Abstract
Apoptosis of neurons and astrocytes is induced by human immunodeficiency type 1 (HIV-1) infection in vitro and has been demonstrated in brain tissue from patients with AIDS. We analyzed a panel of diverse HIV-1 primary isolates for the ability to replicate and induce neuronal and astrocyte apoptosis in primary human brain cultures. Apoptosis was induced three- to eightfold by infection with the blood-derived HIV-1 isolates 89.6, SG3, and ADA. In contrast, the brain-derived HIV-1 isolates YU2, JRFL, DS-br, RC-br, and KJ-br did not induce significant levels of apoptosis. The ability of HIV-1 isolates to induce apoptosis was independent of their replication capacity. Studies of recombinant chimeras between the SG3 and YU2 viruses showed that replacement of the YU2 Env with the SG3 Env was sufficient to confer the ability to induce apoptosis to the YU2 virus. Replacement of the Env V3 regions alone largely conferred the phenotypes of the parental clones. The SG3 Env used CXCR4 and CCR3 as coreceptors for virus entry, whereas YU2 used CCR5 and CCR3. The V3 regions of SG3 and YU2 conferred the ability to use CXCR4 and CCR5, respectively. In contrast, the 3′ region of Env, particularly the C3V4 region, was required in conjunction with the V3 region for efficient use of CCR3. These results provide evidence that Env is a major determinant of neurodegenerative mechanisms associated with HIV-1 infection in vitro and raise the possibility that blood-derived viruses which emerge during the late stages of disease may affect disease progression in the central nervous system.
Human immunodeficiency virus type 1 (HIV-1) infects the brain and frequently causes dementia and other neurologic disorders in patients with AIDS (reviewed in reference 37). HIV-1 enters the brain through the passage of infected mononuclear cells across the blood-brain barrier. Most of the HIV-1-infected cells in the brain are macrophages and microglia (37, 61). Infected astrocytes and brain capillary endothelial cells are infrequently detected (2, 61). Neuropathological abnormalities in the brains of patients with HIV-1 encephalitis include reactive astrocytosis, myelin pallor, microglial nodules, perivascular inflammation, multinucleated giant cells, abnormal blood-brain barrier permeability, and neuronal loss (37, 49). Apoptosis of neurons and possibly other cell types is a likely cause of central nervous system (CNS) injury in AIDS (1, 21, 46, 53, 64). Apoptosis of neurons and astrocytes is induced by HIV-1 infection in vitro (53) and has been demonstrated in autopsy brain tissue from children and adults with AIDS (1, 21, 46, 53, 64). Neurons are not directly infected by HIV-1. Moreover, apoptosis in HIV-1-infected primary brain cultures in vitro is not significantly induced until 1 to 2 weeks after the time of peak viral replication (53). Together, these observations suggest that neuronal apoptosis is induced by soluble factors rather than by direct viral infection. Several candidates for soluble proapoptotic factors that may lead to neuronal cell death in HIV-1 infection have been proposed based on in vitro studies (37); these include the HIV-1 gp120 and Tat proteins, as well as factors secreted by HIV-1-infected or -activated macrophages and microglia, such as tumor necrosis factor alpha, oxygen-free radicals, and excitatory amino acids. However, the in vivo role of these factors in contributing to apoptosis in the brains of AIDS patients has not been established.
The role of strain variability in the pathogenesis of HIV-1 dementia is unknown. The genetic evolution of HIV-1 within the brain is distinct from that in lymphoid tissues and other organs (29, 32, 47, 65, 67). Specific sequences in Env, particularly the V3 region, are associated with brain infection (29, 32, 47, 48, 65, 67). HIV-1 in the brain is typically macrophagetropic (M-tropic) (13, 32, 47). However, specific determinants of HIV-1 neurotropism or neurovirulence have not been identified (58). Infection of the central nervous system (CNS) by M-tropic strains of HIV-1 or simian immunodeficiency virus (SIV) is not sufficient to cause dementia or encephalitis (31, 32, 40, 47), suggesting that neurovirulence is likely to be determined by genetic or biological characteristics that are distinct from M-tropism.
HIV-1 tropism and coreceptor usage play an important role in disease pathogenesis in the immune and central nervous systems (reviewed in references 12, 19, and 38). Several members of the chemokine receptor family are used together with CD4 for HIV-1 entry into target cells (12, 38). T-cell line-tropic (T-tropic) HIV-1 isolates use CXCR4 as a coreceptor, whereas M-tropic viruses use CCR5. A subset of HIV-1 isolates can also use CCR3 or CCR2b (4, 9, 11, 14). Other chemokine receptors such as Gpr1, Gpr15/BOB, STRL33/BONZO, ChemR1/CCR8, V28, or US28 can be used by some HIV-1, HIV-2, or SIV isolates, but their role in HIV-1 infection in vivo is unknown (12, 38, 52). CXCR4, CCR5, and CCR3 are expressed on microglia and other cell types in the brain (19, 23, 27, 34, 55, 64, 70). CCR5 and CCR3 serve as coreceptors for HIV-1 infection of microglia, whereas infection mediated by CXCR4 is relatively inefficient (23, 27, 55). HIV-1 in the brain uses CCR5 and in some cases CCR3 for virus entry (27, 55). Minor use of CXCR4 has been demonstrated for some brain-derived viruses (55).
A panel of diverse HIV-1 primary isolates was investigated for the ability to replicate and induce apoptosis in primary human brain cultures. We demonstrate that HIV-1 isolates differ in the ability to induce apoptosis of neurons and astrocytes in primary brain cultures and that the ability to induce apoptosis is independent of replication capacity. Surprisingly, apoptosis was induced by three blood-derived viruses, whereas five brain-derived viruses did not induce significant levels of apoptosis. Replacement of the env gene was sufficient to confer the ability to induce apoptosis to an otherwise non-apoptosis-inducing virus. Our studies suggest that some HIV-1 isolates which use CXCR4, in addition to CCR5 or CCR3, may be more cytopathic in the CNS. Understanding the role of strain variability, genetic determinants, and coreceptor usage in HIV-1 cytopathicity in the CNS may advance the development of new therapeutic strategies to prevent neurologic injury in patients with AIDS.
MATERIALS AND METHODS
Cell cultures.
Primary human brain cultures were prepared from fetal abortuses at 13 to 18 weeks as previously described (6), plated in 24-well plates (250,000 cells per well), and maintained in Dulbecco modified Eagle medium containing 10% calf serum for 14 days before infection. Tissue was procured by using an approved protocol in compliance with institutional and federal regulations. These cultures contain a mixture of astrocytes (70 to 90%), neurons (10 to 30%), microglial cells (1 to 5%), and fibroblasts (1 to 5%) (53). For detection of apoptosis, cultures were fixed in 4% paraformaldehyde in phosphate-buffered saline–sucrose for 30 min, washed, and stored in phosphate-buffered saline at 4°C. The 293T, U87, Cf2Th, HeLa, and COS-1 cell lines were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. MT-2, CEMx174, PM1, and human peripheral blood mononuclear cells (PBMC) stimulated with interleukin-2 were maintained in RPMI supplemented with 10% calf serum.
