Abstract
Induction of an effective antibody response against human cytomegalovirus (HCMV) is an important defense mechanism since it is potentially capable of neutralizing infectious viruses. We have analyzed the extent of HCMV strain-specific neutralization capacity in human sera. Nine recent HCMV isolates and their corresponding sera were investigated in cross-neutralization assays. We observed differences, independent of the overall neutralization capacity, in the 50% neutralization titers of the sera against individual strains, differences that ranged from 8-fold to more than 60-fold. For one isolate, complete resistance to neutralization by two human sera was observed. The neutralization capacity of human sera was not influenced by the presence of various concentrations (up to 100-fold excess) of noninfectious envelope glycoproteins, an inherent contamination of virus preparations from recent HCMV isolates. This indicated that the decisive parameter for neutralization is the titer of the neutralizing antibodies and that neutralization is largely independent of the concentration of virus. Analysis with transplant patients revealed that during primary infection strain-specific and strain-common antibodies are produced asynchronously. Thus, our data demonstrate that the induction of strain-specific neutralizing antibodies is a common event during infection with HCMV and that it might have important implications for the course of the infection and the development of anti-HCMV vaccines.
Human cytomegalovirus (HCMV) remains a significant pathogen in individuals with an immature or compromised immune system. In contrast, infection of immunocompetent persons has had limited consequences in the vast majority of cases, indicating the importance of a functional immune response in the control of HCMV infections (23). Although the immunological effector functions which control HCMV are incompletely understood, it must be assumed that the humoral immune response represents an important defense mechanism against HCMV. It is well established that seroimmunity to HCMV prior to conception provides substantial protection against symptomatic infection of the newborn (19, 44). In a recent vaccination study it was also demonstrated that protection from reinfection is correlated with the titers of neutralizing antibodies but not of T cells (2). In transplant recipients the absence of viral DNA in the blood is associated with high levels of neutralizing antibodies (39). Moreover, passive transfer of antibodies seems to have a beneficial effect on the clinical outcome of infection (41, 49). In the murine cytomegalovirus model, protection from a lethal challenge can be achieved by using monoclonal antibodies (MAbs) or immune sera directed against glycoproteins B (gB) and H (gH), respectively (18, 34). In addition, antibodies are the limiting factor for the prevention of virus dissemination (25). Collectively, these findings point to a major role of antibodies in limiting the consequences of a HCMV infection.
Although no two HCMV isolates are identical with respect to the restriction endonuclease patterns of the entire genomes, strain variations have been considered to be of little consequence for the host (12, 21). However, in recent years several studies have suggested that strain differences might contribute to the clinical course of the infection. For example, in kidney transplant recipients reinfection with a genetically different donor virus is associated with a higher risk of developing severe HCMV disease than of reactivation of the endogenous virus (22). Likewise, survival rates of bone marrow transplant recipients with HCMV infection have been linked to specific genotypes of the envelope gB (gpUL55) (20). In addition, there is evidence that increased incidence of retinitis in patients with AIDS is associated with the gB genotype (40).
Although the underlying mechanisms for the different clinical outcome of HCMV infections are unexplained, strain-specific immune responses might play an important role in clinical situations where reinfections occur and where the de novo immune response against viral antigens is impaired as, for example, in transplant patients or in individuals infected with human immunodeficiency virus. In addition, strain-specific immune responses might hamper the development of an effective vaccine. Antibodies against envelope glycoproteins could be particularly important since they have been shown to neutralize virus. Thus far, gB and gH (gpU175) have been identified as dominant targets for the humoral immune response, and immunoglobulins reacting with these antigens have been characterized in some detail (for a review, see reference 10). Extensive strain-specific virus neutralization has been observed in the vast majority of studies that have employed against gB and gH in the neutralization of different clinical HCMV isolates, and some of the B-cell epitopes involved have been characterized (6, 32, 35, 45). For polyclonal sera, the situation is less well investigated. When the sera from HCMV-immunized animals were used, significant differences in neutralization capacity against different HCMV strains were observed (47, 48). A potential factor influencing the strain-specific neutralization of a given serum is the amount of noninfectious enveloped particles present in the virus preparations. It is well known that HCMV, depending on the stage of cell culture adaptation, can produce various amounts of noninfectious particles which contain the major envelope glycoproteins and therefore have the capacity to bind neutralizing antibodies (24, 27). The importance of noninfectious particles to the neutralization of infectious virus by MAbs or polyclonal sera has not been investigated.
In this study we have used sera from HCMV-infected persons and analyzed their neutralization capacity against homologous and heterologous HCMV isolates. The impact of noninfectious particles on the neutralization titer of human sera was also investigated. Our data reveal extensive differences in the capacity of human sera to neutralize heterologous HCMV isolates.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts were grown in minimal essential medium (Gibco BRL, Glasgow, Scotland) supplemented with 5% fetal calf serum (FCS), glutamine (100 mg/liter), and gentamycin (350 mg/liter). Virions and dense bodies from cell culture-adapted virus strains were isolated via glycerol-tartrate gradient centrifugation as described previously (3). To obtain infectious virus from highly cell-associated HCMV strains, infected cells were trypsinized and resuspended in phosphate-buffered saline (PBS). After Dounce homogenization, the suspension of the cellular debris was removed by low-speed centrifugation (15 min at 8,000 rpm in a Sorvall centrifuge). Virus particles were pelleted by high-speed centrifugation in a Beckman SW27 rotor (70 min at 23,000 rpm), suspended in PBS, and stored at −80°C. Clinical isolates were obtained from patients after solid-organ (six HCMV-seropositive recipients receiving organs from seronegative donors) or bone marrow (three seronegative recipients receiving marrow from seropositive donors) transplantation and were used between passage 5 and passage 10.
MAbs.
The MAbs that were used in this study have been described previously: gB-specific MAbs included 89-104 (17) and C23 (31), the gH-specific MAb AP86-SA4 (45), and the gp65-specific MAb 14-16A (9).
Neutralization analysis.
