Abstract
In vivo targeting of therapeutic genes to specific tissues has become a major issue in gene therapy, in particular when recombinant adenovirus vectors are used. Restriction of the viral tropism to selected cell types requires the abrogation of the interaction between the viral fiber and its natural cellular receptors and the introduction of a new binding specificity into the virion. In this context, fiberless adenoviruses are attractive vectors, since they may be used as substrates for the insertion of a new ligand in other capsid proteins. In this study, we confirm by using cloned full-length adenovirus genomes with the fiber gene deleted that efficient virus particle formation can occur in the absence of fiber. As expected, the infectivity of such fiberless viruses was severely reduced, but it could be only partially restored when the viruses were produced in cells stably providing the fiber in trans. Although incorporation of penton base into the fiberless particles was normal and binding of the particles to the cellular integrins was functional, several pieces of experimental evidence suggest that later steps in the cell entry process are impaired in correlation with an incorrect maturation of several structural proteins of the fiberless particles. These observations support the hypothesis that the fiber protein may have additional biological functions besides its role in cell binding. Together with the fiber complementation cells, such fiberless vectors constitute unique tools to investigate the role of the fiber in virus assembly, maturation, and cell entry and to explore the possibility of deriving gene transfer vectors with novel target specificities.
Replication-defective human adenoviruses are attractive candidate vectors for the direct in vivo delivery of marker or therapeutic genes to a large variety of dividing or postmitotic cells, including cells from highly differentiated tissues such as liver, skeletal muscle, lung, brain, or heart (6, 13, 40). While their broad host range is considered one of the notable advantages of adenovirus vectors, the absence of cell specificity may also constitute a drawback in some particular circumstances. For example, in various current cancer gene therapy protocols, uncontrolled transduction, and hence expression, of genes encoding immunomodulatory molecules (e.g., interleukin-2 or -12) or cytotoxic products (e.g., thymidine kinase of herpes simplex virus type 1) may potentially be harmful to the patient (7, 22, 65). Moreover, the overall in vivo efficiency of gene delivery may be reduced by a significant dilution of the virus in the organism after the transduction of nontarget cells. The development of recombinant vectors with defined tropisms would therefore greatly improve the safety and efficiency of some current gene therapy protocols.
The specificity and efficiency of infection by human adenoviruses is determined by the biology of the interaction between the virus and its target cells. This interaction involves an initial high-affinity (Kd, 10−9 to 10−10 M) binding of the knob portion of the viral trimeric fiber protein (28, 36) to ubiquitous cell surface receptors (14, 45). Internalization of the virus particles is subsequently mediated through a specific interaction between the viral penton base and cell surface integrins (41, 59). Two distinct proteins belonging to the immunoglobulin superfamily were recently identified as the primary receptors for adenovirus serotype C fibers: the coxsackievirus-adenovirus receptor (5, 55) and the α2 domain of the major histocompatibility complex class I molecule (30). The ubiquitous distribution of these receptors is primarily responsible for the broad cell tropism of the human serotype C adenoviruses. Reciprocally, the absence or reduced expression of these receptors has been shown to correlate with the poor sensitivity of certain cell types (e.g., lymphocytes and smooth muscle cells) to adenovirus transduction (31, 60). This inability of human adenoviruses to efficiently transduce some cellular populations, together with their lack of specificity, has stimulated increasing efforts to redirect the adenovirus tropism from its natural receptors to specifically selected cell surface receptors.
Until now, most attempts to alter the adenovirus tropism were based on the use of bispecific molecules recognizing simultaneously a component of the virus particle (e.g., penton base or fiber) and the targeted cell surface molecule (16, 21, 57, 61, 63). Bispecific antibodies directed to the viral penton base and to either the cellular αv integrins (61) or the CD3 molecules (63) were shown to increase the efficiency of infection of the relatively adenovirus-resistant endothelial or smooth muscle cells or T lymphocytes. Such gene transfer complexes, however, are characterized by an extended tropism and not by a more restricted specificity (32). Abrogation of binding of the fiber to its natural receptors is therefore a prerequisite for any in vivo application. This abrogation was recently indirectly achieved by the use of bispecific antibodies that simultaneously block the knob-receptor interactions and target specific cell surface markers such as the folate receptor (16), the fibroblast growth factor-2 receptor (21) or the epidermal growth factor receptor (57). The major drawbacks of these strategies are the potential clearance of the complexes by Fc receptors and the risk of potential activation of the complement system. Moreover, the production and use of a second molecular component is required, making such approaches relatively complex and expensive to develop. Alternatively, strategies based on the use of viruses whose fiber proteins have been genetically engineered to acquire a novel binding specificity are more attractive, since no other targeting molecular component is required. Adenovirus type 5 (Ad5) vectors harboring the binding specificity of Ad3 and able to transduce cells normally resistant to Ad5 were thus generated by simply replacing the Ad5 knob sequences by their homologous Ad3 sequences (35, 51, 52). Similarly, nonviral ligands (polylysine and RGD peptides) were also successfully inserted in the fiber protein, generating viruses with extended tropism (60, 64). However, the interaction of these virus particles with their natural receptors was still retained.
The major obstacle to generating newly targeted adenoviruses that have simultaneously lost their original tropism is the trimeric nature of the fiber protein. A trimerized fiber protein is absolutely required for the incorporation of the fiber into the virus particle, for the binding to penton base (44), and for the interaction with the cellular receptors. While some specific locations in the fiber protein have been found to be accessible for modification, most mutations heavily disturb its three-dimensional structure. For example, addition of 24 amino acids (aa) containing the gastrin-releasing peptide at the C-terminal end of the fiber did not prevent fiber trimerization (43) and allowed the generation of viable viruses (our unpublished data). Similarly, addition at the same location of peptides of various lengths (17, 21, or 32 aa) was also shown to yield viable viruses (64). In contrast, insertions of peptides of 26 aa (64) or 27 aa (29, 64) were found to fully abrogate the trimerization of the fiber protein (29) and to prevent the generation of viable viruses (64). These data suggest that the sequence of the targeting peptide rather than its absolute length is critical for successful insertion at the fiber C terminus. Although the fiber HI loop was recently shown to be a potential second site for the insertion of foreign peptides (34), no mutation that completely abolishes the interaction of the fiber with its cellular receptors without altering its three-dimensional structure has been yet identified (references 29, 48, and 64 and our unpublished data). The generation of a truly redirected adenovirus vector would therefore require the prior identification of such a putative sequence responsible for binding to the cell and whose mutation does not disturb the structural properties of the fiber.
We describe here an alternative strategy in which the abrogation of the interaction between fiber and its receptors is obtained by the generation of adenovirus particles completely devoid of fiber proteins. The analysis of these fiberless viral species confirms that fiber is dispensable for particle formation but is absolutely necessary for the production of fully infectious and correctly assembled virions. The impact of these findings on the design of targeted vectors is discussed.
MATERIALS AND METHODS
Construction of fiber deletion viral genomes.
All cloning steps were performed by using standard molecular biology techniques (46). The large deletion of the fiber-coding sequences (from nucleotide [nt] 31042 to 32787 [throughout this paper, Ad5 nucleotide numbering is according to reference 10]) was obtained by ligation of two PCR amplification products. The first PCR product extends from nt 30564 to 31041 and was generated by using the OTG7171 (5′-atggttaacttgcaccagtgc-3′) and OTG7155 (5′-gggaagcttctgcaacaacatgaagat-3′) oligonucleotide primers. The second PCR product extends from nt 32788 to 33099 and was generated by using the OTG7048 (5′-gggaagcttagaatcgtttgtgttatgttt-3′) and OTG7049 (5′-ctgcccggggagtttattaat-3′) primers. The two products were then restricted with HindIII (sites are underlined) and ligated. The small deletion in the fiber gene (from nt 31042 to 31128) was similarly generated by ligation of two PCR products: the first product extends from nt 30564 to 31041 and was produced by using the OTG7171 and OTG7275 (5′-gggctcgagctgcaacaacatgaagat-3′) primers, and the second fragment extends from nt 31129 to 33099 and was produced by using the OTG7276 (5′-ccgctcgagactcctccctttgtatcc-3′) and OTG7049 primers. The two products were ligated after digestion by XhoI (sites are underlined). Reconstitution of the fiber deletion Ad5 genomes was achieved by homologous recombination in the Escherichia coli BJ5183 recBC sbcBC strain (9): first, the wild-type fiber gene was replaced by the ligated PCR products by homologous recombination with pTG8533, a transfer plasmid bearing an Ad5 segment extending from nt 21562 to the right-end inverted terminal repeat (ITR); second, purified BstEII fragments (nt 24843 to 35233) containing either the small or the large fiber deletion were introduced into the Ad5 genome by homologous recombination with pTG3602, a plasmid containing the full-length Ad5 genome (9). The resulting recombinant plasmids were named pAd-Fb°min (pTG4607; small deletion) and pAd-Fb°max (pTG4608; large deletion). Plasmids pAd-LacZ/Fb°min (pTG4629) and pAd-LacZ/Fb°max (pTG4630) were derived from pAd-Fb°min and pAd-Fb°max, respectively, by replacing the E1 region with a β-galactosidase expression cassette; replacement was done by homologous recombination by cotransformation of E. coli BJ5183 with pAd-Fb°min or pAd-Fb°max linearized by ClaI (nt 918) and an BsrGI-PstI fragment bearing the β-galactosidase gene under the control of the major late promoter of Ad2 and the simian virus 40 (SV40) polyadenylation signal. The BsrGI-PstI insert was isolated from a transfer plasmid (pTG8526) containing 17% of the viral genomic DNA (nt 1 to 6241) in which the E1 region (nt 459 to 3328) was replaced with the β-galactosidase expression cassette.
