Abstract
Objective
JCOG1106, a randomized phase II trial conducted to compare chemoradiotherapy (S-1 concurrent radiotherapy) with (Arm B) or without (Arm A) induction chemotherapy using gemcitabine in patients with locally advanced pancreatic cancer, showed a more favorable long-term survival in Arm A. This study was aimed at exploring whether some subgroups classified by the systemic inflammatory response might derive greater benefit from either treatment.
Methods
All subjects eligible for JCOG1106 were included in this analysis (n = 51/49 in Arm A/B). This exploratory subgroup analysis was performed by Cox regression analysis to investigate the impact of the systemic inflammatory response, as assessed based on the serum C-reactive protein, serum albumin (albumin), Glasgow Prognostic Score and derived neutrophil–lymphocyte ratio, at the baseline on overall survival. P values <0.1 for the interaction were regarded as denoting significant association.
Results
Glasgow prognostic score showed significant treatment interactions for overall survival. Hazard ratios of Arm B to Arm A were 1.35 (95% confidence interval, 0.82–2.23) in the Glasgow Prognostic Score 0 (C-reactive protein ≤10 mg/L and albumin ≥35 g/L) (n = 44/34 in Arm A/B) and 0.59 (95% confidence interval, 0.24–1.50) in the Glasgow Prognostic Score 1/2 (C-reactive protein >10 mg/L and/or albumin <35 g/L) (n = 7/15) (P-interaction = 0.06). C-reactive protein alone and albumin alone also showed significant treatment interactions for overall survival.
Conclusions
Survival benefits of induction chemotherapy in chemoradiotherapy for locally advanced pancreatic cancer were observed in patients with elevated Glasgow Prognostic Score, high C-reactive protein and low albumin. These results suggest that systemic inflammatory response might be considered to apply induction chemotherapy preceding chemoradiotherapy.
Keywords: Glasgow prognostic score, treatment interaction, S-1 concurrent radiotherapy, gemcitabine
Survival benefits of induction chemotherapy with gemcitabine in chemoradiotherapy for locally advanced pancreatic cancer were observed in patients with elevated Glasgow Prognostic Score, high serum CRP and low serum albumin.
Introduction
Pancreatic cancer (PC) is one of the leading causes of cancer-related death in the world. The prognosis of PC is extremely poor, with a 5-year survival proportion of 10% (1). Despite surgical resection is the only chance for cure, ~80–85% of patients already are unresectable status (1). Nearly one-third of patients are diagnosed at locally advanced disease with vascular invasion, particularly of the superior mesenteric artery or the celiac axis (2). Chemoradiotherapy (CRT) as well as systemic chemotherapy are the treatment options for locally advanced PC (LAPC). The recent phase II trial showed that median overall survival (OS) of LAPC is 18.0–31.4 months (3–5).
JCOG1106 (UMIN000006811) was a randomized phase II trial that was conducted to evaluate the efficacy and safety of CRT (S-1 concurrent radiotherapy) with or without induction chemotherapy (iCT) using gemcitabine in patients with LAPC (6). Based on the final analysis, the 1- and 2-year survival proportions were 66.7 and 36.9% in CRT without iCT and 69.3 and 18.9% in CRT with iCT. The median survival time was 19.0 months in CRT without iCT and 17.2 months in CRT with iCT. The hazard ratio (HR) for death of CRT with iCT to CRT without iCT was 1.26 [95% confidence interval (CI), 0.82–1.93]. After the final analysis, CRT without iCT was selected as the more promising regimen because of the more favorable 2-year OS, despite the poorer 1-year OS and the survival curves crossing at around 1 year (6).
Systemic inflammatory response (SIR), for instance, serum C-reactive protein (CRP), serum albumin, Glasgow Prognostic Score (GPS), neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR) and platelet–lymphocyte ratio (PLR), has been reported as a predictive marker as well as a prognostic marker in patients with advanced cancer, across tumor subtypes. Among components of the SIR, the serum CRP, serum albumin, GPS and NLR have consistently been validated across tumor types, based on objective measures defined by the numerical values (7). The SIR has been also suggested as prognostic factors in patients with pancreatic (8) and other types of cancers (9–11) receiving CRT. Moreover, in the various types of cancers, the SIR is recognized as a predictive factor in patients receiving chemotherapy (12,13) or CRT (14).
In JCOG1106, crossing of the Kaplan–Meier curves for OS of the two arms at 1 year suggested the possibility of inconsistent treatment effect between the two treatment arms. Therefore, we hypothesized that the SIR may modify the effect of iCT, and this study was aimed at exploring subgroups classified according to the SIR that might derive greater benefit from either treatment.
Patients and methods
Patients in CRT without iCT underwent radiotherapy with concurrent S-1, and patients in CRT with iCT received induction gemcitabine for 12 weeks, and thereafter only patients with controlled disease underwent the same CRT as CRT without iCT. After CRT, gemcitabine was continued until disease progression or unacceptable toxicity in both arms. The secondary use of data from JCOG1106 was included in the written informed consent provided by all patients at the enrollment in JCOG1106, and the study was protocol approved from the institutional review board of each of the participating institutions.
Among the subjects enrolled in the JCOG1106, all eligible cases were included in this exploratory subgroup analysis, except for two cases in which distant metastases were found prior to the start of protocol treatment (n = 51 in CRT without iCT and n = 49 in CRT with iCT) (6). The protocol stipulated that blood tests related to the SIR should be allowed up to 7 days before randomization and that treatment should be started within 15 days after randomization. The GPS was determined according to the baseline serum CRP and serum albumin levels. Patients with serum CRP ≤10 mg/L and serum albumin ≥35 g/L were assigned a GPS of 0, those with serum CRP >10 mg/L or serum albumin <35 g/L were assigned a GPS of 1 and those with serum CRP >10 mg/L and serum albumin <35 g/L were assigned a GPS of 2 (15). The baseline value of the derived NLR (dNLR) was calculated as the absolute neutrophil count (ANC) divided by the white blood cell (WBC) minus the ANC (13).
