Abstract
p53 mutation is common and highly related to radiotherapy resistance in rectal cancer. APR-246, as a small molecule, can restore the tumor-suppressor function to mutant p53. As there is currently no existing study on combining APR-246 with radiation in rectal cancer, our objective was to investigate whether APR-246 could enhance the sensitivity of colorectal cancer cells, regardless of their p53 status, to radiation treatment. The combination treatment had synergistic effects on HCT116p53-R248W/− (p53Mut) cells, followed by HCT116p53+/+ [wild-type p53 (p53WT)] cells, and exhibited an additive effect on HCT116p53−/− (p53Null) cells through inhibiting proliferation, enhancing reactive oxygen species, and apoptosis. The results were confirmed in zebrafish xenografts. Mechanistically, p53Mut and p53WT cells shared more activated pathways and differentially expressed genes following the combination treatment, compared with p53Null cells, although the combination treatment regulated individual pathways in the different cell lines. APR-246 mediated radiosensitization effects through p53-dependent and -independent ways. The results may provide evidence for a clinical trial of the combination in patients with rectal cancer.
Introduction
Radiotherapy has improved the outcome of patients with rectal cancer due to reducing local recurrence (1). However, many tumors are resistant to radiotherapy, which is associated with poor patient prognosis (2, 3). Previous studies, including our own and those conducted by other researchers, have demonstrated that patients with rectal cancers carrying mutant p53 gene are more resistant to radiotherapy compared to those with WT p53 rectal cancers (4–6). APR-246 (PRIMA-1Met, eprenetapopt), a small molecule, has been reported to restore tumor-suppressor function to mutant p53, resulting in anticancer activity in several types of cancers (7, 8). Mechanistically, APR-246 is hydrolyzed into its active form, methylene quinuclidinone, inside cells, where it covalently binds to thiol groups of the core domain of mutant p53 protein, thereby resulting in a structural change that restores its active conformation (9). Because p53 mutation occurs in approximately 60% of rectal cancers (10, 11), restoring p53 function by APR-246 and radiation is an attractive strategy for developing new treatments for rectal cancer.
APR-246 has been shown to have synergistic anticancer effects with several chemotherapeutic agents, for example, cisplatin. APR-246 has even been successfully tested in phase I/II clinical trials in patients with hematologic and prostate malignancies, which concluded that APR-246 was safe, with a favorable pharmacokinetic profile (12, 13). In a phase Ib/II clinical trial (NCT03072043), the combination treatment of APR-246 with azacytidine was found to be well-tolerated and resulted in high rates of clinical response and molecular remissions in patients diagnosed with TP53-mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (14). As a result, a phase III clinical trial is currently underway, investigating the use of APR-246 in combination with azacytidine specifically for patients with TP53-mutant MDS (NCT03745716). Furthermore, several types of solid cancers, including esophageal, gastric, bladder, and non–small cell lung carcinoma etc., are included in an ongoing phase I/II clinical trial using APR-246 together with other standard-of-care treatments (NCT04383938, NCT02999893). To date, however, there have been no studies investigating the potential anticancer effects of combining APR-246 with radiation treatment in rectal cancer. Therefore, in the current study, we examined whether APR-246 sensitized colorectal cancer cells with varying p53 status to radiation in vitro and in vivo conditions.
Materials and Methods
Cell culture
The study included three colon cancer cell lines [wild-type p53 (p53WT), p53Mut, and p53Null HCT116], one normal colonic epithelial cell line (CCD841 CoN), and one normal colonic fibroblast cell line (CCD18 Co). p53WT, CCD841 CoN, and CCD18 Co cell lines were purchased from the ATCC (Manassas, VA), and both p53Mut and p53Null HCT116 cell lines were kindly provided by Professor Bert Vogelstein from the CORE Cell Center (Johns Hopkins University, Baltimore, MD). The TP53 statuses were TP53+/+ in p53WT, TP53R248W/− (a hot spot point mutation) in p53Mut, and TP53−/− in p53Null HCT116 cells. p53Mut and p53null cells were generated by knock-in of R248W-mutant TP53 or targeted disruption of endogenous WT TP53 using TP53 recombinant adeno-associated viruses (rAAVs) knock-out and knock-in constructs, and clones selected in a geneticin solution (15, 16). All three colon cancer cell lines and two normal cell lines were grown in McCoy 5A medium and EMEM medium, respectively, supplemented with 10% FBS, and penicillin/streptomycin at 37°C in 5% CO2 and 95% humidity. Western blotting showed that p53 protein expression was markedly higher in p53Mut than that in p53WT cells (P < 0.01) and absent in p53Null cells (Supplementary Fig. S1A).
The study also included three primary cell cultures from surgical specimens. Tissues were taken from patients who underwent colorectal surgery at the West China Hospital, Sichuan University's Department of Gastrointestinal Surgery, for primary colorectal adenocarcinoma. All the patients who donated samples gave their informed consent, and the West China Hospital of Sichuan University Institutional Ethics Committees authorized this study. Ethical permission has been obtained for the use of colorectal cancer patient data and samples (Dnrs: 2012–107–31, 2014–79–3). 1597D cells were isolated and expanded from a colon cancer patient, and 3117D and 3431D cells, respectively, from two patients with rectal cancer, as previously described at our lab in China (17–20). Briefly, colorectal cancer tissue was collected from surgical specimens from West China Hospital of Sichuan University, China, and instantaneously minced on ice, rinsed in DMEM/F12 medium (HyClone, Logan, UT) containing 1% penicillin/streptomycin, and dissociated with collagenase IV (Sigma). The tissue was enzymatically dissociated with collagenase IV (Sigma), filtered, and washed three times with PBS. Contaminating blood cells were removed by incubation in ammonium chloride-potassium phosphate hypotonic buffer. The dissociated single tumor cells were cultured in serum-free DMEM/F12 supplemented with human recombinant EGF (Peprotech, Rocky Hill, NJ) and bFGF (Peprotech) and on Ultra Low Attachment plates (Corning, Corning, NY). To obtain differentiated primary cells (used in the current article), stem cell medium in serum-free DMEM/F12, supplemented with human recombinant EGF, was removed, and replaced with 20% FBS. The p53 statuses of primary cells were detected by whole-exome sequencing at BGI (The Beijing Genomic Institute, China). The results of our study indicated that none of the three primary cell cultures examined had mutations in the coding sequence of TP53. However, we did observe several TP53 mutations in the introns of these cell cultures (Supplementary Table S1). All the primary cells were cultured in 20% FBS-containing DMEM/F12 medium with penicillin/streptomycin at 37°C in 5% CO2 and 95% humidity, as previously described (20).
APR-246
APR-246 was provided by Aprea Therapeutics AB, Stockholm, Sweden. APR-246 was dissolved in DMSO and kept frozen (−20°C) as a stock solution. The percentage of DMSO during drug exposure never surpassed 0.02% for cell and zebrafish experiments.
Radiation
Both cells and zebrafish larvae were irradiated at the Department of Radiation Oncology, Linköping Hospital with the same equipment (True Beam linear accelerator, Varian Medical Systems Inc.) used for patients receiving radiotherapy, and the same radiation oncology medical physicists for treating the patients operated the equipment. Single absorbed doses of radiation were given using a 6-MV photon beam. Controls of cells and zebrafish larvae were mock radiated under the same conditions.
Zebrafish experiments
Zebrafish were maintained at the Zebrafish Core facility of Linköping University as previously described (21). All experiments on zebrafish were done in accordance with the regulations and policies of the Linköping University Animal Care and Use Committee (Dnrs: 104/12, 53/14, 89/15). In brief, adult zebrafish were held on a 14:10 hours light: dark cycle at 28.5°C, following the regulations and policies of the Linköping University Animal Care and Use Committee. The age of zebrafish is indicated throughout this article as days post fertilization (dpf) and days post injection (dpi) for all experimental data. Larvae raised beyond 1 dpf were treated with phenylthiourea (0.003%, w/v; Sigma) to prevent melanization. Larvae were maintained at 35°C after tumor transplantation.
Cells were labeled with Vybrant CM-Dil (Vybrant CM-DiI, Thermo Fisher Scientific), at 3 μmol/L concentration, for 30 minutes at 37°C. After labeling, the cells were collected and injected into the perivitelline space of zebrafish larvae at 2 dpf. After injection, the larvae with labeled cells in the circulation were excluded. Successfully injected larvae were imaged and placed into individual wells in standard 48-well polystyrene tissue culture plates containing E3 medium. At 0.5 dpi, tumor-bearing larvae with xenografts were administered with APR-246 at 20 μmol/L in an E3 medium and treated for 12 hours. At 1 dpi (3 dpf), a single dose of 6 Gy was delivered to tumor-bearing larvae. After radiation treatment, E3 medium in all groups was replaced by fresh E3 medium without APR-246. At 3 dpi (5 dpf), larvae were imaged with a fluorescence microscope (Nikon D-eclipse C1, Tokyo, Japan). Occasionally, a “granular-like” appearance of the primary tumors developed during the 3-day assay period likely indicative of somewhat uneven staining of the initially injected cells. In such cases, the small spaces between tumor cells were included to consider the entire tumor as a coherent mass when calculating the tumor volume. Normalized tumor sizes were calculated as the size at 3 dpi divided by the size of the same tumor at 0 dpi, measured using the ImageJ software, and differences were compared between groups. Animals were euthanized through exposure to tricaine (0.5 mmol/L 3-aminobenzoic acid ethyl ester, 2 mmol/L Na2HPO; Sigma) after the experiment.