Construction of an expression plasmid for the fiber gene.
The wild-type fiber gene was PCR amplified by using the OTG7208 (5′-gggctcgagccaccatgaagcgcgcaagaccgtc-3′) and OTG7209 (5′-gggctcgagcacaaacgattctttattct-3′) primers (the ATG initiation and TAA stop codons are in boldface). Sequences upstream of the start codon were modified to a more favorable translational environment (double underline) according to Kozak (33). Restriction of the amplified product by XhoI (sequence underlined) and cloning into the pTG5355 eucaryotic expression vector generated plasmid pCMV-Fb (pTG4604) with the fiber gene under the control of the human cytomegalovirus (CMV) immediate-early promoter (CMVpro) and the rabbit β-globin splicing and polyadenylation signals. The same plasmid also carries an expression cassette for the hygromycin B phosphotransferase gene.
Transient-trans-complementation experiments.
Five micrograms of the fiber-modified viral genomes (pAd-Fb°min, pAd-Fb°max, pAd-LacZ/Fb°min, and pAd-LacZ/Fb°max) was excised from the plasmid backbone by PacI digestion and transfected into 293 cells (25) in the presence or absence of various concentrations of the fiber-expressing plasmid (pCMV-Fb). The cells were then either overlaid 24 h later with a solution of Dulbecco modified Eagle medium (DMEM), 2% fetal calf serum (FCS), and 1% agar to allow plaque formation or recovered at 9 days posttransfection for further analysis. The presence of infectious virions in the cell extracts was determined by incubating 20 μl of the cell extracts with fresh 293 cells followed by Southern blot analysis of the viral DNA accumulated at 1, 2, 4, and 8 days postincubation, using a 32P-labelled oligonucleotide probe (OTG7436, 5′-gaatgtcagtttcctcct-3′ [nt 30983 to 31000]). Viral DNA was extracted by the Hirt method (20). Alternatively, 293 cells were examined at 24 h postinfection for β-galactosidase expression by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining (47).
Generation of 293 fiber complementation cell lines.
293 cells (106) were transfected by the standard calcium phosphate precipitation method (26) with 10 μg of the pCMV-Fb expression plasmid. Stably transfected cells were selected by addition of hygromycin (Boehringer Mannheim, Meynan, France) at a concentration of 350 μg/ml. Resistant clones were isolated and expanded for further analysis. Expression of the fiber protein was monitored by immunofluorescence staining with a rabbit antiknob polyclonal antibody (kindly provided by R. Gerard [28]) diluted 1:100 in phosphate-buffered saline (PBS). The second antibody was a DTAF-conjugated-F(ab′)2 donkey anti-rabbit immunoglobulin G (Jackson Immuno Research Laboratories, Baltimore, Md.) diluted 1:50 in PBS. The fiber-expressing 293 clones were also tested for their ability to rescue fiberless viruses after infection with viruses produced on 293 cells transiently cotransfected with the pAd-Fb°min and pCMV-Fb plasmids.
Viral growth and titration.
Virus plaques were generated by transfection of 293 or 293-Fb cells with 10 μg of PacI-restricted plasmids containing the full-length adenovirus genomes, as previously described (9). Virus amplification, titration, and storage were as described previously (24). The virus particle concentration (particles per milliliter) was measured by optical density (1 U of optical density at 260 nm corresponds to 1.1 × 1012 particles/ml), infectious titers (infectious units [IU] per milliliter) were determined at 16 to 20 h postinfection of 293 cells by staining for β-galactosidase expression or for expression of the viral DNA binding protein (DBP) protein (37), and titration of the particle-forming units per milliliter was performed by plaque assay on permissive 293 or 293-Fb complementation cells (24). All adenoviruses are named according to the numbering of the parental plasmids (e.g., transfection of plasmid pAd-Fb°min generates virus Ad-Fb°min).
Western blot analysis.
Viral particles (1010) were diluted in 2× Laemmli buffer, incubated for 5 min at 95°C, and loaded onto a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel. Western blot analysis was done with antiknob or anti-penton base rabbit polyclonal antibodies (kindly provided by R. Gerard [28] and P. Boulanger, Montpellier, France, respectively). Bound antibodies were detected by using a horseradish peroxidase-conjugated donkey antirabbit antibody according to the instructions of the manufacturer (ECL detection kit; Amersham, Les Ullis, France).
Electron microscopy of infected cells.
293 and 293-Fb cells were infected with Ad-Fb°max or Ad-LacZ (54) at a multiplicity of infection (MOI) of 10 IU/cell in DMEM–2% FCS. Infected cells were fixed at various times postinfection with 2.5% glutaraldehyde in 0.1 M cacodylate buffer–2% sucrose, pH 7.2. The cells were then harvested and treated as previously described (50). Ultrathin sections were observed under a Philips CM120 Biotwin electron microscope at 120 kV.
Radiolabelling of adenovirus proteins.
293 or 293-Fb cells were infected with Ad-LacZ or Ad-LacZ/Fb° at an MOI of 2 IU/cell. At 20 h postinfection, the cell culture supernatant was removed and replaced for 1 h by a medium depleted of methionine and cysteine (Life Technologies, Cergy-Pontoise, France) and supplemented with 2% dialyzed FCS. The depleted medium was then replaced by a minimal volume of the same medium containing 0.2 mCi of [35S]methionine (Amersham) per ml. At 24, 30, and 36 h postinfection, the cells were harvested and viral progeny was recovered by three rounds of freezing and thawing of the infected cells. The extracts were then loaded on CsCl gradients (24). Gradients were fractionated, and the refractive index and the radioactivity of each fraction were measured. The labelled proteins were denatured and analyzed by SDS–10% polyacrylamide gel electrophoresis (SDS–10% PAGE).
Kinetics of viral mRNA expression.
293 or 293-Fb cells were infected with Ad-LacZ/Fb°min, Ad-LacZ/Fb°max, or Ad-LacZ at an MOI of 10 IU/cell. At various times postinfection, cells were recovered, and total RNA was extracted and analyzed by Northern blotting by standard procedures (46). Hybridizations were performed with specific 32P-labelled oligonucleotide probes for each gene (E2, E4, IVa2, pIX, penton base, hexon, fiber, and protease genes and an open reading frame [ORF] encoding a 10.7-kDa product [10.7K ORF]).
Production and purification of the Ad5 knob in E. coli.
A DNA fragment encoding the knob and the last repeat of the shaft was isolated by PCR from AdTG3602 (9) with the OTG7740 (5′-cggccatgggtgccattacagtaggaaac-3′) and OTG7741 (5′-gggaagcttattcttgggcaatgtatga-3′) primers and cloned into the pARA13 expression plasmid (8) by using the NcoI and HindIII restriction sites (restriction sites are underlined; ATG initiation and TAA stop codons are in boldface). The knob-pARA13 plasmid was transformed into E. coli MC1061 [araD139 Δ(ara-leu)7696 lacX74 galV galK hsr-hsm rpsL] (23), and expression of the knob peptide was induced by growth of the transformed bacterial clone in Luria-Bertani medium supplemented with 0.2% arabinose (Sigma, Saint-Quentin Fallavier, France) for 4 h (49). Purification of soluble knob peptides was performed essentially as previously described (28).
Competition experiments.
293 cell monolayers were incubated for 1 h at 4°C with either PBS, purified Ad5 knob (10 μg/ml in DMEM–2% FCS), anti-αvβ5 antibodies (P1F6 1/100; Life Technologies), or RGD peptides (4 mg/ml, or the control RGE peptide; Life Technologies). Ad-LacZ or Ad-LacZ/Fb° was then added and left for 24 h at 37°C, and cells were then fixed and stained for β-galactosidase expression. In parallel, the β-galactosidase activity of solubilized cell extracts was measured in a luminometer by using a chemiluminescence reporter assay (Clontech, Palo Alto, Calif.).
Conjugation with Polybrene.
Conjugation of Ad-LacZ/Fb° or Ad-LacZ with Polybrene was as previously described (3). The viruses were then added to 293 cells (25) or CHO cells (ATCC CCL-61) at various MOIs. At 24 to 48 h postinfection, cells were fixed and stained for β-galactosidase expression.
RESULTS
Generation of fiberless adenoviruses.
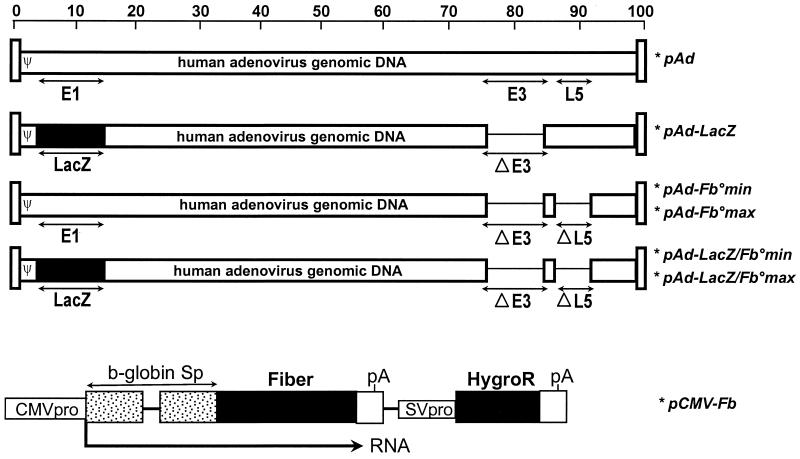
All plasmids containing the modified full-length Ad5 genomes (Fig. 1) were constructed by homologous recombination in E. coli (9). Plasmids pAd-LacZ/Fb°min and pAd-LacZ/Fb°max contain the bacterial β-galactosidase gene substituted for the E1 region, while pAd-Fb°min and pAd-Fb°max contain an intact E1 region. Deletions in the E1 and E3 regions extended from nt 459 to 3328 and from nt 28592 to 30470, respectively. Fiber-coding sequences were fully deleted in plasmids pAd-Fb°max and pAd-LacZ/Fb°max (from nt 31042 to 32787), while only 86 nt (from nt 31042 to 31128) was removed in plasmids pAd-Fb°min and pAd-LacZ/Fb°min, in order to preserve the putative 10.7K ORF in the antisense orientation from nt 31628 to 31158 (1).