Patient sera were obtained between days 100 and 150 after transplantation. The general outline of the neutralization assay was as described earlier (4). Serial serum dilutions (in the range of 1:50 to 1:250,000) were incubated with virus preparations for 4 h at 37°C. Viral titers were adjusted to give 100 to 150 infected cells counted on a fluorescence microscope (Olympus/IMT-2) with a ×200 magnification, which is equivalent to 2,000 infected cells/15,000 total cells. 1.5 × 104 fibroblasts were added, and the mixture was plated on microtiter plates. Infected cells were counted 16 h later by using indirect immunofluorescence with a MAb directed against the immediate-early protein 1 of HCMV. The percent neutralization was calculated as the reciprocal of infectivity, with a maximum infectivity being determined by incubation of the virus without serum. The number of infected cells without the addition of serum also served as a basis for the determination for infectious units (IU). When neutralization assays were carried out in the presence of complement, 2% freshly prepared HCMV-negative human sera were mixed with serial dilutions of inactivated (30 min, 56°C) HCMV-positive sera and virus. The concentration of complement was titrated for the ability to lyse (100% lysis) sensibilized sheep erythrocytes (Boehringer Mannheim) without neutralizing virus. Approximately 2% were found to be sufficient as the complement source.
Enzyme-linked immunosorbent assay (ELISA) to determine relative ratios of gB.
Polystyrene 96-well microtiter plates were coated with 50-μl/well serial dilutions of HCMV infectious virus and noninfectious particles (5 μg to 40 ng) in 6 M Urea and incubated for 16 h at 4°C in a humid chamber. Reaction wells were rinsed three times with washing buffer (PBS, 0.05% Tween 20). Blocking was done for 2 h at 37°C with PBS containing 2% FCS. After incubation with the gB-specific human MAb C23 for 2 h at 37°C and three additional washing steps, the bound antibodies were detected with peroxidase-conjugated anti-human immunoglobulin G (IgG) (Dako, Hamburg, Germany) (45 min, 37°C). After three washing steps, 100 μl of substrate (o-phenylenediamine; 2 mg/ml) was added for 20 min. The reaction was stopped by the addition of 100 μl of 2 N H2SO4, and the optical density was determined at 492 nm.
Individual antigens and the assay procedure for determining antibody titers of human sera with selected antigens of HCMV have been described previously (39). Briefly, selected antigens were expressed either as glutathione S-transferase (GST) fusion proteins (pp65, pp28, pp71, p52, and IE/1) or with β-galactosidase (Sem2) as the fusion partner (pp150, gH/AD169, gH/Towne, gB/AD-1, gB/AD169, and gB/Towne). The peptide gB/AD-2 was chemically synthesized (for more detailed information, see reference 39). The ELISA procedure was performed as described above. The reactivity index (RI) was calculated according to the following formula: RI = (A490 − antigen)/(A490− fusion partner).
PCR amplification and restriction pattern analysis.
Total chromosomal DNA was prepared from 2 × 106 to 3 × 106 HCMV-infected cells as previously described (28). Three regions of glycoproteins B and H were amplified by using the PCR. (i) Primers gB 1319 and gB 1604 amplified a region of high peptide variability of gB between nucleotides 1319 and 1604. Digestion with HinfI and RsaI in two separate reactions was used to differentiate gB types 1 through 4 (16). (ii) Primers 58-20 and 58-21 (28) amplified a fragment of the amino terminus of gB (nucleotides −57 to 848 of the open reading frame), which contains variable regions within the antigenic domain 2 (AD2). NciI, which only cuts the AD169-like fragments, was used to discriminate between “AD169-like” and “Towne-like” virus strains. (iii) Primers NEST 5′ from nucleotides −63 to −43 of the open reading frame of gH (5′-TCTCGGGTGTAACGCCAACCA-3′) and NEST 3′ from nucleotides 225 to 205 (5′-GTTTTCCCTGACGACCGTGCT-3′) amplified the amino terminus of gH (nucleotides −63 to 225), which contains strain-specific regions within the antigenic domain 86 (AD86) (45). AccI, which only cuts the AD169-like fragments, was used to discriminate between the AD169-like and Towne-like virus strains.
RESULTS
Reliability of the neutralization assay.
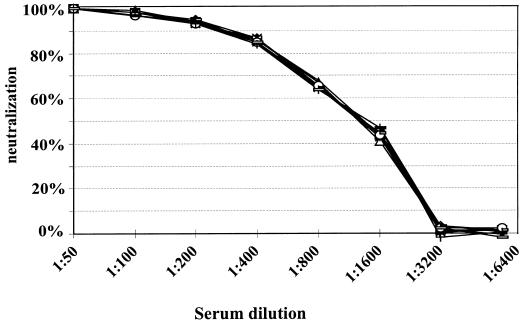
In order to determine reliability of the neutralization assay, several experiments were carried out. In a first set of experiments the reproducibility was determined. Testing a single serum on repeated occasions resulted in identical titration curves (data not shown). Neutralization assays were also performed by using serum samples collected from one healthy donor over a period of several years. The individual serum samples were tested in an ELISA against 12 different previously characterized HCMV-derived recombinant antigens (39). The sera were found to contain constant levels of antibody against the individual antigens (data not shown). When neutralization capacity was assayed, superimposable titration curves were obtained (Fig. 1). These data demonstrate that sera containing a given titer of HCMV-specific antibodies show highly reproducible results in our assays.
FIG. 1.
Neutralizing activity of sequential sera from an HCMV-positive healthy person. Neutralization analysis with HCMV strain AD169 was performed as described in Materials and Methods. The percentage of neutralization is plotted as a function of the serum dilution. Symbols represent sera collected at 0, 2, 4, 8, 11, 17, 22, 28, 30, and 55 months.