Tumorigenesis of primary cells in mouse experiments
To confirm that the primary cells are from malignant tumors, their tumorigenesis was determined using the mouse xenograft experiments as previously described (20). In brief, female nude mice (BALB/c strain, aged 4–6 weeks) were procured from the Beijing Experimental Animal Centre of the Chinese Academy of Sciences (Beijing, China). The experimental procedures were approved by the Sichuan University Institutional Animal Care and Use Committee, ensuring ethical standards were met. Throughout the animal research, the mice were housed in a pathogen-free environment in strict accordance with the approved guidelines (Dnr: 35/17). Primary cells were dissociated using 0.05% trypsin. A 1:1 mixture of 5×105 cells and BD Matrigel (BD Biosciences, San Jose, CA) was administered subcutaneously into the lower flank of naked mice. When the defined criteria for the end-stage disease were met, the mice were euthanized, and the images were acquired.
Cell viability
The cells in the logarithmic growth phase were seeded on 96-well plates at a density of 1,500 cells/well in quadruplicate and allowed 24 hours to attach. To calculate radiosensitizing effects, cells were preincubated with or without APR-246 (0, 2.5, 5, 7.5, 10, 15, and 20 μmol/L, respectively) at gradient concentrations for 12 hours and then exposed to single graded doses of radiation (0, 2, 4, and 6 Gy, respectively). Accordingly, cells were incubated with a drug-free fresh medium for 72 hours (for HCT116 cell lines) or 120 hours (for primary cell lines). Cell viability was assayed by Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) according to the manufacturer's instructions. The inhibition of the cell viability = [1− average optical density (OD) value of the treated group/average OD value of the control group] × 100%. Finally, the semi-log dose-response curves were plotted, and half maximal inhibitory dosage (IC50) was calculated by GraphPad Prism 8.0 software (GraphPad Software, Inc).
Calculation of synergy score between APR-246 and radiation
The combinational inhibitory activity of radiation and APR-246 was examined using a response surface model called Zero Interaction Potency (ZIP). This model proposes a delta score (δ) to characterize the synergy landscape over the full dose-response matrix. Briefly, APR-246 and radiation were examined for their ability to suppress activity both individually and collectively at various dose ratios. Then, the synergistic effect was quantified online using the synergy finder software (https://synergyfinder.fimm.fi/). Calculations for synergy effects were based on the ZIP model. The interaction between two factors was defined as synergistic when the synergy score was greater than 10, as an additive between −10 and 10, and as antagonistic when less than −10.
Colony formation
Logarithmic growth phase cells in each group were incubated in 6-well plates at 400 cells/well density and allowed 24 hours to attach. Cells were preincubated with or without APR-246 at 2.5 μmol/L for 12 hours followed by 2-Gy radiation. Cells were then cultured for 14 days in drug-free fresh media to form visible colonies before being fixed with methanol and stained with 1% crystal violet. Under a microscope, the cell colonies were enumerated using the standard definition of a colony as one that comprises more than 50 cells. The signal intensity ratio between the treatment groups and the untreated group had been used to define the clonogenic survival percent.
Flow cytometry
Cells in the logarithmic growth phase were seeded in 10-cm dishes at a density of 3×105 cells/dish and allowed 24 hours to attach. Cells were then treated with or without APR-246 at 20 μmol/L for 12 hours and then exposed to 6 Gy of radiation. Subsequently, cells were incubated with a drug-free fresh medium for 72 hours. Flow cytometry was conducted using the Gallios Cell flow cytometer (Beckman Coultier, Brea, CA).
To examine the cell cycle, after treatments, cells were trypsinized and fixed in 70% cold ethanol at −20°C overnight. The fixed cells were collected and stained with PBS containing 200 μg/mL RNase A, 20 μg/mL PI, and 0.2% Triton X-100 for 30 minutes in the dark at room temperature. G0–G1, S, and G2–M phases were analyzed using ModFit software 4.0.5 (Verity Software House, Topsham, Maine).
To investigate reactive oxygen species (ROS), after treatments, cells were harvested and washed twice with cold PBS, subjected to BioVision's ROS Detection Assay Kit (K936, BioVision Incorporated, Milpitas, CA) to incubate for 30 minutes at 37°C in the dark. The stained cells were analyzed with flow cytometry within 60 minutes. The percentage of ROS-positive cells was calculated using Kaluza Analysis Software 2.1.
To detect apoptosis, cells were harvested and washed twice with cold PBS and resuspended in binding buffer containing Annexin V-FITC (BD Biosciences, Bedford, MA) and subjected to PI staining for 15 minutes at 4°C in the dark. The stained cells were analyzed with flow cytometry within 60 minutes. The percentage of apoptotic cells was calculated using the Kaluza Analysis Software 2.1. Annexin V-positive and PI-negative (Annexin V-FITC+/PI-) were considered early-stage apoptosis while Annexin V/PI-double-positive (Annexin V-FITC+/PI+) was considered to indicate late-stage apoptosis.
Western blot
Cells in the logarithmic growth phase were seeded in 10-cm dishes at a density of 3×105 cells/dish and allowed 24 hours to attach. Cells were preincubated with or without APR-246 at 20 μmol/L for 12 hours and then exposed to 6 Gy of radiation. Subsequently, cells were incubated with a drug-free fresh medium for 72 hours. An ice-cold RIPA lysis buffer was employed to prepare whole-protein extracts, which were then fortified with a mixture of protease inhibitors (Complete Mini, Roche Diagnostics GmbH, Germany). Total proteins were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes and blocked with 5% nonfat milk and incubated sequentially with primary antibodies against p53 (DO-1; sc-126, Santa Cruz, Germany) and GAPDH (NB300–322SS, Novus Biologicals, UK.) and horseradish peroxidase-conjugated secondary antibodies. Chemiluminescence was used to detect the proteins using a ChemiDoc (Bio-Rad, Hercules, CA), and Bio-Rad Image Lab 6.1 software was used to quantify the signals.
mRNA sequencing libraries preparation and transcriptome sequencing
TRIzol reagent (Invitrogen, MA) was used to isolate total RNA following the manufacturer's protocol. Oligo(dT)-attached magnetic beads were used to purify mRNA. Purified mRNA was fragmented into small pieces with fragment buffer at an appropriate temperature. Then, first-strand cDNA was generated using random hexamer-primed reverse transcription, followed by second-strand cDNA synthesis. Afterward, A-Tailing Mix and RNA Index Adapters were added by incubating to end repair. The cDNA fragments obtained from the previous step were amplified by PCR, and products were purified by Ampure XP Beads, then dissolved in EB solution. The product was validated on the Agilent Technologies 2100 Bioanalyzer for quality control. The final library was constructed by heating, denaturing, and circularizing the double-stranded PCR products from the previous stage using the splint oligo sequence. The single-strand circle DNA was formatted as the last library. The final library was amplified with phi29 to make a DNA nanoball (DNB), which had more than 300 copies of one molecule; DNBs were loaded into the patterned nanoarray, and 100 base-pair paired-end reads were generated on the Illumina HiSeq platform (BGI-Shenzhen, China). Paired-end sequencing with a 150-bp read length and average of 10.34 Gb of data was run for each sample. After the data preprocessing, fragments per kilobase of exon per million fragments mapped (FPKM) were calculated. The data were verified to be of good quality and repeatable, which allowed for further analyses (Supplementary Table S2).
Bioinformatics
Gene set enrichment analysis (GSEA) was performed with the “clusterProfile” package version 3.18.1 within the R software (https://www.r-project.org/; ref. 22), allowing the investigation of whether certain sets of genes exhibit significant differences between two groups. Gene comparison is incapable of disclosing the subtle but noticeable alterations in the expression of the genes associated with the pathway function, which can be acquired by GSEA. The Reactome database was used in pathway enrichment because of its quick update and close relationship with molecular signaling pathways, especially in cancer. Through GSEA-based on the Reactome database, we explored the potential biological pathways and their enriched genes involved in the combinational effect of APR-246 with radiation. Gene sets with |normalized enrichment score (NES)| > 1, nominal (NOM) P value < 0.05, and FDR q-value < 0.25 were considered significantly enriched.
