Abstract
Objective
A specific type of mesenchymal stem/progenitor cells (MSPCs), CD105+ is reported to aid in cartilage regeneration through TGF-β/Smad2-signalling. The purpose of this study was to identify and characterize CD105+ MSPCs in temporomandibular joint (TMJ) cartilage.
Materials and Methods
MSPCs were isolated from mouse TMJ condyle explants and evaluated for their clonogenicity and pluripotential abilities. MSPC were examined for CD105 antigen using immunohistochemistry and flow cytometry.
Results
Immunohistochemistry revealed presence of CD105+ MSPCs in the proliferative zone of condyle’s cartilage. Only 0.2% of isolated MSPCs exhibited CD105, along with the stem cell surface markers CD44 and Sca-1. In CD105+ MSPCs, intracellular immunostaining revealed significantly higher (p < 0.05) protein levels of collagen type 1, 2, proteoglycan 4. Ability for chondrogenic differentiation was found to be significantly higher (p < 0.05) after 4 weeks compared to CD105− cells, using alcian blue staining. CD105+ cells were found to resemble an early MSPC subgroup with significantly higher gene expression of biglycan, proteoglycan 4, collagen type 2, Gli2, Sox5 (p < 0.001) and Sox9 (p < 0.05). In contrast, significantly lower levels of Runx2 (p < 0.05), Osterix, Trps1, Col10a1 (p < 0.01), Ihh (p < 0.001) related to chondrocyte senescence and commitment to osteogenic lineage, were observed compared to CD105− cells.
Conclusion
The study showed the existence of a CD105+ MSPC subgroup within TMJ fibrocartilage that may be activated to aid in fibrocartilage repair.
Keywords: CD105, Mesenchymal stem/progenitor cells, Cartilage regeneration, Temporomandibular joint
Introduction
Temporomandibular joint disorders reflect a broad cluster of degenerative conditions including TMJ osteoarthritis, which can lead to remodeling and/or degeneration and/or functional distortion of the physiological TMJ anatomy, and subsequent disfunction [1, 2].
TMJ degeneration results in an imbalance between the synthesis and degradation of the extracellular matrix (ECM) of articular fibrocartilage and subchondral bone. This can have negative functional implications. Physiologically, chondrocytes percursors and MSPCs, residing in the cartilage proliferative zone, adapt the cartilage to ongoing changes in the application of friction and load onto the cartilage surface [3–6]. MSPCs of this zone express CD44 as well as stem cell antigen 1 (Sca-1) on their surface [7]. These cells also show considerable expression of collagen type 2 (Col2a1, Indian hedgehog (Ihh) and parathyroid-hormone-related protein (PTHrP/PTHLH), signals that regulate chondrogenesis and endochondral ossification [4, 8, 9]. Suzuki and Iwata highlighted the importance of Ihh, which leads in its absence to loss of the articular disc, the lower articular cavity and severe TMJ deformities [10]. Cells of the growth plate proliferative zone show strong expression of SRY (Sex-Determining Region Y)-Box 9 (Sox9) protein, which is required for chondrocyte differentiation and therefore responsible for initiating the development of the mandibular condyle [10–12]. Sox9 is expressed by MSPCs until they become pre-hypertrophic [4]. Sox9 together with Sox5 and Sox6 are termed as the chondrogenic/Sox trio and are essential for early chondrogenesis [13]. The Sox trio provides sufficient intrinsic signaling adequate to form permanent cartilage and also activates specific enhancers in the Col2a1, Col9a1, Col11a2 and aggrecan (Agc1) genes to stimulate chondrogenic differentiation [14–18]. Senescent chondrocytes of the chondroblastic and hypertrophic cartilage zone lose their ability to synthesize collagen type 2 and show increased Col10a1 gene expression [4, 11, 19].
CD105 also known as Endogolin is an accessory part of the transforming growth factor (TGFβ) receptor (TGFβ-R) complex [20, 21]. CD105 was first described by Quackenbush et al. and found in human pre-B leukemia cells [22]. CD105 is expressed on human pre-erythroblasts, macrophages, leukemic cells, the syncytiotrophoblast of the placenta and vascular endothelium [22–25]. In most mesenchymal and epithelial cells the TGFβ-R complex consists of the type I and II receptor type [21].
Previous studies found increased ability for chondrogenic differentiation of CD105+ MSPCs in synovia and adipose tissue, due to the maintenance of transforming growth factor-beta (TGF-β) and Smad2 signaling and the subsequent higher expression of the Col2a1, Sox9 and aggrecan genes [26–28]. Those are main components and transcriptional targets for chondrogenic differentiation.
In contrast, little is known about MSPCs of the TMJ, which are known to be critical for tissue regeneration [29]. We hypothesized that MSPCs would be present in the TMJ condyle’s cartilage, and could be identified using CD105 antigen staining.
Materials and Methods
Mice
The use of mice followed the Institutional Animal Care and Use Committee (IACUC) guidelines approved and conducted by Massachusetts General Hospital (Protocol #2017N000086). Two eight-week-old C57BL/6 female mice were obtained from Charles River Laboratories (Wilmington, MA).
