Abstract
As an immunomodulatory agent with antitumor activity, lenalidomide has been evaluated for its value in diffuse large B-cell lymphoma (DLBCL). We performed a meta-analysis to gain a better understanding of the efficacy and safety of lenalidomide in DLBCL. PubMed, Cochrane Library, and Embase were searched up to March 2022 for potential studies. The pooled hazard ratio (HR) and relative risk (RR) with 95% confidence interval (CI) were estimated by the fixed/random effects model. Overall, 6 randomized controlled trials including 1938 patients were included. The complete response rate (CRR) of the group containing lenalidomide was 47.7% (95%CI 28.5–67.2%), which was higher than the 37.8% (95%CI 16.7–61.5%) of the control group without lenalidomide (RR = 1.11, 95%CI 1.03–1.20, P = 0.008). The overall estimation of survival showed a benefit for progression-free survival (PFS) (HR = 0.77, 95%CI 0.66–0.90, P = 0.001) but not overall survival (OS) or event-free survival (EFS). The lenalidomide group had a significant incidence of grade ≥ 3 hematological adverse events (AEs) involving neutropenia (RR = 1.56, 95%CI 1.15–2.11, P = 0.004) and febrile neutropenia (RR = 1.81, 95%CI 1.31–2.49, P < 0.001), with the incidence of neutropenia (48.3%, 95%CI 37.5–59.1%) being highest. In conclusion, addition of lenalidomide results in a higher CRR and better PFS but a higher incidence of grade ≥ 3 hematological AEs involving neutropenia and febrile neutropenia.
Keywords: Lenalidomide, Diffuse large B-cell lymphoma, Meta-analysis, Clinical trials
Introduction
Diffuse large B-cell lymphoma (DLCBL) is the most common, aggressive non-Hodgkin lymphoma (NHL) subtype, comprising approximately 30–40% of cases [1]. The disease is a highly heterogeneous lymphoma characterized by diffuse structure, mature B-cell phenotype, and cell morphology, with multiple subtypes and genetic profiles. DLCBL is divided according to the Hans classification into a germinal center type (GCB) and non-germinal center type (non-GCB, most of the activated B-cell type, named ABC-type) [2]. Standard treatment is usually immune chemotherapy combined with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Although 50–60% of DLBCL patients can be cured by R-CHOP, the outcome of 40–50% of patients who still have relapsed/refractory (R/R) DLBCL remains poor [3]. Although understanding of the genetic and molecular landscape of DLBCL has increased significantly over the last two decades, there has been limited progress with regard to implementing this knowledge as improved upfront therapies. Recently, increasing attention has focused on the addition of various drugs to improve outcomes.
Lenalidomide is an immunomodulatory agent that is a derivative of thalidomide with fewer side effects, e.g., myelosuppression, which can limit lenalidomide's usage. Preclinical studies have shown that the antineoplastic effects of lenalidomide include direct antineoplastic activity, immunologic effects mediated by inhibition of tumor cell proliferation and angiogenesis, and stimulation of cytotoxicity mediated by T cells and NK cells [4–7]. Moreover, its activity has been demonstrated in a wide spectrum of hematologic malignancies, including myelodysplastic syndromes [8], multiple myeloma [9, 10], and B-cell NHL [11]. Several clinical trials have shown that lenalidomide has efficacy against DLBCL and is well tolerated, and it is expected to become a new treatment option for DLBCL [12–14]. Long-term follow-up combined analysis from two phase II trials showed that the efficacy of lenalidomide combined with R-CHOP (R2CHOP) was maintained over time, with a high rate of progression-free survival (PFS), and overall survival (OS); late toxicity was also low. Furthermore, considering the patients with high-risk features who were included, addition of lenalidomide to R-CHOP appears to mitigate the negative prognostic impact of the non-GCB phenotype [15]. Based on real-world data, lenalidomide plus rituximab may serve as a salvage therapy for R/R DLBCL, with a complete response rate (CRR) of 21% and an overall response rate (ORR) of 38%; the median posttreatment OS and PFS were 7.3 and 1.8 months, respectively [16]. We performed this meta-analysis to comprehensively analyze the efficacy and safety of lenalidomide in DLBCL.
Materials and methods
Search strategy
PubMed, Cochrane Library, and Embase were searched up to March 2022 for potential eligible published studies. We used the following search terms: [(revlimid) OR (lenalidomide)] AND (diffuse large B-cell lymphoma).
Selection criteria
Studies were included if the following inclusion criteria were met: (a) patients: all patients diagnosed with DLBCL; (b) intervention: treatment including lenalidomide; (c) control: treatment not including lenalidomide; and (d) outcomes: primary outcomes of OS, PFS and event-free survival (EFS) and secondary outcomes of the response rate and any potential hematological adverse events (AEs); (e) study design: all included studies with a randomized controlled trial (RCT) design aiming to investigate the efficacy and safety of lenalidomide in DLBCL. The following types of articles were excluded: case reports/case series, conference abstracts/papers, reviews and meta-analyses, preclinical research, notes/letters/short surveys/editorial/comment/brief communication, retrospective/observational studies, single arm studies and studies not providing information about the effectiveness of lenalidomide in DLBCL.