The online analysis of protein and protein interactions (PPI) was performed using STRING version 11.0, a database that compiles, stores, and incorporates all publicly available sources of PPI information.
Patients
To investigate the relationship of radiotherapy with p53 status, cell proliferation, and apoptosis, 163 patients with locally resectable primary rectal cancer from the Swedish Rectal Cancer Trial of Preoperative radiotherapy between 1987 and 1990 (23) were included. In the radiotherapy group, 76 patients received 25 Gy in five fractions followed by surgical resection within 2 weeks. In the nonradiotherapy group, 87 patients received surgery alone. None of the patients received preoperative or adjuvant chemotherapy. Five-μm sections of surgical cancer samples were subjected to IHC procedures and stained with primary antibodies to detect the expression of p53 (antibody PAb 1801, Oncogene Science, Manhasset, New York) and Ki67 (antibody MIB-1, Immunotech SA, Marseilles, France). The Apop-Tag in situ apoptosis detection kit (Oncor, Gaithersburg, MD) was used to detect apoptotic cells. IHC staining of p53 within 5% or more of the cancer cells was considered positive and indicative of a p53 mutant tumor, which was used as a surrogate for mutational analysis in the diagnostic workup of carcinomas of multiple origins, when DNA sequencing was not available (24).
Statistics
Results from in vitro and animal experiments were presented as mean ± SEM or SD based on at least three independent experiments for all assays. The Student t test (two-tailed unpaired t test) was employed for comparison between two groups, and multiple-group data was analyzed using one-way ANOVA with Newman-Keuls post hoc test for continuous variables. All data were analyzed using GraphPad Prism 8.0 software (GraphPad Software, Inc). Asterisks indicate significant difference and were represented as: *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not statistically significant. In patients, the normality of distribution of continuous variables was tested by the Shapiro–Wilk normality test. The relationships of radiotherapy with apoptosis and the Ki-67 index were examined using the Wilcoxon rank-sum test because both the indexes were not normally distributed. The frequencies of categorical variables were compared using Fisher exact test when appropriate. Statistical analysis was performed using R software with different packages. P < 0.05 was considered significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
APR-246 sensitized HCT116 cell lines to radiation in vitro
We first examined IC50 of APR-246 alone and radiation alone in the three HCT116 cell lines with different p53 statuses. IC50 of APR-246 alone in p53Mut was significantly lower than those in p53WT and p53Null (Fig. 1A; Supplementary Fig. S1B). The semi-log dose curves for these three HCT116 cells were shown in Supplementary Fig. S1C and S1D. IC50 of radiation alone was the lowest in p53WT among the three cell lines (Fig. 1B). We then pretreated the three cell lines with APR-246 followed by irradiation and observed that IC50 of radiation decreased with increased APR-246 concentrations. Notably, at APR-246 concentrations of 7.5 μmol/L and 10 μmol/L, the IC50 for radiation treatment was significantly lower in p53Mut cells than those of the other two cell lines (P < 0.05; Fig. 1B). In contrast, the normal epithelial cells (CCD841 CoN) and fibroblasts (CCD18 Co) from human colon tissue were relatively insensitive to combination treatments, if the effects of radiation alone were excluded. The viability of normal colonic epithelial cells and fibroblasts was found to be mainly affected by radiation for doses greater than 2 Gy (Supplementary Fig. S2A–S2C).
Figure 1.
Radiosensitization effects of APR-246 in p53WT, p53Mut, and p53Null HCT116 cells. A–D, p53WT, p53Mut, and p53Null cells were seeded in 96-well plates, after attachment pretreated with or without APR-246 (0, 2.5, 5, 7.5, or 10 μmol/L, respectively) for 12 hours and then exposed to a single dose of radiation (0, 2, 4, or 6 Gy, respectively). At 72 hours after radiation, inhibition of radiation and/or APR-246 were calculated for an IC50 value of APR-246 (A) without radiation and IC50 of radiation (B) under different concentrations of APR-246 in p53WT, p53Mut, and p53Null cells. C, The combinational inhibitory activity of APR-246 and radiation was analyzed using a ZIP model. This model proposes a delta score (δ) to characterize the synergy landscape over the full dose-response matrix. The interaction between two factors was defined as synergistic when the synergy score was larger than 10; as additive, from −10 to 10; and as antagonistic, less than −10. 3D synergy landscape of APR-246 and radiation in p53WT, p53Mut, and p53Null cells. The red area refers to synergy and the green area refers to antagonism. D, Synergy score (δ) of APR-246 and radiation in each cell line shown in histogram. Data represent the mean ± SD (n = 3) obtained from three independent experiments. E and F, Radiosensitization effects of APR-246 determined by colony-formation assay. Cells were seeded in 6-well plates, after attachment pretreated with APR-246 (2.5 μmol/L) for 12 hours and then irradiated at 2 Gy. The representative photos of p53WT, p53Mut, and p53Null cells after treatments and staining with crystal violet (E). The ratio of signal intensity of the plates treated with APR-246 and/or radiation versus control is displayed and compared in histogram and table (F). *, P < 0.05; **, P < 0.01; ***, P < 0.001. n,s., not statistically significant. #, P < 0.05; versus p53Mut cells. All experiments were performed three times.
The combined effects of APR-246 with radiation in each cell line were calculated using the Synergy Finder module with the ZIP model. The red area of the three-dimensional (3D; Fig. 1C) and 2D (Supplementary Fig. S1E–S1G) synergy landscape indicated a synergistic effect of APR-246 with radiation in both p53Mut (11.76 ± 1.90) and p53WT (10.26 ± 1.03), an additional effect in p53Null cells (7.94 ± 1.52; Fig. 1C and D). These results indicate that APR-246 had synergistic cell-killing effects with radiation on the p53Mut and p53WT cell, and an additive effect with radiation on p53Null cells. Notably, the synergistic effect of APR-246 with radiation was not present in normal epithelial cells (CCD841 CoN) and fibroblasts (CCD18 Co; Supplementary Fig. S2D–S2F).
We also investigated the effect of APR-246 alone and radiation alone on cell proliferation using clonogenic survival analysis (Fig. 1E and F). Either of the two single treatments significantly inhibited the proliferation of the three cell lines compared with the control group. Treating the cells with APR-246 alone, the survival fraction of p53Mut cells was significantly lower than that of both the p53WT and p53Null cells. When treating the cells with radiation alone, the survival fraction of p53WT was the lowest among the three cell lines, although the differences did not reach statistical significance. We then examined the combined effect of APR-246 with radiation, compared with the single treatments, on the cells. The combination had a significantly stronger antiproliferative and antisurvival effect compared with the single treatments on all three cell lines (combination vs. APR-246 or radiation; P < 0.05); furthermore, p53Mut had the lowest survival fraction among the three cell lines (P < 0.05 for p53Mut vs. either p53WT or p53Null, Fig. 1F).
We further investigated whether APR-246 increased radiation-induced cell-cycle arrest by flow cytometry. Compared with control, radiation alone induced S-phase arrest in the three cell lines, and APR-246 alone did not have such effects (Fig. 2A; Supplementary Fig. S3A–S3F). However, APR-246 significantly increased radiation-induced S-phase arrest compared with radiation alone in the three cell lines (P < 0.05). The ratio of increased S-phase arrest caused by combination treatment to radiation alone is the highest in p53Mut cells (2.4 ± 0.6) compared with the other two cell lines (p53WT, 2.0 ± 0.3; p53Null, 1.4 ± 0.3; Fig. 2B).
Figure 2.
APR-246 enhanced radiation-induced S-phase arrest, ROS accumulation, apoptosis, and p53 expression in p53WT, p53Mut, and p53Null HCT116 cells. Cells were seeded in 10-cm dishes and treated with control, APR-246 (20 μmol/L), radiation (6 Gy), or a combination. A, Percentages of cells at S-phase in each cell line after treatments based on PI staining. B, Ratio of increased S-phase arrest caused by combination treatment to radiation alone in the three cell lines. C, ROS-positive cells after treatments shown and compared in the histogram. D, Ratio of ROS-positive cells caused by combination treatment compared with radiation alone in p53WT, p53Mut, and p53Null cells. E, Apoptotic cells of p53WT, p53Mut, and p53Null cells under different conditions based on Annexin V-FITC and propidium iodide (PI) staining. F, Ratio of increased apoptosis caused by combination treatment compared with radiation alone in each cell line. G and H, P53 expression levels were examined by Western blotting. p53 protein in p53WT (G) and p53Mut (H) cells were compared in the histogram. GAPDH was used as an interval control. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not statistically significant. Statistical analysis was carried out with one-way ANOVA and Dunnett multiple comparisons test. Mean ± SD. n = 3.
Radiation alone induced ROS production in contrast to control among all three cell lines. Although APR-246 alone did not induce ROS production, APR-246 pretreatment markedly augmented radiation-induced ROS production in all three cell lines, compared with radiation alone (P < 0.05; Fig. 2C; Supplementary Fig. S4A). There were no significant differences among the three cell lines regarding the ratio of ROS positive caused by combination treatment compared with radiation alone (P > 0.05; Fig. 2D).
