Abstract
Background
Shared decision making using patient decision aids (PtDAs) was established over a decade ago, but few studies have evaluated its efficacy in Asian countries. We therefore evaluated the application of PtDAs in a decision conflict between two muscle relaxant reversal agents, neostigmine and sugammadex, and sequentially analyzed the regional differences and operating room turnover rates.
Methods
This multicenter, outcome-assessor-blind, randomized controlled trial included 3,132 surgical patients from two medical centers admitted between March 2020 and August 2020. The patients were randomly divided into the classical and PtDA groups for pre-anesthesia consultations. Their clinicodemographic characteristics were analyzed to identify variables influencing the choice of reversal agent. On the day of the pre-anesthesia consultation, the patients completed the four SURE scale (sure of myself, understand information, risk-benefit ratio, encouragement) screening items. The operating turnover rates were also evaluated using anesthesia records.
Results
Compared with the classical group, the PtDA group felt more confident about receiving sufficient medical information (P < 0.001), felt better informed about the advantages and disadvantages of the medications (P < 0.001), exhibited a superior understanding of the benefits and risks of their options (P < 0.001), and felt surer about their choice (P < 0.001). Moreover, the PtDA group had a significantly greater tendency to choose sugammadex over neostigmine (P < 0.001).
Conclusions
PtDA interventions in pre-anesthesia consultations provided surgical patients with clear knowledge and better support. PtDAs should be made available in other medical fields to enhance shared clinical decision-making.
Keywords: Anesthesia, Decision making, Decision support techniques, Neostigmine, Psychological conflict, Sugammadex
Introduction
Muscle relaxants play a central role in general anesthesia; however, the safe reversal of neuromuscular blocks for tracheal extubation remains challenging. Acetylcholinesterase inhibitors, particularly neostigmine, have traditionally been used for the reversal of nondepolarizing neuromuscular blocking agents, as complete reversal can be achieved in only 15–30 min. However, they entail serious limitations. For instance, their indirect mechanism of action (competing with the same receptors as muscle relaxants) may lead to residual neuromuscular block. Moreover, neostigmine-related muscarinic side effects are common, such as postoperative nausea and vomiting, QT interval prolongation, bradycardia, and bronchospasm [1,2].
Sugammadex, a newer reversal agent, is a modified γ-cyclodextrin that encapsulates a free rocuronium molecule to form a stable complex, thus rapidly reducing the free plasma concentration and reversing the block [3]. It is fast-acting, safe, and has predictable reversal of any degree of block [4–6]. A previous meta-analysis reported significantly greater benefits and fewer adverse events with sugammadex than neostigmine, most notably, a much lower incidence of residual blockade, which could reduce pulmonary complications [7]. However, sugammadex costs 187 United States dollars (USD) for patients under 80 kg and double the price for patients over 80 kg and must be paid out-of-pocket, making its feasibility in routine clinical practice low. Considering its efficacy and safety, however, adverse effects are less likely and the operation turnover rate may be higher; thus, its use could lead to potential economic benefits for the medical system.
Traditionally, healthcare professionals have made decisions regarding medications without patient input; however, as the trend of patient-centered care increases, patients are increasingly participating in clinical decision-making, with medical professionals assuming the role of information providers. Individuals’ choice of medicine may be influenced by many factors, including economic concerns, local culture, personal preferences, and values [8]. In the hospital setting, clinical practitioners make suggestions regarding anesthesia during the pre-anesthesia consultation, which only lasts approximately 20 min. Fully clarifying various medication options to patients is difficult in such a short time.
Shared decision-making, which was introduced in 1982 and further developed by Charles et al. in 1997 [9], includes information provision and preference establishment by both physicians and patients to reach a mutual consensus. However, clinical practitioners usually find it challenging to communicate effectively, particularly when medical knowledge is involved. To enhance shared decision-making, patient decision aids (PtDAs), including printed booklets, videos, and web-based resources, have attracted worldwide attention [10]. These tools are demonstrably efficient in many medical fields for improving patients’ quality of decision-making, but their benefit in Asian countries remain unclear [8,11,12].
In this randomized controlled trial (RCT), we evaluated whether PtDAs could truly provide patients with sufficient information and understandable explanations for different muscle relaxant reversal medications, neostigmine and sugammadex, and allow patients to make appropriate medical decisions. We also analyzed whether the PtDAs would affect the operating room turnover rate, use of sugammadex, and rate of adverse events. Finally, we assessed regional differences in PtDA acceptance in Taiwan.