Harvesting of TMJ Condyle, MSPC Isolation and Colony-Forming Assay
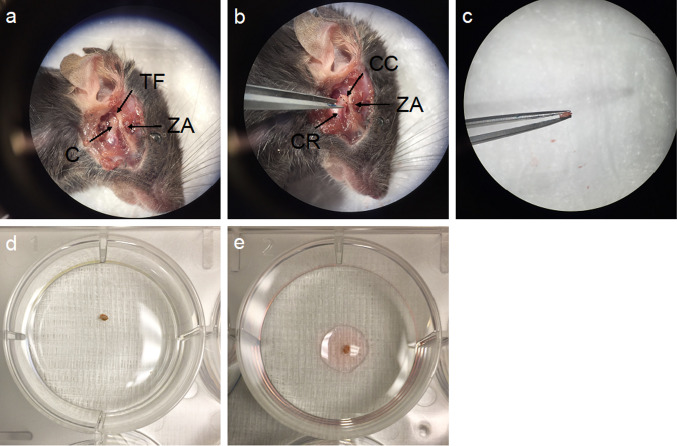
TMJs were removed in a sterile manner and condyles were explanted (n = 4). The fibrocartilage cap was carefully removed from the TMJ condyles, cleaned from surrounding soft tissue and washed using sterile phosphate-buffered saline (PBS). 50 µL of growth media, consisting of 900 mL filtered Dulbecco’s Modified Eagle medium (DMEM) (Life Technologies, Carlsbad, CA) supplemented with 10 mL of 10% fetal bovine serum (FBS) (GenDEPOT, Katy, Tx), 10 mL antibiotic, antimycotic solution (10,000 units/mL penicillin, 10 mg/mL streptomycin, 25 µg/mL amphotericin B) (Sigma-Aldrich, St. Louis, MO) and 10 mL non-essential amino acids (NEAA) (Millipore, Burlington, MA) were applied, to allow the tissue to adhere to the plate. After 2 h (h) of incubating at 37 °C, 5% CO2, 42% humidity, 1.5 mL of DMEM was carefully added to each plate. After a period of 3 days (d) an additional volume of 1.5 mL DMEM was added. When the specimen detached from the plate after 48 h, it was removed under sterile conditions. To remove all detritus of the specimen, the plate was washed gently, using PBS. Then, another 3 mL of fresh DMEM growth medium were added. At a cell confluency of 60—80% cells were transferred onto a 25 cm2 CO2-permeable flask and passaged for further use (Fig. 1a–e).
Fig. 1.
Harvesting of TMJ condyle cartilage. a After removing the muscle layer, TMJ anatomy was exposed, showing the condyle (C) and the temporomandibular fossa (TF), as well as the zygomatic arch (ZA). b TMJ was opened and the condyle was liberated by dissecting adherent muscle and connective tissue. Condyle cartilage (CC) was cut from the condyle ramus (CR). c harvested condyle was rinsed d and left for attachment in e 50 µl regular DMEM
Flasks of 25 cm2 size were seeded with 1000–2000 cells per flask and incubated for 7 d. Nuclei were stained using 0.5% crystal violet staining solution (Sigma-Aldrich, St. Louis, MO). Cells gathering at a number of at least 50 cells, were considered to be a colony (Fig. 1c).
Histology and Immunostaining
Explanted TMJs (n = 4) were stored in 10% neutral buffered formalin (EMD Millipore, Burlington, MA), decalcified and finally embedded in paraffin. Hematoxylin and Eosin (HE) staining and Safranin-O staining were performed from 5 µm thick deparaffinized histological slides.
For immunostaining, deparaffinized sections were initially blocked for endogenous peroxidase activity and unspecific secondary antibody binding applying Bloxall Endogenous Peroxidase blocking solution (VectorLabs, Burlingame, CA) and subsequently 2.5% goat/ horse serum for 20 min each. Sections were incubated in primary antibody solution over night at 4 °C, followed by secondary antibody binding for 1 h at room temperature. Immunohistochemical detection was performed using Vectastain Elite ABC HRP Kit and ImmPACT DAB kit (both VectorLabs, Burlingame, CA) according to the manufacturer’s instructions. Nuclear counterstaining was performed using Hematoxylin QS solution. Primary antibodies used were anti-CD44 (1:100), anti-CD105 (1:100), anti-aggrecan (1:200), anti-collagen-type-I (1:100), anti-Ihh (1:200), anti-PTHLH (1:50) (all Abcam, Cambridge, UK), anti-Sox9 (1:600, Cell Signaling Technology Inc., Danvers, MA) and anti-collagen-type-II (1:200, SouthernBiotech, Birmingham, AL). Secondary antibodies used for immunofluorescence were goat anti-rat (Texas Red, ThermoFisher Scientific, Waltham, MA) and donkey anti-rabbit (Alexa Fluor 488, Abcam, Cambridge, UK).
Flow Cytometry (FACS) Analysis
MSCPCs (P1) were seeded and expanded after reaching approximately 70% confluency on a 25 cm2 Flask. MSPCs were harvested at a cell viability rate over 90%. Cells were resuspended in ice-cold staining buffer (BioLegend, San Diego, CA) at a concentration of 10 × 106 cells/mL per tube. Primary antibodies used to stain for cluster of differentiation (CD) MSPC antigens were: Anti-CD44 (FITC, 1:200), anti-Sca-1 (APC/Cy7, 1:80), anti-CD73 (PE/Cy7, 1:160) and anti-CD105 (Pacific Blue, 1:50). Anti-CD34 (APC, 1:20), displaying hematopoietic stem cells, was chosen for negative control. Stained cells were stored on ice for 20 min in dark, followed by washing the cells 3 times at 350 × g for 5 min each. 7-AAD viability staining solution was added at 5 µL per tube to exclude non-viable cells from further evaluation. Cells were analyzed on a FACSAria IIU machine (BD Biosciences, San Jose, CA) and sorted into two groups, CD34-/CD44 + /Sca-1 + /CD105 + and CD34-/CD44 + /Sca-1 + /CD105-. Groups differed in whether expressing CD105 or not. Surface antigen antibodies were obtained from BioLegend, San Diego, CA. Data analysis was performed using FlowJo software program (BD Biosciences, San Jose, CA).
RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
RNA isolation from first passage (P1) MSPCs was performed using TRIzol RNA isolation reagent (ThermoFisher Scientific, Waltham, MA). RNA concentration and quality were determined using spectrophotometry and gel electrophoresis. Synthesis of cDNA was performed, using SMARTScribe Reverse Transcriptase Kit (Takara Bio USA Inc., Mountain View, CA). qRT-PCR was performed using Platinum SYBR Green qPCR Super Mix (Invitrogen, Carlsbad, CA) on a CFX96 Real-Time PCR System (Bio-Rad Laboratories Inc., Hercules, CA) and primers as outlined in Table 1. Measured values were normalized to cyclophilin B and calculated using the 2-ΔΔCt method. Gene expression was evaluated in an unsorted sample of harvested MSPCs, serving as a control, and also in preselected CD105+ and CD105− subgroups.
Table 1.
Listing of all chosen primer pairs (5′-3′ direction) for measuring gene expression in mus musculus, using qRT-PCR
| Gene | Primer pair (5′-3′ direction) |
|---|---|
| Cyclophilin B |
TTCTTCATAACCACAGTCAAGACC ACCTTCCGTACCACATCCAT |
| Biglycan (Bgn) |
GACAACCGTATCCGCAAAGT GTGGTCCAGGTGAAGTTCGT |
| Insulin-like growth factor (Igf1) |
GACCGAGGGGCTTTTACTTC CATCCACAATGCCTGTCTGA |
| Aggrecan (Acg1) |
CCTGCTACTTCATCGACCCC AGATGCTGTTGACTCGAACCT |
| Collagen Type I (Col1a1) |
TGGTTTGGAGAGAGCATGACCGA TTGGTCGATGTAGGCTACGCTGTT |
| Collagen Type II (Col2a1) |
ACCAAATTCCTGTTCA ACTGGTAAGTGGGGCAAGAC |
| Collagen Type X (Col10a1) |
CTTTGTGTGCCTTTCAATCG GTGAGGTACAGCCTACCAGTTTT |
| Decorin (Dcn) |
GAGGGAACTCCACTTGGACA TTGTTGTTGTGAAGGTAGACGAC |
| Lysyl Oxidase (Lox) |
ATGCGTTTCGCCTGGGCTGTGC CTAATACGGTGAAATTGTGCAGCC |
| SRY-box 5 (Sox5) |
ATGGAAGTCGATGGCAATAAAGT CCACCACATCCGCTAAGCTG |
| SRY-box 6 (Sox6) |
AATGCACAACAAACCTCACTCT AGGTAGACGTATTTCGGAAGGA |
| SRY-box 9 (Sox9) |
CAGCAAGACTCTGGGCAAG TCCACGAAGGGTCTCTTCTC |
| Osterix (Osx) |
AGCGACCACTTGAGCAAACAT GCGGCTGATTGGCTTCTTCT |
| Proteoglycan 4 (Prg4) |
ACTTCAGCTAAAGAGACACGGAGT GTTCAGGTGGTTCCTTGGTTGTAGTAA |
| Runt related transcription factor 2 (Runx2) |
CCACAAGGACAGAGTCAGATTACA TGGCTCAGATAGGAGGGGTA |
| Transcriptional repressor GATA binding 1 (Trps1) |
CAAATCTCAGGCCTGAGTGA GTGAAGAGCTGATATCCTGCAG |
| Indian hedgehog signaling molecule (Ihh) |
GACTCATTGCCTCCCAGAACTG CCAGGTAGTAGGGTCACATTGC |
| GLI family zinc finger 2 (Gli2) |
TGCTGTGGACTAGGAATAGG AACCTTCCGCTCAACCACAA |
Intracellular Immunostaining
CD105± and unsorted MSPCs (all P1) were seeded at a number of 1000 cells/mL on chamber slides and were left for cell attachment overnight. Cell permeabilization and intracellular immunostaining were finally performed according to the protocol reported by Khanna-Jain et al. [30]. Primary collagen-type-1, type-2, aggrecan, Sox9 and Ihh antibodies were used at the same concentrations as previously reported. Proteoglycan 4 primary antibody (Abcam, Cambridge, UK) was used at a concentration of 1:250. Secondary antibodies used were donkey anti-rabbit (Alexa Fluor 488, 1:200) and donkey anti-mouse (Alexa Fluor 568, 1:200) (Abcam, Cambridge, UK). Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI) solution. Primary antibody was omitted in negative controls.
Multi-Differentiation Potential Assays
CD105± cells were used at 2000 cells per 25 cm2 flask and cultured for 7 d. Osteogenic and adipogenic media were produced as described elsewhere [8]. Chondrogenic media was obtained by adding dexamethasone (100 nM), 50 µg/mL of ascorbic acid, L-proline (40 µg/mL) (Sigma-Aldrich, St. Louis, MO), sodium pyruvate (2 mM) (Gibco, Grand Island, NY), 1% Insulin-Transferrin-Selenium (ITS) solution (Corning, Corning, NY) and TGF-β1 at 10 ng/mL (Invitrogen, Carlsbad, CA). After 7 d of cell growth, media was changed to either chondrogenic, osteogenic or adipogenic media and further incubated for 4 weeks. Media was changed every other day. Staining protocols were carried out as described elsewhere [7], using alcian blue, alizarin Red S and oil Red O (Thermo Fisher Scientific, Waltham, MA). Five flasks each, were evaluated for differentiation capatcity of CD105± cells and each lineage was separated from unstained cells using ImageJ software setting the appropriate threshold level, leading to the proportionate area of differentiated cells.
Statistical Analysis
Statistical analysis was performed, using SPSS software (IBM, Armonk, NY). T-Test or Mann–Whitney-U-Test for independent samples was carried out. For evaluation of intracellular immunostaining, results were conservatively estimated using Kruskal–Wallis-Test. Level of significance was set to p < 0.05 for all statistical testing. Results are displayed as mean ± standard deviation (SD). Level of significance: *p < 0.05, **p < 0.01, ***p < 0.001.