We then investigated the impact of APR-246 on radiation-induced apoptosis in the three cell lines using flow cytometry (Fig. 2E and F; Supplementary Fig. S4B). By treating the cells with radiation alone, the highest apoptotic response was observed in the p53WT cells, while p53Mut was the most resistant to radiation compared with the control group (p53WT, 19.5% ± 2.6%; p53Null, 10.3% ± 2.1%; p53Mut, 3.2% ± 1.2%). We did not see significant induction of apoptosis by APR-246 alone, but APR-246 pretreatment significantly increased the apoptosis response to radiation in all three cell lines when compared with radiation alone (P < 0.05; Fig. 2E). The apoptosis caused by combination treatment compared with radiation alone was much higher in p53Mut (4.7 ± 0.7) than in p53WT (1.7 ± 0.2) or in p53Null (2.1 ± 0.4; P < 0.05; Fig. 2F).
We further examined the levels of p53 protein in all three cell lines and confirmed that p53 protein was absent in p53Null cells. In p53WT cells, p53 expression was markedly increased (especially at 48 hours) by radiation alone and lightly by APR-246 alone when compared with the controls (Fig. 2G). In p53Mut cells, p53 expression was also markedly increased by radiation alone (highest at 72 hours), but not by APR-246 (Fig. 2H). Combination of APR-246 and radiation did not further increase p53 protein expression compared with radiation alone in either p53WT or p53Mut cells.
These results suggest that the combination of APR-246 with radiotherapy had apparent synergistic or additive effects on the three HCT116 cell lines, particularly in p53Mut cells, through proliferation inhibition and induction of apoptosis.
APR-246 sensitized radiation effect in primary cancer cell cultures
We next assessed the effect of APR-246 and radiation in primary colorectal cells that recapitulated tumor cell heterogeneity. We first isolated and expanded three primary cancer cell cultures, which displayed a typical epithelial-like tumor cell morphology (Fig. 3A). Furthermore, we confirmed the tumorigenesis of the primary cells in all three cultures by establishing tumor xenografts in mice, which is the gold standard for assessing the primary cells forming malignant tumors (Fig. 3B).
Figure 3.
Radiosensitization effects of APR-246 in three primary colorectal cancer cell cultures. A, Morphology of primary cell cultures 1597D, 3117D, and 3431D cells. Bars = 200 μm. B, The tumorigenesis of primary cells in nude mice. The xenografts of primary cell cultures were pointed with red arrows. C and D, Calculation of synergistic effect between APR-246 and radiation in primary cell cultures. All primary cells were seeded in 96-well plates. In 1597D primary cells, APR-246 was at 0, 10, 25, 30, 35, or 40 μmol/L, respectively, and radiation was at 0, 2, 4, 6, or 8 Gy, respectively. In 3117D and 3431D primary cells, the concentration of APR-246 was 0, 2.5, 5, 15, or 20 μmol/L, while the dose of radiation was 0, 1, 2, 4, 6, or 8 Gy, respectively. The red areas refer to synergy and the green areas refer to antagonism in 3D synergy landscape of APR-246 and radiation (C). Synergy score (δ) of APR-246 and radiation shown in histogram (D). Data represent the mean ± SD (n = 3) obtained from three independent experiments. E and F, Flow cytometry assay for the S-phase distribution (E) and cell apoptosis (F) of 3 primary cell cultures after treatment with radiation alone or combination with APR-246. The dose of APR-246 and radiation was 25 μmol/L and 2 Gy separately in 1597D primary cells, while 20 μmol/L and 2 Gy separately both in 3117D and 3431D primary cells. *, P < 0.05; **, P < 0.01.
Next, we treated the three primary cultures with different doses of radiation alone or a combination with APR-246 to evaluate the corresponding synergistic efficacy in vitro. IC50 values for radiation were significantly lowered by combination treatment with APR-246 (Supplementary Fig. S5A and S5B), while IC50 values for APR-246 were significantly lowered by combination treatment with radiation in primary cultures (Supplementary Fig. S5C and S5D). Combination treatment of APR-246 and radiation resulted in apparent synergism in 3117D or 3431D cells and had an additive effect in 1597D cells (Fig. 3C and D). Cell-cycle analysis demonstrated that the combination significantly increased the proportions of cells in the S-phase compared with radiation alone (P < 0.05; Supplementary Figs. 3E and S5E). Moreover, the combination treatments significantly enhanced the apoptotic effects on these primary cells (P < 0.05; Supplementary Figs. 3F and S5F). Therefore, these results of primary cells further verified the radiosensitization efficacy of APR-246.
APR-246 sensitized radiation effect in zebrafish xenografts with HCT116 cell lines
We performed zebrafish xenograft experiments using the same treatments and the three HCT116 cell lines as we did in the in vitro studies and the corresponding scheme in Fig. 4A. Compared with controls, APR-246 alone or radiation alone reduced the tumor sizes in zebrafish embryos carrying p53WT, p53Mut and p53Null cells. We then examined the combinational effects of APR-246 with radiation on this zebrafish xenograft assay. The combination treatment significantly reduced the tumor sizes beyond that of APR-246 alone or radiation alone for all three cell lines (P < 0.05; Fig. 4B–D). Furthermore, the ratio of inhibition (i.e., the inhibition found after combination treatment divided by the inhibition caused by radiation alone) was the highest for zebrafish carrying p53Mut xenografts, although the difference did not reach statistical significance (Fig. 4E). The results suggest that the combination of APR-246 with radiotherapy had a better effect on reducing the size of xenografts compared with either monotherapy alone, and the effects of the combination treatment were greater for p53Mut tumors.
Figure 4.
Radiosensitization effects of APR-246 in p53WT, p53Mut, and p53Null HCT116 cells in vivo. A, A workflow of zebrafish xenograft experiment. Cells were labeled on 0 dpi (2 dpf), APR-246 (20 μmol/L) was added on 0.5 dpi, and radiation (6 Gy) was done on 1 dpi (3 dpf); the experiment ended on 3 dpi (5 dpf). B–D, Lateral views of live zebrafish embryos after transplantation of fluorescently labeled p53WT (B), p53Mut (C), and p53Null (D) cells. Dots indicated individual tumors. E, The ratio of inhibition (i.e., the inhibition found after combination treatment divided by the inhibition caused by radiation alone) in each cell line. Scale bar: 400 μm. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (Student t test).
p53Mut and p53WT cells shared more deregulated pathways and genes following combination treatment relative to p53null cells
To investigate the how the genes differentially regulated by APR-246, radiation and combination under different p53 status affected cellular signaling pathways, we examined the gene expression in three cell lines with different p53 status posttreatment by APR-246, radiation, and combination. The expression data are available through the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/) and can be accessed via the GSE series number GSE232305. We first analyzed the differences of transcriptional targets of TP53 in the three cell lines with single treatments to clarify the role of TP53. When comparing radiation-alone treatment with control, there were 19, 21, and 19 differentially expressed genes (DEG) (P < 0.05) enriched in transcriptional regulation by TP53 in p53WT, p53Mut, and p53Null cells, respectively. TP53 was enriched in both p53WT and p53Mut cells but not in p53Null cells. Furthermore, typical genes related to transcriptional regulation by p53, such as CDKN1A, BAX, PHLDA3, and TP53I3 were only enriched in p53WT cells. p53Mut cells shared seven DEG with p53WT cells, but they also had 14 DEG that were different from p53WT cells. PLK3 was downregulated in p53Null cells and upregulated in p53WT after radiation treatment (Supplementary Fig. S6 and Supplementary Table S3). When comparing APR-246–alone treatment with control, there were seven, 338, and 418 DEG respectively obtained in p53WT, p53Mut, and p53Null cells comparing APR-246 treatment with control. In p53WT cells, no pathway was enriched because only seven genes were differentially expressed, suggesting that APR-246 alone slightly affected p53WT cells (Supplementary Fig. S7A and Supplementary Files 1). In p53Mut cells, enriched pathways mainly related to cell cycle, cellular response to stress, DNA replication, metabolism, and programmed cell death, etc. (Supplementary Fig. S7B and S7C; Supplementary Table S4). In p53Null cells, enriched pathways were mainly related to cell cycle, cellular response to stimuli, DNA replication, infectious disease, DNA repair, and metabolism of proteins, etc. (Supplementary Fig. S7B and S7C; Supplementary Table S5).