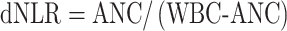 |
This post hoc exploratory subgroup analysis was performed by Cox regression analysis to investigate the impact of the SIR at the baseline on OS, progression-free survival (PFS) and distant metastasis-free survival (DMFS). P values < 0.1 for the interaction were regarded as denoting significant association. Adverse events were evaluated for each SIR for hematologic and non-hematologic toxicity of G3 or greater according to the Common Terminology Criteria for Adverse Events, ver. 4.0 throughout the entire protocol period. All statistical analyses were carried out by using SAS version 9.2 or later version (SAS Institute, Cary, NC, USA).
Results
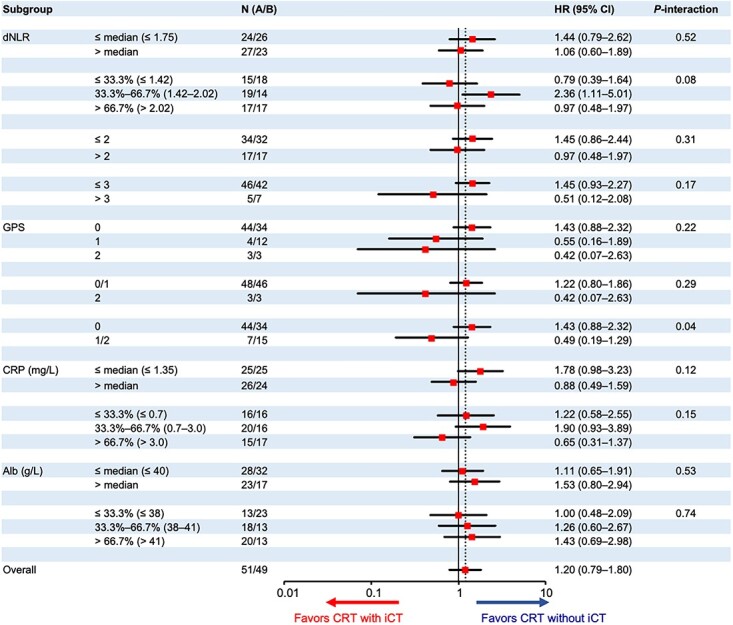
The GPS, serum CRP and serum albumin measured at the baseline showed significant treatment interactions for OS (Fig. 1). The HRs of CRT with iCT to CRT without iCT were as follows: 1.35 (95% CI 0.82–2.23) in the GPS 0 group (n = 44 in CRT without iCT and n = 34 in CRT with iCT) and 0.59 (95% CI, 0.24–1.50) in the GPS 1/2 group (n = 7/15) (P-interaction = 0.06); 2.57 (95% CI, 1.36–4.86) and 0.70 (95% CI, 0.37–1.32) in the low serum CRP (≤1.35 mg/L, n = 25/25) and high serum CRP (>1.35 mg/L, n = 26/24) (P-interaction = 0.01) groups, respectively; 1.62 (95% CI, 0.77–3.40), 2.70 (95% CI, 1.17–6.23) and 0.52 (95% CI, 0.24–1.13) in the first (≤0.7 mg/L, n = 16/16), second (>0.7, ≤3.0 mg/L, n = 20/16) and third (>3.0 mg/L, n = 15/17) (P-interaction = 0.01) tertiles of the serum CRP, respectively; 2.29 (95% CI, 1.11–4.69) and 0.89 (95% CI, 0.51–1.54) in the high serum albumin (>40 g/L, n = 23/17) and low albumin (≤40 g/L, n = 28/32) (P-interaction = 0.04) groups, respectively. For the serum CRP, especially strong interaction (P-interaction = 0.02) was shown by the multivariable analysis with the treatment arm, serum CRP (≤1.35 vs. > 1.35 mg/L), serum albumin (>40 vs. ≤40 g/L), the GPS (0 vs. 1/2) and their treatment interaction (Supplemental Table 1).
Figure 1.
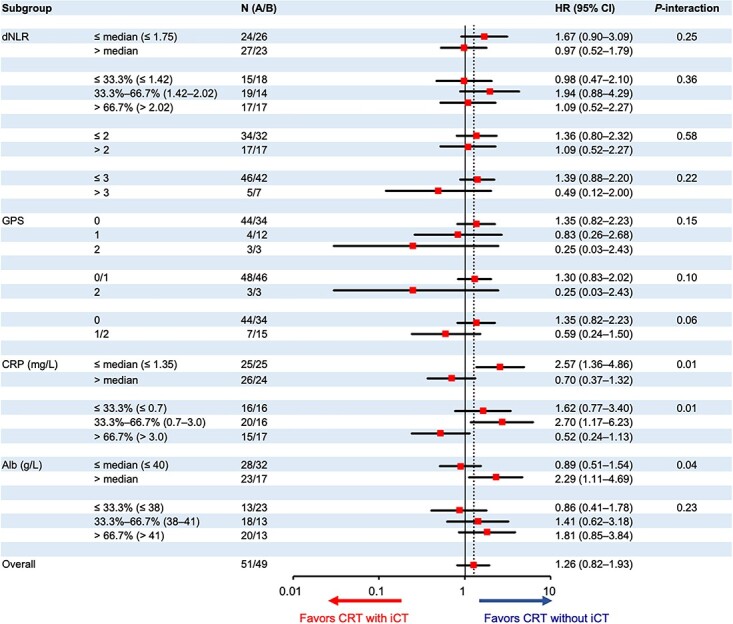
Subgroup analysis for overall survival. dNLR, derived neutrophil–lymphocyte ratio; GPS, Glasgow Prognostic Score; CRP, C-reactive protein; Alb, albumin; HR, hazard ratio; iCT, induction chemotherapy using gemcitabine; CRT, S-1 concurrent radiotherapy.