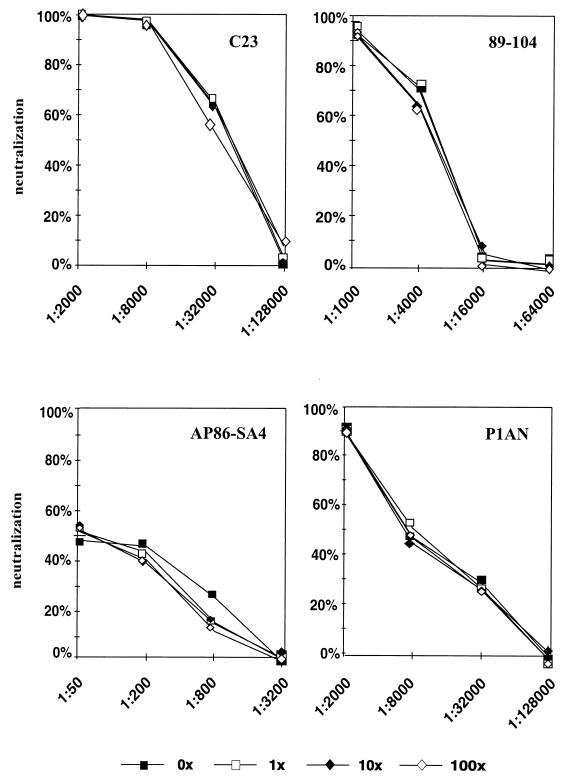
Next, the influence of enveloped noninfectious viral particles on the neutralization capacity of a given serum was analyzed. Low-passage clinical isolates of HCMV are tightly cell associated, and in general it is impossible to purify sufficient amounts of infectious virions by gradient centrifugation for repeated neutralization tests with a number of different sera. It was therefore decided to work with infectious particles obtained from cell lysates after high-speed centrifugation. These preparations, however, will consist of different ratios of infectious to noninfectious HCMV particles. Noninfectious particles include dense bodies and noninfectious enveloped particles, as well as noninfectious virions (24). All of these particles contain an envelope capable of binding glycoprotein-specific antibodies and could potentially influence the titer of neutralizing antibodies in a given serum. We therefore determined the ratio of IU to the amount of gB in our preparations. IU were measured as the number of infected cells at 16 h after infection as determined by indirect immunofluorescence with an antibody specific for the immediate-early protein 1 of HCMV (4). The relative amount of gB was determined by ELISA with MAb C23, which is specific for a conserved epitope in gB (32). gB is the dominant antigen in the envelope of HCMV involved in the induction of neutralizing antibodies (11, 29). The determination of gB can therefore serve as an indirect marker for the amount of neutralization-relevant envelope proteins in the preparation. Table 1 shows a comparison of 11 strains from a single preparation. Relative to the IU, the amount of gB differed by a factor of 30 between strains, with strain Towne having the highest gB/IU ratio and strain F3I having the lowest gB/IU ratio. Similar overall differences were found in additional preparations. However, the gB/IU ratio was not constant for individual strains, indicating that it is influenced by passage in cell culture (data not shown). Whether these different ratios of gB to IU could influence neutralization was subsequently tested. Virions from strain AD169, as well as one low-passage clinical isolate (F3I) from which enough material could be obtained, were gradient purified. Increasing amounts of homologous noninfectious dense bodies were added to each preparation, and neutralization assays were carried out with human sera as well as MAbs specific for gB (89-104 and C23) and gH (AP86-SA4). The results were identical for both strains, and the data obtained with strain AD169 are presented (Fig. 2). Increasing the amount of noninfectious particles up to a 100-fold excess of gB did not result in a difference in the neutralization titer of either the MAbs or the human sera. This effect was independent of the neutralization titer of the respective antibody or antiserum. We conclude from these results that, within a broad range, the ratio of infectious to noninfectious particles did not influence the neutralization titer of a given serum or MAb.
TABLE 1.
Characterization of virus strains
| Strain | DNA,a gB type | Epitopeb, gB type | Epitope,c gH type | gBd | IUe | IU/gB ratio |
|---|---|---|---|---|---|---|
| AD169 | II | AD169 | AD169 | 1.0 | 1.0 | 1.00 |
| Towne | I | Towne | Towne | 7.8 | 23.8 | 3.05 |
| P1AN | III | AD169 | AD169 | 1.4 | 0.5 | 0.36 |
| 236 | IV | AD169 | AD169 | 1.4 | 0.2 | 0.14 |
| 210 | I | Towne | Towne | 1.4 | 0.8 | 0.57 |
| P143 | I | Towne | Towne | 1.8 | 0.9 | 0.50 |
| R2O | I | Towne | Towne | 1.8 | 4.1 | 2.28 |
| 244 | I | Towne | AD169 | 2.8 | 1.3 | 0.46 |
| F3I | I | Towne | Towne | 1.0 | 0.1 | 0.10 |
| 209 | II | AD169 | Towne | 3.5 | 2.0 | 0.57 |
| 252 | III | AD169 | AD169 | 0.7 | 0.12 | 0.17 |
gB genotypes (types I to IV) are as defined by Chou on basis of restriction enzyme pattern of gB (16).
Virus types (AD169-like or Towne-like) were defined on basis of the strain-specific epitope present in the amino terminus of gB.
Virus types (AD169-like or Towne-like) were defined on basis of the strain-specific epitope present in the amino terminus of gH.
The relative concentration of gB is shown. The concentration of AD169 was arbitrarily set at 1.
The IU values relative to strain AD169 are shown. The IU was measured as described in Materials and Methods.
FIG. 2.
Influence of noninfectious HCMV particles on neutralization capacity. Increasing amounts of noninfectious AD169 particles were added to infectious AD169 virions, and the neutralization capacity of the MAbs and a human serum was determined. Panels: C23 and 89-104, gB-specific human MAbs; AP86-SA4, gH-specific murine MAb; P1AN, human serum.
Neutralization of clinical isolates by homologous and heterologous sera.