We also investigated the pathways underlying the radiosensitization effects of APR-246 using GSEA, focusing on comparing the results of combination treatment with radiation alone in all three HCT116 cell lines. The GSEA indicated that 33, 28, and 144 Reactome pathways were significantly enriched between combination treatment and radiation alone in p53WT, p53Mut, and p53Null cells, respectively. Around half of those enriched pathways were shared by p53Mut (15 of 28, 53.6%) and p53WT (15 of 33, 45.5%) cells, while many more and different pathways were enriched in p53Null cells (vs. p53WT: 14 of 144, 9.7%; vs. p53Mut: 12 of 144, 8.2%; Fig. 5A). In detail, the cell cycle and metabolism of RNA pathways were found to be enriched in all three cell lines. Transcriptional regulation by TP53 and apoptosis pathways were only increased in p53WT and p53Mut cells, while autophagy, DNA replication, and base excision repair pathways were only enriched in p53Null cells (Supplementary Table S6-S8). The log2fold change (FC) value of genes obtained by comparing the radiation group with the combination group in each cell line were submitted as Supplementary files 1–3.
Figure 5.
The Venn diagrams of significantly enriched pathways and genes between combination and radiation-alone treatment in three HCT116 cell lines. Cells were seeded in 10-cm dishes and treated with 6-Gy radiation alone or in combination (20 μmol/L APR-246 + 6 Gy radiation). After cell harvest, RNA sequencing was performed as described in the Materials and Methods section. The potential biological pathways and their enriched genes involved in the combinational effect of APR-246 with radiation in each cell line were obtained by performing GSEA with the clusterProfile package version 3.18.1 within the R software based on the Reactome database. Gene sets with |NES| > 1, nominal (NOM) P value < 0.05, and FDR q-value < 0.25 were considered significantly enriched. A, The Venn diagrams of deregulated pathways were drawn for the three cell lines. B–E, The Venn diagrams of differentially expressed genes in cell cycle (B), mRNA splicing (C), apoptosis (D), and transcriptional regulation by TP53 (E) pathways between combination and radiation-alone treatment were further drawn for the three cell lines.
We then compared all the enriched genes of the three cell lines in selected pathways involved with radiosensitivity in cancers. A total of 67, 47, and 44 genes within the cell-cycle pathway were enriched in p53WT, p53Mut, and p53Null cells, respectively, but only five of these genes were shared by all three cell lines (Fig. 5B). In addition, more differentially expressed genes in the cell-cycle pathway were shared by p53WT (34 of 67, 50.7%) and p53Mut (34 of 47, 72.3%) compared with p53Null cells (vs. p53WT, nine of 44, 20.5%; vs. p53Mut, 13 of 44, 29.5%). Similarly, more differentially expressed genes in the mRNA splicing pathway were shared by p53WT (31 of 38, 81.6%) and p53Mut (31 of 45, 68.9%) compared with p53Null cells (vs. p53WT, 27 of 53, 50.9%; vs. p53Mut, 32 of 53, 60.4%, Fig. 5C). Although most enriched genes in the apoptosis pathway were present in both p53WT (26 of 37) and p53Mut cells (26 of 29), some genes were still only present in p53WT (11 of 37) or p53Mut (three of 29) cells, and none of the genes in p53Null cells (Fig. 5D). Similar results were also found for the enriched genes within the transcriptional regulation by TP53 pathway, where 26 of 39 and 26 of 35 were shared by p53WT and p53Mut cells, respectively (Fig. 5E), and none of these genes were enriched in p53Null cells. There are 37, six, and 19 genes that were enriched within the DNA replication, base excision repair, and autophagy pathways, respectively, which were not found in p53WT or p53Mut cells. Actinomorphic diagrams were used to show the enriched genes and their FC within selected pathways in each cell line (Supplementary Fig. S8A–S8C). The PPI of these enriched genes in cell cycle, mRNA Splicing, apoptosis, transcriptional regulation by TP53, DNA replication, Base Excision Repair, and autophagy pathways in each cell line were then further analyzed using the STRING online tool and shown in Supplementary Fig. S9 and S10.
These results suggest that p53WT and p53Mut cells were derived from a molecular standpoint, responding to an APR-246 radiosensitization effect through pathways and genes that were more similar compared with the response of p53Null cells, although all the cell lines had unique pathways and genes enriched.
Negative p53 expression related to better response to radiotherapy in patients with rectal cancer
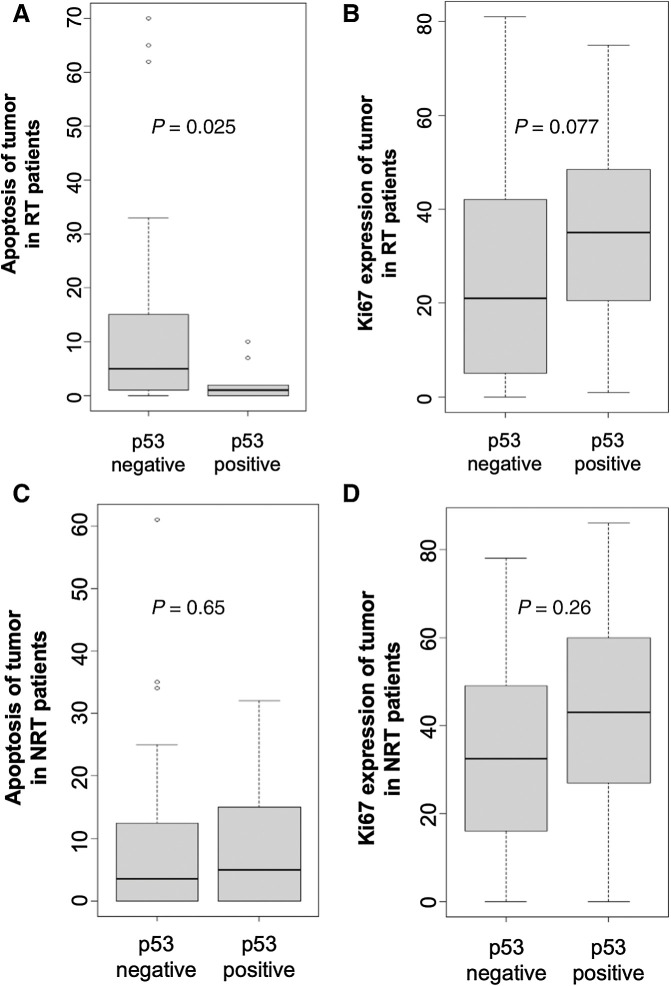
The results above showed that p53 reactivation by APR-246 increased apoptosis and reduced proliferation in HCT116 cell lines and primary cell cultures. We wanted to see whether this reflected to some extent, a clinically relevant response to radiotherapy in patients. Here, we examined how p53 status was related to apoptosis and proliferation in patients with rectal cancer with preoperative radiotherapy even though none of these patients received APR-246 administration. We produced a surrogate for p53 mutational analysis by IHC staining because DNA sequencing was not feasible. Negative p53 expression in a tumor is usually considered an indicator of p53WT (25–27). In 76 patients who underwent radiotherapy, negative p53 expression was significantly related to increased apoptosis index (sum of ranks 1903 and 177 in p53-negative and -positive groups, respectively; P = 0.025; Fig. 6A), in surgical resected tumor samples. While cell proliferation (Ki-67 expression) appeared to be reduced in patients who had received preoperative radiotherapy, the difference was not significant (sum of ranks 761.5 and 834.5 in the p53-negative and -positive group, respectively; P = 0.077; Fig. 6B). There were no such correlations in patients who did not undergo radiotherapy (Figs. 6C and D). The data indicated that p53WT was related to a better response to radiotherapy through apoptosis and proliferation inhibition in patients with rectal cancer and that it is important to reactivate mutated p53 in tumors treated with radiotherapy.
Figure 6.
Negative p53 expression correlated with better response to radiotherapy (RT) in tumors of patients with rectal cancer with RT. A and B, The relationship of p53 status with apoptosis (A) and Ki67 expression (B) in resected tumor samples from patients with rectal cancer with RT. C and D, The relationship of p53 status with apoptosis (C) and Ki67 expression (D) in resected tumor samples from patients without RT (NRT).
Discussion
This is the pioneer investigation that reports the radiosensitizing effects of APR-246 in colorectal cancer through in vitro and in vivo studies. The results derived from three HCT116 cell lines with different p53 statuses showed that the combination of APR-246 with radiation had a significant synergistic effect on p53Mut cells, followed by p53WT cells and an additive effect on p53Null cells. Furthermore, radiosensitizing results of APR-246 were confirmed in vivo using zebrafish xenografts and in primary colorectal cancer cells. Mechanistically, APR-246 appeared to mediate radiosensitization effects on the present models via p53-dependent and -independent ways.