Results
Colony-Forming Assay
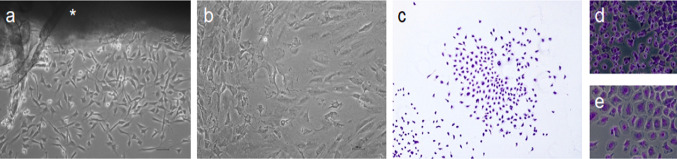
Cell escape from the explant (n = 4) was observed after 3 d of culturing (Fig. 2a) showing the typical spindle-shape of MSPCs. Violet nuclear staining showed the formation of MSCP colonies (> 50 cells/colony) over all the 25 cm2 plate after 7 d of incubation. Colony forming ability of the harvested cells revealed clonogenicity of the isolated stem cells (Fig. 2b,c). Cells differed in phenotype and were observed at small (Fig. 2d) and large cell (Fig. 2e) size. Cultured CD105+ and CD105− cells showed similar proliferation rates and displayed only homogenous small cell phenotype.
Fig. 2.
Culturing of explanted condyle, colony forming assay. a Cell outgrowth from explanted condyle (*) at third day. b Dense cell layer after 14 d of culturing. Bright-field microscopy (Mag = 10x). c Representative cell colony, consisting of > 50 cells after 7 d of incubation (Mag = 10x). Isolated cells differed in small d and large e phenotype (Mag = 20x). Scale bar = 50 µm
Histology and Immunostaining
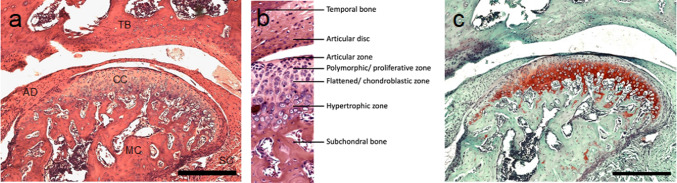
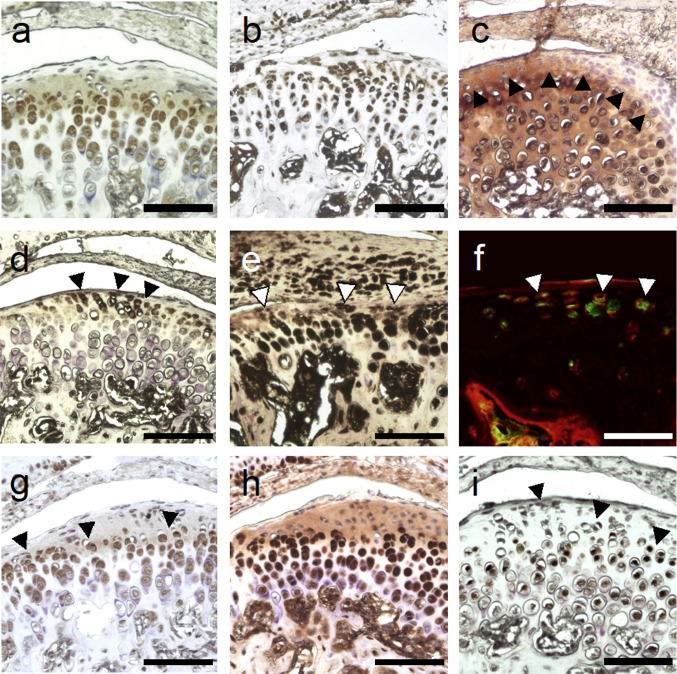
Organization of the condyle articular cartilage in 4 distinct layers was observed (Fig. 3a–c). The proliferative zone was identified through immunohistochemical analysis, revealing absence of collagen type 2, but positive Sox9 antigen staining (Fig. 4b, i). CD44 positive MSPCs were localized in the proliferative zone (Fig. 4d). Collagen Type 2 and aggrecan were mainly distributed in the chondroblastic zone (Fig. 4a, c). CD44 positivity decreased dramatically in deeper cartilage layers towards subchondral bone. CD105 presence was observed over all cartilage layers (Fig. 4e). Double immunofluorescence staining certified presence of CD44 and CD105 positive cells in the proliferative zone (Fig. 4f). Proliferative zone and chondroblastic zone were also found to be rich in Ihh, and PTHLH, and Sox9 (Fig. 4g–i).
Fig. 3.
Histology of mouse TMJ and distinct zonal organization of the cartilage layer. a H&E Staining (Mag = 10x) of the temporal bone (TB), mandibular condyle (MC), articular disc (AD), cartilage of condyle (CC), synovial capsule (SC). b Histological organization in distinct layers of the articular cartilage (Mag = 20x). c Safranin O/fast green staining of the condyle (Mag = 10x). Proteoglycans and chondrocytes are stained red. Scale bar = 100 µm
Fig. 4.
Immunohistochemistry/Immunofluorescence of TMJ condyle’s cartilage (Mag = 20x, scale bar = 100 µm). Zonal distribution of aggrecan a, collagen type 1 b. Collagen type 2 c distribution defines border of upper localized proliferative zone. CD44 positive d, CD105 positive e, Ihh positive g, PTHLH positive h, and Sox9 positive i cells in the TMJ condyle of 8-weeks-old mice. CD44 (red)/CD105 (green) positive f cells within the proliferative cartilage zone
FACS Analysis
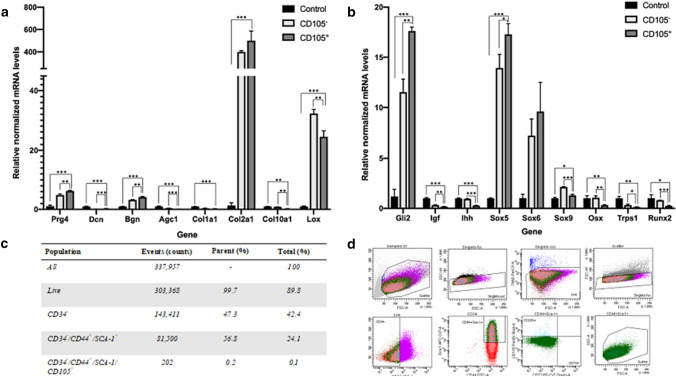
It was found that 42.4% of isolated MSPCs (P1) were CD34 negative (−). CD34− cell population was further screened for expressing both CD44 and Sca-1, which was found in 56.8% of the sample. Only 0.2% of CD34−/CD44+/Sca-1+ cells expressed CD105 antigen and 0.1% of the non-preselcted sample. CD73+ expression was not observed within the isolated cell population. CD34−/CD44+/Sca-1+/CD105− and CD34−/CD44+/Sca-1+/CD105+ cell populations were separately sorted and subsequently cultured for further qRT-PCR analysis (Fig. 5c).