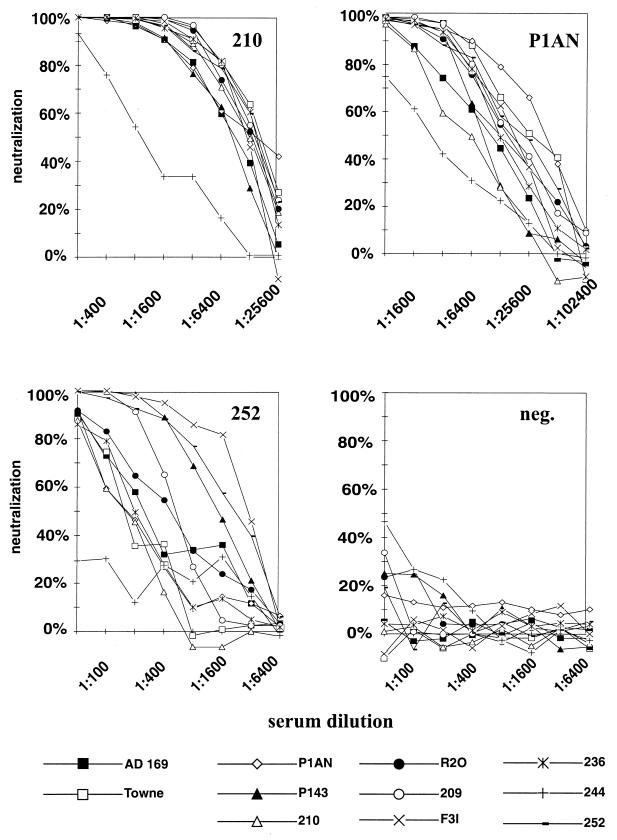
A total of 11 virus strains, including the laboratory-adapted isolates AD169 and Towne, as well as 9 fresh clinical isolates, were tested with their corresponding sera in cross-neutralization experiments. The minimal serum dilution used in these experiments was 1:50. Lower dilutions of some sera had toxic effects on the cell monolayer and, in addition, some sera from HCMV-seronegative donors started to show nonspecific reduction of input infectivity at lower dilutions (see Fig. 3D for an example). Results of representative sera are shown in Fig. 3, and the data are summarized in Table 2.
FIG. 3.
Neutralization capacity of three HCMV-positive human sera (210, P1AN, and 252) and one HCMV-negative serum (neg.) against homologous and heterologous virus strains. The percentage of neutralization is plotted as a function of the serum dilution.
TABLE 2.
50% neutralization titers of sera against homologous and heterologous virus strains
| Serum | Results, 50% neutralization capacitya
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Homologous strain | Maximum | Minimum | Ratiob | Minimumc (−strain 244) | Ratioc | Ratio, (+/−C′) AD169d | Ratio, (+/−C′) 244d | |
| P1AN | 1:40,000 | 1:40,000 (P1AN) | 1:2,500 (244) | 16 | 1:6,400 (210) | 6.3 | 1.8 | 1.7 |
| 236 | 1:22,000 | 1:32,000 (236) | 1:2,400 (244) | 13.3 | 1:2,400 (210) | 13.3 | 1.5 | 1.8 |
| 210 | 1:12,800 | 1:13,000 (Towne) | 1:1,000 (244) | 13 | 1:9,000 (P143) | 1.4 | 1 | 1 |
| P143 | 1:3,000 | 1:3,200 (Towne) | 1:350 (244) | 9.1 | 1:1,200 (AD169) | 2.7 | ND | ND |
| R20 | 1:6,400 | 1:7,000 (236) | 1:600 (244) | 11.7 | 1:1,200 (AD169) | 5.8 | ND | ND |
| 244 | 1:300 | 1:3,500 (P143) | 1:300 (244) | 1.7 | 1:700 (AD169) | 5 | ND | ND |
| F3I | 1:1,300 | 1:1,300 (F3I) | 1:160 (244) | 8.1 | 1:350 (P1AN) | 3.7 | ND | ND |
| 209 | 1:380 | 1:1,000 (P143) | <1:50 (244/AD169) | >20 | <1:50 (AD169) | >20 | 1 | 4 |
| 252 | 1:2,500 | 1:3,000 (F3I) | <1:50 (244) | >60 | 1:150 (210) | 20 | ND | ND |
Neutralization was measured as described in Materials and Methods. The neutralization titer refers to the serum dilution, with the respective strain given in parentheses. ND, not determined.
Ratios were calculated according to the following formula: maximum neutralization capacity/minimum neutralization capacity.
Ratios were calculated according to the following formula: maximum neutralization capacity/minimum neutralization capacity without strain 244.
Neutralization assay was performed in the presence (+C′) or absence (−C′) of 2% human complement with strain AD169 or strain 244. The ratios were calculated according to the following formula: neutralization with complement/neutralization without complement.
In most cases the homologous isolate was among the strains that were most effectively neutralized (Table 2). We observed differences, independent of the overall neutralizing capacity, in the 50% neutralization titer of sera against strains that ranged from 8-fold (serum F3I) to more than 60-fold (serum 252). In fact, strain 244 was not neutralized by sera 252 and 209. The isolate that was least effectively neutralized by all of the sera was strain 244, and this was seen even with the homologous serum. The resistance of strain 244 to neutralization by human sera was probably due to a phenomenon other than strain specificity, and therefore 50% neutralization titers were also calculated without the inclusion of strain 244. Still, differences between 1.4-fold (serum 210) and >20-fold (serum 209) were seen (Table 2).
The influence of complement on the neutralization capacity was also tested. Four sera (P1AN, 210, 236, and 209) with different neutralization titers, as well as differences in strain specificity, were chosen (see Table 2). Neutralizing capacity was tested on isolates AD169 and 244. Only marginal differences (up to 1.8-fold) in the 50% neutralization titer were seen for both strains in the presence or absence of complement (Table 2). The only exception was serum 209 which, in the presence of complement, showed a fourfold greater neutralization capacity against strain 244 than without added complement. However, the neutralization titer of the serum against AD169 was not influenced by complement. As a control for the activity of the exogenously added human complement, neutralization tests were carried out with the MAb IgM 14-16A, which is specific for HCMV gp65 and which requires complement for effective neutralization (9). This antibody was completely dependent on the addition of complement for virus neutralization, thus demonstrating the presence of active complement in our assays (data not shown). Collectively, these data suggest that production of strain-specific neutralizing antibodies is common following natural infection, since we have tested only 11 HCMV strains and found significant strain specificity of the neutralizing response, as well as a strain which exhibited a neutralization-resistant phenotype.
Correlation between virus types and susceptibility to neutralization.
Although no two HCMV isolates are identical when analyzed with restriction fragment length polymorphism, only very crude tools are available for grouping virus strains. Based on the DNA sequence heterogeneity of a small region of the gB gene (nucleotides 1319 to 1604 of the open reading frame), Chou has reported on the identification of four discrete genotypes (gB1 to -4) (16). Alternatively, HCMV isolates can be grouped according to the presence of the known strain-specific neutralizing epitopes on gB and gH (32, 45). These epitopes are located between amino acids (aa) 34 and 43 on gH and aa 27 and 84 on gB and are represented by AD169 and Towne as prototypes, respectively. It should be noted, however, that these characterized epitopes most likely represent only a fraction of the total number of strain-specific epitopes on the envelope of HCMV. According to these criteria our strain collection, including strains AD169 and Towne, comprised six gB1, two gB3 and gB2, and one gB4 genotypes. With respect to neutralizing epitopes, the strains could be classified as four AD169-like isolates, five Towne-like isolates, as well as two isolates showing a mixed phenotype (Table 1).