FIG. 1.
Structures of the adenovirus vectors and fiber expression plasmid. All indicated adenovirus vectors are derived from full-length adenovirus genomes cloned and manipulated in E. coli as bacterial plasmids (9). pAd contains the wild-type Ad5 genome, while all other vectors have been modified as described in Materials and Methods. The pCMV-Fb plasmid is an expression vector for the Ad5 fiber in which the fiber gene is under the control of the human CMV promoter and rabbit β-globin splicing signals. The polyadenylation signal (pA) is from SV40. This plasmid also contains the hygromycin resistance gene (HygroR) under the control of the SV40 promoter (SVpro) and polydenylation sequences.
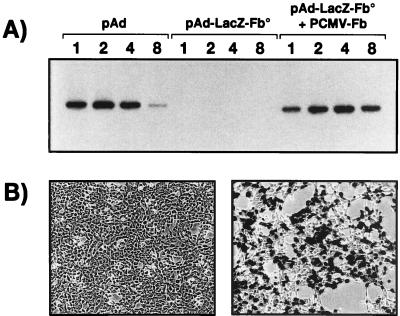
The biological impact of the fiber deletions was first investigated by transient trans-complementation experiments. Transfection of the fiber-modified viral genomes into 293 cells did not allow the development of any viral plaques. In contrast, cotransfection of the fiber deletion genomes together with pCMV-Fb, an expression plasmid carrying the fiber gene (nt 31042 to 32787) under the control of the CMV early promoter (Fig. 1), led to the development of numerous virus plaques at 13 days posttransfection (Table 1). Such viral plaques are not the result of contamination by a virus that had recovered the fiber sequences by homologous recombination in the transfected cells, since all DNA homologies between the fiber deletion virus genomes and the fiber sequences in the pCMV-Fb plasmid were eliminated. The absence of growth of fiberless viruses and the ability of the fiber protein to rescue their growth in trans were further demonstrated by analysis of viral DNA accumulation and transgene expression (Fig. 2). No viral DNA (Fig. 2A) or LacZ expression (Fig. 2B) was found in cells treated with supernatant recovered from cells transfected with pAd-LacZ/Fb° alone. In contrast, viral DNA and transgene expression were easily detectable when supernatant recovered from cells transfected either with pAd (positive control) or with both the pAd-LacZ/Fb°max and pCMV-Fb plasmids was used. No clear differences in DNA replication and virus generation were observed between the adenoviruses with the entire fiber sequence deleted or with the putative 10.7K ORF preserved (data not shown).
TABLE 1.
trans complementation of the fiber deletion Ad5 genomesa
| Ad5 genome | Fiber expression cassette | Molar ratio (Ad5/fiber) | No. of plaques/ 6-cm-diameter dishb |
|---|---|---|---|
| pAd-LacZ/Fb°min | Absent | 0, 0 | |
| pAd-LacZ/Fb°max | Absent | 0, 0 | |
| pAd-LacZ/Fb°min | Present | 1/10 | Lysed, lysed |
| pAd-LacZ/Fb°max | Present | 1/10 | Lysed, lysed |
| pAd-LacZ/Fb°min | Present | 1/1 | Lysed, lysed |
| pAd-LacZ/Fb°max | Present | 1/1 | Lysed, lysed |
| pAd-LacZ/Fb°min | Present | 10/1 | 4, 4 |
| pAd-LacZ/Fb°max | Present | 10/1 | 3, 13 |
293 cells were transfected with 5 μg of the fiber deletion Ad5 genomes in the presence or absence of the fiber expression cassette pCMV-Fb. Cells were examined for plaque formation at 13 days posttransfection.
Results from two independent experiments are shown.
FIG. 2.
Infectivity of the fiberless viruses generated by transient trans complementation. 293 cells transfected with pAd, pAd-LacZ/Fb°max, or pAd-LacZ/Fb°max together with pCMV-Fb were recovered 9 days later and lysed by repeated freeze-thawing. Twenty microliters of cell extract was used to infect fresh 293 cells, which were analyzed at 1, 2, 4, and 8 days postinfection for viral DNA replication (A) or for β-galactosidase expression (B). (B) Left panel, 293 cells treated with extracts purified from 293 cells transfected with pAd-LacZ/Fb°max alone; right panel, 293 cells treated with extracts isolated from 293 cells cotransfected with pAd-LacZ/Fb°max and pCMV-Fb.
Production of fiberless adenoviruses in 293 fiber complementation cells.
The demonstration of an efficient transient trans-complementation of the fiberless viruses prompted us to generate complementation cell lines constitutively expressing the fiber gene. 293 cells were transfected with pCMV-Fb, and 85 cell clones were selected for resistance to hygromycin. Among them, 26 were shown to express the Ad5 fiber (data not shown) and to complement the fiber deletion Ad5 genomes for DNA replication and plaque formation (data not shown). These data are consistent with a recent observation that fiber complementation cells that support the growth of ts fiber mutant adenovirus can be derived from 293 cells (56). One of these cell clones (clone 38) was selected for further analysis based on its higher expression of the fiber protein (similar to the expression level found in cells infected with wild-type Ad5 at an MOI of 1 [data not shown]).
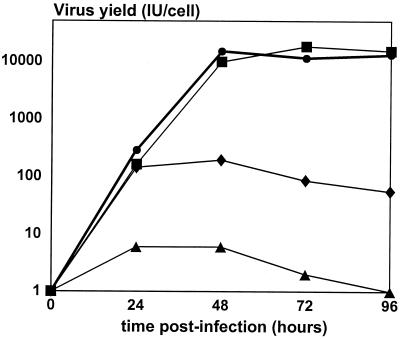
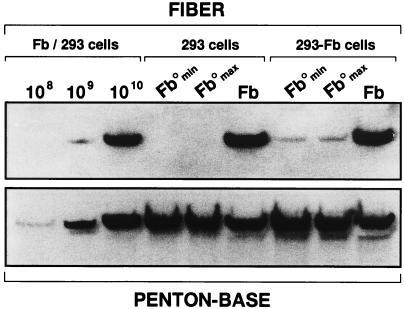
Transfection of the pAd-LacZ/Fb°max and pAd-LacZ/Fb°min plasmids into this 293-Fb clone allowed the production of large stocks of Ad-LacZ/Fb° containing the recombinant fiber-deletion Ad5 genome (Table 2). DNA restriction and PCR analysis confirmed the absence of fiber DNA in such virus preparations, excluding any possible contamination by viruses with wild-type fiber sequences (data not shown). Analysis of Ad-LacZ/Fb° (large and small deletions) and Ad-LacZ virions revealed that the total number of Ad-LacZ/Fb° particles produced by the fiber complementation cell line was roughly identical to the number of Ad-LacZ particles generated on 293 cells. Interestingly, elevated amounts of fiberless virus particles were also obtained, suggesting that virus assembly occurs efficiently in the absence of fiber (Table 2). However, the total number of infectious Ad-LacZ/Fb° virions produced by 293-Fb and 293 cells was reduced by 100- and 10,000-fold, respectively, compared to that for Ad-LacZ (Table 2). In agreement with these observations, a comparison of the growth kinetics of Ad-LacZ and Ad-LacZ/Fb° in 293 and 293-Fb cells showed that the production of Ad-LacZ/Fb° infectious virions by 293-Fb and 293 cells was 100 times and 5,000 times lower, respectively, than the production of infectious Ad-LacZ (Fig. 3). A protein analysis of purified Ad-LacZ and Ad-LacZ/Fb° virions grown on 293 and 293-Fb cells confirmed that similar amounts of virus particles were produced: 1010 purified Ad-LacZ and Ad-LacZ/Fb° particles contain identical quantities of penton base proteins (Fig. 4, lower panel). As expected, no fiber signal could be detected for the Ad-LacZ/Fb° produced in 293 cells (Fig. 4, upper panel), confirming the absence of fiber in these virus particles. In contrast, a substoichiometric amount of fiber protein was found in Ad-LacZ/Fb° particles grown on 293-Fb cells compared to that of Ad-LacZ virions (Fig. 4). This observation suggests that complementation of the fiber deletion adenovirus genome in the fiber complementation cell lines is suboptimal, leading to the production of normal amounts of total adenovirus particles, among which only a small proportion (1%) is fully infectious (Table 2). These data also indicate that adenovirus particle assembly is efficient even in the absence of fiber proteins, but only 1 in 105 particles is infectious (Table 2), allowing a weak but reproducible propagation of fiberless particles in 293 cells (Fig. 3).
