative treatments would also be enhanced by physician recommendation. The ideal microbiome restoration therapeutic would be consistently highly effective, orally administered (thus reducing the need for invasive procedures), safe, and with minimal side effects.
There are limitations to this SLR. First, given that reference lists were hand-searched, it is possible that some articles were unintentionally omitted. However, this SLR clearly demonstrates the consistently documented and devastating effect CDI has on all domains of HRQoL and that meaningful HRQoL improvements can be attained with successful treatment. Second, the search was limited to studies published in the English language and as a result the generalizability of the findings applies predominantly to North America and Europe. There were limited studies with patients with CDI from non-English speaking regions (Table 2). Lastly, where available, the impact of treatment on rCDI versus pCDI was presented. Unfortunately, the history of CDI diagnosis was often not reported in identified PED studies.
Conclusion
Patient experience data identified from this SLR demonstrate the profound impact of CDI is far-reaching. These negative impacts on HRQoL were shown to persist well beyond the acute CDI episode. pCDI has a devastating impact on HRQoL, and even more so with recurrences [22, 25, 46], across physical, mental, social, and role (work activities/professional life) functioning and overall HRQoL that endured over time beyond the clearance of CDI [46]. The thematic analysis of this SLR provided added insights to the quantitative analysis. Patients were aware of the unpredictability of recurrence, and almost all continued being afraid of recurrences that could be precipitated by certain foods and antibiotic use. These feelings endured independently of the resolution of CDI or history of recurrences, believing they would never be free of CDI [46]. The impact of CDI on HRQoL was more traumatic for rCDI than pCDI with the rise of community-associated infections in younger adults [58] resulting in devastating effects on patients’ professional lives [46], in addition to financial impacts [46]. The results of this SLR suggest that CDI is a devastating condition in need of better prevention strategies, improved psychological support, and treatments that address the microbiome disruption to break the cycle of recurrence. Because of the overwhelming burden of rCDI on patients, there is an urgent need for better therapies that can reduce rCDI. Given the lasting impact of CDI after clearance, further research is warranted to assess the long-term impact of new therapies, such as FMT and newer microbiome therapies, on HRQoL and recurrence.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This work was supported by Seres Therapeutics (award number not applicable). No Seres Therapeutics product was included in this analysis. Aimmune Therapeutics, a Nestlé Health Science company funded the rapid service fee.
Editorial Assistance
Editorial assistance was provided by Cheryl Casterline, MA (Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA), and funded by Aimmune Therapeutics, a Nestlé Health Science company.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Edward P. Armstrong, Daniel C. Malone, Duska M. Francic, Sissi V. Pham, Dan Gratie made substantial contributions to the conception or design of the work and analysis and interpretation of data. Edward P. Armstrong and Daniel C. Malone were responsible for the literature search and first draft of the manuscript. Data acquisition by hand searching articles was conducted by Edward P. Armstrong, Daniel C. Malone, and Duska M. Francic. Edward P. Armstrong, Daniel C. Malone, Duska M. Francic, Sissi V. Pham, Dan Gratie, and Alpesh Amin have reviewed and approved the final version of the manuscript.
Disclosures
Sissi V. Pham, Dan Gratie, Duska M. Franic, and Alpesh Amin are consultants to Seres Therapeutics. In addition, Alpesh Amin has been PI or Co-I of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion. Alpesh Amin has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Spero, Eli Lilly, Millennium, HeartRite, Aseptiscope, and Sprightly. Daniel C. Malone and Edward P. Armstrong are consultants to Seres Therapeutics through Strategic Therapeutics, LLC. Seres Therapeutics has an investigational product being studied in the treatment of Clostridioides difficile infections; however, no study assessed in this analysis used a Seres Therapeutics product.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balsells E, Shi T, Leese C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health. 2019;9(1):010407. doi: 10.7189/jogh.09.010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessa FC, Gould CV, McDonald LC. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65–70. doi: 10.1093/cid/cis319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2019 AR Threats Report. https://www.cdc.gov/drugresistance/biggest-threats.html#cdiff. Accessed 15 June 2023.