Plasmids.
The SG3, YU2, DH123, and HXB2 plasmids encode full-length infectious HIV-1 proviruses (25, 35, 54). The chimeric HIV-1 proviral clones SG29 and SG52 were constructed by substituting the KpnI-to-KpnI (nucleotides 6348 to 9010) and BglII-to-MstII (7033 to 7301) fragments, respectively, of the YU2 env gene into the corresponding segment of the SG3 env gene. SG26, SG57, SG68, and SG84 were constructed by substituting the KpnI-to-KpnI (5871 to 8551), BglII-to-MstII (6573 to 6842), BglII-to-KpnI (6573 to 8551), and BglII-to-AflIII (6573 to 7027) fragments, respectively, of the SG3 env gene into the corresponding segment of the YU2 env gene. The AflIII site in YU2 was created by PCR without altering the amino acid sequence. The env genes of all chimeric clones were sequenced in their entirety to confirm that no errors were introduced during the PCR amplification. Env expression plasmids were constructed by replacement of the 2.7-kb KpnI-to-KpnI env fragment in pSVIIIenv, which expresses HXB2 Env and Rev under the control of the HIV-1 long terminal repeat (9). Env expression was confirmed by Western blotting of lysates of 293T cells transfected with Env expression plasmids by the calcium phosphate method using a rabbit anti-gp120 antibody raised against the full-length HXB2 envelope (kindly provided by Richard Wyatt and Joseph Sodroski) and an Amersham ECL detection kit.
Virus stocks.
YU2, SG3, DH123, HXB2, SG26, SG29, SG52, and SG57 virus stocks used for infection of primary brain cultures were produced by transfection of 293T cells with 20 μg of proviral DNA plasmids by the calcium phosphate method (27). Supernatants containing virus were collected 48 h after transfection, filtered (0.45-μm-pore-size filter), quantified by reverse transcriptase (RT) assay using [3H]TTP incorporation, (50), and stored at −70°C. YU2, SG3, SG26, SG29, SG52, SG57, SG68, and SG84 virus stocks used for infection of PBMC, CEMx174 cells, and MT-2 cells were similarly produced by calcium phosphate transfection of COS-1 or HeLa cells (25) and quantified by RT assay using [35S]TTP (35), except that [35S]thymidine incorporation was quantified with a scintillation counter. 89.6 and ELI virus stocks (obtained from the NIH AIDS Research and Reference Reagent Program; donated by Ronald Collman and by Jean-Marie-Bechet and Luc Montagnier, respectively) (10, 45) were prepared from the supernatants of infected PM1 and CEMx174 cells, respectively. JR-FL and ADA virus stocks (obtained from the NIH AIDS Research and Reference Reagent Program; donated by Irvin Chen and Lee Ratner, respectively) (22, 44, 66) were prepared from supernatants of infected phytohemagglutinin-stimulated PBMC or PM1 cells. KJ-br, RC-br, and DS-br virus stocks were prepared from the supernatants of infected peripheral blood-derived monocytes/macrophages (20). Control supernatants from uninfected 293T, PM1 cells, PBMC, or monocytes/macrophages were similarly prepared and used for mock infections.
HIV-1 infections.
Primary brain cultures were infected by incubation with virus stocks normalized for equivalent amounts of RT activity (20,000 or 50,000 3H cpm RT units) or mock infected with the same volume of control supernatant. After 16 h of incubation at 37°C, the medium was removed and the cultures were washed twice before addition of fresh medium. A 50% medium change was performed every 7 days. Productive HIV-1 infection was confirmed by monitoring RT activity in the culture supernatants, using [3H]dTTP incorporation (50) every 7 days. Phytohemagglutinin-stimulated PBMC (107), CEMx174 cells (106), and MT-2 cells (106) were infected with equivalent amount of virus (250,000 35S cpm RT units). After 12 h of incubation at 37°C, the medium was removed and the cultures were washed before addition of fresh medium. Virus replication was measured by monitoring RT activity in the culture supernatants, using [35S]thymidine incorporation (35) every third day. Syncytium formation was observed daily by light microscopy. Double-immunofluorescence staining of fixed primary brain cultures with rabbit anti-P24 (Intracell) or rabbit anti-Nef (53) and mouse anti-CD68 (EBM 11; Dako) or mouse anti-glial fibrillary acidic protein (GFAP; Sigma) monoclonal antibodies followed by fluorescein isothiocyanate- or rhodamine- conjugated secondary antibodies (Sigma) was performed as described elsewhere (27, 53, 64).
Detection of apoptosis.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) staining was performed with an ApopTag kit (Oncor) (53). Quantitation of TUNEL staining was performed by counting cells in 20 random microscope fields, using a 20× objective. TUNEL staining was combined with double-immunofluorescence staining as described elsewhere (53). Propidium iodide staining was performed by incubation of fixed cells in 3 mM sodium citrate buffer (pH 7.0) containing propidium iodide at 50 μg/ml and 0.1% Triton X-100 for 1 h at 37°C (53). The percentage of apoptotic cells was determined by counting the number of nuclei with morphologic features characteristic of apoptosis (chromatin condensation and nuclear fragmentation), using a 20× objective. Histone-associated DNA fragmentation was detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocol (Cell Death Detection ELISA; Boehringer Mannheim Biochemicals).
HIV-1 entry assay.
An env complementation assay was used to quantitate HIV-1 entry as described previously (9). Briefly, recombinant HIV-1 chloramphenicol acetyltransferase (CAT) reporter constructs were generated by cotransfection of 293T cells with 20 μg of pHXBΔenvCAT, which contains an HIV-1 provirus with a deletion in the env gene and a replacement of the nef gene with a gene encoding CAT, and 4 μg of pSVIIIenv plasmids, which encode different HIV-1 Env proteins and Rev, using the calcium phosphate method. U87 cells to be used as target cells were transfected with 10 μg of plasmid pcDNA3-CD4 and 20 μg of plasmid pcDNA3 containing CXCR4, CCR5, or CCR3 (9) by lipofection with DOTAP (Boehringer Mannheim). Alternatively, Cf2Th cells were cotransfected with the same plasmids by the calcium phosphate method. Approximately 48 h after transfection, cells were infected by incubation with 40,000 3H cpm RT units of recombinant CAT reporter viruses. CAT viruses with no Env protein were used as a negative control. Sixty hours later, cells were harvested and assayed for CAT activity.