Data collection and quality assessment
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [17] was used as a guide and template for every step of this study. The quality of the evidence was assessed using the Joanna Briggs Institute (JBI) reviewers' manual for RCTs and quasi-experimental studies [18]. The evidence level of the RCTs was level 1. The following items were extracted among treated patients from each study: authors, publication year, country, sample size, median age, sex ratio, disease status, enrollment period, phase, response rate and survival. The data extraction was conducted independently by two authors. Information was examined and adjudicated independently by an additional author referring to the original studies.
Statistical analysis
Statistical heterogeneity was assessed using Cochran Q statistics and I2 statistics, with I2 statistics categorized as low (I2 ≤ 25%), moderate (I2 ≤ 50%), high (I2 ≤ 75%), or considerable (I2 > 75%) heterogeneity. If there was significant heterogeneity between studies (P < 0.10 or I2 > 50%), the random effects model was used; otherwise, the fixed effects model was chosen. A meta-analysis of proportions with 95% confidence interval (95%CI) was conducted after the data were transformed by Freeman-Tukey double arcsine transformation. The pooled hazard ratio (HR) and its 95%CI were used to evaluate survival in relation to lenalidomide in DLBCL. The pooled relative risk (RR) with 95%CI was used to assess the response rate and grade ≥ 3 hematological toxicity. Egger’s linear regression test and Begg & Mazumdar’s rank correlation tests were performed to detect publication bias. Visual inspection of funnel plot was conducted. Sensitivity analysis was conducted by sequential omission of each included study. All analyses were performed using R 4.1.1, and P < 0.05 was considered statistically significant for all included studies.
Results
Study selection and characteristics
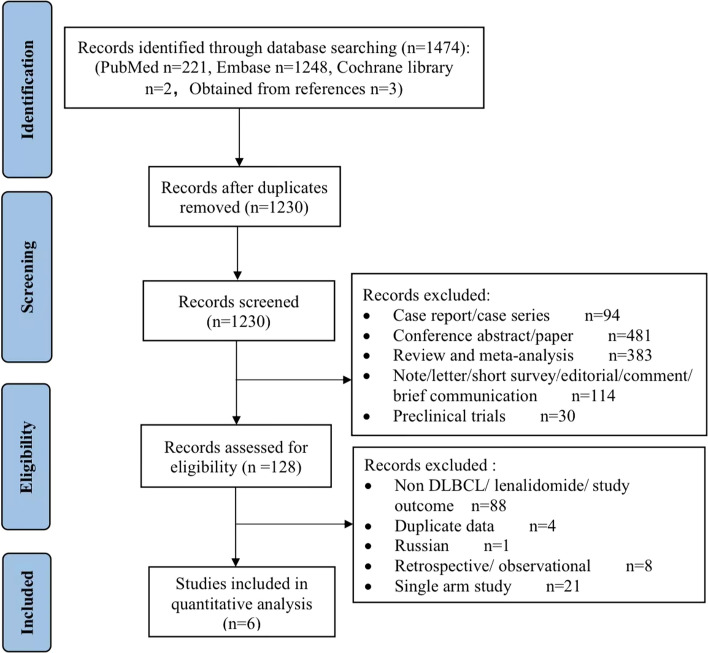
Our initial literature search yielded 1474 studies. After duplicates were removed, 1230 articles remained. A total of 1102 studies were excluded due to irrelevance after screening. The remaining 128 studies were retrieved for eligibility, 88 were excluded due to non-DLBCL/lenalidomide/study outcomes, 8 studies were retrospective/observational studies, 1 study was in Russian, 4 studies involved duplicate data, and 21 studies were single-arm studies. Eventually, 6 randomized controlled trials including 1938 patients were included in the present meta-analysis (Fig. 1) [19–24]. Information related to the population characteristics, and trial-reported results was summarized in Table 1. Among the included patients, 4 included untreated patients, and 2 included R/R cases. There were 2 phase II studies, 1 phase II/III study, and 3 phase III studies. The studies were published between 2017 and 2021 and were mainly initiated by researchers in Europe and America. The sample sizes ranged from 39 to 645. The median age of most patients was greater than 65 years, with the oldest being over 80 years.
Fig. 1.
Literature search and selection
Table 1.