- 4.Ma GK, Brensinger CM, Wu Q, Lewis JD. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med. 2017;167(3):152–158. doi: 10.7326/M16-2733. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert JA. Microbiome therapy for recurrent Clostridioides difficile. Lancet Microbe. 2022;3(5):e334. doi: 10.1016/S2666-5247(22)00096-9. [DOI] [PubMed] [Google Scholar]
- 6.Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. 2022;386(3):220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 7.Guh AY, Yi SH, Baggs J, et al. Comparison of the risk of recurrent Clostridioides difficile infections among patients in 2018 versus 2013. Open Forum Infect Dis. 2022;9(9):ofacc422. doi: 10.1093/ofid/ofac422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guh AY, Mu Y, Winston LG, et al. Trends in US burden of Clostridioides difficile infection and outcomes. N Engl J Med. 2020;382(14):1320–1330. doi: 10.1056/NEJMoa1910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allegretti JR, Marcus J, Storm M, et al. Clinical predictors of recurrence after primary Clostridioides difficile infection: a prospective cohort study. Dig Dis Sci. 2020;65(6):1761–1766. doi: 10.1007/s10620-019-05900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verheyen E, Dalapathi V, Arora S, et al. High 30-day readmission rates associated with Clostridium difficile infection. Am J Infect Control. 2019;47(8):922–927. doi: 10.1016/j.ajic.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Scaria E, Powell WR, Birstler J, et al. Neighborhood disadvantage and 30-day readmission risk following Clostridioides difficile infection hospitalization. BMC Infect Dis. 2020;20(1):762. doi: 10.1186/s12879-020-05481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zilberberg MD, Shorr AF, Micek ST, Kollef MH. Clostridium difficile recurrence is a strong predictor of 30-day rehospitalization among patients in intensive care. Infect Control Hosp Epidemiol. 2015;36(3):273–279. doi: 10.1017/ice.2014.47. [DOI] [PubMed] [Google Scholar]
- 13.Enoch DA, Murray-Thomas T, Adomakoh N, et al. Risk of complications and mortality following recurrent and non-recurrent Clostridioides difficile infection: a retrospective observational database study in England. J Hosp Infect. 2020;106(4):793–803. doi: 10.1016/j.jhin.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Madeo M, Boyack M. Using the lived experiences of patients with Clostridium difficile infection to improve care. Nurs Times. 2010;106(36):10–13. [PubMed] [Google Scholar]
- 15.Kelly CR, Yen EF, Grinspan AM, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT National Registry. Gastroenterology. 2021;160(1):183–192. doi: 10.1053/j.gastro.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao D, Wong K, Franz R, et al. The effect of a microbial ecosystem therapeutic (MET-2) on recurrent Clostridioides difficile infection: a phase 1, open-label, single-group trial. Lancet Gastroenterol Hepatol. 2021;6(4):282–291. doi: 10.1016/S2468-1253(21)00007-8. [DOI] [PubMed] [Google Scholar]
- 17.Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16–24. doi: 10.5009/gnl18071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(8):1351–1358. doi: 10.1093/cid/ciy721. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Zhu J, McCarty TR, et al. Cost-effectiveness analysis of sequential fecal microbiota transplantation for fulminant Clostridioides difficile infection. J Gastroenterol Hepatol. 2021;36(9):2432–2440. doi: 10.1111/jgh.15483. [DOI] [PubMed] [Google Scholar]
- 20.Wu Z, Bandini A, Brazeau AS, Rabasa-Lhoret R. Patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs), it's time to give more credits to patients' voice in research: the example of assessing hypoglycemia burden. Diabetes Metab. 2023;49(2):101417. doi: 10.1016/j.diabet.2022.101417. [DOI] [PubMed] [Google Scholar]
- 21.Schuster ALR, Hampel H, Paskett ED, Bridges JFP. Rethinking patient engagement in cancer research. Patient. 2023;16(2):89–93. doi: 10.1007/s40271-022-00604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol. 2016;50(8):631–637. doi: 10.1097/MCG.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ePROVIDE. 2023. https://eprovide.mapi-trust.org/. Accessed 16 June 2023.