When the neutralization capacity was calculated in connection with the virus type, neither the gB genotype nor the presence of the known strain-specific epitopes on gB or gH were found to be related to the differences in neutralization titer by human sera (data not shown).
Synthesis of strain-specific antibodies during natural infection.
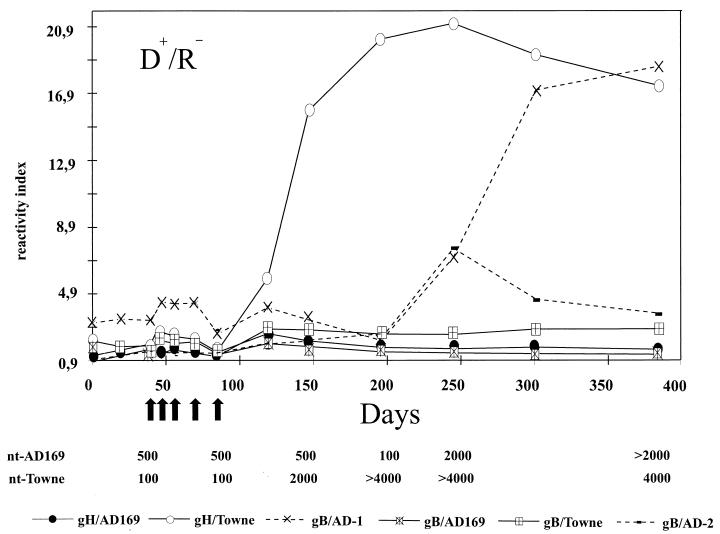
Lastly, we were interested in the kinetics of the development of strain-specific neutralizing antibodies in human sera. To this end, antibodies directed against strain-common and strain-specific epitopes on the viral gB and gH were tested in serum specimens from bone marrow transplant patients in an ELISA with recombinant proteins (39). Strain-common antigens included gB/AD-1 (aa 484 to 650) and gB/AD-2 (aa 67 to 84) of gB; strain-specific antigens included gB/AD169 (aa 28 to 67) or gB/Towne (aa 12 to 57) on gB, as well as gH/Towne (aa 14 to 43) or gH/AD169 (aa 15 to 142) of gH (39). A total of more than 600 serum samples from 31 patients were analyzed in this ELISA. In nine patients (29%), strain-specific antibodies were detected in the absence of antibodies to cross-reactive epitopes, and four of these patients (13%) also exhibited 50% neutralization capacities which differed by a factor of more than two between the two strains. An example is shown in Fig. 4. This patient tested positive for HCMV DNA in the peripheral blood between days 45 and 80 posttransplantation, indicating an active viral infection. At around day 100 posttransplantation, antibodies reacting with the neutralizing epitope on gH strain Towne (antigen gH/Towne) but not AD169 (antigen gH/AD169) were detectable. At the same time the 50% virus neutralization titer against strain Towne began to rise, with titers of more than 1:2,000 compared to 1:500 against strain AD169. This strain specificity was detected until day 200, after which time the appearance of cross-neutralizing anti-gB antibodies (antigen gB/AD-1) abolished the difference. Similar findings were observed when the remaining four patients were tested for strain-specific neutralizing antibodies. In five patients the presence of antibodies to strain-specific epitopes was not reflected by a difference in neutralization capacity towards strain AD169 and Towne (data not shown). These data indicate that in the posttransplant period the strain-specific neutralizing antibody response can persist for extended periods of time. Because the lack of ELISA-reactive strain-specific antibodies does not necessarily reflect the lack of strain-specific neutralization activity, the four patients which exhibited strain-specific virus neutralizing antibodies most likely reflect a minimal estimate of the frequency of such response.
FIG. 4.
HCMV-specific antibody response in a bone marrow transplant patient. Antibody titer measured against gB- and gH-specific antigens is shown at various time points (days after transplantation). The solid lines represent antibody reactivity against strain-specific epitopes on gB or gH. The dashed lines show antibody reactivity against strain-common epitopes. The arrows indicate time points at which the PCR was positive for HCMV. The 50% neutralization titer against strains AD169 and Towne are shown at the analyzed time points. D+, donor, HCMV seropositive; R−, recipient, HCMV seronegative.
DISCUSSION
Studies which compared different clinical HCMV isolates invariably came to the conclusion that differences exist between viral strains. At the level of the genome, no two clinical isolates are identical when analyzed for restriction fragment length polymorphism (12, 26). When specific regions of the genome, such as the genes for glycoproteins, are compared different strains can be distinguished (14, 16). The clinical relevance of strain variations, as well as the impact on active and passive immunoprophylaxis, is incompletely defined. A few reports have linked HCMV genotypes to different clinical courses of the infection (20, 40). We have investigated the consequences of HCMV strain variation for the virus neutralizing antibody response. Our data clearly show that the neutralizing capacity of human sera against heterologous HCMV isolates can vary over a wide range, leading in some cases to an inability to neutralize heterologous isolates.
Strain-specific neutralization by using different clinical isolates has been investigated previously with animal sera or MAbs. The vast majority of these studies have reported extensive strain specificity (6, 30, 45, 47, 48). However, the immune response that develops during a long-term persistent infection is different from short-term immunization, and it cannot be assumed that during natural HCMV infection a strain-specific humoral immune response would persist. Therefore, we have tested two cell culture-adapted strains, as well as nine low-passage clinical isolates, with their corresponding sera in cross-neutralization experiments. Besides sera which showed little strain specificity (e.g., serum 210), we could identify sera which, at the same dilution, neutralized some strains completely and yet failed to neutralize other strains at all (e.g., serum 252). This effect was independent of the presence of complement in the assays, a finding which is in line with results showing that HCMV has developed mechanisms to control the activity of complement (42, 43). Thus, our data extend the previous findings in that we have shown that also during natural infection neutralizing antibodies with a wide spectrum of strain specificity are induced. The fact that the strains and sera in our study were derived from immunosuppressed patients does not influence this conclusion, since we have previously shown that a suppressed immune system develops the same antibody specificities as does a competent immune system (39).