We used HCT116 cells because of their different p53 statuses as a model system in the current study. p53Mut cells originally seemed to exhibit less apoptosis in response to radiation than both p53WT and p53Null cells. However, the p53Mut cells increased in radiosensitivity after pretreating with APR-246. The evidence was supported by in vivo experiments using zebrafish xenografts, where the tumor sizes of zebrafish xenografts carrying the three HCT116 cell lines were decreased by combination treatment compared with radiation alone and the most significant in p53Mut. The p53 protein levels were higher in p53Mut cells than in p53WT cells with or without radiation treatment. These results are consistent with previous reports in which mutated p53 protein commonly accumulates in the cancer cells due to its longer half-life than p53WT protein (28–30). Observably, p53Mut cells with accumulated p53 protein are much less sensitive to radiation than p53WT cells and even more resistant than p53Null cells where p53 is absent. This indicates that p53 mutation not only loses response to radiation but also gains the ability to be resistant to radiation, which is called loss of function and gain of function (31, 32). Therefore, the reactivation of already accumulated p53 mutation protein is necessary and has the potential to be an efficient approach for treating cancers with p53 mutation. When pretreated with APR-246 to reactivate p53 mutation, p53Mut cells became more sensitive to radiation through cell-cycle arrest and apoptosis. These results were confirmed by RNA sequencing: that cell cycle and apoptosis pathways are significantly enriched between combination treatment and radiation alone in p53Mut cells. It needs to be noted that transcriptional regulation of the TP53 pathway is involved in the radiosensitizing effect of APR-246 in p53Mut cells. Furthermore, p53Mut cells and p53WT cells shared much more common dysregulated pathways and genes than p53Null cells. Altogether, these results indicate that APR-246 radiosensitized p53Mut cells by restoring them to WT function. These results are also consistent with previous reports that APR-246 could restore normal function of p53WT through direct binding and stabilizing the p53 core domain to promote WT folding, leading to restoration of specific DNA binding and transcription of p53 target genes (33–36).
Interestingly, our study on HCT116 cells and primary cells showed S-phase arrest following radiation with or without APR-246 treatment, although the canonical cell-cycle consequence of radiotherapy was a G2–M accumulation. It is believed that different cell lines may have different reactions to different treatments, including molecules, drugs, and radiotherapy, which may depend on many factors such as gene features of cells. It has been reported that radiation-induced DNA damage triggers the activation of G1–S, G2–M, and intra-S cell-cycle checkpoints, consequently slowing the progress of radiation-exposed cells in the cell cycle (37). The response of a cell to radiation-induced DNA double-strand breaks formation is dependent on the phases of the cell cycle where the cells are irradiated. The G1–S checkpoint exists to prevent cells from entering S-phase in the presence of DNA damage. The intra-S-phase checkpoint exists for monitoring cells that escape the G1–S checkpoint or are in the S-phase at the time of irradiation to prevent the replication of damaged DNA and proteins necessary for mitosis. Cells can escape the intra S-phase checkpoint and progress into G2 in the presence of radiation-induced damage (38). G2–M arrest prevents cells from entering the M (mitosis) phase, and this cell-cycle arrest in G2 allows the coordinated repair of the damage. G1–S- and intra-S-phase checkpoints (cyclin E and cyclin A protein levels) might occur in a dose-dependent manner, while G2–M delay (cyclin B1 protein levels) might happen in a threshold-dependent way (39). For example, by combination of in vitro experiments and mathematical modeling, Matsuya and colleagues (35) found that CHO-K1 cells show the following responses after exposure to X-ray: (i) accumulation of cells in G2 during exposure with 1.0 Gy/h and (ii) delay of DNA synthesis and accumulation of the cells in S and G2 during exposure with 3.0 Gy/h. It was explained that the increase of cells in the S-phase during the exposure to 3.0 Gy/h might be attributed to the DNA damage response in the S-phase checkpoint (40). Furthermore, a study in breast cancer cell lines treated by APR-246 in combination with CX-5461 (an RNA polymerase I inhibitor) shows that both SUM159PT and MDA-MB-231 cell lines having an increase in S-phase and in G2–M phase arrest, but not MCF7 cells (41). In gastric cancer cells, sulforaphane (a protein extracted from broccoli) induces cell-cycle arrest in the S-phase and apoptosis in a p53-dependent manner (42). There is the possibility that some proteins upstream of p53 mediate this effect as a study done with sophoridine, which caused ROS-dependent ERK and JNK activation leading to S-phase arrest and apoptosis in prostate cancer cells (43). ERK and JNK function upstream of p53, which could explain how even HCT116 with p53WT had this increase in S-phase arrest and apoptosis when treated with APR-246 and radiation. Although p53 plays a central role in the arrest in G1 and the G2–M phase of the cell cycle following DNA damage, one of the downstream proteins, p21, can inhibit DNA replication in cells that have already entered the S-phase (44).
Transcriptional regulation of the TP53 pathway was involved in radiosensitizing effects of APR-246 in p53WT cells, suggesting that APR-246 sensitized p53WT cells to radiation via p53-dependent pathways as well. It should be noticed that, although p53Mut and p53WT cells shared a more similar transcriptomic response to the combination treatment compared with p53Null cells, they both still had their own individual pathways. Furthermore, p53WT and p53Mut cells shared only a part of deregulated genes, even in the same pathways. For example, MDM2, a critical negative regulator of p53 (45), is only present in p53WT cells but not in p53Mut cells between combination and radiation-alone treatment.
APR-246 also sensitized p53Null cells to radiation, but this was less profound than in p53Mut cells and p53WT cells. Combination treatment promoted cell-cycle arrest and apoptosis compared with radiation alone in p53Null cells. Intriguingly, while the cell-cycle pathway was enriched when comparing combination treatment and radiation alone in p53Null cells, the apoptosis pathway was not. However, because apoptosis was induced by the combination treatment well beyond radiation treatment alone in p53Null cells, it is likely that APR-246 might promote apoptosis of p53Null cells via nonclassic apoptosis pathways. As DNA replication, base excision repair and autophagy pathways were specifically deregulated in p53Null cells; we hypothesize that other proteins involved in detecting and mediating responses to radiation-induced DNA damage may also be targeted by APR-246, at least in colorectal cancer cells. Furthermore, the transcriptional regulation of TP53 pathway, playing pivotal roles in p53Mut and p53WT cells, was not enriched in p53Null cells between combination and radiation-alone treatment. These results suggested that APR-246 could sensitize cancer cells even without p53 expression to some extent via a p53-independent mechanism, which is consistent with previous reports that a combination of APR-246 with DNA damage reagents exhibited anticancer effects even in the absence of p53 expression (46–48). Our results suggest that APR-246 can mediate radiosensitization effects on colorectal cancers with different p53 status through p53-dependent and -independent ways. As shown in experiments, synergism between APR-246 and radiation only was observed at low doses of radiation. The main reason for using the low doses of radiation was that the higher doses of radiation alone already showed great cytotoxic effect and led to the lack of synergy of these doses in the combinations in cell lines.
We noticed that the regulation of HMOX1 expression and the activity pathway, which is related to oxidoreductase activity (49–51) and ferroptosis (52–54), was enriched both in p53WT and p53Null cells, but not in p53Mut cells. Reports from other cancer cells have shown that APR-246 treatment potently induced the generation of ROS by disturbing the cellular redox balance (55–59). In the current study, we similarly found that ARP-246 pretreatment augmented radiation treatment–induced ROS production, which is likely considerable for the radiotherapy sensitization effects of APR-246 also in colorectal cancer. ROS seemed to play a more important role in p53WT and p53Null cells than in p53Mut cells, where p53 reactivation was the main mechanism. The ability of APR-246 to increase radiation-induced ROS in all cell lines with different p53 statuses may also, in part, explain the radiation-sensitizing activity of APR-246 in the p53WT and p53Null cells. Furthermore, ferroptosis is reported to mediate p53 function in tumor radiosensitivity (60, 61). Therefore, ferroptosis might also play a role in the radiosensitizing effects of APR-246 in colorectal cancers. More experiments need to be performed to further understand the mechanisms of distinguished radiosensitization of cells with different p53 statues.
Furthermore, APR-246 had been demonstrated to interact with a wide range of p53 mutant proteins (such as R175H, R273H, R248Q, R280K, and P278R) in various cancer types, for example, hematopoietic malignancy (14, 62), breast cancer (63), colorectal cancer (64), glioblastoma (65), ovarian cancer (27), and small and non–small cell lung cancer cells (5, 66). We supposed that the radiosensitization effect of APR-246 would also work with different TP53 mutations in rectal cancers. Because no one has done the experiment of APR-246 combination with radiotherapy in colorectal cancer, we have planned to perform a further project on APR-246 in combination with both radiotherapy and chemotherapy together in colorectal cancer. This coming project will include more commercial cell lines and primary cell lines with different biological features, such as various statues of p53, KRAS, E2F transcription factor 1 (E2F1), ribosomal protein L15 (RPL15), and urokinase-type plasminogen activator (uPA).