RESULTS
Replication and cytopathicity of HIV-1 isolates derived from blood and brain.
To examine the role of strain variability in apoptosis induced by HIV-1 infection, we initially analyzed a panel of well-characterized primary HIV-1 isolates derived from blood or brain for the ability to replicate and induce apoptosis in primary human brain cultures. SG3 is a highly cytopathic syncytium-inducing T-tropic isolate cloned from PBMC of an AIDS patients (25). The dualtropic 89.6 and M-tropic ADA isolates were also derived from blood of AIDS patients (10, 22). YU2 and JRFL are non-syncytium-inducing M-tropic viruses derived from brains of AIDS patients with dementia (36, 44). YU2 was cloned directly from brain tissue (35, 36), and JRFL was isolated from the frontal lobe by coculturing with PBMC (44).
Primary brain cultures were infected with the different isolates, and virus replication was monitored by RT assay of the culture supernatants. Peak levels of replication were detected between days 7 and 21 (Fig. 1A). ADA and JRFL replicated at high levels, while SG3 replicated at low levels. 89.6 and YU2 showed somewhat lower levels of replication than ADA and JRFL but more efficient replication than SG3. SG3 virus could easily be rescued by coculturing with PM1 cells at 21 to 28 days after infection, indicating the presence of infectious virus despite low levels of RT activity. Double-immunofluorescence staining with anti-HIV-1 p24 or anti-HIV-1 Nef and the microglial cell marker anti-CD68 or astrocyte marker anti-GFAP showed that microglia were the only cell type expressing these HIV-1 antigens (not shown).
FIG. 1.
Apoptosis induced by infection of primary brain cultures with different HIV-1 isolates. Cultures were infected by incubation with 89.6, SG3, ADA, YU2, or JRFL virus stock (20,000 3H cpm RT units) or mock infected with control supernatants. (A) Virus replication measured by RT assay of culture supernatants (means of triplicate samples). The background level for RT assays in mock infections was ≤200 3H cpm RT units/ml. (B to D) Detection of apoptosis on day 30 after infection by TUNEL staining (B), propidium iodide staining (C), and detection of cytosolic histone-associated DNA fragments by ELISA (D) (mean ± SD, n = 2). ∗, P < 0.05 by ANOVA with Bonferroni posttest correction compared with mock-infected control cultures. Results are representative of three independent experiments. O.D., optical density.
Apoptosis was quantified 30 days after initiation of infection, since we previously demonstrated that apoptosis is not significantly induced by the 89.6 virus until 28 to 30 days after infection (53). Three different assays were used to quantitate apoptosis. TUNEL staining was used to detect DNA fragmentation in situ, propidium iodide staining was used to detect apoptotic nuclear morphology, and an ELISA was used to determine the release of histone-associated DNA fragments into the cytoplasmic fraction (53). By all three methods, apoptosis (represented as mean ± standard deviation [SD]) was shown to be three- to eightfold greater in 89.6-, SG3-, and ADA-infected cells than in mock-infected control cultures (Fig. 1B to D) (P < 0.05 by analysis of variance [ANOVA] with Bonferroni posttest correction). In contrast, YU2 and JRFL induced minor increases in apoptosis that were not statistically significant and were not enhanced with longer culture times of up to 40 days (not shown). Inhibition of virus replication by addition of the RT inhibitor zidovudine (2.5 μg/ml) after infection with ADA abolished the induction of apoptosis at 30 days after infection (not shown). TUNEL staining in combination with double-immunofluorescence staining using the neuron-specific marker anti-Tau and astrocyte marker anti-GFAP showed that infection with 89.6, SG3, and ADA induced apoptosis in both neurons and astrocytes. Approximately 30 to 40% of the TUNEL-positive cells were neurons and 30% were astrocytes (not shown), consistent with results of a previous study (53). Rare TUNEL-positive cells (1 to 5%) stained positively for the microglial cell marker anti-CD68. The remaining TUNEL-positive cells were not labeled with either anti-Tau or anti-GFAP; these most likely represent cells in the late stages of apoptotic degeneration. Since all three methods of apoptosis detection gave similar results (Fig. 1B to D), the TUNEL staining method was used for subsequent quantitation of apoptosis. These results show that HIV-1 isolates differ in the ability to induce apoptosis in primary brain cultures and further demonstrate that the ability to induce apoptosis requires virus replication but is independent of replication capacity.
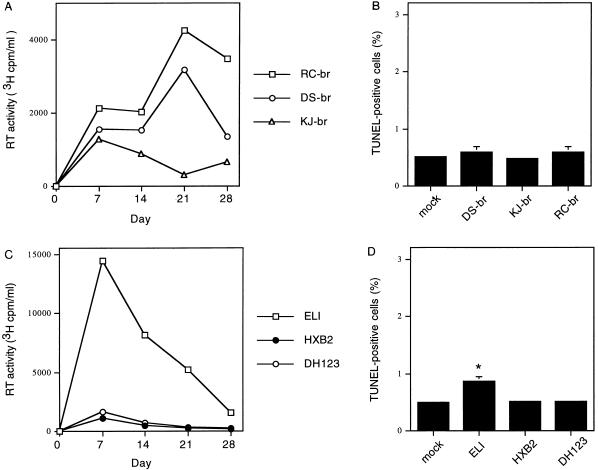
The preceding experiments demonstrate that the brain-derived JRFL and YU2 viruses replicated to high levels but did not induce significant levels of apoptosis in primary brain cultures. To examine the induction of apoptosis by other primary neurotropic isolates, we performed similar experiments using three viruses isolated directly from the brain tissue of AIDS patients. DS-br and RC-br were isolated from two adult AIDS patients with dementia, and KJ-br was isolated from a pediatric AIDS patient with encephalopathy by coculturing brain tissue with peripheral blood monocyte-derived macrophages (20). All three isolates replicated in primary brain cultures (Fig. 2A), although at lower levels than the ADA, YU2, and JRFL viruses (Fig. 1A). Infection with KJ-br consistently resulted in lower levels of replication compared to DS-br and RC-br, in accord with a previous study (55). Infection with these primary brain isolates did not induce a significant increase in apoptosis (Fig. 2B). In further experiments using additional blood-derived isolates, we found that the primary T-tropic ELI isolate (45), but not the primary dualtropic DH123 (8, 54) or laboratory-adapted T-tropic HXB2 and NL4-3 isolates, induced a minor but statistically significant increase in apoptosis (Fig. 2C and D and data not shown) (P < 0.05). ELI consistently replicated to significantly higher levels in primary brain cultures than the other blood-derived isolates. Thus, there is significant heterogeneity among primary M- and T-tropic isolates for replication in macrophages and microglia, consistent with previous studies (24, 57, 60). These results together with the preceding experiments suggest that HIV-1 isolates derived from the brain do not necessarily induce apoptosis in primary human brain cultures and raise the possibility that some blood-derived T-tropic or dualtropic viruses are cytopathic in the CNS.