Fig. 5.
qRT-PCR analysis. a Gene expression of genes relevant for building up the extracellular matrix of fibrocartilage. b genes relevant for stimulation of skeletogenesis, and cell differentiation. All gene expression is normalized to cyclophilin B expression. All gene expression of CD105+ and CD105− cells is displayed relative to gene expression of unsorted cells (control). Error are means ± SD. c Flow cytometry analysis of MSPCs. d Ratio and gating strategy of detected antigens labeled within the sample. %Total gives the ratio of antigens within the whole sample, while %Parent outlines the ratio of each antigen-subgroup
RNA Isolation and qRT-PCR Analysis
Comparing CD105+ and CD105− MSPC subgroups, gene expression of Bgn, Prg4 (p < 0.01) and Dcn (p < 0.001) were found significantly higher in CD105+ cells (Fig. 5a). Agc1 expression was higher in the CD105− cells (Fig. 5a). Gene expression of Col2a1 was significantly higher in CD105+ cells compared to the unsorted sample (p < 0.001), Col1a1 did not differ significantly between CD105+ and negative cells (Fig. 5a). Significant Col2a1 upregulation correlated with increased Sox9 levels of CD105+ cells, compared to the unsorted control sample. Col10a1 gene was found to be significantly increased (p < 0.01) in the unsorted and CD105− sample, compared to CD105+ cells (Fig. 5a). Sox 5, 6, 9, Runx2, Transcriptional repressor GATA binding 1 (Trps1) and Gli2 were observed at significantly increased levels (p < 0.001) in the CD105+ sample (Fig. 5b). Sox5 and 9 were significantly expressed (p < 0.001 and p < 0.05) in preselected cells compared to the unsorted control sample. Osx (p < 0.01), Runx2 (p < 0.05), Ihh (p < 0.001), Trps1 (p < 0.01) relevant for osteogenesis were found to be significantly downregulated in CD105+ cells (Fig. 5b).
Intracellular Protein Expression
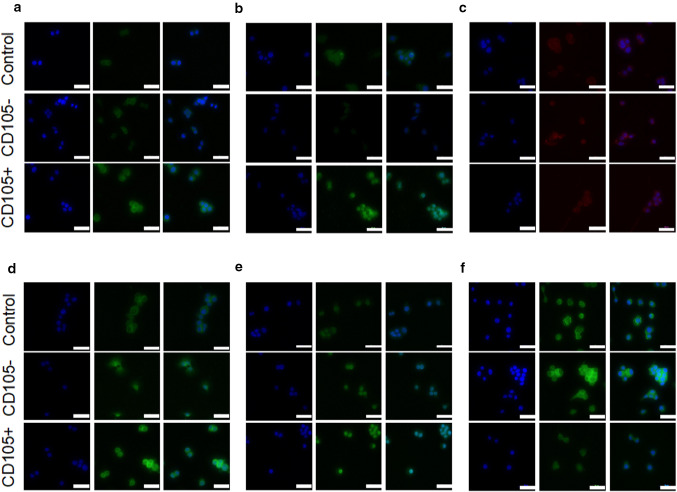
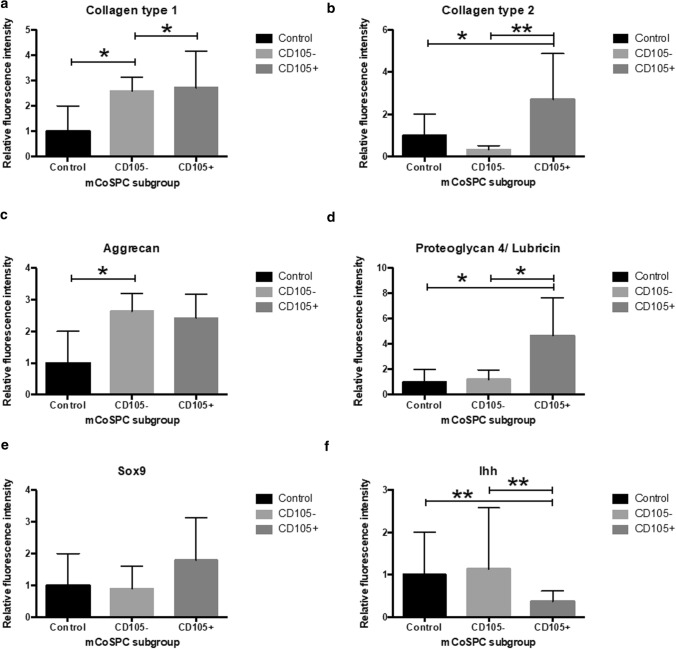
Significantly higher intracellular collagen type 1 was observed comparing CD105+ and CD105− to the control sample (p < 0.05) (Fig. 6a, 7a). Collagen type 2 protein expression aligned with qPCR findings of the Col2a1 gene. Collagen type 2 protein levels of CD105+ cells were found at significantly increased levels compared to CD105− cells (p < 0.001) and the control (p < 0.05) (Fig. 6b, 7b). Levels of aggrecan were found approximately two times higher in CD105± cohorts compared to the control, but only levels of aggrecan were found significantly elevated for CD105− group (p < 0.05) (Fig. 6c, 7c). Between CD105± groups no significant difference in aggrecan protein expression was found. In accordance to the qPCR findings of proteoglycan 4, intracellular staining revealed a significant increase (p < 0.05) in CD105+ cells (Fig. 6d, 7d). Intranuclear Sox9 levels did not differ significantly between groups (Fig. 6e, 7e), while Ihh protein expression was significantly increased in the control and CD105− cohort compared to CD105+ cells, in line with the previously described gene expression (Fig. 6f, 7f).