In the current study, Fig. 5 and Supplementary Figs. S6–S8 showed the key pathways and molecules mediating radiosensitization effects of APR-246 in colorectal cancer cells with different p53 statuses. The information may be used for the following experiments and treatments: (i) researchers, including ourselves, will examine more commercial and primary cell lines with different p53 (and also other genes) status to determine the synergistic effects of APR-246 combining with radiotherapy, and the results will be confirmed in more animal models; (ii) based on the current results, we will perform spatial proteomics in zebrafish and patients as well as Western blotting in cells to verify molecules playing important roles in radiosensitivity. Some proteins might become predictive and prognostic biomarkers for radiotherapy targets and prognostic evaluation in patients with rectal cancer; and (iii) the results will provide evidence for a clinical trial by taking a tissue biopsy and/or a liquid sample from a tumor before radiotherapy for identifying not only p53 status but also intra-tumoral heterogeneity. The findings will provide significant knowledge for clinicians to design a more efficient radiotherapy strategy for precision medicine in patients.
Radiotherapy would increase expression and stabilization of p53 in WT and mutant cells, but this change would fade 2 days after radiation in WT cells. According to results from WB, p53WT protein was going down from about 2 days after irradiation, implying a recovery from stress reaction. For these included patients (taken from Stockholm II clinical trial of preoperative radiotherapy in patients with rectal cancer), it usually took 3 to 7 days waiting for surgery. We compared p53 protein expression of biopsies before radiotherapy with that of surgical samples after radiotherapy from the same patients; p53 expression did not significantly change between the biopsies and surgical samples (neither in non-radiotherapy patients; P > 0.05). Our previous research already showed that negative p53 by IHC had a better local outcome after radiotherapy in patients with rectal cancer (4), as some comparison studies showed that most negative p53 expression in tumors was composed of p53WT, although some null and nonsense mutations also existed. When we focused on these patients again, a significant relationship between negative p53 expression in tumor samples and increased apoptosis was found in only 76 patients who underwent radiotherapy, rather than those who did not. This finding might explain the results of the clinical trial and implied that WT (mainly negative in IHC) p53 was related to a better response to radiotherapy through apoptosis and proliferation inhibition in patients with rectal cancer. Therefore, it is meaningful to reactivate mutated p53 in tumors treated with radiotherapy, although no clinical trial was carried out in rectal cancer. In addition, we produced a surrogate for p53 mutational analysis by IHC staining because DNA sequencing was not feasible. Thus, the current study should be interpreted within the context of its limitations. Although the negative p53 expression in a tumor is usually considered as an indicator of p53WT, p53 null tumors would be misclassified as p53WT due to lack p53 expression. Meanwhile, potentially p53WT tumors may be misclassified as p53 mutant if p53 is overexpressed in response to radiotherapy or other cellular stress, and this stress-induced p53 overexpression does not fade when the tumors were resected. Thus, it is necessary to identify the types of p53 mutants by DNA sequencing in future studies.
In summary, APR-246 sensitized colorectal cancers with different p53 statuses to radiation via p53-dependent and -independent ways. Our findings provided evidence for a possible clinical trial for the combination of APR-246 with radiotherapy in patients with rectal cancer. Eventually, the results may improve therapy response and approach precision medicine, leading to better clinical outcome and limit side effects in patients.
Supplementary Material
Supplementary tables
p53Wt_log2FC of genes
p53Mut_log2FC of genes
p53Null_log2FC of genes
Supplementary figure 1
Supplementary figure 2
Supplementary figure 3
Supplementary figure 4
Supplementary figure 5
Supplementary figure 6
Supplementary figure 7
Supplementary figure 8
Supplementary figure 9
Supplementary figure 10
Supplementary Figure Legends
Acknowledgments
This work was supported by grants from Swedish Cancer Foundation (CAN2016/341 and 190322Pj), Liu Cancer (2020–0331 and 2020–11–25), Post-Doctor Research Project of Sichuan University (grant No. 2022SCU12025), and Natural Science Foundation of Sichuan Province (grant No. 2022NSFSC0764).
The authors acknowledge Klas G. Wiman at Karolinska Institute for his suggestions on the study design and comments on the manuscript; Lars Abrahmsen at Aprea Therapeutics AB for providing APR-246 and valuable advice on this study; Jyotirmoy Das and Massimiliano Volope at Clinical Genomics Linköping, Science for Life Laboratory, Dept. of Biomedical and Clinical Sciences, Linköping University, for aiding in bioinformatic analysis; and Sara Kidane at Linköping University for her help in Western blotting. We appreciate Professor Surajit Pathak for carefully revising the manuscript, checking the English grammar using Grammarly Premium Software, and enhancing its overall readability. We also acknowledge the valuable contribution of our university-approved interpreting and translation agency, “Språkservice,” for their professional expertise in refining the English grammar of this manuscript.
The publication costs of this article were defrayed in part by the payment of publication fees. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Footnotes
Note: Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Authors' Disclosures
L.D. Jensen reports other support from BioReperia AB outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
X. Xie: Conceptualization, formal analysis, investigation, writing–original draft. C. Fan: Conceptualization, formal analysis, investigation, writing–original draft. B. Luo: Formal analysis, investigation, writing–original draft. J. Zhang: Conceptualization, investigation, writing–review and editing. L.D. Jensen: Formal analysis, investigation, writing–review and editing. J. Burman: Formal analysis, investigation, writing–review and editing. C. Jönsson: Investigation. A. Ljusberg: Methodology. P. Larsson: Methodology. Z. Zhao: Conceptualization. X. Sun: Conceptualization, resources, supervision, funding acquisition, writing–original draft, writing–review and editing.
References
- 1. Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644–50. [DOI] [PubMed] [Google Scholar]
- 2. Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, et al. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol 2019;25:4850–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 4. Adell G, Sun XF, Stal O, Klintenberg C, Sjodahl R, Nordenskjold B. p53 status: an indicator for the effect of preoperative radiotherapy of rectal cancer. Radiother Oncol 1999;51:169–74. [DOI] [PubMed] [Google Scholar]
- 5. Deben C, Lardon F, Wouters A, Op de Beeck K, Van den Bossche J, Jacobs J, et al. APR-246 (PRIMA-1(MET)) strongly synergizes with AZD2281 (olaparib) induced PARP inhibition to induce apoptosis in non–small cell lung cancer cell lines. Cancer Lett 2016;375:313–22. [DOI] [PubMed] [Google Scholar]
- 6. Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer 2005;92:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor-suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med 2002;8:282–8. [DOI] [PubMed] [Google Scholar]
- 8. Zache N, Lambert JM, Wiman KG, Bykov VJ. PRIMA-1MET inhibits growth of mouse tumors carrying mutant p53. Cell Oncol 2008;30:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ceder S, Eriksson SE, Cheteh EH, Dawar S, Corrales Benitez M, Bykov VJN, et al. A thiol-bound drug reservoir enhances APR-246-induced mutant p53 tumor cell death. EMBO Mol Med 2021;13:e10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soussi T, Wiman KG. TP53: an oncogene in disguise. Cell Death Differ 2015;22:1239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakayama M, Oshima M. Mutant p53 in colon cancer. J Mol Cell Biol 2019;11:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lehmann S, Bykov VJ, Ali D, Andren O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol 2012;30:3633–9. [DOI] [PubMed] [Google Scholar]
- 13. Deneberg S, Cherif H, Lazarevic V, Andersson PO, von Euler M, Juliusson G, et al. An open-label phase I dose-finding study of APR-246 in hematological malignancies. Blood Cancer J 2016;6:e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol 2021;39:1584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998;282:1497–501. [DOI] [PubMed] [Google Scholar]
- 16. Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat Protoc 2007;2:2734–46. [DOI] [PubMed] [Google Scholar]