Figure 2 presents the Kaplan–Meier curves for OS in the two treatment arms classified according to the GPS into the GPS 0 and GPS 1/2 groups. In the group with GPS 0, as shown in Fig. 2A, the survival curves of both arms crossed at 1 year as in the final analysis, and CRT without iCT exceeded CRT with iCT after 1 year. On the other hand, the survival curves in the group with GPS 1/2, shown in Fig. 2B, indicate that the survival in CRT without iCT was consistently lower than that in CRT with iCT.
Figure 2.
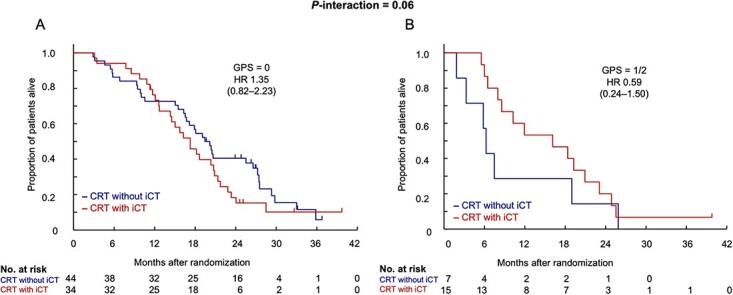
Overall survival according to the GPS. GPS, Glasgow Prognostic Score; HR, hazard ratio; CRT without iCT, S-1 concurrent radiotherapy without induction chemotherapy using gemcitabine; CRT with iCT, S-1 concurrent radiotherapy with induction chemotherapy using gemcitabine.
We also investigated the treatment effect of each of dNLR using several cutoff values, GPS (0, 1 and 2, 0/1 and 2) and serum albumin [first (≤38 g/L), second (>38, ≤41 g/L) and third tertile (>41 g/L)] for OS. No significant differences in the treatment effect were observed across the subgroups.
Furthermore, the GPS at the baseline also showed significant treatment interactions for PFS (Fig. 3). The HRs of CRT with iCT to CRT without iCT were 1.18 (95% CI, 0.74–1.88) in the GPS 0 group (n = 44 in CRT without iCT, and 34 in CRT with iCT) and 0.53 (95% CI, 0.21–1.37) in the GPS 1/2 (n = 7/15) (P-interaction = 0.07). The serum CRP (≤1.35 mg/L/>1.35 mg/L) and dNLR [first (≤1.42)/second (>1.42, ≤2.02)/third (>2.02) tertile] also showed significant treatment interactions for PFS. For the serum CRP, especially strong interaction (P-interaction = 0.05) was shown by the multivariable analysis with the treatment arm, serum CRP (≤1.35 vs. > 1.35 mg/L), serum albumin (>40 vs. ≤40 g/L), the GPS (0 vs. 1/2) and their treatment interaction (Supplemental Table 2).
Figure 3.
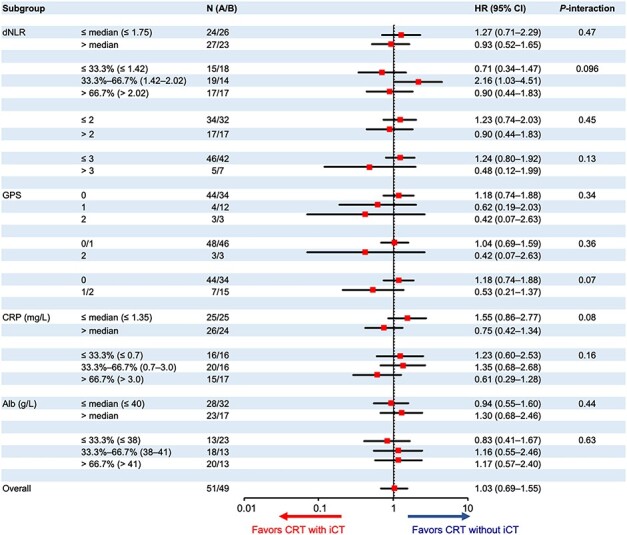
Subgroup analysis for progression-free survival. PFS, progression-free survival; dNLR, derived neutrophil–lymphocyte ratio; GPS, Glasgow Prognostic Score; CRP, C-reactive protein; Alb, albumin; HR, hazard ratio; iCT, induction chemotherapy using gemcitabine; CRT, S-1 concurrent radiotherapy.
Figure 4 presents the Kaplan–Meier curves of PFS for the two treatment arms according to the GPS (0 and 1/2). In Fig. 4A, showing the results for the GPS 0 group, the survival curves in both treatment arms almost overlapped. On the other hand, in Fig. 4B, showing the results for the GPS 1/2 group, the survival curve of CRT without iCT was consistently lower than the survival curve of CRT with iCT.
Figure 4.