TABLE 2.
Physical characteristics of the viruses lacking E1 (Ad-LacZ) or E1 and fiber produced on 293 and 293-Fb cellsa
| Cells | Virus | Particles/ml | IU/ml | IU/particle |
|---|---|---|---|---|
| 293 | Ad-LacZ | 1.1 × 1012 | 1 × 1011 | 1/10 |
| Ad-LacZ/Fb°max | 4.4 × 1011 | 4.3 × 106 | 1/105 | |
| Ad-LacZ/Fb°min | 8.8 × 1011 | 6.65 × 106 | 1/105 | |
| 293-Fb | Ad-LacZ/Fb°max | 5.4 × 1011 | 4.1 × 108 | 1/103 |
| Ad-LacZ/Fb°min | 8.8 × 1011 | 7.4 × 108 | 1/103 |
Human 293 or 293-Fb cells (3 × 108) were infected with the indicated viruses at 3 IU/cell. At 40 to 72 h postinfection, the cells were recovered and the viruses were extracted and purified. The results shown are representative of three independent productions.
FIG. 3.
Growth characteristics of viruses lacking E1 or E1 and fiber on 293 and 293-Fb cells. 293 cells and 293-Fb cells were infected at an MOI of 1 IU/cell with Ad-LacZ or Ad-LacZ/Fb°max. Infected cells and supernatant were harvested at 24, 48, 72, and 96 h postinfection and were treated by three freeze-thawing cycles to release virus particles. Titers of released viruses on 293 cells were determined by β-galactosidase staining. Each data point represents the average for duplicate infected cultures. ■, 293 cells infected with Ad-LacZ; •, 293-Fb cells infected with Ad-LacZ; ⧫, 293-Fb cells infected with Ad-LacZ/Fb°max; ▴, 293 cells infected with Ad-LacZ/Fb°max.
FIG. 4.
Protein analysis of purified virus particles. Ad-LacZ/Fb°min, Ad-LacZ/Fb°max, and Ad-LacZ (Fb) produced on 293 or 293-Fb cells were purified on cesium chloride gradients (density of 1.34 g/ml). Purified particles (1010) were subjected to SDS–12% PAGE and transferred to nitrocellulose. Ad-LacZ particles (108, 109, and 1010) were used as positive controls. Filters were hybridized with either an anti-penton base serum (lower panel) or an antifiber antibody (upper panel) and were then treated with a secondary horseradish peroxidase-conjugated donkey antirabbit antibody.
Assembly of adenovirus particles in the absence of fiber.
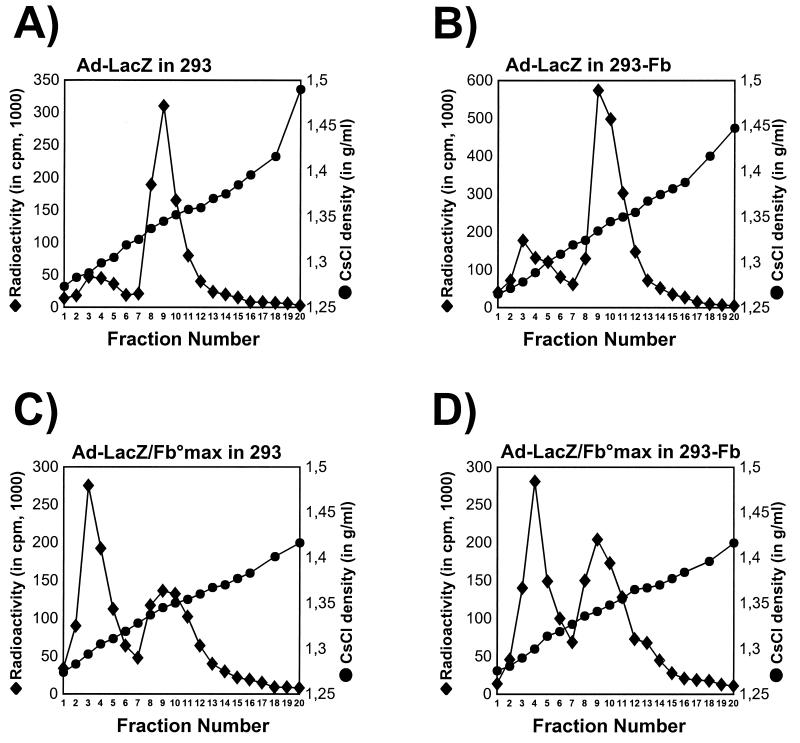
In order to determine more precisely the role of the fiber, and the impact of its absence, in the physical assembly of adenovirus particles, Ad-LacZ/Fb° and Ad-LacZ labelled with [35S]methionine were produced on both 293 and 293-Fb cells and analyzed for their density in isopycnic cesium chloride gradients (Fig. 5). All four virus preparations were characterized by the presence of two different types of virus populations: a population of immature empty particles with a density of 1.30 g/ml (15) and a population of more mature virions with the expected density of 1.34 g/ml (Fig. 5). However, the proportion of immature to mature particles was strikingly greater for the fiberless viruses (Fig. 5C and D). Interestingly, the growth of the fiberless viruses on the fiber complementation cell line seemed to increase the proportion of mature particles (Fig. 5D), in correlation with the 100-fold-increased virus infectivity determined for this virus preparation (Table 2). These results indicate that virus assembly occurs in the absence of fiber, but this process is probably disturbed, leading to a significant increase in the proportion of immature virions.
FIG. 5.
Densities of purified Ad-LacZ and Ad-LacZ/Fb° viruses. [35S]methionine-labelled Ad-LacZ/Fb°max and Ad-LacZ were extracted from 293 and 293-Fb cells 36 h after infection (MOI of 2 IU/cell) and loaded on isopycnic cesium chloride gradients. Gradients were fractionated from the top, and the individual fractions were analyzed for radioactivity and CsCl density. (A) Ad-LacZ produced on 293 cells; (B) Ad-LacZ produced on 293-Fb cells; (C) Ad-LacZ/Fb°max produced on 293 cells; (D) Ad-LacZ/Fb°max virus produced on 293-Fb cells.
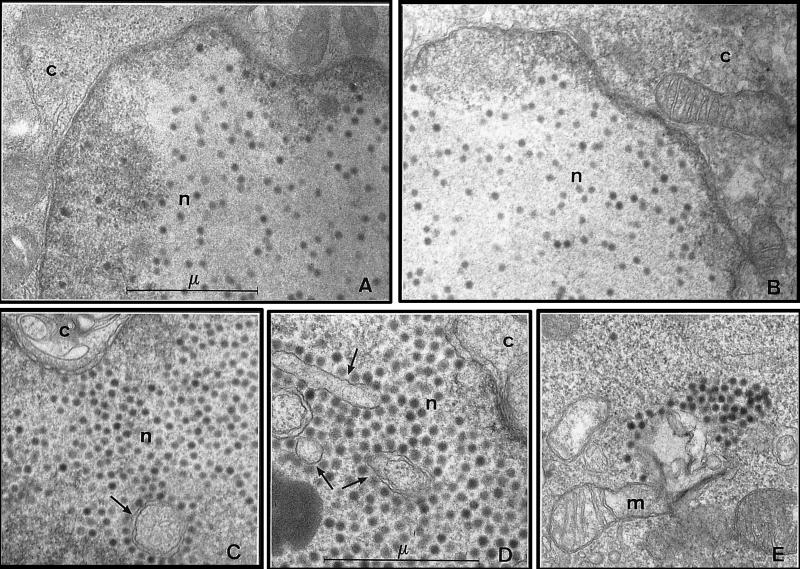
The existence of efficient particle formation in the absence of fiber was confirmed by electron microscopy analysis of 293 and 293-Fb cells infected at an MOI of 10 IU/cell with Ad5, Ad-LacZ, or Ad-LacZ/Fb° previously produced on 293 and 293-Fb cells, respectively (Fig. 6). Similar to cells infected with Ad5 or Ad-LacZ, all cells infected with fiberless viruses were found to contain numerous virus particles within the nuclear compartment at 29 h postinfection. Interestingly, these fiberless particles were often associated with nuclear membrane structures that were not observed in cells infected with viruses expressing the wild-type fiber protein (Fig. 6C and D). Moreover, an abundant cytoplasmic accumulation of virus particles was also observed in cells infected with fiberless viruses (Fig. 6E), while this phenomenon was rare in cells infected with Ad5 or Ad-LacZ (Fig. 6A and B and data not shown). Finally, close examination by electron microscopy of 293 or 293-Fb cells infected with Ad-LacZ or Ad-LacZ/Fb° and harvested at various time points revealed an acceleration of the viral replication cycle for the fiberless viruses (data not shown). Together, these studies indicate that the fiber protein is dispensable for particle formation but may play a direct or indirect role in the control of the viral replication cycle and/or in the viral particle maturation process.
FIG. 6.
Electron microscopy analysis of 293 cells infected with fiberless viruses. 293 and 293-Fb cells were infected at an MOI of 10 IU/cell with Ad5, Ad-LacZ, and Ad-LacZ/Fb°max produced on 293-Fb cells. Infected cells were fixed with glutaraldehyde at 29 h postinfection, and ultrathin sections were stained with uranyl acetate. (A) Nuclear accumulation of Ad-LacZ particles in 293 cells. Magnification, ×20,000. (B) nuclear accumulation of Ad5 particles in 293-Fb cells. Magnification, ×20,000. (C to E) Infection of 293 (C) or 293-Fb (D and E) cells with Ad-LacZ/Fb°max leads to an important nuclear (C and D) and cytoplasmic (E) accumulation of fiberless virus particles. Such particles are often associated with unusual nuclear membrane structures (arrows) (C and D). Magnifications, ×20,000 (C and E) and ×26,000 (D). c, cytoplasm; n, nucleus; m, mitochondria; μ, micrometer.