Characteristics of the included trials
| Author | Year | Country | Study | Disease status | Enrollment period | Phase | Median follow-up (months) | Lenalidomide/control group | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | Median age (range) | Female/Male | ||||||||
| Czuczman et al. [19] | 2017 | America | DLC-001 | R/R | – | II/III | – | LEN | 69 (28–84) | 21/30 |
| Investigator’s Choice | 65 (20–84) | 20/31 | ||||||||
| Thieblemont et al. [20] | 2017 | France | REMARC | Untreated | 2009.05–2014.05 | III | 52 | LEN | 69 (58–80) | 140/183 |
| Placebo | 68 (59–80) | 147/180 | ||||||||
| Kühnl et al. [21] | 2020 | UK | LEGEND | R/R | 2013.10–2016.11 | II | 21.5 for living pts | LEN+R-GEM | 58 (21–75) | 8/13 |
| R-GEM-P | 59 (21–77) | 5/14 | ||||||||
| Nowakowski et al. [22] | 2021 | America | ECOG-ACRIN E1412 | Untreated | 2013.08–2017.01 | II | 36 | LEN+R-CHOP | 67 (24–88) | 51/94 |
| R-CHOP | 66 (37–92) | 59/76 | ||||||||
| Oberic et al. [23] | 2021 | France | SENIOR | Untreated | 2014.08–2017.09 | III | 25.1 | LEN+R-MiniCHOP | ≥ 80 | 65/57 |
| R-MiniCHOP | ≥80 | 71/56 | ||||||||
| Nowakowski et al. [24] | 2021 | America | ROBUST | Untreated | 2015.02–2017.08 | III | 27.1 (0–47) for living pts | LEN+R-CHOP | 65 (21–82) | 121/164 |
| Placebo+R-CHOP | 65 (28–83) | 142/143 | ||||||||
R/R relapsed/refractory, LEN lenalidomide, pts: patients, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, R-GEM rituximab, methylprednisolone and gemcitabine, R-GEM-P rituximab, methylprednisolone, gemcitabine, cisplatin, R-MiniCHOP standard attenuated dose of R-CHOP
Response rate
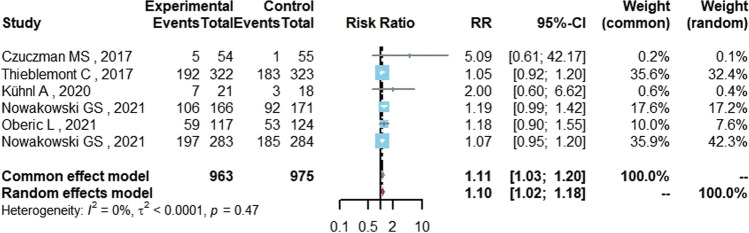
Among 963 DLBCL patients in the group containing lenalidomide, ORR was 67% (95%CI 45.7–85.3%), CRR was 47.7% (95%CI 28.5–67.2%), and the partial response rate (PRR) was 16.3% (95%CI 10.6–23.0%). In the control group without lenalidomide, which included 975 DLBCL patients, the ORR was 56.9% (95%CI 31.4–80.6%), the CRR was 37.8% (95%CI 16.7–61.5%), and the PRR was 15.6% (95%CI 10.1–21.9%). The CRR in the lenalidomide group was significantly higher than that in the control group (RR = 1.11, 95%CI 1.03–1.20, P = 0.008) (Table 2, Fig. 2). Statistical significance was not found for ORR or PRR.
Table 2.
Response rate and safety of lenalidomide in DLBCL
| Primary outcomes | Lenalidomide group | Control group | No of studies | No. of patients | I2 (%) | P value for heterogeneity | RR (95%CI) | P value for effects model | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Pooled rate (%, 95%CI) | I2 (%) | Pooled rate (%, 95%CI) | I2 (%) | |||||||
| Response rate | ||||||||||
| ORR | 67.0 (45.7, 85.3) | 96.5 | 56.9 (31.4, 80.6) | 97.5 | 6 | 1938 | 61.2 | 0.024 | 1.09 (0.99, 1.20) | 0.080 |
| CRR | 47.7 (28.5, 67.2) | 94.5 | 37.8 (16.7, 61.5) | 96.1 | 6 | 1938 | 0 | 0.468 | 1.11 (1.03, 1.20) | 0.008 |
| PRR | 16.3 (10.6, 23.0) | 87.7 | 15.6 (10.1, 21.9) | 85.7 | 6 | 1938 | 0 | 0.423 | 0.96 (0.79; 1.17) | 0.674 |
| Safety | ||||||||||
| Neutropenia | 48.3 (37.5, 59.1) | 86.6 | 31.7 (19.7, 44.9) | 94.6 | 6 | 1938 | 86.0 | < 0.001 | 1.56 (1.15, 2.11) | 0.004 |
| Thrombocytopenia | 13.7 (5.7, 24.2) | 95.2 | 10.5 (1.9, 24.1) | 94.8 | 6 | 1938 | 75.5 | 0.001 | 1.55 (0.71, 3.37) | 0.272 |
| Anemia | 17.3 (9.9, 26.1) | 81.7 | 16.0 (8.3, 25.5) | 82.8 | 5 | 1293 | 53.9 | 0.070 | 1.21 (0.79, 1.87) | 0.383 |
| Febrile neutropenia | 11.9 (5.2, 20.6) | 87.1 | 5.8 (1.8, 11.5) | 81.7 | 5 | 1293 | 0 | 0.891 | 1.81 (1.31, 2.49) | < 0.001 |
Italic values indicate P < 0.05
ORR overall response rate, CRR complete response rate, PRR partial response rate, 95%CI 95% confidence interval, RR relative risk
Fig. 2.