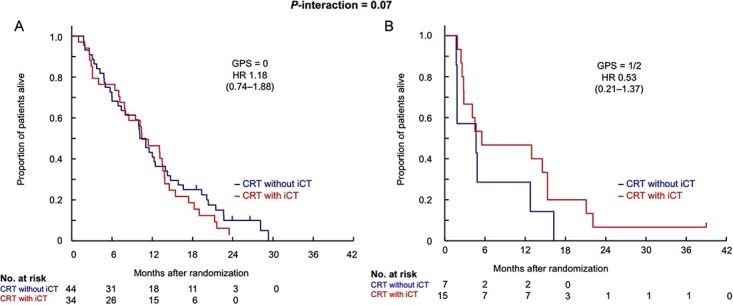
Progression-free survival according to the GPS. GPS, Glasgow Prognostic Score; HR, hazard ratio; CRT without iCT, S-1 concurrent radiotherapy without induction chemotherapy using gemcitabine; CRT with iCT, S-1 concurrent radiotherapy with induction chemotherapy using gemcitabine.
The GPS at the baseline also showed significant treatment interactions for DMFS (Fig. 5). The HRs of CRT with iCT to CRT without iCT were 1.43 (95% CI, 0.88–2.32) in the GPS 0 group (n = 44 in CRT without iCT, and 34 in CRT with iCT) and 0.49 (95% CI, 0.19–1.29) in the GPS 1/2 (n = 7/15) (P-interaction = 0.04). The dNLR [first (≤1.42)/second (>1.42, ≤2.02)/third (>2.02) tertile] also showed significant treatment interactions for DMFS. No significant differences in the treatment effect were observed in the other subgroups for DMFS. No strong interaction was shown by the multivariable analysis with the treatment arm, serum CRP (≤1.35 vs. > 1.35 mg/L), serum albumin (>40 vs. ≤40 g/L), the GPS (0 vs. 1/2) and their treatment interaction (Supplemental Table 3).
Figure 5.
Subgroup analysis for distant metastasis-free survival. dNLR, derived neutrophil–lymphocyte ratio; GPS, Glasgow Prognostic Score; CRP, C-reactive protein; Alb, albumin; HR, hazard ratio; iCT, induction chemotherapy using gemcitabine; CRT, S-1 concurrent radiotherapy.
Figure 6 presents the Kaplan–Meier curves of DMFS according to the GPS (0 and 1/2). In Fig. 6A, showing the results for the GPS 0 group, the DMFS of CRT with iCT tended to be shorter than that of CRT without iCT. On the other hand, in Fig. 6B, showing the results for the GPS 1/2 group, the DMFS of CRT with iCT tended to be longer than that of CRT without iCT. Furthermore, more cases with early distant metastasis were observed in GPS 1/2 when comparing GPS 0 and GPS 1/2 regardless of iCT (Fig. 6C).
Figure 6.
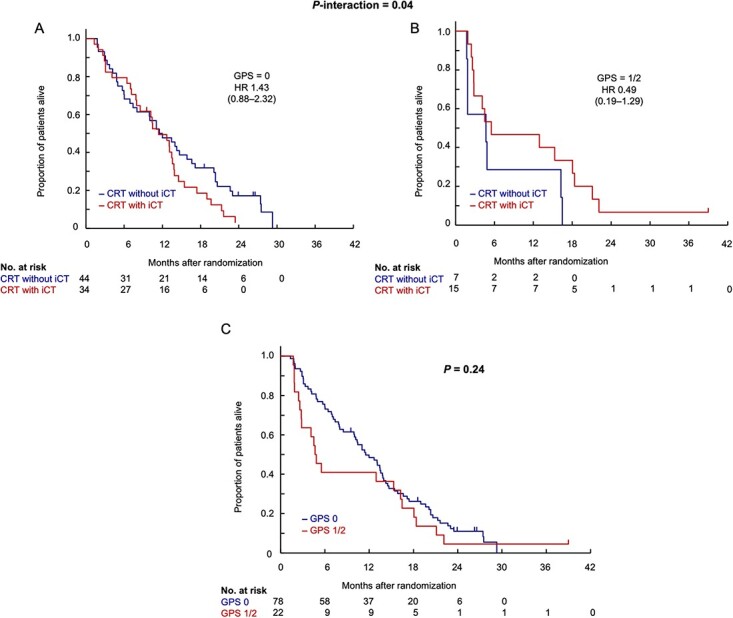
Distant metastasis-free survival according to GPS. GPS, Glasgow Prognostic Score; HR, hazard ratio; CRT without iCT, S-1 concurrent radiotherapy without induction chemotherapy using gemcitabine; CRT with iCT, S-1 concurrent radiotherapy with induction chemotherapy using gemcitabine.
The incidence of grade 3 or higher hematologic toxicities was not different between the two groups, and the incidence of grade 3 or higher non-hematologic toxicities was generally similar between the two groups (Supplemental Tables 4 and 5).
Discussion
Current analysis revealed that the GPS, serum CRP level and serum albumin level showed significant treatment interactions for OS in the JCOG1106 cohort. Patients with a high GPS, high serum CRP level and/or a low serum albumin level showed less survival benefit of upfront S-1 concurrent radiotherapy (S-1/RT) as compared with induction systemic chemotherapy with gemcitabine followed by S-1/RT. Thus, although the JCOG1106 failed to show the efficacy of iCT, our results of this study suggest that patients with elevated serum CRP levels (>1.35 mg/L) and patients with a GPS of 1/2 may derive benefit from iCT with gemcitabine prior to S-1/RT as compared with upfront S-1/RT.
SIR has been recognized as a prognostic factor in many tumor types, including PC (12,16–18). To the best of our knowledge, there are no studies to date that have examined the SIR as a potential predictor of the efficacy of iCT in patients with PC receiving CRT.