Neutralization assays with low-passage HCMV isolates are not a straightforward experimental problem. During the early passages in cell culture the viruses are tightly cell associated, preventing the preparation of sufficient quantities of infectious virions via gradient purification. This limitation can be overcome by repeated passages in cell culture, a process which usually leads to the emergence of more-cytopathic virus variants which can be gradient purified from cell culture supernatant. Although the underlying mechanism for the cell culture adaptation process is unknown, it might involve alterations in the viral envelope that could affect neutralization by human sera. We therefore decided to work with unpurified viral preparations from HCMV strains which are still tightly cell associated and which contained noninfectious as well as infectious particles. According to our results, these preparations differ in the ratio of envelope glycoproteins to infectious units to a considerable extent and consequently could mimic strain-specific differences in neutralization assays. To our knowledge, different ratios of infectious to noninfectious particles have not been considered in previous investigations, although even purified virion preparations of lytic strains of HCMV show a considerable variability in the ratio of envelope glycoprotein to infectious units (27a). However, as our data clearly show, this ratio does not need to be considered for in vitro neutralization, since even a 100-fold excess of noninfectious particles did not result in a change in the neutralizing titer of a given serum or MAb. This result was somewhat unexpected, but it is best explained by the so-called “percentage law,” which was proposed for bacteriophages and has been shown to extend to nonenveloped animal viruses when tested with polyclonal and MAbs (5, 8). The law states that, regardless of virus concentration (up to 108 PFU/ml), a constant percentage will be neutralized by a fixed amount of antibodies. At virus concentrations over 108 PFU/ml, the neutralization is proportional to the virus or antibody concentration. The law still defies explanation. If applied to our situation (2 × 104 PFU/ml), the law would predict that the total concentration of envelope protein, be it on infectious virions or noninfectious particles, would be irrelevant within a wide range. In our analysis neither the addition of a 100-fold excess of noninfectious envelope protein nor the increase of infectious virions (up to 106 PFU/ml [27a]) influenced the neutralization titer of human sera or MAbs. It must therefore be concluded that the percentage law also applies to a complex enveloped virus such as HCMV. If neutralization tests in vitro have any relevance for the in vivo situation, this could mean that a sufficiently high antibody titer is the single most important parameter for virus neutralization. Recent data from the Zinkernagel group have confirmed this for the in vivo situation, since they reported that protection against vesicular stomatitis virus infection in mice depended simply on a minimum serum concentration (7). If the percentage law applies to the in vivo situation, it might also explain the lack of protection from reinfection or HCMV disease in vaccinees immunized with the Towne virus (1, 33). In fact, results from a recent vaccination trial by Adler et al. showed that protection from reinfection in women is associated with high levels of neutralizing antibodies. These levels were not provided by vaccination with the Towne strain but by natural infection (2). The controversial results on the beneficial effects of passively transferred immunoglobulin preparations to limit the clinical consequences of HCMV replication in transplant patients might also be explained by the same mechanism. It is clear that these preparations greatly vary in the titer of HCMV neutralizing antibodies and that the neutralization capacities in patient sera after infusion remains low, thus explaining the lack of efficacy of some of these preparations (13, 38).
Which molecules on the viral envelope could be involved in the induction of strain-specific neutralizing antibodies? The envelope of HCMV contains a minimum of three complexes of glycoproteins with at least six individual members (10). So far, gB and gH have been identified as major targets for the neutralizing humoral immune response in human sera. When recombinant gB and gH were used, between 0 and 98% (gB) and between 0 and 58% (gH) of the total neutralizing capacity could be removed from human sera by preadsorption (11, 29, 46). This wide range could be explained in at least two ways. (i) Some sera do not contain neutralizing antibodies directed against gB or gH. This seems highly unlikely since 97 and 82% of convalescent sera contain antibodies against gB and gH, respectively (39). (ii) The preadsorption experiments were performed using AD169-derived antigen as well as AD169 virus for the subsequent neutralization tests. Consequently, non-AD169 neutralizing antibodies could not be determined, resulting in a low estimate of the gB- or gH-directed neutralizing capacity in human sera. In our sample group, serum 209 would most probably be unaffected by preadsorption with AD169-derived antigens, since this serum did not neutralize AD169. However, preadsorption with strain P143-derived antigens would most probably have resulted in a considerable reduction in the neutralization capacity of serum 209.
Theoretically, a strain-specific neutralizing antibody response could be clinically relevant in situations where reinfection occurs and the ability of the immune system to mount a de novo response against the infecting strain is impaired. Such situations could include transplantation of solid organs or bone marrow from an HCMV-seropositive donor to a seropositive recipient. Grundy et al. reported a correlation between proven reinfection (six cases) in renal transplant patients and clinical symptoms, whereas Chou in a study of heart and kidney recipients involving 16 reinfections could not confirm this finding (15, 22). Chou also determined the neutralizing antibody response to the reinfecting strain and found that levels of neutralizing antibodies varied up to fourfold. Both studies evaluated only a small number of patients. HCMV replication in previously HCMV-seropositive kidney recipients results in clinical symptoms in only 19 to 69% of patients (23). Thus, in both studies insufficient numbers of patients were analyzed to obtain statistically significant data. Larger studies need to be carried out in order to investigate the potential correlation between the neutralizing antibody response to reinfecting HCMV strains and the clinical course of the infection. A further aspect of a strain-specific neutralizing antibody response that could be relevant to the clinical situation is the importance of virus neutralizing antibodies in limiting virus dissemination. Studies in the MCMV system have shown that antibodies can limit dissemination and reduce viral titers in organs (25). Viral load has been shown to be an important parameter for reactivation and the risk of recurrent cytomegalovirus disease (36). The lack of strain-specific neutralizing antibody responses as seen in the bone marrow transplant patients could increase the likelihood of dissemination and increased viral load. Whether this chain of events is important for the clinical situation will be difficult to prove and can only be answered by larger clinical studies. Alternatively, the HCMV system could be exploited to address this question.