- 17. Fan CW, Lu R, Fang C, Zhang XL, Lv ZY, Li Y, et al. Expression profile, molecular functions, and prognostic significance of miRNAs in primary colorectal cancer stem cells. Aging 2021;13:12067–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shangguan W, Fan C, Chen X, Lu R, Liu Y, Li Y, et al. Endothelium originated from colorectal cancer stem cells constitute cancer blood vessels. Cancer Sci 2017;108:1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu R, Fan C, Shangguan W, Liu Y, Li Y, Shang Y, et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct Target Ther 2017;2:16036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan CW, Chen T, Shang YN, Gu YZ, Zhang SL, Lu R, et al. Cancer-initiating cells derived from human rectal adenocarcinoma tissues carry mesenchymal phenotypes and resist drug therapies. Cell Death Dis 2013;4:e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gnosa S, Capodanno A, Murthy RV, Jensen LD, Sun XF. AEG-1 knockdown in colon cancer cell lines inhibits radiation-enhanced migration and invasion in vitro and in a novel in vivo zebrafish model. Oncotarget 2016;7:81634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu GWL, Han Y, He Q. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 2012;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Swedish Rectal Cancer T, Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980–7. [DOI] [PubMed] [Google Scholar]
- 24. Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol 2011;24:1248–53. [DOI] [PubMed] [Google Scholar]
- 25. Oh HJ, Bae JM, Wen X, Jung S, Kim Y, Kim KJ, et al. p53 expression status is associated with cancer-specific survival in stage III and high-risk stage II colorectal cancer patients treated with oxaliplatin-based adjuvant chemotherapy. Br J Cancer 2019;120:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robles AI, Harris CC. Clinical outcomes and correlates of TP53 mutations and cancer. Cold Spring Harb Perspect Biol 2010;2:a001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fransson A, Glaessgen D, Alfredsson J, Wiman KG, Bajalica-Lagercrantz S, Mohell N. Strong synergy with APR-246 and DNA-damaging drugs in primary cancer cells from patients with TP53 mutant high-grade serous ovarian cancer. J Ovarian Res 2016;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012;26:1268–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol 2010;2:a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strano S, Dell'Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene 2007;26:2212–9. [DOI] [PubMed] [Google Scholar]
- 31. Mantovani F, Collavin L, Del SG. Mutant p53 as a guardian of the cancer cell. Cell Death Differ 2019;26:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 2009;9:701–13. [DOI] [PubMed] [Google Scholar]
- 33. Aryee DN, Niedan S, Ban J, Schwentner R, Muehlbacher K, Kauer M, et al. Variability in functional p53 reactivation by PRIMA-1(Met)/APR-246 in Ewing sarcoma. Br J Cancer 2013;109:2696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bao W, Chen M, Zhao X, Kumar R, Spinnler C, Thullberg M, et al. PRIMA-1Met/APR-246 induces wild-type p53–dependent suppression of malignant melanoma tumor growth in 3D culture and in vivo. Cell Cycle 2011;10:301–7. [DOI] [PubMed] [Google Scholar]
- 35. Ali D, Jonsson-Videsater K, Deneberg S, Bengtzen S, Nahi H, Paul C, et al. APR-246 exhibits antileukemic activity and synergism with conventional chemotherapeutic drugs in acute myeloid leukemia cells. Eur J Haematol 2011;86:206–15. [DOI] [PubMed] [Google Scholar]
- 36. Russo D, Ottaggio L, Foggetti G, Masini M, Masiello P, Fronza G, et al. PRIMA-1 induces autophagy in cancer cells carrying mutant or wild-type p53. Biochim Biophys Acta 2013;1833:1904–13. [DOI] [PubMed] [Google Scholar]
- 37. Sia J, Szmyd R, Hau E, Gee HE. Molecular mechanisms of radiation-induced cancer cell death: a primer. Front Cell Dev Biol 2020;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakeman TP, Kim WJ, Callens S, Chiu A, Brown KD, Xu B. The ATM-SMC1 pathway is essential for activation of the chromium[VI]-induced S-phase checkpoint. Mutat Res 2004;554:241–51. [DOI] [PubMed] [Google Scholar]
- 39. Cariveaua MJ, Kalmus GW, Johnke RM, Allison RR, Evans M, Holbert D. Correlations between radiation-induced double-strand breaks and cell-cycle checkpoints in X-irradiated NIH/3T3 fibroblasts. Anticancer Res 2006;26:3311–6. [PubMed] [Google Scholar]
- 40. Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S-phase checkpoint is regulated by parallel pathways. Nat Genet 2002;30:290–4. [DOI] [PubMed] [Google Scholar]
- 41. Makhale A, Nanayakkara D, Raninga P, Khanna KK, Kalimutho M. CX-5461 enhances the efficacy of APR-246 via induction of DNA damage and replication stress in triple-negative breast cancer. Int J Mol Sci 2021;22:5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Y, Wu H, Dong N, Su X, Duan M, Wei Y, et al. Sulforaphane induces S-phase arrest and apoptosis via p53-dependent manner in gastric cancer cells. Sci Rep 2021;11:2504. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Xu Z, Zhang F, Bai C, Yao C, Zhong H, Zou C, et al. Sophoridine induces apoptosis and S-phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J Exp Clin Cancer Res 2017;36:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waga S, Hannon GJ, Beach D, Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 1994;369:574–8. [DOI] [PubMed] [Google Scholar]
- 45. Hock AK, Vousden KH. The role of ubiquitin modification in the regulation of p53. Biochim Biophys Acta 2014;1843:137–49. [DOI] [PubMed] [Google Scholar]
- 46. Cory AH, Chen J, Cory JG. Effects of PRIMA-1 on wild-type L1210 cells expressing mutant p53 and drug-resistant L1210 cells lacking expression of p53: necrosis vs. apoptosis. Anticancer Res 2006;26:1289–95. [PubMed] [Google Scholar]
- 47. Saha MN, Jiang H, Yang Y, Reece D, Chang H. PRIMA-1Met/APR-246 displays high antitumor activity in multiple myeloma by induction of p73 and Noxa. Mol Cancer Ther 2013;12:2331–41. [DOI] [PubMed] [Google Scholar]
- 48. Supiot S, Zhao H, Wiman K, Hill RP, Bristow RG. PRIMA-1(met) radiosensitizes prostate cancer cells independent of their MTp53-status. Radiother Oncol 2008;86:407–11. [DOI] [PubMed] [Google Scholar]
- 49. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016;73:3221–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chiang SK, Chen SE, Chang LC. A dual role of heme oxygenase-1 in cancer cells. Int J Mol Sci 2018;20:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vitek L, Schwertner HA. The heme catabolic pathway and its protective effects on oxidative stress-mediated diseases. Adv Clin Chem 2007;43:1–57. [DOI] [PubMed] [Google Scholar]
- 52. Roh JL, Kim EH, Jang H, Shin D. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol 2017;11:254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hassannia B, Wiernicki B, Ingold I, Qu F, Van Herck S, Tyurina YY, et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J Clin Invest 2018;128:3341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11–7085 induced ferroptosis. Cancer Lett 2018;416:124–37. [DOI] [PubMed] [Google Scholar]
- 55. Tessoulin B, Descamps G, Moreau P, Maiga S, Lode L, Godon C, et al. PRIMA-1Met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood 2014;124:1626–36. [DOI] [PubMed] [Google Scholar]
- 56. Ali D, Mohammad DK, Mujahed H, Jonson-Videsater K, Nore B, Paul C, et al. Antileukemic effects induced by APR-246 are dependent on induction of oxidative stress and the NFE2L2/HMOX1 axis that can be targeted by PI3K and mTOR inhibitors in acute myeloid leukemia cells. Br J Haematol 2016;174:117–26. [DOI] [PubMed] [Google Scholar]
- 57. Ceder S, Eriksson SE, Cheteh EH, Dawar S, Benitez MC, Bykov VJN, et al. A thiol-bind drug reservoir enhances APR-246-induced mutant p53 tumor cell death. EMBO Mol Med 2021;13:e10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu DS, Duong CP, Haupt S, Montgomery KG, House CM, Azar WJ, et al. Inhibiting the system xC(-)/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nat Commun 2017;8:14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Peng X, Zhang MQ, Conserva F, Hosny G, Selivanova G, Bykov VJ, et al. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis 2013;4:e881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lei G, Zhang YL, Hong T, Zhang XD, Liu XG, Mao C, et al. Ferroptosis as a mechanism to mediate p53 function in tumor radiosensitivity. Oncogene 2021;40:3533–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lang X, Green MD, Wang W, Yu J, Choi JE, Jiang L, et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov 2019;9:1673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Maslah N, Salomao N, Drevon L, Verger E, Partouche N, Ly P, et al. Synergistic effects of PRIMA-1(Met) (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica 2020;105:1539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liang Y, Besch-Williford C, Cook MT, Belenchia A, Brekken RA, Hyder SM. APR-246 alone and in combination with a phosphatidylserine-targeting antibody inhibits lung metastasis of human triple-negative breast cancer cells in nude mice. Breast Cancer 2019;11:249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li XL, Zhou J, Chan ZL, Chooi JY, Chen ZR, Chng WJ. PRIMA-1met (APR-246) inhibits growth of colorectal cancer cells with different p53 status through distinct mechanisms. Oncotarget 2015;6:36689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patyka M, Sharifi Z, Petrecca K, Mansure J, Jean-Claude B, Sabri S. Sensitivity to PRIMA-1MET is associated with decreased MGMT in human glioblastoma cells and glioblastoma stem cells irrespective of p53 status. Oncotarget 2016;7:60245–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zandi R, Selivanova G, Christensen CL, Gerds TA, Willumsen BM, Poulsen HS. PRIMA-1Met/APR-246 induces apoptosis and tumor growth delay in small cell lung cancer expressing mutant p53. Clin Cancer Res 2011;17:2830–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables
p53Wt_log2FC of genes
p53Mut_log2FC of genes
p53Null_log2FC of genes
Supplementary figure 1
Supplementary figure 2
Supplementary figure 3
Supplementary figure 4
Supplementary figure 5
Supplementary figure 6
Supplementary figure 7
Supplementary figure 8
Supplementary figure 9
Supplementary figure 10
Supplementary Figure Legends
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.