An explanation of SIR as a predictor is the possible presence of occult metastases at the baseline. Higher GPS can be associated with a more advanced tumor stage in patients with head and neck cancer (9). Approximately 30% of patients with LAPC have occult metastases (19) that progress rapidly within a few months, resulting in unsuitable for CRT. One concept of the JCOG1106 was to screen patients for occult metastases during iCT so as to spare those with rapidly progressive disease from the potentially ineffective and unsuitable for upfront CRT. The GPS and serum CRP also showed treatment interactions for PFS, and among patients with a higher GPS, PFS tended to be shorter in CRT without iCT. The GPS also showed treatment interactions for DMFS, and among patients with a higher GPS, DMFS tended to be shorter in CRT without iCT. Furthermore, more cases with early distant metastasis were observed in patients with a higher GPS regardless of iCT. These findings suggest that patients with a high GPS and elevated serum CRP in the JCOG1106 cohort might have had occult metastases at the baseline. Although systemic chemotherapy would be appropriate for those patients with high GPS and elevated serum CRP, iCT with gemcitabine may not be effective to control these patients.
Serum CRP, serum albumin and the peripheral blood neutrophil count, and scores calculated from these parameters, such as the GPS/modified GPS and NLR, have all been well validated. However, the role of SIR in predicting the treatment effect in different treatment modalities was not understood. This study showed that SIR may not only serve as a prognostic factor, but also aid in selection of the appropriate treatment across the different modalities. Other SIRs include LMR and PLR. However, the lymphocyte and/or monocyte counts are necessary for LMR and PLR calculations, but these were not collected in JCOG1106.
The GPS is known to reflect the degree of tumor-associated inflammation and cachexia (7). Patients with PC often have latent cachexia at diagnosis, and alterations in metabolic parameters such as lipids, body weight and blood glucose are observed (20). Elevated GPS is reported as a poor prognostic factor in patients with esophageal cancer receiving CRT. In the supplementary analysis of a phase II trial conducted to evaluate the safety and efficacy of concurrent CRT with S-1 plus cisplatin in patients with unresectable locally advanced head and neck squamous cell carcinoma (JCOG0706), the presence of cachexia prior to the start of CRT was identified as a poor prognostic factor in patients with head and neck cancer (21). The complete response and proportion of completion of treatment were also reported to be poorer in these patients (21). However, in the JCOG1106 subjects, there was no significant difference in both hematologic and non-hematologic toxicity between the two groups in terms of SIR strength. Therefore, we suppose that the effect of toxicity on the difference in survival in the two groups due to the strength of SIR was limited.
The strength of this study is that we identified simple inflammation-based scores/parameters, such as the GPS, serum albumin level and serum CRP measured at the baseline as showing treatment interactions prior to CRT in patients with LAPC. High serum CRP, low serum albumin, high GPS and high dNLR were defined as ‘strong SIR’ in this study, indicating a highly inflammatory state. Conversely, low serum CRP, high serum albumin, low GPS and low dNLR were defined as weak SIR. Potentially ineffective upfront CRT can be avoided in patients showing a strong SIR at the baseline. Among GPS, CRP and albumin, GPS is a well-validated and robust categorical variable compared with continuous variables CRP, and albumin is easy to use in clinical practice. In multivariable analyses, CRP was a significant factor for OS and PFS. On the other hand, the cutoffs for serum CRP and serum albumin were set as median values, which may not be replicated in other studies or other cohorts.
JCOG1106 used gemcitabine, a less toxic drug, as iCT, which may have contributed to these results. FOLFIRINOX (22) and gemcitabine/nab-paclitaxel (23) have been demonstrated to show superior efficacy to gemcitabine monotherapy in patients with metastatic disease; therefore, these regimens may also show superior efficacy in patients with locally advanced disease. We conducted another randomized phase II trial, JCOG1407, comparing modified FOLFIRINOX and gemcitabine/nab-paclitaxel and reported that both regimens are promising for LAPC (4). Although not commonly used, these strong regimens may be promising as iCT. In cases with strong SIR, control of potential distant metastases may provide the effect of CRT, but tolerability to FOLFIRINOX and gemcitabine/nab-paclitaxel is a concern. On the other hand, from the results of this study, even for weak SIR cases in which iCT with gemcitabine was ineffective, iCT with these strong regimens may be effective. Some clinical studies are in progress to assess the efficacy of FOLFIRINOX or gemcitabine/nab-paclitaxel as iCT for LAPC prior to CRT (NCT01921751, NCT01827553, NCT02024009). Despite various factors, SIR is an important factor in the treatment strategy for CRT for LAPC.
Based on the results of final analysis of JCOG1106, we selected radiotherapy with concurrent S-1 without iCT as a test regimen for a future phase III trial. The phase III trial will compare radiotherapy with concurrent S-1 without iCT with the gemcitabine/nab-paclitaxel selected in JCOG 1407. However, this exploratory subgroup analysis suggests that patients with high GPS, high CRP and low albumin may be unsuitable for CRT without iCT. Therefore, we will consider excluding high GPS, high CRP and low albumin as exclusion criteria in the phase III trial, and also consider the need for a clinical trial specifically for LAPC with high GPS, high CRP and low albumin.
There are several limitations to this exploratory analysis. First, this is a post hoc subgroup analysis of a randomized phase II trial with a small number of cases and no prior statistical settings. Therefore, it is difficult to draw a concrete conclusion from the results of the analysis of this study alone. Second, gemcitabine, the standard of care for unresectable PC at the time of designing the JCOG1106, was used as iCT, which may not have been sufficient. Third, cachexia was not evaluated in the JCOG1106, and the relevance of cachexia to the results of this analysis is only speculative.
In conclusion, survival benefits of iCT in CRT for LAPC were observed in patients with elevated GPS, high CRP and low albumin. These results suggest that SIR might be considered to apply iCT preceding CRT. Therefore, SIR might be considered as an inclusion criterion in future phase III trials.