The forest plot of CRR
PFS, EFS and OS
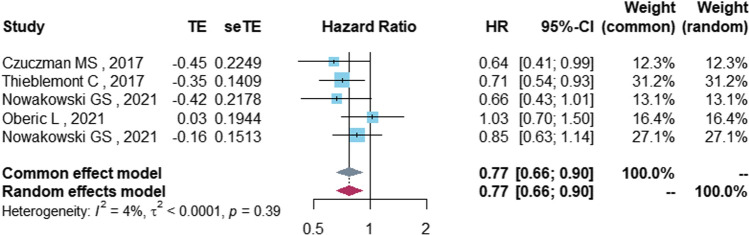
Regarding meta-analysis evaluating survival, five studies with 1899 patients analyzed the PFS of DLBCL patients treated with lenalidomide. Low heterogeneity was found among the included studies (I2 = 3.6%). The overall estimation in the fixed effects model showed a PFS benefit in favor of the control group not treated with lenalidomide (HR = 0.77, 95%CI 0.66–0.90, P = 0.001) (Fig. 3, Table 3). Subgroup analysis showed a survival benefit in the untreated (HR = 0.79, 95%CI 0.67–0.94, P = 0.006), R-CHOP-based (HR = 0.75, 95%CI 0.62–0.90, P = 0.002), and ≥ 65-year-old (HR = 0.77, 95%CI 0.66–0.90, P = 0.001) populations. There was no significant benefit in GCB (HR = 0.70, 95%CI 0.48–1.03, P = 0.070) or non-GCB (HR = 0.83, 95%CI 0.66–1.05, P = 0.125) patients.
Fig. 3.
The forest plot of PFS
Table 3.
Survival analysis of lenalidomide in DLBCL
| Secondary outcomes | No. of studies | No. of patients | I2 (%) | P value for heterogeneity | HR (95%CI) | P value for effects model |
|---|---|---|---|---|---|---|
| PFS | ||||||
| ALL | 5 | 1899 | 3.6 | 0.386 | 0.77 (0.66, 0.90) | 0.001 |
| Disease status | ||||||
| Untreated | 4 | 1790 | 10.1 | 0.343 | 0.79 (0.67, 0.94) | 0.006 |
| Regimen | ||||||
| R-CHOP based | 3 | 1549 | 0 | 0.550 | 0.75 (0.62, 0.90) | 0.002 |
| Median age | ||||||
| ≥ 65 | 5 | 1899 | 3.6 | 0.386 | 0.77 (0.66, 0.90) | 0.001 |
| Subtype | ||||||
| Non-GCB | 3 | 834 | 47.6 | 0.148 | 0.83(0.66, 1.05) | 0.125 |
| GCB | 3 | 350 | 0 | 0.484 | 0.70 (0.48, 1.03) | 0.070 |
| OS | ||||||
| ALL | 5 | 1601 | 0 | 0.592 | 0.99 (0.83, 1.20) | 0.950 |
| EFS | ||||||
| ALL | 3 | 847 | 0 | 0.818 | 0.99 (0.81, 1.21) | 0.927 |
Italic values indicate P < 0.05
EFS event-free survival, PFS progression-free survival, OS overall survival, 95%CI 95% confidence interval, HR hazard ratio, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, GCB germinal center type
Also, 1601 DLBCL patients from five trials were available for analysis of OS, with 847 patients from 3 trials for EFS. The estimation of OS and EFS were similar in the two groups, with pooled HRs of 0.99 (95%CI: 0.83–1.20, I2 = 0, P = 0.950) and 0.99 (95%CI: 0.81–1.21, I2 = 0, P = 0.927), respectively.