Maturation of the fiberless adenovirus particles.
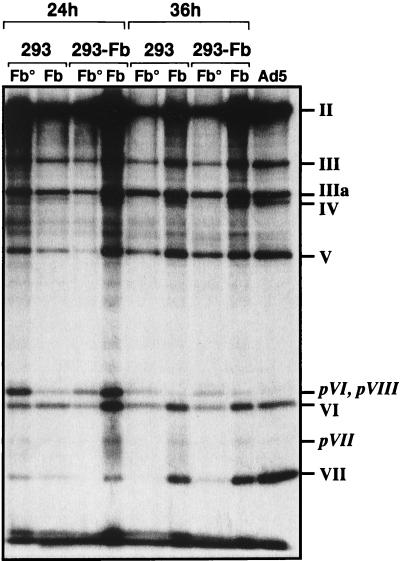
The state of maturation and protein content of fiberless, fiber-complemented (fiber deletion vectors produced on 293-Fb cells), and wild-type adenovirus particles were compared by SDS-PAGE analysis of [35S]methionine-labelled virus preparations (Fig. 7). These virus preparations were isolated by isopycnic cesium chloride gradients in order to purify and analyze the infectious virus population with a density of 1.34 g/ml and eliminate the empty particles with a density of 1.30 g/ml (Fig. 5). As expected, no fiber protein was detectable in the purified fiberless particles, while fiber was correctly incorporated in Ad5 and Ad-LacZ virions. Purified Ad-LacZ/Fb° particles grown on the 293-Fb cells were also mostly devoid of detectable fiber. These results are in agreement with a more accurate Western blot analysis of purified virus particles, showing an absence and a substoichiometric amount of fiber in fiberless viruses grown on 293 and 293-Fb cells, respectively (Fig. 4). The SDS-PAGE analysis of the [35S]methionine-labelled viruses also confirmed that the maturation of the virus particles is slightly altered in the absence of fiber. No particular differences between Ad-LacZ/Fb° and Ad-LacZ were observed at 24 h postinfection. Both types of particles are still relatively immature and contain large amounts of pVI, pVII, and pVIII precursor proteins. However, Ad-LacZ particles progress into more mature forms at 36 h postinfection, with a complete processing of pVI, pVII, and pVIII proteins, while Ad-LacZ/Fb° particles remain immature. The maturation of the fiberless particles is not improved when these viruses are grown on 293-Fb cells, indicating that incorporation of small amounts of fiber is not sufficient to allow correct capsid processing (Fig. 7). This incomplete maturation of fiberless virions suggests that the biological activity of the L3-23K protein, the adenovirus-encoded protease responsible for the processing of the viral precursors (11, 58), is reduced in the absence of fiber.
FIG. 7.
Protein composition and maturation of Ad-LacZ and Ad-LacZ/Fb°max. Ad-LacZ and Ad-LacZ/Fb°max were labelled with [35S]methionine from 20 to 24 or from 30 to 36 h after infection of 293 or 293-Fb cells. The labelled viruses were then purified on cesium chloride gradients (density of 1.34 g/ml) and analyzed by SDS–10% PAGE for protein contents and state of maturation of the protein precursors. II, hexon; III, penton base; IIIa, peripentonal protein; IV, fiber; V, major core protein; VI, hexon-associated protein; VII, minor core protein; pVI, pVII, and pVIII protein precursors for VI, VII, and VIII, respectively.
Kinetics of viral transcription in fiberless viruses.
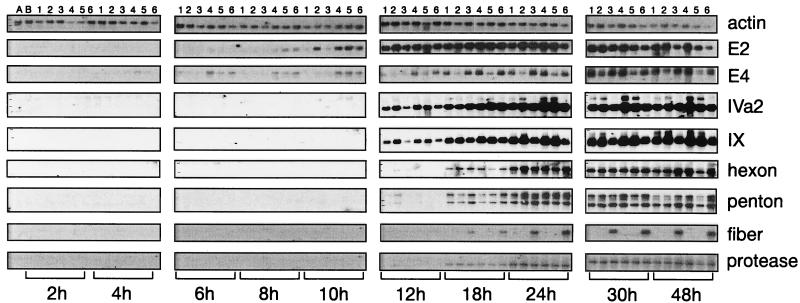
Electron microscopic examination of cells infected with Ad-LacZ/Fb° and Ad-LacZ at 12, 17, 23, and 29 h postinfection showed a clear accelerated assembly of viral particles for the fiberless vectors (data not shown). In order to gain insight into the molecular mechanisms responsible for this apparent accelerated viral particle formation but impaired maturation, a kinetic analysis of the viral mRNA expression was performed for the different early (E2 and E4), intermediate (IVa2 and IX), and late (hexon, penton base, fiber, and protease) adenovirus transcription units. 293 and 293-Fb cells were infected at an MOI of 10 IU/cell with Ad-LacZ/Fb°min, Ad-LacZ/Fb°max, or Ad-LacZ, and viral gene expression was analyzed from 2 to 48 h postinfection (Fig. 8). As expected, no fiber RNA was detectable in cells infected with the fiberless viruses, and the order of appearance of the early, intermediate, and late RNAs was normal. However, no significant differences between the viruses in the kinetics of appearance and levels of expression of the different transcription units were observed. These data also indicate that the impaired maturation of the fiberless virions is not a consequence of reduced expression of the 23K protease. The only anomaly observed in cells infected with the fiberless viruses was a stronger early expression of the intermediate IX and IVa2 transcripts, whose encoded proteins have been shown to regulate the activity of the virus major late promoter (38, 39). In addition, no differences were again found between the viruses carrying the small and large fiber deletions. The fact that no transcript was found when the same RNAs were analyzed for the expression of the putative 10.7K ORF indicates that this ORF is most probably not functional (data not shown).
FIG. 8.
Kinetics of viral mRNA expression in cells infected with fiberless viruses. 293 and 293-Fb cells were infected with Ad-LacZ, Ad-LacZ/Fb°min, or Ad-LacZ/Fb°max at an MOI of 2 IU/cell, and total RNA was extracted at the indicated times for analysis by Northern blotting. 32P-labelled oligonucleotide probes specific for the human actin transcript (to quantitate the loaded RNAs) and for each tested viral transcript were used for hybridization. Early viral transcription units, E1 and E4; intermediate viral transcription units, IVa2 and IX; late viral transcription units, hexon, penton base, fiber, and protease. Lanes: A, mock-infected 293 cells; B, mock-infected 293-Fb cells; 1, 293 cells infected with Ad-LacZ/Fb°min; 2, 293 cells infected with Ad-LacZ/Fb°max; 3, 293 cells infected with Ad-LacZ; 4, 293-Fb cells infected with Ad-LacZ/Fb°min; 5, 293-Fb cells infected with Ad-LacZ/Fb°max; 6, 293-Fb cells infected with Ad-LacZ.
Mechanisms of cell entry of the fiberless viruses.
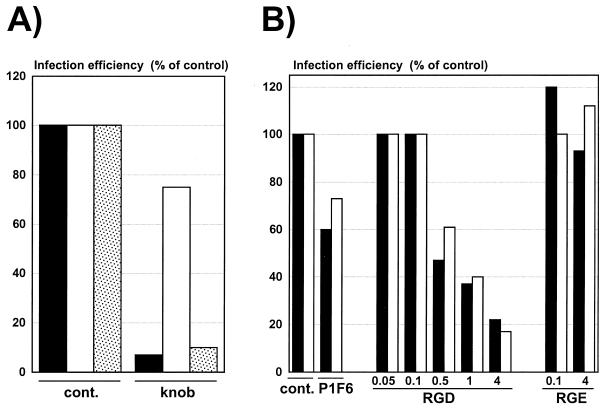
Although they are 10,000-fold less infectious than normal virions, fiberless viruses can still transduce human cells (Table 2). In order to characterize their mechanism of cell entry, 293 cells were infected with fiberless viruses, fiber-complemented viruses, or wild-type viruses in the presence or absence of molecules that specifically block the fiber-receptor or the penton base-integrin interactions (28, 31, 59) (Fig. 9). As expected, addition of soluble Ad5 knob at 10 μg/ml efficiently neutralized all fiber-bearing viruses but did not inhibit infection with fiberless virions (Fig. 9A). In contrast, infection with fiberless or fiber-bearing viruses was similarly inhibited by addition of the GRGDSP peptide, indicating that the cellular entry of the fiberless virions is mediated by the interaction of the penton base with the αvβ3 and αvβ5 integrins. The reduced viral sensitivity of cells treated with the anti-αvβ5 integrin P1F6 monoclonal antibody further supports this conclusion (Fig. 9B). Addition of a control GRGESP peptide that does not bind to the cellular integrins had no effect on infection of 293 cells (Fig. 9B). These data also suggest that the absence of fiber does not impair the ability of the penton base to bind to cellular integrins.
FIG. 9.