Supplementary Material
Acknowledgements
We thank the patients, their families and all the investigators who participated in the study for their contribution. We also thank the members of the JCOG Data Center and JCOG Operations Office for their support in preparing the manuscript (Dr Kenichi Nakamura), data management (Ms Ayaka Nakano, Ms Kyoko Hasegawa) and oversight of the study management (Dr Haruhiko Fukuda).
Contributor Information
Nobumasa Mizuno, Department of Gastroenterology, Aichi Cancer Center Hospital, Nagoya, Japan.
Tatsuya Ioka, Oncology Center, Yamaguchi University Hospital, Yamaguchi, Japan; Department of Cancer Survey and Gastrointestinal Oncology, Osaka International Cancer Institute, Osaka, Japan.
Gakuto Ogawa, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Satoaki Nakamura, Division of Radiation Oncology, Kansai Medical University Hospital, Osaka, Japan.
Nobuyoshi Hiraoka, Division of Pathology and Clinical Laboratories, National Cancer Center Hospital, Tokyo, Japan.
Yoshinori Ito, Department of Radiation Oncology, Showa Universtity School of Medicine, Tokyo, Japan.
Hiroshi Katayama, JCOG Data Center/Operations Office, National Cancer Center Hospital, Tokyo, Japan.
Ryoji Takada, Department of Hepatobiliary and Pancreatic Oncology, Osaka International Cancer Institute, Osaka, Japan.
Satoshi Kobayashi, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Masafumi Ikeda, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital East, Kashiwa, Japan.
Haruo Miwa, Gastroenterological Center, Yokohama City University Medical Center, Yokohama, Japan.
Naohiro Okano, Department of Medical Oncology, Kyorin University Faculty of Medicine, Mitaka, Japan.
Hidekazu Kuramochi, Department of Chemotherapy and Palliative Care, Tokyo Women's Medical University, Tokyo, Japan.
Mitsugu Sekimoto, Department of Surgery, Kansai Medical University, Osaka, Japan.
Takuji Okusaka, Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center Hospital, Tokyo, Japan.
Masato Ozaka, Department of Hepato-Biliary-Pancreatic Medicine, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan.
Akiko Todaka, Division of Gastrointestinal Oncology, Shizuoka Cancer Center, Sunto-gun, Japan.
Kunihito Gotoh, Department of Surgery, National Hospital Organization Osaka National Hospital, Osaka, Japan.
Kazutoshi Tobimatsu, Division of Gastroenterology, Department of Internal Medicine, Graduate School of Medicine, Kobe University, Kobe, Japan.
Hironori Yamaguchi, Department of Clinical Oncology, Jichi Medical University, Shimotsuke, Japan.
Toshio Nakagohri, Department of Gastroenterological Surgery, School of Medicine Tokai University, Isehara, Japan.
Shinya Kajiura, Department of Clinical oncology, Toyama University Hospital, Toyama, Japan.
Kentaro Sudo, Department of Gastroenterology, Chiba Cancer Center, Chiba, Japan.
Keiya Okamura, Department of Bilio-Pancreatology, Sapporo Kosei General Hospital, Sapporo, Japan.
Satoshi Shimizu, Department of Gastroenterology, Saitama Cancer Center, Kita-adachi-gun, Japan.
Hirofumi Shirakawa, Department of Hepatobiliary–Pancreatic Surgery, Tochigi Cancer Center, Utsunomiya, Japan.
Naoya Kato, Department of Gastroenterology, Chiba University School of Medicine, Chiba, Japan.
Keiji Sano, Department of Surgery, Teikyo University School of Medicine, Tokyo, Japan.
Tomohisa Iwai, Department of Gastroenterology, Kitasato University School of Medicine, Sagamihara, Japan.
Nao Fujimori, Department of Medicine and Bioregulatory Science, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan.
Makoto Ueno, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan.
Hiroshi Ishii, Clinical Research Center, Chiba Cancer Center, Chiba, Japan.
Junji Furuse, Department of Gastroenterology, Kanagawa Cancer Center, Yokohama, Japan; Department of Medical Oncology, Kyorin University Faculty of Medicine, Mitaka, Japan.
Conflict of interest statement
Nobumasa Mizuno has received all support for the present manuscript to their institution from the Ministry of Health, Labour and Welfare, Japan, grants and non-financial support from the Japan Agency for Medical Research and Development (AMED) and the Japan Society for the Promotion of Science (JSPS); has received grants or contracts from any entity from to their institution from Yakult Honsha, Novartis, MSD, ASLAN Pharmaceuticals, Incyte, Ono Pharmaceutical, Seagen, Taiho Pharmaceutical, Dainippon Sumitomo Pharma; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Yakult Honsha, AstraZeneca, Novartis, FUJIFILM Toyama Chemical, MSD and has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca.
Tatsuya Ioka has received grants or contracts from any entity from to their institution from Taiho; has received consulting fees from Taiho and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Taiho.
Gakuto Ogawa has received all support for the present manuscript to their institution from the Ministry of Health, Labour and Welfare, Japan, grants and non-financial support from the Japan Agency for Medical Research and Development (AMED).
Satoaki Nakamura has nothing to disclose.
Nobuyoshi Hiraoka has nothing to disclose.
Yoshinori Ito has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai Pharma, Ono Pharmaceutical, Taiho Pharmaceutical, Bristol-Myers Squibb Japan, AstraZeneca, Eisai.
Hiroshi Katayama has received all support for the present manuscript to their institution from the Ministry of Health, Labour and Welfare, Japan, grants and non-financial support from the Japan Agency for Medical Research and Development (AMED).
Ryoji Takada has nothing to disclose.