Fig. 6.
Intracellular immunostaining (Mag = 40x, scale bar 50 µm). Protein levels of collagen type 1 a, collagen type 2 b, aggrecan c, proteoglycan 4 d, Sox9 e and Ihh f were quantified by intracellular staining
Fig. 7.
Bar graphs (mean ± SD) visualize relative protein expression of CD105± cohorts as measured fluorescence intensity signal to the control
Multi-Differentiation Potential Assay
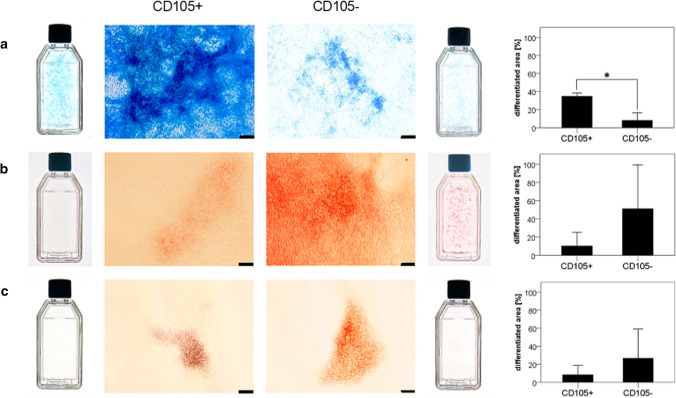
Alcian blue staining visualized extracellular glycosaminoglycan secretion of differentiated cells. Extracellular free calcium deposits within the colonies were visualized by deep red and bright orange ECM staining, after applying alizarin red staining solution. Adipogenic differentiation was observed, and appeared as red vacuoles and reddish colony staining as oil red O binds to triglycerides of fatty acids. CD105+ cells were found to show stronger chondrogenic differentiation than CD105− cells (p < 0.05) after 4 weeks (Fig. 8a). In contrast, CD105− cells trended to show higher osteogenic and adipogenic differentiation without reaching a significant level (Fig. 8b,c).
Fig. 8.
Multi-differentiation potential assay (Mag = 4x, scale bar = 200 µm). Bar graphs (mean ± SD) visualize area [%] found to be differentiated after 4 weeks of cell differentiation, comparing CD105+ and CD105− cell subgroups. a Chondrogenic differentiation, b osteogenic differentiation and c adipogenic differentiation
Discussion
Application of MSPCs is widely studied for regeneration and/or repair of many tissues, including fibrocartilage. Numerous authors agree that autologous cells would be the best choice for restoring damaged tissue. However, it appears that in situ cells are also required to maintain organ hemostasis [31–35]. Underlining this fact, it was found that tissue resident stem cells possess increased organ hemostasis ability [29].
Gamer et al. found that fibrocartilage of the mouse knee harbors different subpopulations of MSPCs [7]. Moreover, CD105+ MSPCs, derived from the synovium or adipose tissue, are reported to have increased chondrogenic differentiation potential, due to TGF-β/ Smad2 signaling, as CD105 is an integral part of the TGF-β receptor complex [26–28].
We hypothesized that the triad of CD44, Sca-1 and CD105 and the lack of CD34 antigen identify MSPCs of the TMJ’s fibrocartilage, similar to fibrocartilage of the mouse knee joint [7]. Immunostaining of the TMJ revealed CD44+ and CD105+ MSPCs in the proliferative zone. After harvesting the TMJ fibrocartilage cap, clonogenicity, tissue outgrowth and multi-differentiation potential were investigated to show that MSPCs were isolated as a first step and, subsequently, verified sorting for CD44, Sca-1, CD73 and CD105, using FACS as reported by Gamer et al. [7].
As no single MSPC marker exists, the triad of CD105, CD73 and CD90 and the lack of CD34 are proposed to identify mesenchymal stem cells in humans as shown by Dominici et al. [36]. Mouse TMJ MSPCs lacked specific surface antigen markers that human species demonstrate. Stem cells harvested from the mouse condyle were found not to express CD73 (0%) in CD34− subgroup; and, almost no CD105 (0.2%), similar to the findings reported by Gamer et al., who identified and characterized adult mouse meniscus stem/progenitor cells [7]. Low expression rates of CD73 is consistent with the idea that surface antigen markers differ between species; CD73 expression is also negative in mouse MSPCs [37, 38].
Gene expression of CD105− preselected cells was defined through increased Col10a1, Col1a1, Sox9 and Ihh levels, together with elevated levels of Osx, Runx2, Ihh, Trps1 genes, which are related to chondrocyte maturation, subsequently leading to osteoblastic differentiation and endochondral ossification as shown elsewhere [12, 39–44]. Elevated gene expression of Runx2 correlated with findings of Runx2 upregulation in chondrocytes populating the OA affected joint [45]. Hence, Runx2 upregulation is accepted as an important player in cartilage maturation and in the pathogenesis of OA [45]. Those findings may validate the assumption, that CD105− cells were identified as matured MSPCs of the chondroblastic or hypertrophic zone, expressing osteogenesis driving genes at a higher level while in the process of endochondral ossification.
In contrast, CD105+ antigen is associated with an earlier MSPC state, due to significant downregulation of Osx, Runx2, Ihh, Trps1 and Col10 gene expression and considerable high expression of Col2a1 and Prg4, compared to the control (unsorted MSPCs) [4].