Mechanisms of infection of human cells by fiberless, fiber-complemented, and wild-type viruses. 293 cells were incubated for 1 h at 4°C with saturating concentrations of Ad5 knob to block the Ad5 cell receptor (A) or with the P1F6 monoclonal antibody to block the cellular αvβ5 integrins or increasing amounts of the RGD peptides (0.05 to 4 mg/ml) to block all αv integrins (B). The nonactive RGE peptide was used as a control. Ad-LacZ (black bars), Ad-LacZ/Fb°max purified on 293 cells (white bars), or Ad-LacZ/Fb°max purified on 293-Fb cells (stippled bars) was then added at the appropriate dilution. At 24 h postinfection, the β-galactosidase activity of the cells lysates was monitored as described in Materials and Methods. Infection efficiency is expressed as the percentage of the β-galactosidase activity in the absence of competitor (cont.).
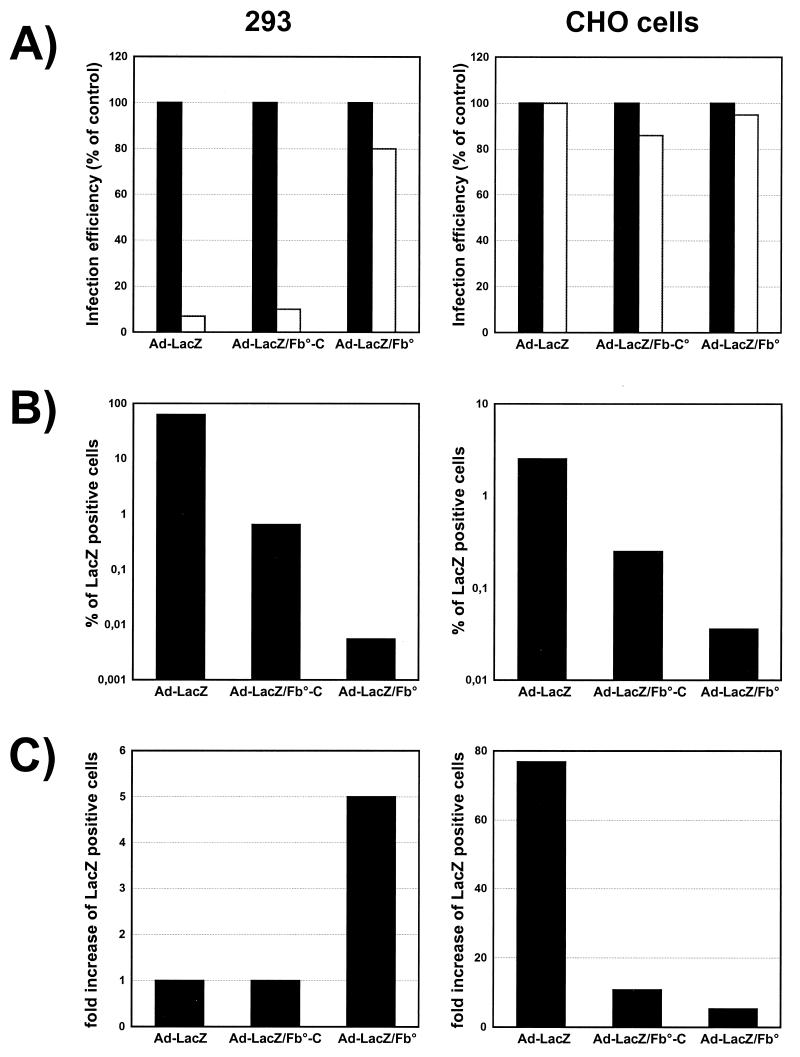
The reduced viral infectivity of the fiberless particles (Table 2) could be a direct consequence of the elimination of the high-affinity interactions between the fiber and its cellular receptors or, alternatively, could be due to deficient biological steps later in the viral entry process. To address this issue, we compared the abilities of fiberless viruses, fiber-complemented viruses, and wild-type viruses to transduce CHO cells lacking the receptors for the Ad5 fiber (5, 48, 51) (Fig. 10). In the absence of fiber receptors, fiberless and fiber-bearing viruses should use the same pathway to enter CHO cells, as supported by the observation that addition of Ad5 knob did not inhibit the infection of these cells by any of the tested viruses (Fig. 10A). However, these experiments also show that CHO cells were 100-fold less sensitive to fiberless viruses than to normal viruses (Fig. 10B), suggesting that the reduced infectivity of fiberless virions is not only the consequence of their poor efficiency of binding to the cells. In parallel, the observation that 293 cells were 10,000-fold less sensitive to fiberless viruses than to nonmodified viruses (Fig. 10B; Table 2) supports the hypothesis that the reduced infectivity of the fiberless viruses is a consequence of both the absence of fiber-receptor interactions and an alteration of a later step in the cell entry process. This hypothesis is further supported by the demonstration that infectivity of Ad-LacZ/Fb° towards CHO cells can be increased 5-fold by addition of Polybrene, a polycation known to improve the interactions of viruses with cell membranes (3), while infectivity of normal viruses was increased 80-fold (Fig. 10C). As expected, no increase of infectivity was observed for Ad-LacZ on 293 cells, which are known to express high levels of the adenovirus receptors, while a fivefold increase was again found for the fiberless viruses (Fig. 10C). Together, these data suggest that fiberless viruses are less infectious as a consequence of both an absence of efficient binding to the cells and an impaired cell entry pathway.
FIG. 10.
Mechanisms of infection of cells expressing or lacking the fiber receptors by fiberless, fiber-complemented, and wild-type viruses. (A) Neutralization of the fiber receptors. 293 and CHO cells (lacking the receptors for Ad5) were incubated for 30 min at 37°C with saturating concentrations of Ad5 knob; Ad-LacZ, Ad-LacZ/Fb°max purified on 293-Fb cells (Ad-Lac/Fb°-C), or Ad-LacZ/Fb°max was then added at the appropriate dilution. At 24 h (293 cells) and 48 h (CHO cells) postinfection, the cells were stained for β-galactosidase expression. The efficiency of infection was expressed as the percentage of β-galactosidase positive cells in absence of knob. Black bars, control without knob; white bars, presence of knob). (B) Viral sensitivity of 293 cells and CHO cells. 293 cells and CHO cells were incubated with a multiplicity of infection of 10 and 104 particles, respectively, of Ad-LacZ, Ad-Lac/Fb°-C, or Ad-LacZ/Fb°max purified on 293 cells. The percentage of infected cells was determined by X-Gal staining at 24 h (293 cells) and 48 h (CHO cells) postinfection. (C) Influence of Polybrene on virus infectivity. In order to optimize the attachment of the virus particles to the cell membranes, 293 and CHO cells were incubated with Ad-LacZ, Ad-LacZ/Fb°max-C, or Ad-LacZ/Fb°max in the presence or absence of Polybrene at 4 μg/ml. At 48 h postinfection, the cells were stained for β-galactosidase expression and β-galactosidase positive cells were counted to determine the influence of Polybrene on virus infectivity.
DISCUSSION
Adenoviruses are characterized by a number of features that make them attractive tools for in vivo gene therapy (32). In particular, they are able to efficiently infect most quiescent and dividing human cells. However, this broad host range also constitutes a disadvantage in some specific applications, such as the transfer of genes encoding immunomodulatory or toxic proteins into tumor cells (7, 22, 65). Specifically redirecting the natural tropism of adenovirus vectors to cancer tissues should therefore significantly reduce the toxicity and increase the efficiency of anticancer gene therapy treatments by concentrating the vectors in the tumor area. Moreover, redirected adenoviruses may also be useful in achieving improved transduction of particular cell types (e.g., hematopoietic, endothelial, or smooth muscle cells) that express low levels of the fiber receptors on their surfaces (31, 60). Therefore, the utility of adenovirus vectors for gene therapy can theoretically be improved by the development of viruses specifically altered in their natural cellular tropism.
Redirecting the adenovirus cellular specificity requires the abrogation of the natural interaction of the fiber with its receptors and provision of a novel binding specificity to the virus particles. While this can be indirectly achieved by conjugating the virus with bispecific molecules that simultaneously neutralize the viral knob and bind to a selected cell surface marker (16, 21, 57), all attempts to directly genetically manipulate the capsid proteins have failed (29, 34, 35, 43, 52, 60, 64); to date, no specific fiber sequences whose mutation would abolish the binding of the fiber to its cellular receptors without preventing its trimerization and/or incorporation in the virus particle have been identified. Consequently, all reported insertions of novel nonviral ligands in the adenovirus fiber led to the generation of vectors with an extended, and not a more restricted, tropism (32, 35, 52, 60, 64).
Since the most radical way to prevent the binding of the virus to its receptors consists of the complete elimination of the fiber from the viral particles, we attempted to generate fiberless virions that could then be used as substrates for the incorporation of an exogenous ligand at another location in the virus capsid. Successful insertions of exogenous DNA sequences into the penton base or the hexon genes were already reported (12, 62), and the possibility of generating fiberless virus particles was previously suggested by Falgout and Ketner (18). Those authors reported in 1988 that expression of the fiber gene was nonessential for virion assembly. However, their observation was weakened by the presence in the fiberless virus stocks of low levels of contamination (0.1 to 0.8%) of a helper virus with a wild-type fiber gene (17, 18). Using our previously described method for the manipulation of the cloned full-length Ad5 genome by homologous recombination in E. coli (9), we generated a viral genome with the whole fiber-coding sequence precisely deleted and analyzed the biological properties of the corresponding virus. Since a previous report suggested the existence in the antisense orientation of a putative 10.7K ORF overlapping the fiber gene (1), we also generated a viral genome in which the fiber gene was partially deleted but in which the 10.7K ORF was preserved.