Satoshi Kobayashi has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Chugai Pharmaceutical, Taiho Pharmaceutical, Elli Lilly, Yakult Honsha, Takeda Pharmaceutical and Ono Pharmaceutical.
Masafumi Ikeda has received grants or contracts from any entity from to their institution from Bristol Myers Squibb, MSD, Chugai, Bayer, Eisai, Eli Lilly Japan, Takeda; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Chugai, Eisai, Eli Lilly Japan, Takeda and has participated on a Data Safety Monitoring Board or Advisory Board for Chugai, Eisai, Eli Lilly Japan, Takeda, MSD.
Haruo Miwa has nothing to disclose.
Naohiro Okano has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Taiho Pharmaceutical, Eli Lilly Japan, Eisai, Bayer Yakuhin, Chugai Pharma, Ono Pharmaceutical, Takeda Pharmaceutical, Daiichi Sankyo and has participated on a Data Safety Monitoring Board or Advisory Board for GlaxoSmithKline.
Hidekazu Kuramochi has nothing to disclose.
Mitsugu Sekimoto has nothing to disclose.
Takuji Okusaka has received grants or contracts from any entity from to their institution from AstraZeneca, Bristol-Myers Squibb Company, MSD, K.K., Syneos, EP-crsu, Eisai, Incyte jp., Dainippon Sumitomo Pharma; has received consulting fees from Meiji Seika Pharma, Takara Bio Inc, Nippon Shinyaku Co., Ltd; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from MSD, K.K, AstraZeneca, Incyte jp., Eisai, Ono Pharmaceutical Co., Ltd, Teijin Pharma Ltd, Taiho Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Bayel, Yakult Honsha Co., Ltd, Johnson & Johnson K.K., Daiichi Sankyo Co., Ltd, Nihon Servier Co., Ltd., Novartis Pharma K.K., Eli Lilly Japan K.K. and has participated on a Data Safety Monitoring Board or Advisory Board for AstraZeneca, Incyte jp., Ono Pharmaceutical Co., Lt., Pfizer Japan Inc, Nihon Servier Co., Ltd, Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K, Mundipharma K.K.
Masato Ozaka has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Taiho, Yakult, MSD, Bayer, Ono, Servier, Pfizer.
Akiko Todaka has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Yakult Honsha, Taiho Pharmaceutical, Ono Pharmaceutical, Nihon Servier.
Kunihito Gotoh has nothing to disclose.
Kazutoshi Tobimatsu has nothing to disclose.
Hironori Yamaguchi has nothing to disclose.
Toshio Nakagohri has nothing to disclose.
Shinya Kajiura has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Daiichi Sankyo Company, Ltd.
Kentaro Sudo has received grants to their institution from Bristol-Myers Squibb/Ono Pharmaceutical, Eisai, Incyte, and has received honoraria for presentations from Ono Pharmaceutical, Yakult Honsha.
Keiya Okamura has nothing to disclose.
Satoshi Shimizu has received grants or contracts from any entity from to their institution from AstraZeneca, Incyte Corporation, Delta-Fly Pharma.
Hirofumi Shirakawa has nothing to disclose.
Naoya Kato has received grants from AbbVie G.K., Ohtsuka Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Corporation, Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, EA Pharma Co., Ltd, Shionogi & Co., Ltd, Boston Scientific Corporation, Eisai Co., Ltd, Aska Pharmaceutical Co., Ltd, Tsumura & Co., Mochida Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd, Eli Lilly Japan K.K., MEDICO’S HIRATA INC and JIMRO Co., Ltd; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Gilead Sciences Inc, AbbVie G.K., MSD K.K., Bristol-Myers Squibb Company, Ohtsuka Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Bayer Yakuhin Ltd, Chugai Pharmaceutical Co., Ltd, AstraZeneca K.K., Mitsubishi Tanabe Pharma Corporation, Sumitomo Pharma Co., Ltd, Takeda Pharmaceutical Co., Ltd, Mylan EPD G.K., Zeria Pharmaceutical Co., Ltd, EA Pharma Co., Ltd, Astellas Pharma Inc, Olympus Corporation, Shionogi & Co., Ltd, Eisai Co., Ltd, Aska Pharmaceutical Co., Ltd, Tsumura & Co., Mochida Pharmaceutical Co., Ltd, Miyarisan Pharmaceutical Co., Ltd, Nippon Kayaku Co., Ltd, Covidien Japan Inc, Eli Lilly Japan K.K., FUJIFILM Medical Co., Ltd, Nobelpharma Co., Ltd, Abbott Japan LLC, Kowa Company, Ltd, Incyte Biosciences Japan GK, Yakult Honsha Co., Ltd, Olympus Marketing, Inc, Taisho Pharmaceutical Co., Ltd and Janssen Pharmaceutical K.K..
Keiji Sano has nothing to disclose.
Tomohisa Iwai has nothing to disclose.
Nao Fujimori has nothing to disclose.
Makoto Ueno reports grants and personal fees from Taiho Pharmaceutical, grants and personal fees from AstraZeneca, grants and personal fees from Merck Biopharma, grants and personal fees from MSD, personal fees from Yakult Honsha, personal fees from Nihon Servier, grants from Astellas Pharma, grants from Eisai, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Incyte, grants and personal fees from Chugai Pharma, grants from DFP, grants from Daiichi Sankyo, grants from Novartis, grants and personal fees from Boehringer Ingelheim, grants and personal fees from J-pharma, outside the submitted work.
Hiroshi Ishii has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis Japan, Ono Pharmaceutical Co., Ltd, Taiho Pharmaceutical Co., Ltd, Eli Lilly Japan; and has participated on a Data Safety Monitoring Board or Advisory Board for Ono Pharmaceutical Co., Ltd.