FIG. 2.
Detection of apoptosis in primary brain cultures infected with primary HIV-1 isolates derived from brain tissue (A and B) or blood (C and D) of AIDS patients. (A and B) Cultures were infected with RC-br, DS-br, or KJ-br (20,000 3H cpm RT units) or mock infected with control supernatants. (A) Virus replication measured by RT assay of culture supernatants (means of quadruplicate samples). The background level for RT assays in mock infections was ≤200 3H cpm RT units/ml. (B) Apoptosis on day 30 after infection, detected by TUNEL staining (mean ± SD, n = 2). (C and D) Cultures were infected with ELI, DH123, or HXB2 or mock infected with control supernatants, and virus replication (C) and apoptosis (D) were detected as for panels A and B. ∗, P < 0.05 by ANOVA with Bonferroni posttest correction compared with mock-infected control cultures. Similar results were obtained in two independent experiments.
Molecular determinants of HIV-1 replication and cytopathicity in the env gene.
Molecular determinants for HIV-1 tropism, as well as replication efficiency and cytopathicity in PBMC and T-cell lines, are in the env gene, particularly within the V3 loop region of gp120 (30, 44, 66). To examine the role of Env in HIV-1 replication and apoptosis in primary brain cultures, we constructed chimeric proviruses between the blood-derived SG3 (apoptosis-inducing) and brain-derived YU2 (nonapoptosis-inducing) viruses. As shown in Fig. 3, different regions of the SG3 and YU2 env genes were exchanged. The parental and chimeric viruses replicated with similar kinetics in PBMC (Fig. 4A). As expected, the viruses showed significant differences in the ability to replicate in CEMx174 cells (Fig. 4B). YU2, or chimeras containing the entire env gene or V3 region of YU2 Env (SG29 and SG52), did not show significant replication above background levels in CEMx174 cells. In contrast, SG3 and chimeras SG26, SG57, and SG84, with the entire SG3 env gene, SG3 V3 region, and SG3 V3V4 region, respectively, replicated to high levels. SG68 showed similar replication kinetics as SG84 in PBMC and CEMx174 cells (not shown). SG3 induced syncytium formation in CEMx174 and MT-2 cells, while YU2 showed a non-syncytium-inducing phenotype (Fig. 3), consistent with previous studies (25, 35). As expected, chimeric viruses with the V3 region or larger regions of the SG3 env gene induced syncytia in both cell lines, whereas chimeric clones that contained the V3 region or the entire env gene of YU2 showed a non-syncytium-inducing phenotype. The ability of SG57 to induce syncytia was lower in CEMx174 cells and somewhat delayed in MT-2 cells compared to SG3. The syncytium-inducing phenotype of the chimeric viruses was also tested in HeLa-T4 cells by using a vaccinia virus-based assay (43), which gave results similar to those obtained for MT-2 and CEMx174 cells (not shown). These results are consistent with previous studies which have shown that Env, particularly the V3 region, is the major determinant for tropism and syncytium induction in T-cell lines (30, 44, 66).
FIG. 3.
Structure of SG3/YU2 envelope chimeras. Chimeric env genes were constructed by using the indicated restriction enzyme sites in the HIV-1 genome (top). Scores for syncytium formation in CEMx174 or MT2 cells infected with the indicated viruses as described in Materials and Methods are shown at the right as follows: 1+, 1 to 10%; 2+, 10 to 25%; 3+, 25 to 50%; 4+, >50%. Similar results were obtained in three independent experiments.
FIG. 4.
Replication of the SG3/YU2 chimeric viruses in PBMC and CEMx174 cells. PBMC (A) and CEMx174 cells (B) were infected with equal amounts (250,000 35S cpm RT units) of YU2, SG3, SG26, SG29, SG52, SG57, or SG84 virus stock. Virus replication was monitored by measuring RT activity in culture supernatants every third day. The background level for RT assays in mock infections was ≤1,500 35S cpm RT units/25 μl. Results are representative of three independent experiments.
The chimeric viruses were analyzed for the ability to replicate and induce apoptosis in primary brain cultures. YU2 replicated to higher levels than SG3 (Fig. 5A). SG52, which contains the V3 region of the YU2 Env, replicated at lower levels than YU2, whereas SG57, which contains the V3 region of the SG3 Env, replicated with slightly higher efficiency than SG3. Thus, replacement of the Env V3 regions alone largely but not fully conferred the replication capacity of the parental clone. The SG29 virus, which contains the YU2 Env in the context of the SG3 virus, showed slightly lower replication efficiency than YU2. Additionally, SG26, which contains the SG3 Env in the context of the YU2 virus, showed slightly lower levels of replication than SG3. Replacement of the YU2 Env with the SG3 Env was sufficient to confer the ability to induce apoptosis to the otherwise non-apoptosis-inducing YU2 virus (Fig. 5B). Conversely, replacement of the SG3 Env with the YU2 Env significantly reduced the ability of the cytopathic SG3 virus to induce apoptosis. Exchange of the V3 regions alone largely but not fully conferred the phenotypes of the parental clones. These results suggest that the V3 region as well as other regions of Env are important determinants of HIV-1 replication and cytopathicity in primary brain cultures.
FIG. 5.
Induction of apoptosis in primary brain cultures infected with SG3/YU2 chimeric viruses. Cultures were infected with YU2, SG3, SG26, SG29, SG52, or SG57 virus stock normalized for equivalent amounts of RT activity. (A) Virus replication measured by RT activity of culture supernatants (means of triplicate samples). The background level for RT assays in mock-infected cultures was ≤200 3H cpm RT units. (B) Detection of apoptosis on day 30 after infection by TUNEL staining. ∗, P < 0.05 by ANOVA with Bonferroni posttest correction compared with mock-infected control cultures. Similar results were obtained in three independent experiments.
Coreceptor usage.
The preceding experiments show that molecular determinants for HIV-1 replication and cytopathicity in primary brain cultures are in the env gene, particularly the V3 region. The V3 region is also an important determinant of coreceptor usage (3, 9, 11). YU2 has been shown to use CCR5 and CCR3 as coreceptors for virus entry (9, 27). The coreceptor usage of SG3 is unknown. To examine the relationship between coreceptor usage and cytopathicity, the SG3/YU2 parental and chimeric Envs were used in an env complementation assay. The env gene of each virus was cloned into the pSVIIIenv expression vector. Western blotting of 293T cells transfected with the different pSVIIIenv plasmids demonstrated comparable levels of Env expression and processing of gp160 to gp120 and gp41 (Fig. 6).