That CD105+ subpopulation is able to deliver crucial proteins of the ECM and for physiological joint function at increased levels was shown by significant higher intracellular immunostaining of collagen type 1, type 2 and proteoglycan 4, as well as, due to increased intensity of alcian blue staining, probably correlating with higher secretion of glycosaminoglycans related to increased proteoglycan 4 secretion and higher expression of Bgn and Dcn gene. Considerable higher levels of the chondrogenic trio, Sox5, 6 and 9, on gene level and increased chondrogenic turnover potential deliver promising features for investigating further improvements in restoring fibrocartilage. From a preliminary in vivo study conducted by Guastaldi et al. we have promising findings that MSPCs aid in the restoration process of damaged TMJ cartilage [46]. Furthermore administered MSPCs to the damaged TMJ did not lead to increased inflammation vs. a sham group in which only a defect to the TMJ was created, without using any cell therapy [46]. This finding is in accordance to reports that MSCs have immunosuppressive capacities, like inhibition of T-Cell proliferation and are reported to decrease initiate acute immune reaction, when being transplanted [47–49]. Those abilities make the application of MSPCs favorable in tissue engineering and regenerative medicine.
In conclusion, fibrocartilage of the mouse TMJ is populated by a CD105+ MSPC subgroup, a finding that is consistent with previous investigations of mesenchymal tissue [7, 26–28]. The characteristics of TMJ-resident CD105+ MSPCs suggest that this subpopulation might be a favorable source of tissue resident stem cells for future improvements in stem cell-based therapies. To fully understand the role of CD105+ MSPCs and their behavior in maintaining and/or recovering hemostasis of the TMJ fibrocartilage, further studies will need to investigate if this minor population can still be found in mice older than 8 weeks that have undergone a TMJ stress/ alteration process. If isolated CD105+ MSPCs are also found to possess homing ability, using fluorescent protein-transfected cultures, a CD105+ stem-cell-based injection therapy could be a possible next step for delivering CD105+ MSPCs to the OA affected joint.
Acknowledgements
This study was funded in part from grants: MGH-Department of Oral and Maxillofacial Surgery Education Research Fund (Boston, MA), Jean Foundation (NH), Fondation Bertarelli (Gstaadt, Switzerland) and MGH-Walter C. Guralnick Fund (Haseotes-Bentas Foundation, Boston, MA).
Authors’ Contributions
JRT contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data. YJ contributed to the acquisition of data, analysis and interpretation of data. MLM contributed to the acquisition of data, analysis and interpretation of data. VR contributed to analysis and interpretation of data. MJT contributed to the conception and design of the study. FPSG contributed to the conception and design of the study, acquisition of data, analysis and interpretation of data. JRT and MLM drafted the manuscript. YJ, VR, MJT and FPSG revised the manuscript. All authors contributed to final approval of the version to be submitted.
Declarations
Conflict of interest
The authors report no declarations of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Department of Health and Human Services NIoH, National Institute of Mental Health. TMJ Disorders. Bethesda, MD: U.S. Government Printing Office.; 2017. Report No.: NIH Publication No. 17-3487
- 2.McNeill C, Mohl ND, Rugh JD, Tanaka TT. Temporomandibular disorders: diagnosis, management, education, and research. J Am Dent Assoc. 1990;120(3):253. doi: 10.14219/jada.archive.1990.0049. [DOI] [PubMed] [Google Scholar]
- 3.Robinson J, O'Brien A, Chen J, Wadhwa S. Progenitor cells of the mandibular condylar cartilage. Curr Mol Biol Rep. 2015;1(3):110–114. doi: 10.1007/s40610-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22(3):597–609. doi: 10.1016/j.devcel.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 6.Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. J Dent Educ. 2008;72(8):930–947. [PMC free article] [PubMed] [Google Scholar]
- 7.Gamer LW, Shi RR, Gendelman A, Mathewson D, Gamer J, Rosen V. Identification and characterization of adult mouse meniscus stem/progenitor cells. Connect Tissue Res. 2017;58(3–4):238–245. doi: 10.1080/03008207.2016.1271797. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W, Chen J, Zhang S, Ouyang HW. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair. Arthritis Res Ther. 2012;14(4):221. doi: 10.1186/ar4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Andre P, Ye L, Yang YZ. The Hedgehog signalling pathway in bone formation. Int J Oral Sci. 2015;7(2):73–79. doi: 10.1038/ijos.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki A, Iwata J. Mouse genetic models for temporomandibular joint development and disorders. Oral Dis. 2016;22(1):33–38. doi: 10.1111/odi.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236(2):426–434. doi: 10.1002/dvdy.21036. [DOI] [PubMed] [Google Scholar]
- 12.Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 14.Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16(2):174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre V, Huang W, Harley VR, Goodfellow PN, de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. 1997;17(4):2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Li H, Tanaka K, Tsumaki N, Yamada Y. Identification of an enhancer sequence within the first intron required for cartilage-specific transcription of the alpha2(XI) collagen gene. J Biol Chem. 2000;275(17):12712–12718. doi: 10.1074/jbc.275.17.12712. [DOI] [PubMed] [Google Scholar]
- 17.Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, et al. SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem. 2000;275(15):10738–10744. doi: 10.1074/jbc.275.15.10738. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Jimenez SA, Stokes DG. Regulation of human COL9A1 gene expression. Activation of the proximal promoter region by SOX9. J Biol Chem. 2003;278(1):117–123. doi: 10.1074/jbc.M208049200. [DOI] [PubMed] [Google Scholar]