Safety analysis
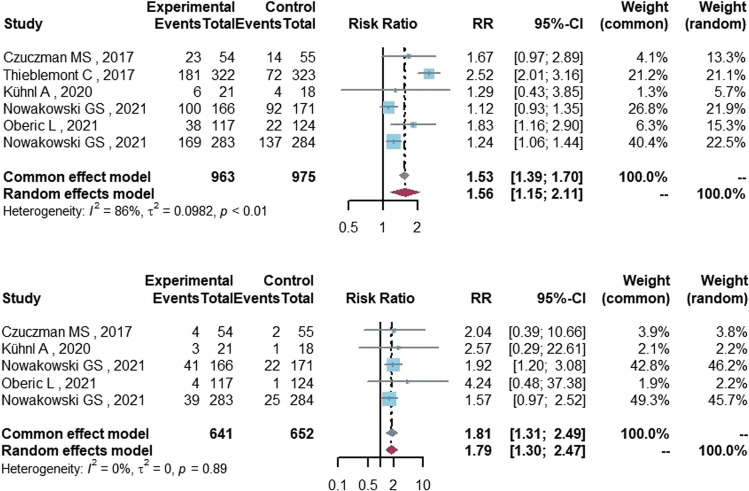
As shown in Table 2, the incidence of neutropenia in the lenalidomide group (48.3%, 95%CI 37.5–59.1%) was higher than that of anemia (17.3%, 95%CI 9.9–26.1%), thrombocytopenia (13.7%, 95%CI 5.7–24.2%) and febrile neutropenia (11.9%, 95%CI 5.2–20.6%). The lenalidomide group had a significant incidence of grade ≥ 3 hematological AEs involving neutropenia (RR = 1.56, 95%CI 1.15–2.11, P = 0.004, Fig. 4) and febrile neutropenia (RR = 1.81, 95%CI 1.31–2.49, P < 0.001, Fig. 4). The incidence of anemia and thrombocytopenia was similar between the lenalidomide group and the control group (RR = 1.21, 95%CI 0.79–1.87, P = 0.383; RR = 1.55, 95%CI 0.71–3.37, P = 0.272, respectively).
Fig. 4.
The forest plot of neutropenia and febrile neutropenia
Sensitivity analysis
Sensitivity analysis was conducted by omitting one study at a time and analyzing the remaining studies. The results are shown in Fig. 5, with no substantial changes, showing the reliability and stability of our results.
Fig. 5.
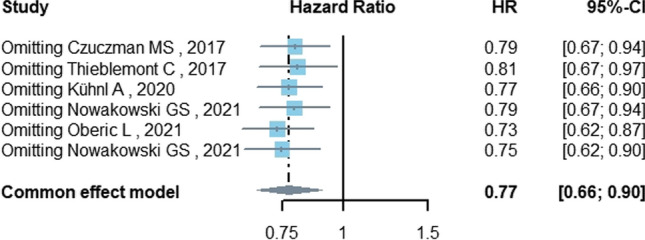
Sensitivity analysis for PFS
Publication bias
Based on the results of Begg & Mazumdar's (P = 0.327) and Egger's (P = 0.809) tests, there was no significant publication bias (Fig. 6).
Fig. 6.
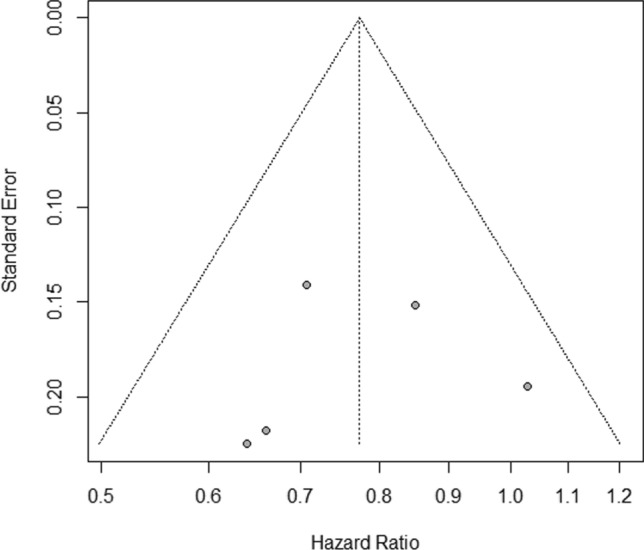
Publication bias based on funnel plot
Discussion
Lenalidomide’s antineoplastic effects have shown a good synergistic effect when combined with anti-CD20 monoclonal antibodies, as the agent enhances the NK-cell and antibody-dependent cell-mediated cytotoxicity of the anti-CD20 monoclonal antibody [25, 26]. The efficacy and safety of lenalidomide have been investigated extensively since the Mayo Clinic first reported a phase I study in which lenalidomide was combined with R-CHOP as front-line treatment in DLBCL patients and safely combined with R-CHOP without affecting the dose intensity of chemoimmunotherapy [27].