FIG. 6.
Expression of HIV-1 envelope chimeras. 293T cells were transfected with expression plasmids containing the SG3, YU2, SG52, SG57, SG68, or SG84 chimeric env gene as shown in Fig. 3. Cell lysates were analyzed by Western blotting using rabbit anti-gp120. Positions of gp160 and gp120 are indicated on the right; positions of molecular weight markers are indicated on the left.
We then determined coreceptor usage of the different Env proteins with transfected U87 cells as target cells. SG3 used CXCR4 and CCR3 but not CCR5 as coreceptors (Fig. 7 and Table 1). CAT reporter viruses without Env or with the CXCR4-tropic HXB2 Env were included as controls. The V3 region of YU2 conferred the ability to use CCR5 as a coreceptor to SG3 and abolished usage of CXCR4. Conversely, the V3 region of SG3 conferred the ability to use CXCR4 to YU2 and abolished usage of CCR5. Exchange of the V3 regions produced chimeric Envs that utilized CCR5 or CXCR4 less efficiently than the parental Envs. Exchange of the V3V4 region from SG3 marginally increased CXCR4 tropism compared to exchange of the V3 region alone. However, exchange of the entire 3′ end of Env along with the V3 region from SG3 was sufficient to fully restore entry of the resulting chimera into CXCR4-expressing cells. Both SG3 and YU2 used CCR3 as a coreceptor, although YU2 did so more efficiently than SG3. The usage of CCR3 was less efficient than the usage of CXCR4 or CCR5, which might reflect the relatively low surface expression of CCR3 (9, 52). Exchange of the V3 regions between SG3 and YU2 produced chimeric Envs with very inefficient usage of CCR3. However, exchange of C3V4 or the entire 3′ end of Env along with the V3 region from SG3 largely or fully restored entry into CCR3-expressing cells. U87 cells expressing either CXCR4, CCR5, or CCR3 in the absence of CD4 could not support entry of any of the HIV-1 CAT reporter viruses (not shown). The env complementation assay was repeated with canine thymocyte Cf2Th cells as target cells. Cf2Th and U87 cells gave similar patterns of coreceptor usage for the chimeric HIV-1 viruses (Table 1). However, Cf2Th cells gave consistently higher levels of CAT activity, probably due to higher levels of chemokine receptor expression (9). Taken together, these results show that the V3 region largely confers the ability to use CXCR4 or CCR5. However, regions outside V3 influence the efficiency of the interaction between Env and these coreceptors, consistent with previous reports (3, 8, 51, 59). In contrast, efficient use of CCR3 requires sequences in the 3′ region of Env, particularly the C3V4 region, in conjunction with the V3 region.
FIG. 7.
CAT activities in transfected U87 cells infected with HIV-1 reporter viruses. U87 cells cotransfected with a plasmid expressing CD4 and a plasmid expressing the chemokine receptor CCR5, CCR3, or CXCR4 were infected with CAT viruses with either no Env protein or Env proteins of HXB2, SG3, YU2, SG52, SG57, SG68, or SG84. Results of the CAT assay performed with the U87 cell lysates are shown. Similar results were obtained in three independent experiments.
TABLE 1.
CAT activities in transfected Cf2Th and U87 cells infected with HIV-1 CAT reporter viruses
| HIV-1 envelope | % Conversion of chloramphenicol to acetylated formsa
|
|||||
|---|---|---|---|---|---|---|
| Cf2Th
|
U87
|
|||||
| CCR5 | CXCR4 | CCR3 | CCR5 | CXCR4 | CCR3 | |
| None | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 |
| HXB2 | 0.4 | 56.8 | 0.4 | 0.2 | 14.4 | 0.3 |
| SG3 | 0.2 | 78.5 | 4.3 | 0.4 | 38.4 | 3.2 |
| YU2 | 91.4 | 0.2 | 27.3 | 29.8 | 0.1 | 19.2 |
| SG52 | 45.4 | 0.3 | 1.5 | 5.3 | 0.1 | 1.1 |
| SG57 | 0.2 | 26.3 | 1.5 | 0.1 | 10.2 | 1.0 |
| SG68 | 0.2 | 52.7 | 6.2 | 0.3 | 35.4 | 5.3 |
| SG84 | 0.2 | 29.8 | 3.2 | 0.3 | 14.8 | 1.7 |
Determined after incubation of equivalent amounts of lysates from Cf2Th or U87 cells cotransfected with plasmids expressing CD4 and either CCR5, CXCR4, or CCR3 and infected with HIV-1 CAT reporter viruses pseudotyped with different HIV-1 Env proteins as for Fig. 7. Similar results were obtained in three independent experiments.
DISCUSSION
In this study, we demonstrate that HIV-1 isolates differ in the ability to induce apoptosis of neurons and astrocytes in primary brain cultures and that the ability to induce apoptosis is independent of replication capacity. Apoptosis was induced by the blood-derived viruses SG3, 89.6, ADA, and ELI, which replicated with variable efficiency in primary brain cultures. Surprisingly, the brain-derived viruses YU2, JRFL, DS-br, RC-br, and KJ-br replicated but did not induce significant levels of apoptosis. The apoptosis-inducing viruses exhibited variable tropism and syncytium-inducing phenotypes, while the non-apoptosis-inducing viruses were all M-tropic and non-syncytium inducing. Thus, M-tropism is neither necessary nor sufficient for a virus to cause apoptosis in the CNS. A further implication of our studies is that HIV-1 isolates in the brain are not necessarily cytopathic in the CNS. Consistent with this prediction, M-tropic strains of HIV-1 or SIV that infect the CNS are not sufficient to cause dementia or encephalitis (31, 40, 47, 58). Our results do not exclude the existence of brain-derived viruses that induce apoptosis or other cytopathic effects in neurons or other cell types (24, 48, 55, 60), since only a limited number of brain-derived viruses were analyzed. However, our findings raise the possibility that blood-derived HIV-1 strains which emerge during the late stages of disease affect disease progression in the CNS. V3 region sequences with characteristics of T-tropic or dualtropic HIV-1 strains have been detected in the brain, albeit at low frequency (7, 32). Furthermore, phylogenetic analysis of blood- and brain-derived Env sequences implies trafficking of virus from blood into the brain in a subset of patients (7, 32, 65, 67). It will be important to analyze a larger series of blood- and brain-derived viruses in future studies to elucidate the relationship between tissue-specific variants, viral phenotypes, and HIV-1 pathogenicity in the CNS.