- 24.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han Z, Lapin B, Garey KW, Donskey CJ, Deshpande A. Impact of Clostridioides difficile infection on patient-reported quality of life. Infect Control Hosp Epidemiol. 2021;2021:1–6. doi: 10.1017/ice.2021.413. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox MH, Ahir H, Coia JE, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72(9):2647–2656. doi: 10.1093/jac/dkx174. [DOI] [PubMed] [Google Scholar]
- 27.Talbot GH, Kleinman L, Davies E, et al. Clostridium difficile Infection-Daily Symptoms (CDI-DaySyms) questionnaire: psychometric characteristics and responder thresholds. Health Qual Life Outcomes. 2019;17(1):77. doi: 10.1186/s12955-019-1142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinman L, Talbot GH, Hunsche E, Schuler R, Nord CE. The CDI-DaySyms: content development of a new patient-reported outcome questionnaire for symptoms of Clostridium difficile infection. Value Health. 2018;21(4):441–448. doi: 10.1016/j.jval.2017.08.3017. [DOI] [PubMed] [Google Scholar]
- 29.Heinrich K, Harnett J, Vietri J, et al. Impaired quality of life, work, and activities among adults with Clostridium difficile infection: a multinational survey. Dig Dis Sci. 2018;63(11):2864–2873. doi: 10.1007/s10620-018-5222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul S, Vickers R, Garey K. Quality of life changes in patients with Clostridium difficile infection (CDI): a randomized, double-blind trial of ridinilazole (RDZ) compared with vancomycin (VAN) Open Forum Infect Dis. 2019;6:S306. doi: 10.1093/ofid/ofz360.736. [DOI] [Google Scholar]
- 31.Wolinsky FD, Wyrwich KW, Nienaber NA, Tierney WM. Generic versus disease-specific health status measures. An example using coronary artery disease and congestive heart failure patients. Eval Health Prof. 1998;21(2):216–243. doi: 10.1177/016327879802100205. [DOI] [PubMed] [Google Scholar]
- 32.Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care. 1989;27(3 Suppl):S217–S232. doi: 10.1097/00005650-198903001-00018. [DOI] [PubMed] [Google Scholar]
- 33.Higgins JT, Chandler J, Cumpston M, et al. Cochrane handbook for systematic reviews of interventions version 6.3, 2022. The Cochrane Collaboration. https://training.cochrane.org/handbook. Accessed 16 June 2023.
- 34.Borenstein M. Introduction to meta-analysis. Hoboken: Wiley; 2009. [Google Scholar]
- 35.Mayring P. Qualitative content analysis: theoretical foundation, basic procedures and software solution. Klagenfurt; 2014. https://www.ssoar.info/ssoar/handle/document/39517. Accessed 16 June 2023.