The phase II MC078E study showed that lenalidomide in combination with standard frontline treatment R-CHOP produced high response rates; the ORR in the intent-to-treat population was 97% (32/33), 29 (88%) had CR, and 3 had PR [28]. In a phase I study of lenalidomide plus R-CHOP, the ORR was 90%, with 81% of untreated, elderly patients with DLBCL achieving CR [29]. The CRR and ORR of lenalidomide in combination with R-ESHAP (rituximab, etoposide, cisplatin, cytarabine, methylprednisolone) in patients with R/R DLBCL were reported to be 47.4% and 78.9%, respectively [30]. As a second-line treatment for DLBCL, 38.9% of patients achieved CR with R-GEM-L (rituximab, methylprednisolone gemcitabine, and lenalidomide) [21]. The ORR for lenalidomide monotherapy in R/R patients was 33.3% [31]. Lenalidomide plus ibrutinib and rituximab have promising activity in R/R DLBCL, with an ORR of 44% (CRR, 28%) [32]. Dual translocation of MYC and BCL2 in patients with DLBCL is termed “double-hit lymphoma” (DHL), and dual protein overexpression of MYC and BCL2 without underlying translocations is termed “double-expressor lymphoma” (DEL). Both DHL and DEL are recognized as a distinct subset of non-Hodgkin lymphoma that is associated with very poor outcomes [33–35]. The combination of lenalidomide with dose-adjusted (DA)-EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) for DLBCL treatment-naive patients shows evidence of DHL or DEL. The best responses after induction were 13 complete responses (87%) and 1 partial response (7%), with 1 case of progressive disease (7%) [36]. Among the included studies, the SENIOR study presented the proportion of patients with expression or rearrangement of MYC and BCL2, and the ORR at the end of treatment was 73% in the R-miniCHOP arm and 82% in the R2-miniCHOP arm [23]. And the vast majority of patients were newly diagnosed and treated with R-CHOP/R-miniCHOP. The ORR in the lenalidomide group was 67%, the CRR 47.7%, and the PRR 16.3% among 963 DLBCL patients, higher than in the control group. In the control group, the ORR was 56.9%, the CRR was 37.8%, and the PRR was 15.6%. However, only the CRR was significantly higher in the lenalidomide group than in the control group.
The prognosis of elderly patients with newly diagnosed DLBCL is worse than that of young patients. Comorbidities and physiological organ function impairment often result in unmanageable toxicities and limit optimal chemotherapy. Our quantitative analysis showed that addition of lenalidomide resulted in a statistically significant improvement in PFS but failed to improve OS and EFS. Subgroup analysis showed survival benefits in the untreated, R-CHOP-treated, and ≥ 65-year-old populations. In the ECOG-ACRIN E1412 study with a median age of 66 years old [22], R2CHOP was associated with a 34% reduction in the risk of progression or death compared with R-CHOP. The 1-, 2-, and 3-year PFS rates were 84% versus 73%, 76% versus 69%, and 73% versus 62% for R2CHOP versus R-CHOP, respectively. The phase III REMARC study showed that lenalidomide maintenance for 24 months after obtaining CR or PR with R-CHOP significantly prolonged PFS in untreated elderly patients with DLBCL. The 2-year PFS was improved from 75% (95%CI 70–80%) to 80% (95%CI 75–84%) in the lenalidomide group [20]. These results were similar to our results.
It is well known that the prognosis of non-GCB is worse than that of GCB in the R-CHOP era [37, 38]. An increasing number of studies have also shown that lenalidomide combined with R-CHOP overcomes the negative impact of the non-GCB phenotype in untreated DLBCL and has promising clinical activity in DLBCL [15]. A retrospectively assessed 123 R/R DLBCL patients showed that lenalidomide is more efficient in non-GCB DLBCL, with complete remission was achieved in 32% and a partial remission in 33% non-GCB patients compared with 0% and 3% in the GCB group [39]. In a phase II trial, the addition of lenalidomide appears to mitigate a negative impact of non-GCB phenotype on patient outcome [40]. There was no significant benefit in either GCB or non-GCB patients in our study. The possible reason is the different typing methods based on Hans and gene expression profiling (GEP) among the included trials. Alternatively, more cases may be needed.
The addition of a new drug to chemoimmunotherapy raises concerns about increased toxicity, especially in older patients. Wang M et al. [41] reported common grades 3–4 hematological adverse events (≥ 10 events), including neutropenia (53%), lymphopenia (40%), thrombocytopenia (33%), leukopenia (27%) and anemia (18%). Ferreri et al. [42] found lenalidomide was well tolerated, especially in this elderly population, with the exception of neutropenia, grade-4 toxicities occurred in < 1% of courses. Our study summarized grade ≥ 3 hematological toxicity events. The results show that the pooled incidence of neutropenia was higher than that of thrombocytopenia, anemia, and febrile neutropenia. Compared to the control group without lenalidomide, the lenalidomide group had a significant incidence of grade ≥ 3 hematological AEs involving neutropenia and febrile neutropenia.
This study has several limitations. First, the results may be affected by heterogeneity caused by many factors, such as different inclusion criteria for the individual studies, inconsistent induction therapy. Second, some stratified analyses according to study or patient characteristics were not performed because several treatments were reported without more information. Therefore, the results should be considered cautiously. Further investigation is essential to provide reliable proof.