Our studies of chimeras between the SG3 and YU2 viruses show that replacement of the Env is sufficient to confer the ability to induce apoptosis to an otherwise non-apoptosis-inducing virus. The V3 region was shown to be an important determinant for the apoptosis-inducing phenotype. However, regions outside V3 also contributed to this cytopathic effect of Env. Thus, the Env is a major determinant of HIV-1-induced apoptosis in primary brain cultures. This conclusion is consistent with previous studies which demonstrated that expression of Env is sufficient to induce apoptosis in T-cell lines (33, 39). Moreover, soluble gp120 is neurotoxic and can induce neuronal apoptosis in vitro (37, 42). Furthermore, SIV neurovirulence largely maps to the env gene (16, 40). Although SG3 is T-tropic, its tropism and other biological characteristics may differ in some respects from those of other T-tropic viruses, based on its extraordinary cytopathicity and the observation that it replicates more efficiently in chimpanzee lymphocytes than in human lymphocytes (25). Eight amino acids in the V3 loop differ between the SG3 and YU2 Env proteins. Notably, the SG3 V3 region contains at positions 6 and 7 two adjacent basic residues (Lys-Lys) that are not found in YU2 or HXB2 (25). Our results do not exclude the possibility that additional determinants for the apoptosis-inducing phenotype are outside the env gene. For example, the HIV-1 Nef and Tat proteins have been proposed to have neurotoxic activity (37). However, our studies suggest that Vpu is not required for HIV-1 replication or cytopathicity in primary brain cultures, since both YU2 and SG3 are vpu negative (25, 35).
CCR3 can mediate infection of microglia by some neurotropic isolates and therefore is likely to be important for HIV-1 pathogenesis in the CNS (27). The apoptosis-inducing SG3 and ELI (9) viruses use CXCR4 and CCR3 but not CCR5. It is likely that SG3 mainly uses CXCR4 for entry into microglia, since a chimera with reduced CCR3 use (SG57) replicated at levels comparable to the parental SG3 virus. The demonstration that T-tropic HIV-1 isolates can replicate in microglia albeit at low levels (60) and recent studies demonstrating that CXCR4 can mediate entry into macrophages (56) are consistent with this prediction. SG3 and ELI replicated to higher levels in primary brain cultures compared to the laboratory-adapted T-tropic NL4-3 and HXB2 viruses, which use only CXCR4. This observation raises the possibility that the ability of SG3 and ELI to use both CXCR4 and CCR3 for virus entry reflects particular features of the Env that increase replication capacity in microglia relative to other T-tropic viruses (44a). CCR3 use per se did not correlate with the ability of isolates to induce apoptosis in primary brain cultures. Together, these findings suggest that CCR3 use may facilitate microglial infection by some HIV-1 isolates (27) but is not necessarily associated with HIV-1 cytopathicity in the CNS.
The finding that SG3 can use both CXCR4 and CCR3 but not CCR5 is of interest, since only a few viruses with this pattern of coreceptor usage have been reported. Among these are HIV-1 ELI, BH8, BK132, and UG21 (9, 52), which are T-tropic and syncytium-inducing strains. Our studies of SG3/YU2 chimeras show that V3 largely confers the ability to use CXCR4 or CCR5, although regions outside V3 influence the efficiency of the interaction, consistent with previous studies of other strains (9, 63, 68). In contrast, we found that sequences in the 3′ region of Env, particularly the C3V4 region, are required in conjunction with V3 for efficient use of CCR3. Thus, regions of the Env that are important for CCR3 use overlap but are distinct from those that are important for CCR5 or CXCR4 use. The finding that exchange of the V3 region between SG3 and YU2 nearly abolished the ability of either virus to use CCR3 is consistent with previous studies which demonstrate that Env-coreceptor interactions are highly strain dependent (3, 8, 59). Our finding that the C3V4 region contains determinants that influence CCR3 usage is consistent with a recent study which demonstrated that CCR3 use is determined by sequences within V3 through V5 (59). Another recent study (51) reported that CCR3 tropism requires an M-tropic V3 region in conjunction with a CCR3 using V1-V2 region. The difference between these results and our findings probably reflects the particular HIV-1 strains tested. The present study together with previous reports (3, 8, 51, 59) support a model in which multiple regions of the HIV-1 Env influence coreceptor usage in a manner that is both strain and coreceptor dependent.
Changes in coreceptor use and cytopathicity correlate with disease progression in HIV-1-infected individuals (4, 11, 62). Viruses isolated in the early stages of infection usually exhibit a nonsyncytium-inducing M-tropic phenotype and utilize only CCR5 (4). In contrast, viruses isolated from patients who have progressed to AIDS frequently exhibit a phenotypic switch to a T-tropic or dualtropic syncytium-inducing phenotype and generally use CXCR4 in addition to CCR5, and in some cases CCR3 and other coreceptors (4, 11, 38). However, not all patients with AIDS harbor syncytium-inducing viruses, suggesting that other characteristics of HIV-1 are important for its pathogenicity (62, 71). The role of coreceptor usage in disease progression in the CNS is unknown. Our studies showed that several viruses which use CXCR4 for virus entry in addition to CCR5 or CCR3 (i.e., SG3, 89.6, and ELI) can induce apoptosis in primary brain cultures. In contrast, viruses which use CCR5 and in some cases CCR3, but not CXCR4 (9, 27, 55), did not induce significant levels of apoptosis. ADA has been shown to induce direct killing of primary T cells (22, 69). Interestingly, we and others (72) found that ADA induced significant levels of apoptosis in primary brain cultures. An Env clone of ADA uses CCR5 and CCR3 but not CXCR4 (8, 9, 52). We confirmed the absence of CXCR4 usage in the uncloned ADA virus stock used for our studies by MAGI assays, syncytium assays, and the inability to infect Jurkat and CEMx174 cells (44a). This finding together with the demonstration that DH123, NL4-3, and HXB2 did not induce significant levels of apoptosis suggests that CXCR4 usage is neither necessary nor sufficient to cause apoptosis in primary brain cultures. Taken together, these observations raise the possibility that the ability to use CXCR4, in addition to CCR5 or CCR3, is a factor that contributes to but is not necessary for HIV-1 pathogenicity in the CNS. However, the in vivo role of viral cytopathicity or apoptosis in HIV-1 dementia remains to be established. Viruses that use CXCR4 arise in the later stages of disease, which is the time when HIV-1 dementia occurs. However, HIV-1 dementia or encephalopathy also occurs in individuals who progress to AIDS in the absence of syncytium-inducing viruses (5), particularly in children (18). Disruption of the blood-brain barrier (49) may increase CNS entry of blood-derived viruses in individuals with advanced disease.