The ability of a prior immune response to neutralize infecting HCMV strains is an important question in vaccine development efforts. Current efforts have focused on gB from strains AD169 or Towne (37). It remains to be seen whether, in accordance with the percentage law, these vaccines will induce sufficiently high titers of cross-neutralizing antibodies in order to protect from infection with antigenically different HCMV strains. Our analysis does not provide information that addresses this question since we were not able to correlate the known gB types with neutralization capacity in human sera. However, our results clearly indicate that antigens from some strains (e.g., strain 210) are superior to others (e.g., strain 252) for the induction of cross-neutralizing antibodies.
In conclusion, our data show that for the neutralization of HCMV the most important parameter is the titer of the neutralizing antibodies and that this neutralization is largely independent of the concentration of virus. On the other hand, the effective neutralization titer of a given serum against heterologous HCMV strains varies considerably. For the clinical situation this could result in insufficient protection from reinfection. With respect to vaccine development, our results indicate that care should be taken to choose antigens for vaccination which induce sufficiently high titers of cross-neutralizing antibodies.
ACKNOWLEDGMENTS
We would like to thank U. Meyer-König for providing virus strains and sera.
This work was supported by grants from the Deutsche Forschungsgemeinschaft, the BMBF, and the Johannes und Frieda Marohn-Stiftung.
REFERENCES
- 1.Adler S P. Current prospects for immunization against cytomegaloviral disease. Infect Agents Dis. 1996;5:29–35. [PubMed] [Google Scholar]
- 2.Adler S P, Starr S E, Plotkin S A, Hempfling S H, Buis J, Manning M L, Best A M. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis. 1995;171:26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 3.Almeida J, Lang D, Talbot P. Herpesvirus morphology: visualization of a structural subunit. Intervirology. 1978;10:318–320. doi: 10.1159/000148994. [DOI] [PubMed] [Google Scholar]
- 4.Andreoni M, Faircloth M, Vugler L, Britt W J. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J Virol Methods. 1989;23:157–167. doi: 10.1016/0166-0934(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 5.Andrews C H, Elford W J. Observations on anti-phage sera. 1: “The Percentage Law.”. Br J Exp Pathol. 1933;14:367–375. [Google Scholar]
- 6.Baboonian C, Blake K, Booth J C, Wiblin C N. Complement-independent neutralising monoclonal antibody with differential reactivity for strains of human cytomegalovirus. J Med Virol. 1989;29:139–145. doi: 10.1002/jmv.1890290212. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann M F, Kalinke U, Althage A, Freer G, Burkhart C, Roost H-P, Aguet M, Hengartner H, Zinkernagel R M. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 8.Brioen P, Boeye A. Poliovirus neutralization and the percentage law. Brief report. Arch Virol. 1985;83:105–111. doi: 10.1007/BF01310968. [DOI] [PubMed] [Google Scholar]
- 9.Britt W J, Auger D. Identification of a 65,000-dalton virion envelope protein of human cytomegalovirus. Virus Res. 1985;4:31–36. doi: 10.1016/0168-1702(85)90018-8. [DOI] [PubMed] [Google Scholar]
- 10.Britt W J, Mach M. Human cytomegalovirus glycoproteins. Intervirology. 1996;39:401–412. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- 11.Britt W J, Vugler L, Butfiloski E J, Stephens E B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990;64:1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandler S H, McDougall J K. Comparison of restriction site polymorphisms among clinical isolates and laboratory strains of human cytomegalovirus. J Gen Virol. 1986;67:2179–2192. doi: 10.1099/0022-1317-67-10-2179. [DOI] [PubMed] [Google Scholar]
- 13.Chehimi J, Peppard J, Emanuel D. Selection of an intravenous immune globulin for the immunoprophylaxis of cytomegalovirus infections: an in vitro comparison of currently available and previously effective immune globulins. Bone Marrow Transplant. 1987;2:395–402. [PubMed] [Google Scholar]
- 14.Chou S. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J Infect Dis. 1992;166:604–607. doi: 10.1093/infdis/166.3.604. [DOI] [PubMed] [Google Scholar]
- 15.Chou S W. Neutralizing antibody responses to reinfecting strains of cytomegalovirus in transplant recipients. J Infect Dis. 1989;160:16–21. doi: 10.1093/infdis/160.1.16. [DOI] [PubMed] [Google Scholar]
- 16.Chou S W, Dennison K M. Analysis of interstrain variation in cytomegalovirus glycoprotein B sequences encoding neutralization-related epitopes. J Infect Dis. 1991;163:1229–1234. doi: 10.1093/infdis/163.6.1229. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich P H, Harfeldt K E, Justice J C, Moustafa Z A, Ostberg L. Rhesus monkey responses to multiple injections of human monoclonal antibodies. Hybridoma. 1987;6:151–160. doi: 10.1089/hyb.1987.6.151. [DOI] [PubMed] [Google Scholar]
- 18.Farrell H E, Shellam G R. Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J Gen Virol. 1991;72:149–156. doi: 10.1099/0022-1317-72-1-149. [DOI] [PubMed] [Google Scholar]
- 19.Fowler K B, Stagno S, Pass R F, Britt W J, Boll T J, Alford C A. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 20.Fries B C, Chou S, Boeckh M, Torok Storb B. Frequency distribution of cytomegalovirus envelope glycoprotein genotypes in bone marrow transplant recipients. J Infect Dis. 1994;169:769–774. doi: 10.1093/infdis/169.4.769. [DOI] [PubMed] [Google Scholar]
- 21.Grillner L, Strangert K. A prospective molecular epidemiological study of cytomegalovirus infections in two day care centers in Sweden: no evidence for horizontal transmission within the centers. J Infect Dis. 1988;157:1080–1083. doi: 10.1093/infdis/157.5.1080. [DOI] [PubMed] [Google Scholar]
- 22.Grundy J E, Lui S F, Super M, Berry N J, Sweny P, Fernando O N, Moorhead J, Griffiths P D. Symptomatic cytomegalovirus infection in seropositive kidney recipients: reinfection with donor virus rather than reactivation of recipient virus. Lancet. 1988;2:132–135. doi: 10.1016/s0140-6736(88)90685-x. [DOI] [PubMed] [Google Scholar]
- 23.Ho M. Cytomegalovirus: biology and infection. New York, N.Y: Plenum Medical Book; 1993. [Google Scholar]
- 24.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 25.Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski U H. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med. 1994;179:1713–1717. doi: 10.1084/jem.179.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilpatrick B A, Huang E-S, Pagano J S. Analysis of cytomegalovirus genomes with restriction endonucleases HinD III and EcoR-1. J Virol. 1976;18:1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klages S, Ruger B, Jahn G. Multiplicity-dependent expression of the predominant phosphoprotein pp65 of human cytomegalovirus. Virus Res. 1989;12:159–168. doi: 10.1016/0168-1702(89)90061-0. [DOI] [PubMed] [Google Scholar]
- 27a.Klein, M. Unpublished data.