We report here that introduction of a cloned Ad5 genome with the E1 and fiber genes deleted into 293 cells or 293 cells stably expressing the fiber in trans leads to the intracellular accumulation of large amounts of fiberless virus capsids. Such particles are unable to propagate in 293 cells, while propagation is restored in 293-Fb complementation cells. Given the fact that a cloned full-length virus genome was used to generate these particles, these results confirm without any ambiguity that the fiber protein is dispensable for virus particle formation but is necessary for efficient virus propagation. Analysis of the virus particles by isopycnic cesium chloride gradients and electron microscopy provided further evidence that formation of adenovirus capsids is efficient in the absence of fiber: accumulation of large quantities of fiberless virus particles of the expected density (1.34 g/ml) was found in the nuclei of 293 cells. However, these experiments also revealed some intriguing abnormalities. (i) While virus assembly was exclusively nuclear for the viruses with wild-type fiber sequences, a massive cytoplasmic accumulation of virus particles was observed for the fiberless capsids; moreover, these fiberless viruses were often associated with abnormal nuclear membrane structures. (ii) The proportion of immature particles with a density of 1.30 g/ml was greatly increased in the purified preparations of fiberless viruses compared to viruses with a wild-type fiber gene. (iii) An SDS-PAGE protein analysis of purified viruses with a density of 1.34 g/ml showed that the processing of the viral pVI, pVII, and pVIII precursor proteins was much less effective in the fiberless particles, leading to the accumulation of mostly immature viruses; this result is in agreement with an earlier observation by Falgout and Ketner, who reported that viruses lacking the fiber gene and segments of either E3 or E4 regions contain poorly processed precursors (18). (iv) Despite clearly impaired maturation of the fiberless particles, the adenovirus L3 transcription unit encoding the 23K protease responsible for the processing of the viral precursors (58) is correctly expressed. (v) A detailed analysis of fiberless virus particles produced on 293 cells shows that only 1 of 105 purified fiberless particles is infectious, compared to 1 of 10 for the vectors with wild-type fiber sequences; providing the fiber protein in trans partially restores the infectivity, since 1 of 103 purified fiberless particles is infectious when grown on the 293-Fb complementation cell line.
These results confirm that virus assembly is efficient in the absence of fiber but also indicate that fiber has additional functions besides its role in the stabilization of the viral capsid and binding to cellular receptors. These observations, together with the demonstration that the 23K protease is required for virus entry into host cells (11, 27), raised the question whether the 10,000-fold reduction in infectivity of the fiberless viruses is only a consequence of the inability of the virions to bind to the cells or whether other biological events involved in the cell entry process were also affected. To address this issue, the mechanisms of binding to and infection of 293 cells (expressing Ad5 receptors) and CHO cells (lacking Ad5 receptors) (5, 48, 51) were analyzed by using specific competitors for binding to the fiber receptors or to the cellular integrins. These studies showed that the residual infectivity of the fiberless viruses is mediated by the direct interaction of the penton base with the αvβ3 and αvβ5 cellular integrins, triggering virus entry into the cells. Given the lower affinity of the penton base-integrins interaction than of the fiber-receptor interaction (59), the reduced infectivity of the fiberless viruses is therefore, at least in part, the consequence of the absence of fiber. However, experiments using CHO cells demonstrated that an additional block at a later stage of infection also significantly reduces the infectivity of these fiberless viruses. Although CHO cells lack Ad5 receptors and should be equally sensitive to fiberless and wild-type viruses, fiberless viruses are still 100-fold less infectious than viruses with wild-type fiber sequences. Moreover, fiberless viruses grown on 293-Fb complementation cells (which have therefore incorporated substoichiometric amounts of fiber proteins) have a 10-fold-increased infectivity towards the CHO cells compared to fiberless viruses grown on 293 cells. Finally, the efficiency of infection of CHO cells by wild-type control viruses could be increased by 80-fold when the attachment of the virus particles to the cell membrane was improved by addition of Polybrene (3). In contrast, only 5- and 10-fold increases were observed for the fiberless viruses and the fiberless viruses grown on 293-Fb cells, respectively. This modest improvement in the efficiency of infection by the fiberless viruses supports the hypothesis that the reduced infectivity of these viruses is not only due to the absence of attachment to the cells but that later stages in the virus entry process are also impaired.
Together, these data demonstrate that fiber not only is essential for the high-affinity binding of the virions to their target cells but is also necessary for the correct maturation of the virus particles. The fact that the L3 transcription unit encoding the 23K protease is expressed at normal levels in cells infected with the fiberless viruses suggests that the biological activity, and not the expression of the protease, is altered in the absence of fiber. Further studies are in progress to investigate the relationship between the fiber, the protease, and the virus maturation process. Finally, these data also stress the fact that these fiberless viruses may become interesting candidates for the development of novel targeted gene transfer vectors if their infectivity can be restored to near-normal levels. Such fiberless viruses may indeed constitute targeted gene transfer vectors on their own, given their ability to specifically bind to the cellular αvβ3 or αvβ5 integrins. αv integrins are selectively expressed on the surfaces of some tumor cell types (2, 19) and on tumor blood vessels (42), and αvβ3 integrins have been shown to be preferentially expressed on vascular endothelial cells and smooth muscle cells at sites of atherosclerotic lesions (4, 53). These viruses are thus potential candidates for cancer and cardiovascular gene therapy applications. Alternatively, fiberless viruses with a restored infectivity would also constitute ideal substrates for the addition of selected novel nonviral ligands into other capsid proteins (e.g., hexon or penton base). A better understanding of the mechanisms responsible for the altered maturation of the fiberless virions is therefore necessary in order to correct the processing of the structural proteins and improve the viral infectivity.
ACKNOWLEDGMENTS
We are grateful to P. Boulanger and R. Gerard for the gifts of the anti-penton base and antiknob polyclonal antibodies, respectively. We thank F. Puvion-Dutilleul for expert advice and R. Rooke, L. Grave, M. Lusky, and M. Courtney for critical reading of the manuscript.
This work was supported in part by the Convention Industrielle pour la Formation par la Recherche CIFRE (grant 512/94), by the Association Française contre la Mucoviscidose (AFLM) and by the Association Française contre les Myopathies (AFM).
REFERENCES
- 1.Akusjärvi G, Wadell G. Genetic maps of human and animal adenoviruses. In: O’Brien S J, editor. Genetic maps 1987, a compilation of linkage and restriction maps of genetically studied organisms. Vol. 4. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1987. pp. 78–85. [Google Scholar]
- 2.Albelda S M, Mette S A, Elder D E, Stewart R, Damjanovich L, Herlyn M, Buck C A. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 3.Arcosoy S M, Latoche J D, Gondor M, Pitt B R, Pilewski J M. Polycations increase the efficiency of adenovirus-mediated gene transfer to epithelial and endothelial cells in vitro. Gene Ther. 1997;4:32–38. doi: 10.1038/sj.gt.3300349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assoian R, Marcantonio E. The extracellular matrix as a cell cycle control element in atherosclerosis and restenosis. J Clin Invest. 1997;100:S15–S18. [PubMed] [Google Scholar]
- 5.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Bramson J, Graham F, Gauldie J. The use of adenoviral vectors for gene therapy and gene transfer in vivo. Curr Opin Biotechnol. 1995;6:590–595. doi: 10.1016/0958-1669(95)80097-2. [DOI] [PubMed] [Google Scholar]
- 7.Bui L A, Butterfield L H, Kim J Y, Ribas A, Seu P, Lau R, Glaspy J A, McBride W H, Economou J S. In vivo therapy of hepatocellular carcinoma with a tumor-specific adenoviral vector expressing interleukin-2. Hum Gene Ther. 1997;8:2173–2182. doi: 10.1089/hum.1997.8.18-2173. [DOI] [PubMed] [Google Scholar]
- 8.Cagnon C, Valverde V, Masson J. A new family of sugar-inducible expression vectors for Escherichia coli. Prot Eng. 1991;4:843–847. doi: 10.1093/protein/4.7.843. [DOI] [PubMed] [Google Scholar]
- 9.Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996;70:4805–4810. doi: 10.1128/jvi.70.7.4805-4810.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chroboczek J, Bieber F, Jacrot B. The sequence of the genome of adenovirus type 5 and its comparison with the genome of adenovirus type 2. Virology. 1992;186:280–285. doi: 10.1016/0042-6822(92)90082-z. [DOI] [PubMed] [Google Scholar]
- 11.Cotten M, Weber J M. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213:494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- 12.Crompton J, Toogood C I, Wallis N, Hay R T. Expression of a foreign epitope on the surface of the adenovirus hexon. J Gen Virol. 1994;75:133–139. doi: 10.1099/0022-1317-75-1-133. [DOI] [PubMed] [Google Scholar]
- 13.Crystal R G. Transfer of genes to humans: early lessons and obstacles to success. Science. 1995;270:404–410. doi: 10.1126/science.270.5235.404. [DOI] [PubMed] [Google Scholar]