Materials and Methods
This RCT was approved by the Joint Institutional Review Board of Taipei Medical University (Approval Number: N201909073). The trial was registered prior to patient enrollment at ClinicalTrials.gov (identifier: NCT04272177). Trial reporting was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) 2010 guidelines.
Inclusion criteria
From March 2020 to August 2020, participants were recruited from Shuang Ho Hospital, Taipei Medical University (located in Northern Taiwan) and Chimei Medical Center (located in Southern Taiwan). All surgical patients aged > 20 years who had a routine general anesthesia assessment planned before surgery were approached for possible enrollment.
Exclusion criteria
We excluded patients who received spinal anesthesia or nerve blocks without general anesthesia; those who needed sugammadex according to their medical concerns (e.g., myasthenia gravis), as evaluated by an anesthesiologist; those who could not communicate in Mandarin or Minnanese; those who underwent an emergency surgery without a pre-anesthesia assessment; and those with a planned delay of extubation in the intensive care unit.
Randomization
The patients were randomly divided into either the classical or PtDA group before the pre-anesthesia assessments. Randomization was performed using computer-generated random numbers with stratification according to the participating center. One blinded registered nurse marked the patient files with classical or PtDA groups before introducing them to the anesthesiologist. The anesthesiologist providing pre-anesthesia consultation introduced the two muscle relaxant reversal agents to the patients (or patients’ families if they could not make medical decisions by themselves). The classical group received the standard explanation and the PtDA group received a set of PtDAs explaining the medications and their benefits and disadvantages (Supplementary 1 for the English version and Supplementary 2 for the Chinese version). On the day of surgery, the anesthesiologists who administered general anesthesia used neostigmine or sugammadex as the reversal agent based on patient preference but were blinded to the decision-making process. Post-anesthetic complications and assessments were performed the day after the operation by an anesthetic nurse blinded to the choice of reversal agent and patient grouping.
Development of PtDAs
The PtDAs were developed based on the International Patient Decision Aid Standards (IPDAS) [13]. The following steps were followed: design, alpha testing with patient and physician expert groups, beta testing (field testing) with patients and clinicians, and several steering group meetings. Several consensus meetings were held with five anesthesiologists from two hospitals. The first prototype of the PtDAs was developed and assessed in pre-anesthetic consultations three months before this study. After three revisions, the final version was published and officially used during our consultations.
Outcome assessment
Blinded outcome assessors evaluated the patients’ degree of satisfaction with the decision process in the PtDA and classical groups and assessed for postoperative complications the day after surgery. They used a questionnaire that included the four SURE (sure of myself, understand information, risk-benefit ratio, encouragement) screening test items, one item for baseline information, and one item related to medical knowledge [14]. The SURE test assessed whether the patient felt well-informed (“I learned enough information about the muscle relaxant reversal agents during this consultation”), understood the important messages (“I learned the advantages and disadvantages of muscle relaxant reversal agents during this consultation”), received appropriate support and suggestions (“I received necessary support and suggestions while deciding on the muscle relaxant reversal agent”), and felt confident in their decisions (“my choice of muscle relaxant reversal agent feels right to me”) [15]. The baseline item evaluated the patient’s baseline knowledge of the muscle relaxant reversal agent (“I had full knowledge about the muscle relaxant reversal agents before this pre-anesthetic consultation”), and the final item was designed to evaluate how clear the PtDAs were (“I fully understand the benefits and risks of the muscle relaxant reversal agent options”). All items were rated on a 5-point Likert scale (1 = strongly disagree, 2 = disagree, 3 = neither agree nor disagree, 4 = agree, and 5 = strongly agree) and were translated into Mandarin and modified to fit the anesthesia situation and Taiwanese culture. All the items were translated using the forward-backward translation method. Moreover, the duration of drug to extubation, drug to operating room discharge, drug to postoperative anesthesia care unit (PACU) entry and stay, total surgical duration, and anesthesia duration were evaluated according to the anesthesia records.