In conclusion, DLBCL patients treated with lenalidomide have a higher CRR, resulting in better PFS but a higher incidence of grade ≥ 3 hematological AEs involving neutropenia and febrile neutropenia.
Author contribution
Study design: QY, JL. Data collection or management: RM, XG. Data analysis and interpretation: TL, LC. Preparation of manuscript: JL, RM, QY. All authors approved the final version of the manuscript.
Funding
This study was funded by the Joint Project of Medical Science and Technology of Henan Province.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jia Liu, Email: zlyyliujia3889@zzu.edu.cn.
Ruihua Mi, Email: miruihua2008@163.com.
Lin Chen, Email: chenlinms@yeah.net.
Xiaoli Guo, gxlemail@126.com.
Taotao Liang, Email: 13838174792@163.com.
Qingsong Yin, Email: jnyinqingsong@163.com.
References
- 1.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas F, Shah B. Advances in diagnosis and management of diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2017;17(12):783–796. doi: 10.1016/j.clml.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grygorowicz MA, Borycka IS, Nowak E, et al. Lenalidomide potentiates CD4+CD25+Treg-related suppression of lymphoma B-cell proliferation. Clin Exp Med. 2017;17(2):193–207. doi: 10.1007/s10238-016-0411-8. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi AK, Kang J, Naziruddin S, et al. Lenalidomide inhibits proliferation of Namalwa CSN.70 cells and interferes with Gab1 phosphorylation and adaptor protein complex assembly. Leuk Res. 2006;30:849–858. doi: 10.1016/j.leukres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Wu L, Adams M, Carter T, et al. Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- 8.Lewis R, Bewersdorf JP, Zeidan AM. Clinical management of anemia in patients with myelodysplastic syndromes: an update on emerging therapeutic options. Cancer Manag Res. 2021;13:645–657. doi: 10.2147/CMAR.S240600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson GH, Davies FE, Pawlyn C, et al. Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2019;20(1):57–73. doi: 10.1016/S1470-2045(18)30687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenbeck KL, Wildes TM. Updated perspectives on the management of multiple myeloma in older patients: focus on lenalidomide. Clin Interv Aging. 2020;15:619–633. doi: 10.2147/CIA.S196087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garciaz S, Coso D, Schiano de Colella JM, et al. Lenalidomide for the treatment of B-cell lymphoma. Expert Opin Investig Drugs. 2016;25(9):1103–1116. doi: 10.1080/13543784.2016.1208170. [DOI] [PubMed] [Google Scholar]
- 12.Vose JM, Habermann TM, Czuczman MS, et al. Single-agent lenalidomide is active in patients with relapsed or refractory aggressive non-Hodgkin lymphoma who received prior stem cell transplantation. Br J Haematol. 2013;162(5):639–647. doi: 10.1111/bjh.12449. [DOI] [PubMed] [Google Scholar]
- 13.Chamuleau MED, Burggraaff CN, Nijland M, et al. Treatment of patients with MYC rearrangement positive large B-cell lymphoma with R-CHOP plus lenalidomide: results of a multicenter HOVON phase II trial. Haematologica. 2020;105(12):2805–2812. doi: 10.3324/haematol.2019.238162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dührsen U, Tometten M, Kroschinsky F, et al. Phase I/II trial of lenalidomide, methotrexate, leucovorin, cytarabine, and rituximab (LeMLAR) in relapsed or refractory diffuse large B cell lymphoma. Blood Cancer J. 2021;11(5):95. doi: 10.1038/s41408-021-00485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castellino A, Chiappella A, LaPlant BR, et al. Lenalidomide plus R-CHOP21 in newly diagnosed diffuse large B-cell lymphoma (DLBCL): long-term follow-up results from a combined analysis from two phase 2 trials. Blood Cancer J. 2018;8(11):108. doi: 10.1038/s41408-018-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee YP, Hong JY, Yoon SE, et al. Real-world, single-center data for lenalidomide plus rituximab in relapsed or refractory diffuse large B-Cell lymphoma and transformed follicular lymphoma. Cancer Manag Res. 2021;13:4241–4250. doi: 10.2147/CMAR.S309092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Joanna Briggs Institute. Joanna Briggs Institute reviewers’ manual: 2014 edition. Australia: The Joanna Briggs Institute, 2014.