- 14.Defer C, Belin M-T, Caillet-Boudin M-L, Boulanger P. Human adenovirus-host cell interactions: comparative study with members of subgroups B and C. J Virol. 1990;64:3661–3673. doi: 10.1128/jvi.64.8.3661-3673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Halluin J-C. Virus assembly. In: Doerfler W, Böhm P, editors. The molecular repertoire of adenoviruses I—virion structure and infection. Berlin, Germany: Springer-Verlag; 1995. pp. 47–66. [Google Scholar]
- 16.Douglas J, Rogers B, Rosenfeld M, Michael S, Feng M, Curiel D. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 17.Falgout B, Ketner G. Adenovirus early region 4 is required for efficient virus particle assembly. J Virol. 1987;61:3759–3768. doi: 10.1128/jvi.61.12.3759-3768.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falgout B, Ketner G. Characterization of adenovirus particles made by deletion mutants lacking the fiber gene. J Virol. 1988;62:622–625. doi: 10.1128/jvi.62.2.622-625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladson C L, Cheresh D A. Glioblastoma expression of vitronectin and the αvβ3 integrin: adhesion mechanism for transformed glial cells. J Clin Invest. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluzman Y, van Doren K. Palindromic adenovirus type 5-simian virus 40 hybrid. J Virol. 1983;45:91–103. doi: 10.1128/jvi.45.1.91-103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman C K, Rogers B E, Douglas J T, Sosnowski B A, Ying W B, Siegal G P, Baird A, Campain J A, Curiel D T. Targeted gene delivery to Kaposi’s sarcoma cells via the fibroblast growth factor receptor. Cancer Res. 1997;57:1447–1451. [PubMed] [Google Scholar]
- 22.Goodman J, Trask T, Chen S-H, Woo S, Grossman R, Carey K, Hubbard G, Carrier D, Rajagopalan S, Aguilar-Cordova E, Shine H. Adenoviral-mediated thymidine kinase gene transfer into the primate brain followed by systemic ganciclovir: pathologic, radiologic, and molecular studies. Hum Gen Ther. 1996;7:1241–1250. doi: 10.1089/hum.1996.7.10-1241. [DOI] [PubMed] [Google Scholar]
- 23.Gough J, Murray N. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983;166:1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- 24.Graham F, Prevec L. Manipulation of adenovirus vectors. Methods Mol Biol. 1991;7:109–128. doi: 10.1385/0-89603-178-0:109. [DOI] [PubMed] [Google Scholar]
- 25.Graham F, Smiley J, Russell W, Nairn R. Characteristics of a human cell line transformed by DNA from human Ad5. J Gen Virol. 1977;75:133–139. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 26.Graham F, Van der Eb A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 27.Greber U F, Webster P, Weber J, Helenius A. The role of the adenovirus protease in virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 28.Henry L, Xia D, Wilke M, Deisenhofer J, Gerard R. Characterization of the knob domain of the adenovirus type 5 fiber protein expressed in Escherichia coli. J Virol. 1994;68:5239–5246. doi: 10.1128/jvi.68.8.5239-5246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong J, Engler J. Domains required for assembly of adenovirus type 2 fibers trimers. J Virol. 1996;70:7071–7078. doi: 10.1128/jvi.70.10.7071-7078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S S, Karayan L, Tournier J, Curiel D T, Boulanger P A. Adenovirus type 5 fiber knob binds to MHC class I α2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang S, Kamata T, Takada Y, Ruggeri Z, Nemerow G. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovesdi I, Brough D E, Bruder J T, Wickham T J. Adenoviral vectors for gene transfer. Curr Opin Biotechnol. 1997;8:583–589. doi: 10.1016/s0958-1669(97)80033-x. [DOI] [PubMed] [Google Scholar]
- 33.Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci USA. 1986;83:2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krasnykh V, Dmitriev I, Mekheeva G, Miller C R, Belousova N, Curiel D T. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krasnykh V, Mikheeva G, Douglas J, Curiel D. Generation of recombinant adenovirus vectors with modified fibers for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louis N, Fender P, Barge A, Kitts P, Chroboczek J. Cell binding domain of adenovirus serotype 2 fiber. J Virol. 1994;68:4104–4106. doi: 10.1128/jvi.68.6.4104-4106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lusky M, Christ M, Rittner K, Dieterle A, Dreyer D, Mourot B, Schutz H, Stoeckel F, Pavirani A, Mehtali M. In vitro and in vivo biology of recombinant adenovirus vectors with E1, E1/E2A, or E1/E4 deleted. J Virol. 1998;72:2022–2032. doi: 10.1128/jvi.72.3.2022-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutz P, Kedinger C. Properties of the adenovirus Iva2 gene product, an effector of late-phase-dependent activation of the major late promoter. J Virol. 1996;70:1396–1405. doi: 10.1128/jvi.70.3.1396-1405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz P, Rosa-Calatrava M, Kedinger C. The product of the adenovirus intermediate gene IX is a transcriptional activator. J Virol. 1997;71:5102–5109. doi: 10.1128/jvi.71.7.5102-5109.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcel T, Grausz J D. The TMC worldwide gene therapy enrollment report, end 1996. Hum Gen Ther. 1997;8:775–800. doi: 10.1089/hum.1997.8.6-775. [DOI] [PubMed] [Google Scholar]
- 41.Mathias P, Wickham T, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Max R, Gerritsen R R, Nooijen P T, Goodman S L, Sutter A, Keilholz U, Ruiler D J, De Waal R M. Immunohistochemical analysis of αvβ3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71:320–324. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Michael S, Hong J, Curiel D, Engler J. Addition of a short ligand peptide to the adenovirus fiber protein. Gene Ther. 1995;2:660–668. [PubMed] [Google Scholar]
- 44.Novelli A, Boulanger P. Deletion analysis of functional domains in baculovirus-expressed adenovirus type 2 fiber. Virology. 1991;185:365–376. doi: 10.1016/0042-6822(91)90784-9. [DOI] [PubMed] [Google Scholar]
- 45.Philipson L, Lonberg-Holm K, Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968;2:1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 47.Sanes J, Rubenstein J, Nicolas J. Use of a retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santis, G., V. Legrand, S. S. Hong, E. Davison, I. Kirby, J.-L. Imler, R. W. Finberg, J. M. Bergelson, M. Mehtali, and P. Boulanger. Molecular determinants and serotype specificity of adenovirus fiber binding to its high affinity receptors CAR. Submitted for publication. [DOI] [PubMed]
- 49.Slos P, Speck D, Accart N, Kolbe H, Schubnel D, Bouchon B, Bishoff R, Kieny M-P. Recombinant cholera toxin B in Escherichia coli: high level secretion, purification and characterization. Prot Expr Purif. 1994;5:518–526. doi: 10.1006/prep.1994.1071. [DOI] [PubMed] [Google Scholar]
- 50.Spehner D, Kirn A, Drillien R. Assembly of nucleocapsid-like structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J Virol. 1991;65:6296–6300. doi: 10.1128/jvi.65.11.6296-6300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson S, Rollence M, White B, Weaver L, McLelland A. Human adenovirus serotypes 3 and 5 bind to two different cellular receptors via the fiber head domain. J Virol. 1995;69:2850–2857. doi: 10.1128/jvi.69.5.2850-2857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevenson S C, Rollence M, Marshall-Neff J, McClelland A. Selective targeting of human cells by a chimeric adenovirus vector containing a modified fiber protein. J Virol. 1997;71:4782–4790. doi: 10.1128/jvi.71.6.4782-4790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stouffer G A, Hu Z, Sajid M, Li H, Jin G, Nakada M T, Hanson S R, Runge M S. β3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation. 1998;97:907–915. doi: 10.1161/01.cir.97.9.907. [DOI] [PubMed] [Google Scholar]
- 54.Stratford-Perricaudet L, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomko R P, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Von Seggern D J, Kehler J, Endo R I, Newerow G R. Complementation of a fiber adenovirus mutant by packaging cell lines expressing the adenovirus type 5 fiber protein. Virology. 1998;79:1461–1468. doi: 10.1099/0022-1317-79-6-1461. [DOI] [PubMed] [Google Scholar]
- 57.Watkins S J, Mesyanzhinov V V, Kurochkina L P, Hawkins R E. The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther. 1997;4:1004–1012. doi: 10.1038/sj.gt.3300511. [DOI] [PubMed] [Google Scholar]
- 58.Weber J M. Adenovirus endopeptidase and its role in virus infection. In: Doerfler W, Böhm P, editors. The molecular repertoire of adenoviruses I—virion structure and infection. Berlin, Germany: Springer-Verlag; 1995. pp. 227–235. [Google Scholar]
- 59.Wickham T, Mathias P, Cheresh D, Nemerow G. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 60.Wickham T, Roelvink P, Brough D, Kovesdi I. Adenovirus targeted to heparan-containing receptors increases its gene delivery efficiency to multiple cell types. Nat Biotechnol. 1996;14:1570–1573. doi: 10.1038/nbt1196-1570. [DOI] [PubMed] [Google Scholar]
- 61.Wickham T, Segal D, Roelvink P, Carrion M, Lizonova A, Lee G, Kovesdi I. Targeted adenovirus gene transfer to endothelial and smooth muscle cells by using bispecific antibodies. J Virol. 1996;70:6831–6838. doi: 10.1128/jvi.70.10.6831-6838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wickham T J, Carrion M E, Kovesdi I. Targeting of adenovirus penton base to new receptors through replacement of its RGD motif with other receptor-specific peptide motifs. Gene Ther. 1995;2:750–756. [PubMed] [Google Scholar]
- 63.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickham T J, Tzeng E, Shears II L L, Roelvink P W, Il Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yee D, McGuire S, Brünner N, Kovelsky T, Allred D, Chen S, Woo S. Adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase in an ascites model of human breast cancer. Hum Gene Ther. 1996;7:1251–1257. doi: 10.1089/hum.1996.7.10-1251. [DOI] [PubMed] [Google Scholar]