HIV-1 pathogenesis in the CNS is likely to be determined by complex interactions between virus and host factors. The expression of CXCR4 and other chemokine receptors on neurons and other cell types in the brain (19, 28, 34, 38, 64, 70) may contribute to mechanisms of CNS injury that are independent of direct viral infection, such as injury mediated by soluble forms of the HIV-1 Env protein (28, 72). CXCR4-mediated mechanisms of neuronal injury may not necessarily require virus replication. For example, gp120 binding to CXCR4 on the surface of macrophages or microglia (34, 64) could activate production of a neurotoxin. A low level of CD4-independent infection of astrocytes and possibly capillary endothelial cells occurs in the CNS (2, 26, 41). We and others (9, 14) found that CXCR4, CCR5, and CCR3 usage by the apoptosis-inducing SG3, 89.6, and ADA viruses requires CD4. However, whether these or other naturally occurring HIV-1 isolates in blood or brain can use certain coreceptors in the absence of CD4 (15, 17), as demonstrated for a neurovirulent strain of SIV (16), remains to be determined. Understanding the role of strain variability and coreceptor usage in HIV-1 neurotropism and neurovirulence may advance the development of new therapeutic strategies to inhibit HIV-1 replication in the CNS and prevent neurologic injury in AIDS patients.
ACKNOWLEDGMENTS
We acknowledge Bruce Yankner for discussions and primary brain cultures, Richard Wyatt and Joseph Sodroski for discussions and anti-gp120, Beatrice Hahn for discussions and pYU2, Riri Shibata and Malcolm Martin for pDH123, and the NIH AIDS Research and Reference Reagent Program for the ADA, JRFL, 89.6, and ELI isolates, plasmid pNL4-3, and cell line PM1.
This work was supported by NIH NS35734 and NS37277 and by gifts from the G. Harold and Leila Mathers Charitable Foundation and the Dana-Farber Friends 10. Core facilities were supported by the Center for AIDS Research (AI28691) and Center for Cancer Research (AO6514). A.O. was supported by the Swedish Medical Research Council and the Swedish Institute. J.H. was supported in part by NIH AIDS training grant AI07386. K.H. is a Howard Hughes Medical Institute predoctoral fellow. S. Gartner was supported by NS35736. D.G. is an Elizabeth Glaser Scientist supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Adie-Biassette H, Levy Y, Colombel M, Poron F, Natcher S, Keohane C, Gray F. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–227. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 2.Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner J P, Tawadros R, Pomerantz R. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Bieniasz P D, Fridell R A, Aramori I, Ferson S S G, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björndal Å, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brew B J, Evans L, Byrne C, Pemberton L, Hurren L. The relationship between AIDS dementia complex and the presence of macrophage tropic and non-syncytium inducing isolates of human immunodeficiency virus type 1 in the cerebrospinal fluid. J Neurovirol. 1996;2:152–157. doi: 10.3109/13550289609146877. [DOI] [PubMed] [Google Scholar]
- 6.Busciglio J, Yeh J, Yankner B A. β-Amyloid neurotoxicity in human cortical culture is not mediated by excitotoxins. J Neurochem. 1993;61:1565–1668. doi: 10.1111/j.1471-4159.1993.tb13658.x. [DOI] [PubMed] [Google Scholar]
- 7.Chang J, Jozwiak R, Wang B, Ng T, Ge Y C, Bolton W, Dwyer D E, Randle C, Osborn R, Cunningham A C, Saksena N D. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res Hum Retroviruses. 1998;14:25–30. doi: 10.1089/aid.1998.14.25. [DOI] [PubMed] [Google Scholar]
- 8.Cho M W, Lee M E, Carney M C, Berson J F, Doms R W, Martin M A. Identification of determinants on a dualtropic human immunodeficiency virus type 1 envelope glycoprotein that confer usage of CXCR4. J Virol. 1998;72:2509–2515. doi: 10.1128/jvi.72.3.2509-2515.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, MacKay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 13.Donaldson Y K, Bell J E, Holmes E C, Hughes E S, Brown H K, Simmonds P. In vivo distribution and cytopathology of variants of human immunodeficiency virus type 1 showing restricted sequence variability in the V3 loop. J Virol. 1994;68:5991–6005. doi: 10.1128/jvi.68.9.5991-6005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 15.Dumonceaux J, Nisole S, Chanel C, Quivet L, Amara A, Baleux F, Briant P, Hazan U. Spontaneous mutations in the env gene of the human immunodeficiency virus type 1 NDK isolate are associated with a CD4-independent entry phenotype. J Virol. 1998;72:512–519. doi: 10.1128/jvi.72.1.512-519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edinger A L, Mankowski J C, Doranz B J, Margulies B J, Lee B, Rucker J, Sharron M, Hoffman T L, Benson J F, Zink M C, Hirsch V M, Clements J E, Doms R W. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci USA. 1997;94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgibbon J E, Gaur S, Gavai M, Gregory P, Frenkel L D, John J F., Jr Effect of the HIV-1 syncytium-inducing phenotype on disease stage in vertically-infected children. J Med Virol. 1998;55:56–63. doi: 10.1002/(sici)1096-9071(199805)55:1<56::aid-jmv10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Gabuzda D, He J, Ohagen A, Vallat A V. Chemokine receptors in HIV-1 infection of the central nervous system. Semin Immunol. 1998;10:203–213. doi: 10.1006/smim.1998.0133. [DOI] [PubMed] [Google Scholar]
- 20.Gartner S, Popovic M. Macrophage tropism of HIV-1. AIDS Res Hum Retroviruses. 1990;6:1017–1021. doi: 10.1089/aid.1990.6.1017. [DOI] [PubMed] [Google Scholar]
- 21.Gelbard H A, James H J, Sharer L R, Perry S W, Saito Y, Kazee A M, Blumberg B M, Epstein L M. Apoptotic neurons in brains from pediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol Appl Neurobiol. 1995;21:208–217. doi: 10.1111/j.1365-2990.1995.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 22.Gendelman H E, Orenstein J M, Martin M A, Ferrua C, Mitra R, Phipps T, Wahl L A, Lane H C, Fauci A S, Burke D S, Skillman D, Meltzer M S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghorpade A, Xia M Q, Hyman B T, Persidsky Y, Nukuna A, Bock P, Che M, Limoges J, Gendelman H E, MacKay C R. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J Virol. 1998;72:3351–3361. doi: 10.1128/jvi.72.4.3351-3361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghorpade A, Nukuna A, Che M, Haggerty S, Persidsky Y, Carter E, Carhart L, Shafer L, Gendelman H E. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J Virol. 1998;72:3340–3350. doi: 10.1128/jvi.72.4.3340-3350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh S K, Fultz P N, Keddie E, Saag M S, Sharp P M, Hahn B H, Shaw G M. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology. 1993;194:858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- 26.Harouse J M, Kunsch C, Hartle H T, Laughlin M A, Hoxie J A, Wigdahl B, Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989;63:2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Buscigilo J, Yang X, Hofmann W, Newman W, MacKay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 28.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 29.Hughes E S, Bell J E, Simmonds P. Investigation of the dynamics of the spread of human immunodeficiency virus to brain and other tissues by evolutionary analysis of sequences from the p17gag and env genes. J Virol. 