Sample size estimation
G*Power version 3.1.9.2 was used to estimate the sample size for this trial. Since no similar studies have been conducted on this topic based in eastern societies, we assumed that the difference between the two groups would be minimal. Presuming that the score of the 5-point rating scale for knowledge and decision satisfaction between the PtDA and classical group might differ by 0.13 with a standard deviation (SD) of 1.5, each group had to have at least 1,463 participants. Assuming a dropout rate of 6% (175 patients), a target sample size of 1550 per group was determined as necessary to achieve a power of 0.95 with an alpha value of 0.05.
Statistical analysis
For between-group comparisons of the clinicodemographic variables, the independent two-sample t-test, chi-square test, and Fisher’s exact test were used for the American Society of Anesthesiologists (ASA) status and the chi-square test was used for the remaining categories, including the sex of the patients and decision-makers, surgical specialties, decision-makers’ relationship with the patients, education level of the decision-makers, location of the hospital, and medication choice. The Student’s t-test was also used to compare differences in baseline, decision regret, and decision conflict between the two groups. Categorical variables were expressed as numbers and percentages. Odds ratios (ORs) were estimated to compare categorical variables between the two groups. In formal tests for interaction, continuous variables were reported as the mean difference (MD) with the SD. Statistical significance was set at P < 0.05. Logistic regression was used to examine the individual contribution of each demographic and clinical variable on the choice of sugammadex. Variables with significant contributions in the univariate logistic regression analysis were further analyzed using multiple logistic regression. All analyses were conducted using SPSS for Windows version 28.0 (SPSS Inc., USA).
Results
A total of 3,132 surgical patients signed informed consent and were randomly allocated to either the classical (n = 1,529) or PtDA (n = 1,594) group using computer-generated random numbers. Among them, 137 were excluded due to age (< 20 years), postponement of the operation, or failure to complete our survey (classical group = 59, PtDA group = 78) (Fig. 1).
Fig. 1.
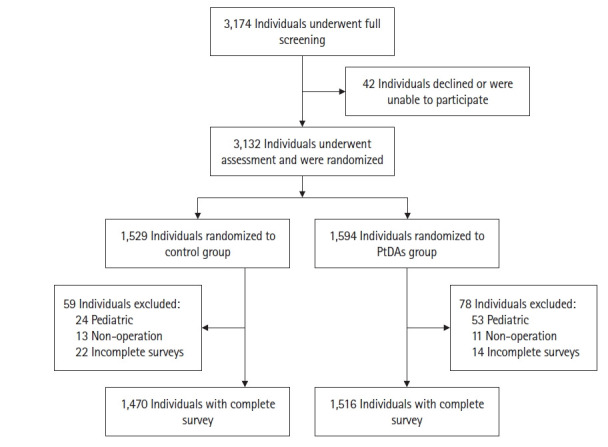
Flowchart of patient enrollment.
Demographic and clinical variables
The distribution of the clinicodemographic variables between the classical and PtDA groups are summarized in Table 1. No differences were found between the two groups in terms of age, sex, ASA status, surgical specialty, hospital location, and baseline characteristics of the decision-makers (age, sex, relationship with the patient, and degree of education). The only exception was the choice of reversal agents: compared with the classical group, the PtDA group had a significantly greater tendency to choose sugammadex (56.73% vs. 71.32%, P < 0.001).
Table 1.