- 19.Yahara Y, Takemori H, Okada M, Kosai A, Yamashita A, Kobayashi T, et al. Pterosin B prevents chondrocyte hypertrophy and osteoarthritis in mice by inhibiting Sik3. Nat Commun. 2016;7:10959. doi: 10.1038/ncomms10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274(2):584–594. doi: 10.1074/jbc.274.2.584. [DOI] [PubMed] [Google Scholar]
- 21.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, et al. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267(27):19027–19030. [PubMed] [Google Scholar]
- 22.Quackenbush EJ, Letarte M. Identification of several cell surface proteins of non-T, non-B acute lymphoblastic leukemia by using monoclonal antibodies. J Immunol. 1985;134(2):1276–1285. [PubMed] [Google Scholar]
- 23.Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990;265(15):8361–8364. [PubMed] [Google Scholar]
- 24.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, et al. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22(2):393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- 25.Pierelli L, Bonanno G, Rutella S, Marone M, Scambia G, Leone G. CD105 (endoglin) expression on hematopoietic stem/progenitor cells. Leuk Lymphoma. 2001;42(6):1195–1206. doi: 10.3109/10428190109097744. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Li J, Wang Y, Pan J, Li S, Zhu L, et al. CD105 promotes chondrogenesis of synovium-derived mesenchymal stem cells through Smad2 signaling. Biochem Biophys Res Commun. 2016;474(2):338–344. doi: 10.1016/j.bbrc.2016.04.101. [DOI] [PubMed] [Google Scholar]
- 27.Chang CB, Han SA, Kim EM, Lee S, Seong SC, Lee MC. Chondrogenic potentials of human synovium-derived cells sorted by specific surface markers. Osteoarthr Cartil. 2013;21(1):190–199. doi: 10.1016/j.joca.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Jiang T, Liu W, Lv X, Sun H, Zhang L, Liu Y, et al. Potent in vitro chondrogenesis of CD105 enriched human adipose-derived stem cells. Biomaterials. 2010;31(13):3564–3571. doi: 10.1016/j.biomaterials.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 29.Goichberg P. Current understanding of the pathways involved in adult stem and progenitor cell migration for tissue homeostasis and repair. Stem Cell Rev. 2016;12(4):421–437. doi: 10.1007/s12015-016-9663-7. [DOI] [PubMed] [Google Scholar]
- 30.Khanna-Jain R, Mannerstrom B, Vuorinen A, Sandor GK, Suuronen R, Miettinen S. Osteogenic differentiation of human dental pulp stem cells on beta-tricalcium phosphate/poly (l-lactic acid/caprolactone) three-dimensional scaffolds. J Tissue Eng. 2012;3(1):2041731412467998. doi: 10.1177/2041731412467998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qu D, Zhu JP, Childs HR, Lu HH. Nanofiber-based transforming growth factor-beta3 release induces fibrochondrogenic differentiation of stem cells. Acta Biomater. 2019;93:111–122. doi: 10.1016/j.actbio.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Fang D, Jin P, Huang Q, Yang Y, Zhao J, Zheng L. Platelet-rich plasma promotes the regeneration of cartilage engineered by mesenchymal stem cells and collagen hydrogel via the TGF-beta/SMAD signaling pathway. J Cell Physiol. 2019;234(9):15627–15637. doi: 10.1002/jcp.28211. [DOI] [PubMed] [Google Scholar]
- 33.Gupte MJ, Swanson WB, Hu J, Jin X, Ma H, Zhang Z, et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018;82:1–11. doi: 10.1016/j.actbio.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacer S, Shafaei H, Soleimani RJ. An investigation on the regenerative effects of intra articular injection of co-cultured adipose derived stem cells with chondron for treatment of induced osteoarthritis. Adv Pharm Bull. 2018;8(2):297–306. doi: 10.15171/apb.2018.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza TR, Takamori ER, Menezes K, Carias RBV, Dutra CLM, de Freitas AM, et al. Temporomandibular joint regeneration: proposal of a novel treatment for condylar resorption after orthognathic surgery using transplantation of autologous nasal septum chondrocytes, and the first human case report. Stem Cell Res Ther. 2018;9(1):94. doi: 10.1186/s13287-018-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Anderson P, Carrillo-Galvez AB, Garcia-Perez A, Cobo M, Martin F. CD105 (endoglin)-negative murine mesenchymal stromal cells define a new multipotent subpopulation with distinct differentiation and immunomodulatory capacities. PLoS ONE. 2013;8(10):e76979. doi: 10.1371/journal.pone.0076979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boxall SA, Jones E (2012) Markers for characterization of bone marrow multipotential stromal cells. Stem Cells Int [DOI] [PMC free article] [PubMed]
- 39.Fu H, Doll B, McNelis T, Hollinger JO. Osteoblast differentiation in vitro and in vivo promoted by Osterix. J Biomed Mater Res A. 2007;83(3):770–778. doi: 10.1002/jbm.a.31356. [DOI] [PubMed] [Google Scholar]
- 40.Tu Q, Valverde P, Chen J. Osterix enhances proliferation and osteogenic potential of bone marrow stromal cells. Biochem Biophys Res Commun. 2006;341(4):1257–1265. doi: 10.1016/j.bbrc.2006.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komori T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- 42.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 43.Suemoto H, Muragaki Y, Nishioka K, Sato M, Ooshima A, Itoh S, et al. Trps1 regulates proliferation and apoptosis of chondrocytes through Stat3 signaling. Dev Biol. 2007;312(2):572–581. doi: 10.1016/j.ydbio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest. 2001;107(3):295–304. doi: 10.1172/JCI11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Komori T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol. 2018;149(4):313–323. doi: 10.1007/s00418-018-1640-6. [DOI] [PubMed] [Google Scholar]
- 46.Guastaldi FPS, Hakim MA. Are stem cells useful in the regeneration and repair of cartilage defects in the TMJ condyle? An In Vivo Study. J Dent Oral Disord. 2021;7(2):1159. [Google Scholar]
- 47.Kim N, Cho SG. Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol. 2016;103(2):129–137. doi: 10.1007/s12185-015-1918-6. [DOI] [PubMed] [Google Scholar]
- 48.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 49.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262(5):509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]