Junji Furuse has received all support for the present manuscript to their institution from the National Cancer Center, the Ministry of Health, Labour and Welfare of Japan; has received grants or contracts from any entity from to their institution from Ono Pharmaceutical, MSD, Merck Bio, J-Pharma, Takeda, Chugai Pharma, AstraZeneca, Eisai, Mochida, Sanofy, Sumitomo Dainippon Bayer, Astellas, Incyte Japan; has received consulting fees from Fuji film, Mudi Pharma, Merck Bio, Ono Pharmaceutical, MSD, Taiho Pharmaceutical, Chugai Pharma, Astellas, Astra Zeneca, Delta-Fly-Pharma, Incyte Japan and has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Ono Pharmaceutical, Bayer, Eisai, Eli Lilly Japan, MSD, Chugai Pharma, Novartis Pharma, Astra Zeneca, Pfizer, Takeda, Taiho Pharmaceutical, Sannofy, Mylan EPD, EA Pharma, Kyowa Hakko Kirin, Teijin pharma, Servier Japan, Incyte Japan.
Funding
This work was supported by the National Cancer Center Research and Development Fund (23-A-16, 23-A-22, 26-A-4, 29-A-3, 2020-J-3, 2023-J-03), Health and Labour Sciences Research Grants for Clinical Cancer Research (H23-006) from the Ministry of Health, Labour and Welfare of Japan, Japan Agency for Medical Research and Development (AMED) under Grant Number JP15ck0106084, and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP19K08433.
Authors’ contributions
N.M., G.O., H.K., M.I., T.O., M.O., M.U., H.I. and J.F. contributed to the conception and design of the study. N.M., T.I., R.T., S.K., M.I., H.M., N.O., H.K., M.S., T.O., M.O., A.T., K.G., K.T., H.Y., T.N., S.K., K.S., K.O., S.S., H.S., N.K., K.S., T.I., N.F., M.U., H.I. and J.F. contributed to the acquisition of data. S.N. and Y.I. contributed to the quality control of radiotherapy. N.H. contributed to the central review of pathological diagnosis. N.M., T.I., G.O., M.U., H.I. and J.F. analysed and interpreted the data. All authors read and approved the final manuscript.
References
- 1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020;395:2008–20. [DOI] [PubMed] [Google Scholar]
- 2. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v56–68. [DOI] [PubMed] [Google Scholar]
- 3. Murphy JE, Wo JY, Ryan DP, et al. Total Neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:1020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ozaka M, Nakachi K, Kobayashi S, et al. A randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab-paclitaxel for locally advanced pancreatic cancer (JCOG1407). Eur J Cancer 2023;181:135–44. [DOI] [PubMed] [Google Scholar]
- 5. Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): a multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol 2020;5:285–94. [DOI] [PubMed] [Google Scholar]
- 6. Ioka T, Furuse J, Fukutomi A, et al. Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106. Jpn J Clin Oncol 2021;51:235–43. [DOI] [PubMed] [Google Scholar]
- 7. Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta-analysis. Crit Rev Oncol Hematol 2017;116:134–46. [DOI] [PubMed] [Google Scholar]
- 8. Kurahara H, Maemura K, Mataki Y, et al. Prognostication by inflammation-based score in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Pancreatology 2015;15:688–93. [DOI] [PubMed] [Google Scholar]
- 9. Chang PH, Yeh KY, Wang CH, et al. Impact of the pretreatment Glasgow prognostic score on treatment tolerance, toxicities, and survival in patients with advanced head and neck cancer undergoing concurrent chemoradiotherapy. Head Neck 2017;39:1990–6. [DOI] [PubMed] [Google Scholar]
- 10. Okuno T, Wakabayashi M, Kato K, et al. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol 2017;22:1042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li KJ, Xia XF, Su M, Zhang H, Chen WH, Zou CL. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer 2019;19:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grenader T, Nash S, Plotkin Y, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol 2015;26:1910–6. [DOI] [PubMed] [Google Scholar]
- 13. Grenader T, Waddell T, Peckitt C, et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 2016;27:687–92. [DOI] [PubMed] [Google Scholar]
- 14. Hasegawa S, Eguchi H, Tomokuni A, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett 2016;11:1560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al Murri AM, Bartlett JM, Canney PA, Doughty JC, Wilson C, McMillan DC. Evaluation of an inflammation-based prognostic score (GPS) in patients with metastatic breast cancer. Br J Cancer 2006;94:227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Imaoka H, Mizuno N, Hara K, et al. Evaluation of modified Glasgow Prognostic Score for pancreatic cancer: a retrospective cohort study. Pancreas 2016;45:211–7. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein D, El-Maraghi RH, Hammel P, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 18. Dell'Aquila E, Cremolini C, Zeppola T, et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol 2018;29:924–30. [DOI] [PubMed] [Google Scholar]
- 19. Furuse J, Kinoshita T, Kawashima M, et al. Intraoperative and conformal external-beam radiation therapy with protracted 5-fluorouracil infusion in patients with locally advanced pancreatic carcinoma. Cancer 2003;97:1346–52. [DOI] [PubMed] [Google Scholar]
- 20. Sah RP, Sharma A, Nagpal S, et al. Phases of metabolic and soft tissue changes in months preceding a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology 2019;156:1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsuzuka T, Kiyota N, Mizusawa J, et al. Clinical impact of cachexia in unresectable locally advanced head and neck cancer: supplementary analysis of a phase II trial (JCOG0706-S2). Jpn J Clin Oncol 2019;49:37–41. [DOI] [PubMed] [Google Scholar]
- 22. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 23. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