1997;71:1272–1280. doi: 10.1128/jvi.71.2.1272-1280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 31.Joag S V, Stephens E B, Galbreath D, Zhu W, Li Z, Foresman L, Zhao L-J, Pinson D M, Narayan O. Simian immunodeficiency virus SIVmac chimeric virus whose env gene was derived from SIV-encephalitic brain is macrophage-tropic but not neurovirulent. J Virol. 1995;69:1367–1369. doi: 10.1128/jvi.69.2.1367-1369.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korber B T M, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 34.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie J A, González-Scarano F. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Path. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, Hui H, Burgess C J, Price R W, Sharp P M, Hahn B H, Shaw G M. Complete nucleotide sequence, genome organization, and biological properties of human immunodeficiency virus type 1 in vivo: evidence for limited defectiveness and complementation. J Virol. 1992;66:6587–6600. doi: 10.1128/jvi.66.11.6587-6600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipton S A, Gendelman H E. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 38.Littman D R. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 39.Lu Y-Y, Koga Y, Tanaka K, Sasaki M, Kimura G, Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994;68:390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mankowski J L, Flaherty M T, Spelman J P, Hauer D A, Didier P J, Amedee A M, Murphey-Corb M, Kirstein L M, Munoz A, Clements J E, Zink M C. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol. 1997;71:6055–6060. doi: 10.1128/jvi.71.8.6055-6060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moses A L, Bloom F E, Pauza C D, Nelson J A. Human immunodeficiency virus infection of human brain capillary endothelial cells occurs via a CD4/galactosylceramide-independent mechanism. Proc Natl Acad Sci USA. 1993;90:10474–10478. doi: 10.1073/pnas.90.22.10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller W, Schroder H C, Ushjima H, Dapper J, Bormann J. gp120 of HIV-1 induces apoptosis in rat cortical cell cultures: prevention by memantine. Eur J Pharmacol. 1992;226:209–214. doi: 10.1016/0922-4106(92)90063-2. [DOI] [PubMed] [Google Scholar]
- 43.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside of the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 44a.Ohagen, A., and D. Gabuzda. Unpublished data.
- 45.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1 LAI, HIV-1 MAL, and HIV-1 ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 46.Petito C K, Roberts B. Evidence of apoptotic cell death in HIV encephalitis. Am J Pathol. 1995;146:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 47.Power C, McArthur J C, Johnson R T, Griffin D E, Glass J D, Perryman S, Chesebro B. Demented and nondemented patients with AIDS differ in brain-derived human immunodeficiency virus type 1 envelope sequences. J Virol. 1994;68:4643–4649. doi: 10.1128/jvi.68.7.4643-4649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power C, McArthur J C, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson R T, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power C, Kong P-A, Crawford T O, Wesselingh S, Glass J D, McArthur J C, Trapp B D. Cerebral white matter changes in acquired immunodeficiency syndrome dementia: alterations of the blood-brain barrier. Ann Neurol. 1993;34:339–350. doi: 10.1002/ana.410340307. [DOI] [PubMed] [Google Scholar]
- 50.Rho H M, Poiesz B, Ruscetti W, Gallo R C. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981;112:355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- 51.Ross, T. M., and B. R. Cullen. The ability of human immunodeficiency virus type 1 to utilize CCR-3 as a coreceptor is controlled by envelope V1/V2 sequences acting in conjunction with a CCR-5 tropic loop. Proc. Natl. Acad. Sci. USA 95:7682–7686. [DOI] [PMC free article] [PubMed]
- 52.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi B, De Girolami U, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Investig. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibata R, Hoggan M D, Broscius C, Englund G, Theodore T S, Buckler-White A, Arthur L O, Israel Z, Schultz A, Lane H C, Martin M A. Isolation and characterization of a syncytium-inducing macrophage/T-cell line-tropic human immunodeficiency virus type 1 isolate that readily infects chimpanzee cells in vitro and in vivo. J Virol. 1995;69:4453–4462. doi: 10.1128/jvi.69.7.4453-4462.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shieh J T C, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simmons G, Reeves J D, McKnight A, Dejucq N, Hibbitts S, Power C A, Aarons E, Schols D, De Clercq E, Proudoot A E I, Clapham P R. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J Virol. 1998;72:8453–8457. doi: 10.1128/jvi.72.10.8453-8457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simmonds P. Neurotropism of HIV type 1? AIDS Res Hum Retroviruses. 1996;12:469–470. [Google Scholar]
- 59.Smyth R J, Yi Y, Singh A, Collman R G. Determinants of entry cofactor utilization and tropism in a dualtropic human immunodeficiency virus type 1 primary isolate. J Virol. 1998;72:4478–4484. doi: 10.1128/jvi.72.5.4478-4484.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strizki J M, Albright A V, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7654–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi K, Wesselingh S L, Griffin D E, McArthur J E, Johnson R T, Glass J D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 62.Tersmette M, Gruters B A, DeWolf F, DeGoede R E Y, Lang J M A, Schellekens P T A, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequence HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 64.Vallat A V, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 coreceptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 65.van’t Wout A B, Ran L J, Kuiken C L, Kootstra N A, Pals S T, Schuitemaker H. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J Virol. 1998;72:488–496. doi: 10.1128/jvi.72.1.488-496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westervelt P, Gendelman H E, Ratner C. Identification of a determinant within the human immunodeficiency virus surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci USA. 1991;88:3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong J K, Ignacio C C, Torriani F, Havlir D, Fitch N J S, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu L, Gerard N, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A, Desjardins E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 69.Yu X, McLane M F, Ratner L, O’Brien W, Collman R, Essex M, Lee T-H. Killing of primary CD4+ T cells by non-syncytium-inducing macrophage-tropic human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1994;91:10237–10241. doi: 10.1073/pnas.91.21.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, He T, Talal A, Wang G, Frankel S S, Ho D D. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y-J, Fadeel B, Hodara V, Fenyo E M. Induction of apoptosis by primary HIV-1 isolates correlates with productive infection in peripheral blood mononuclear cells. AIDS. 1997;11:1219–1225. doi: 10.1097/00002030-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Zheng, J., M. R. Thylin, A. Ghorpade, H. Xiong, R. Cotter, D. Niemann, M. H. Che, Y.-C. Zeng, R. B. Shepard, J. M. Swartz, Y. Peridsky, and H. E. Gendelman. Linkages between intracellular CXCR4 signaling, neuronal apoptosis, and the neuropathogenic mechanisms for HIV-1-associated dementia. Submitted for publication. [DOI] [PubMed]