- 28.Lehner R, Stamminger T, Mach M. Comparative sequence analysis of human cytomegalovirus strains. J Clin Microbiol. 1991;29:2494–2502. doi: 10.1128/jcm.29.11.2494-2502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall G S, Rabalais G P, Stout G G, Waldeyer S L. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis. 1992;165:381–384. doi: 10.1093/infdis/165.2.381. [DOI] [PubMed] [Google Scholar]
- 30.Masuho Y, Matsumoto Y, Sugano T, Fujinaga S, Minamishima Y. Human monoclonal antibodies neutralizing human cytomegalovirus. J Gen Virol. 1987;68:1457–1461. doi: 10.1099/0022-1317-68-5-1457. [DOI] [PubMed] [Google Scholar]
- 31.Masuho Y, Matsumoto Y, Tomiyama T, Sugano T, Ono S. Characterization of human anti-cytomegalovirus monoclonal antibody as biologics. Dev Biol Stand. 1990;71:127–136. [PubMed] [Google Scholar]
- 32.Meyer H, Sundqvist V A, Pereira L, Mach M. Glycoprotein gp116 of human cytomegalovirus contains epitopes for strain-common and strain-specific antibodies. J Gen Virol. 1992;73:2375–2383. doi: 10.1099/0022-1317-73-9-2375. [DOI] [PubMed] [Google Scholar]
- 33.Plotkin, S. A., S. E. Starr, H. M. Friedman, E. Gonczol, and K. Brayman. 1990. Vaccines for the prevention of human cytomegalovirus infection. Rev. Infect. Dis. 12(Suppl. 7):827–838. [DOI] [PubMed]
- 34.Rapp M, Messerle M, Buhler B, Tannheimer M, Keil G M, Koszinowski U H. Identification of the murine cytomegalovirus glycoprotein B gene and its expression by recombinant vaccinia virus. J Virol. 1992;66:4399–4406. doi: 10.1128/jvi.66.7.4399-4406.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen L E, Nelson R M, Kelsall D C, Merigan T C. Murine monoclonal antibody to a single protein neutralizes the infectivity of human cytomegalovirus. Proc Natl Acad Sci USA. 1984;81:876–880. doi: 10.1073/pnas.81.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddehase M J, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski U H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med. 1994;179:185–193. doi: 10.1084/jem.179.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roizman B. The family Herpesviridae: a brief introduction. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 1–9. [Google Scholar]
- 38.Schmitz H, Essuman S. Comparison of the neutralizing and ELISA antibody titres to human cytomegalovirus (HCMV) in human sera and in gamma globulin preparations. J Med Virol. 1986;20:177–182. doi: 10.1002/jmv.1890200209. [DOI] [PubMed] [Google Scholar]
- 39.Schoppel K, Kropff B, Schmidt C, Mach M. The humoral immune response against the human cytomegalovirus is characterized by a delayed synthesis of glycoprotein specific antibodies. J Infect Dis. 1997;175:533–544. doi: 10.1093/infdis/175.3.533. [DOI] [PubMed] [Google Scholar]
- 40.Shepp D H, Match M E, Ashraf A B, Lipson S M, Millan C, Pergolizzi R. Cytomegalovirus glycoprotein B groups associated with retinitis in AIDS. J Infect Dis. 1996;174:184–187. doi: 10.1093/infdis/174.1.184. [DOI] [PubMed] [Google Scholar]
- 41.Snydman D R. Prevention of cytomegalovirus-associated diseases with immunoglobulin. Transplant Proc. 1991;23:131–135. [PubMed] [Google Scholar]
- 42.Spear G T, Lurain N S, Parker C J, Ghassemi M, Payne G H, Saifuddin M. Host cell-derived complement control proteins CD55 and CD59 are incorporated into the virions of two unrelated enveloped viruses. Human T cell leukemia/lymphoma virus type I (HTLV-I) and human cytomegalovirus (HCMV) J Immunol. 1995;155:4376–4381. [PubMed] [Google Scholar]
- 43.Spiller O B, Morgan B P, Tufaro F, Devine D V. Altered expression of host-encoded complement regulators on human cytomegalovirus-infected cells. Eur J Immunol. 1996;26:1532–1538. doi: 10.1002/eji.1830260719. [DOI] [PubMed] [Google Scholar]
- 44.Stagno S, Pass R F, Dworsky M E, Henderson R E, Moore E G, Walton P D, Alford C A. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med. 1982;306:945–949. doi: 10.1056/NEJM198204223061601. [DOI] [PubMed] [Google Scholar]
- 45.Urban M, Britt W, Mach M. The dominant linear neutralizing antibody-binding site of glycoprotein gp86 of human cytomegalovirus is strain specific. J Virol. 1992;66:1303–1311. doi: 10.1128/jvi.66.3.1303-1311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urban M, Klein M, Britt W J, Hassfurther E, Mach M. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J Gen Virol. 1996;77:1537–1547. doi: 10.1099/0022-1317-77-7-1537. [DOI] [PubMed] [Google Scholar]
- 47.Waner J L, Weller T H. Analysis of antigenic diversity among human cytomegaloviruses by kinetic neutralization tests with high-titered rabbit antisera. Infect Immun. 1978;21:151–157. doi: 10.1128/iai.21.1.151-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zablotney S L, Wentworth B B, Alexander E R. Antigenic relatedness of 17 strains of human cytomegalovirus. Am J Epidemiol. 1978;107:336–343. doi: 10.1093/oxfordjournals.aje.a112549. [DOI] [PubMed] [Google Scholar]
- 49.Zaia, J. A. 1993. Prevention and treatment of cytomegalovirus pneumonia in transplant recipients. Clin. Infect. Dis. 17(Suppl. 2):392–399. [DOI] [PubMed]