- 36.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8:45. doi: 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbut F, Galperine T, Vanhems P, et al. Quality of life and utility decrement associated with Clostridium difficile infection in a French hospital setting. Health Qual Life Outcomes. 2019;17(1):6. doi: 10.1186/s12955-019-1081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Misra B, Ramesh M, Sobcinski M. Evaluation of health-related quality of life in patients treated with RBX2660 (microbiota suspension) for recurrent C. difficile infection: 652. Am J Gastroenterol. 2014;109:S188. doi: 10.14309/00000434-201410002-00652. [DOI] [Google Scholar]
- 39.Lee C, Kim P, Smith E. Outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection on quality of life. Gastroenterology. 2017;152:S949. doi: 10.1016/S0016-5085(17)33230-4. [DOI] [Google Scholar]
- 40.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318(20):1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver FM, Trick WE, Evans CT, et al. The impact of recurrent Clostridium difficile infection on patients' prevention behaviors. Infect Control Hosp Epidemiol. 2017;38(11):1351–1357. doi: 10.1017/ice.2017.208. [DOI] [PubMed] [Google Scholar]
- 42.Zellmer C, De Wolfe TJ, Van Hoof S, Blakney R, Safdar N. Patient perspectives on fecal microbiota transplantation for Clostridium difficile infection. Infect Dis Ther. 2016;5(2):155–164. doi: 10.1007/s40121-016-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillemin I, Marrel A, Lambert J, et al. Patients' experience and perception of hospital-treated Clostridium difficile infections: a qualitative study. Patient. 2014;7(1):97–105. doi: 10.1007/s40271-013-0043-y. [DOI] [PubMed] [Google Scholar]
- 44.Vent-Schmidt J, Attara GP, Lisko D, Steiner TS. Patient experiences with Clostridioides difficile infection: results of a Canada-wide survey. Patient Prefer Adherence. 2020;14:33–43. doi: 10.2147/PPA.S229539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pakyz AL, Moczygemba LR, VanderWielen LM, Edmond MB. Fecal microbiota transplantation for recurrent Clostridium difficile infection: the patient experience. Am J Infect Control. 2016;44(5):554–559. doi: 10.1016/j.ajic.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 46.Lurienne L, Bandinelli PA, Galvain T, et al. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. 2020;4(1):14. doi: 10.1186/s41687-020-0179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gill M, Blacketer C, Chitti F, et al. Physician and patient perceptions of fecal microbiota transplant for recurrent or refractory Clostridioides difficile in the first 6 years of a central stool bank. JGH Open. 2020;4(5):950–957. doi: 10.1002/jgh3.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zipursky JS, Sidorsky TI, Freedman CA, Sidorsky MN, Kirkland KB. Patient attitudes toward the use of fecal microbiota transplantation in the treatment of recurrent Clostridium difficile infection. Clin Infect Dis. 2012;55(12):1652–1658. doi: 10.1093/cid/cis809. [DOI] [PubMed] [Google Scholar]
- 49.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 50.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 51.HealthMeasures. PROMIS® Score Cut Points. 2021. https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/promis-score-cut-points. Accessed 16 Sept 2022.
- 52.Elsman EBM, Roorda LD, Crins MHP, Boers M, Terwee CB. Dutch reference values for the Patient-Reported Outcomes Measurement Information System Scale v1.2—Global Health (PROMIS-GH) J Patient Rep Outcomes. 2021;5(1):38. doi: 10.1186/s41687-021-00314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stark RG, Reitmeir P, Leidl R, Konig HH. Validity, reliability, and responsiveness of the EQ-5D in inflammatory bowel disease in Germany. Inflamm Bowel Dis. 2010;16(1):42–51. doi: 10.1002/ibd.20989. [DOI] [PubMed] [Google Scholar]
- 54.Huppertz-Hauss G, Aas E, Lie Hoivik M, et al. Comparison of the multiattribute utility instruments EQ-5D and SF-6D in a Europe-wide population-based cohort of patients with inflammatory bowel disease 10 years after diagnosis. Gastroenterol Res Pract. 2016;2016:5023973. doi: 10.1155/2016/5023973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franic DM, Pathak DS, Gafni A. Quality-adjusted life years was a poor predictor of women's willingness to pay in acute and chronic conditions: results of a survey. J Clin Epidemiol. 2005;58(3):291–303. doi: 10.1016/j.jclinepi.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Franic D, Vandenberg R, Gafni A. Are health states timeless? A test of the utility independence assumption: comparing a repeated measures design and latent growth modeling. Value Health. 2010;13(3):A4. doi: 10.1016/S1098-3015(10)72000-3. [DOI] [Google Scholar]
- 57.DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med. 2019;381(21):2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 58.Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63–72. doi: 10.2147/IDR.S46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.