- 19.Czuczman MS, Trněný M, Davies A, et al. A phase 2/3 multicenter, randomized, open-Label study to compare the efficacy and safety of lenalidomide versus investigator's choice in patients with relapsed or refractory diffuse large B-Cell lymphoma. Clin Cancer Res. 2017;23(15):4127–4137. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thieblemont C, Tilly H, Gomes da Silva M, et al. Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B-Cell lymphoma treated with first-Line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2017;35(22):2473–2481. doi: 10.1200/JCO.2017.72.6984. [DOI] [PubMed] [Google Scholar]
- 21.Kühnl A, Peckitt C, Patel B, et al. R-GEM-Lenalidomide versus R-GEM-P as second-line treatment of diffuse large B-cell lymphoma: results of the UK NRCI phase II randomised LEGEND trial. Ann Hematol. 2020;99(1):105–112. doi: 10.1007/s00277-019-03842-4. [DOI] [PubMed] [Google Scholar]
- 22.Nowakowski GS, Hong F, Scott DW, et al. Addition of lenalidomide to R-CHOP improves outcomes in newly diagnosed diffuse large B-cell lymphoma in a randomized phase II US Intergroup Study ECOG-ACRIN E1412. J Clin Oncol. 2021;39(12):1329–1338. doi: 10.1200/JCO.20.01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberic L, Peyrade F, Puyade M, et al. Subcutaneous rituximab-miniCHOP compared with subcutaneous rituximab-miniCHOP plus lenalidomide in diffuse large B-cell lymphoma for patients age 80 years or older. J Clin Oncol. 2021;39(11):1203–1213. doi: 10.1200/JCO.20.02666. [DOI] [PubMed] [Google Scholar]
- 24.Nowakowski GS, Chiappella A, Gascoyne RD, et al. ROBUST: a phase III study of lenalidomide plus R-CHOP versus placebo plus R-CHOP in previously untreated patients with ABC-type diffuse large B-cell lymphoma. J Clin Oncol. 2021;39(12):1317–1328. doi: 10.1200/JCO.20.01366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, et al. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 26.Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45. doi: 10.1111/j.1365-2141.2007.06841.x. [DOI] [PubMed] [Google Scholar]
- 27.Nowakowski GS, LaPlant B, Habermann TM, et al. Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia. 2011;25(12):1877–1881. doi: 10.1038/leu.2011.165. [DOI] [PubMed] [Google Scholar]
- 28.Desai SH, LaPlant B, Macon WR, et al. Lenalidomide in combination with R-CHOP produces high response rates and progression-free survival in new, untreated diffuse large B-cell lymphoma transformed from follicular lymphoma: results from the Phase 2 MC078E study. Blood Cancer J. 2021;11(9):160. doi: 10.1038/s41408-021-00542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiappella A, Tucci A, Castellino A, et al. Lenalidomide plus cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab is safe and effective in untreated, elderly patients with diffuse large B-cell lymphoma: a phase I study by the Fondazione Italiana Linfomi. Haematologica. 2013;98(11):1732–1738. doi: 10.3324/haematol.2013.085134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín A, Redondo AM, Dlouhy I, et al. Lenalidomide in combination with R-ESHAP in patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 1b study from GELTAMO group. Br J Haematol. 2016;173(2):245–252. doi: 10.1111/bjh.13945. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmaiah KC, Rachan Shetty KS, Sathyanarayanan V, et al. Lenalidomide in relapsed refractory non-Hodgkin's lymphoma: an Indian perspective. J Cancer Res Ther. 2015;11(4):857–861. doi: 10.4103/0973-1482.151418. [DOI] [PubMed] [Google Scholar]
- 32.Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024–1036. doi: 10.1182/blood.2018891598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166:891–901. doi: 10.1111/bjh.12982. [DOI] [PubMed] [Google Scholar]
- 34.Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021–4250. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godfrey JK, Nabhan C, Karrison T, et al. Phase 1 study of lenalidomide plus dose-adjusted EPOCH-R in patients with aggressive B-cell lymphomas with deregulated MYC and BCL2. Cancer. 2019;125(11):1830–1836. doi: 10.1002/cncr.31877. [DOI] [PubMed] [Google Scholar]
- 37.Marcus C, Maragkos GA, Alterman RL, et al. GCB-type is a favorable prognostic factor in primary CNS diffuse large B-cell lymphomas. J Clin Neurosci. 2021;83:49–55. doi: 10.1016/j.jocn.2020.11.031. [DOI] [PubMed] [Google Scholar]
- 38.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 39.Mondello P, Steiner N, Willenbacher W, et al. Lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: is it a valid treatment option? Oncologist. 2016;21(9):1107–1112. doi: 10.1634/theoncologist.2016-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251–257. doi: 10.1200/JCO.2014.55.5714. [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Fowler N, Wagner-Bartak N, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27(9):1902–1909. doi: 10.1038/leu.2013.95. [DOI] [PubMed] [Google Scholar]
- 42.Ferreri AJM, Sassone M, Angelillo P, et al. Long-lasting efficacy and safety of lenalidomide maintenance in patients with relapsed diffuse large B-cell lymphoma who are not eligible for or failed autologous transplantation. Hematol Oncol. 2020;38(3):257–265. doi: 10.1002/hon.2742. [DOI] [PubMed] [Google Scholar]