Distribution of Demographic and Clinical Variables between the Classical and PtDA Groups
| Variable | Classical group (n =1,470) | PtDA group (n = 1,516) | P value |
|---|---|---|---|
| Age (yr) | 56.42 ± 16.03 | 55.12 ± 16.23 | 0.029* |
| Sex | 0.460† | ||
| Male | 696 (47.35) | 698 (46.01) | |
| Female | 774 (52.65) | 819 (53.99) | |
| ASA | 0.100‡ | ||
| I | 231 (15.71) | 267 (17.60) | |
| II | 1108 (75.37) | 1137 (74.95) | |
| III | 128 (8.71) | 113 (7.45) | |
| IV | 3 (0.20) | 0 (0.00) | |
| Surgical specialty | 0.160† | ||
| General surgery | 266 (18.10) | 336 (22.15) | |
| Thoracic surgery | 55 (3.74) | 47 (3.10) | |
| Colorectal surgery | 47 (3.20) | 58 (3.82) | |
| Orthopedic surgery | 417 (28.37) | 373 (24.59) | |
| Otolaryngology | 139 (9.46) | 137 (9.03) | |
| Gynecological surgery | 110 (7.48) | 124 (8.17) | |
| Neurosurgery | 140 (9.52) | 152 (10.02) | |
| Urology | 185 (12.59) | 180 (11.87) | |
| Plastic surgery | 46 (3.13) | 45 (2.97) | |
| Others | 65 (4.42) | 65 (4.28) | |
| Decision-maker | |||
| Age (yr) | 53.55 ± 14.22 | 52.37 ± 14.13 | 0.022* |
| Sex | 0.160† | ||
| Male | 710 (48.30) | 694 (45.75) | |
| Female | 760 (51.70) | 823 (54.25) | |
| Relationship with patient | 0.430† | ||
| Patient | 1180 (80.27) | 1235 (81.41) | |
| Other | 290 (19.73) | 282 (18.59) | |
| Education | 0.036† | ||
| Junior high school or below | 456 (31.02) | 409 (26.96) | |
| Senior high or vocational school | 502 (34.15) | 519 (34.21) | |
| University or college | 462 (31.43) | 520 (34.28) | |
| Graduate school | 50 (3.40) | 69 (4.55) | |
| Location of hospital | 0.037† | ||
| North | 1283 (87.28) | 1361 (89.72) | |
| South | 187 (12.72) | 156 (10.28) | |
| Medication choice | < 0.001† | ||
| Sugammadex | 834 (56.73) | 1082 (71.32) | |
| Neostigmine | 636 (43.27) | 435 (28.68) |
Values are presented as mean ± SD and number (%). PtDA: patient decision aid, ASA: American Society of Anesthesiologists physical status. *t-test, †Chi-square test, ‡Fisher’s exact test.
SURE scale
No significant between-group differences were found in the baseline question (item 1) regarding background knowledge before pre-anesthetic assessment or item 6 regarding encouragement from medical providers during the consultation.
However, significant differences were found for the other three items according to the Student’s t-test. The classical group scored significantly lower than the PtDA group for item 2 regarding learning enough information (3.67 ± 0.88 vs. 4.07 ± 0.77, MD: −0.408, 95% CI [−0.467, −0.349], P < 0.001), item 3 regarding learning the advantages and disadvantages of the different muscle relaxant reversal agents (3.80 ± 0.85 vs. 4.21 ± 0.70, MD: −0.417, 95% CI [−0.473, −0.361], P < 0.001), item 4 regarding fully understanding the benefits and risks of the choice of muscle relaxant reversal agent (3.89 ± 0.85 vs. 4.23 ± 0.69, MD: −0.341, 95% CI [−0.396, −0.285], P < 0.001), and item 5 regarding feeling confident in the choice of muscle relaxant reversal agent (4.10 ± 0.85 vs. 4.25 ± 0.83, MD: −0.148, 95% CI [−0.208, −0.088], P < 0.001) (Table 2).
Table 2.
Comparison of Differences in the SURE Scale Score between the Classical and PtDA Groups
| Question | Location | Classical group (n =1,470) | PtDA group (n = 1,516) | Difference (95% CI) | P value |
|---|---|---|---|---|---|
| 1. I had full knowledge about muscle relaxant reversal agents before this pre-anesthetic consultation [Baseline]. | North | 1.43 ± 0.85 | 1.48 ± 0.89 | −0.053 (−0.119, 0.013) | 0.120 |
| South | 1.38 ± 1.05 | 1.41 ± 1.07 | −0.033 (−0.260, 0.194) | 0.780 | |
| Total | 1.42 ± 0.87 | 1.48 ± 0.91 | −0.052 (−0.116, 0.012) | 0.110 | |
| 2. I learned enough information about the muscle relaxant reversal agents during this consultation [Extra question]. | North | 3.63 ± 0.86 | 4.06 ± 0.75 | −0.429 (−0.490, −0.367) | <0.001 |
| South | 3.90 ± 1.01 | 4.19 ± 0.87 | −0.291 (−0.493, −0.088) | 0.005 | |
| Total | 3.67 ± 0.88 | 4.07 ± 0.77 | −0.408 (−0.467, −0.349) | <0.001 | |
| 3. I learned the advantages and disadvantages of the muscle relaxant reversal agents during this consultation [Understanding information]. | North | 3.78 ± 0.83 | 4.22 ± 0.69 | −0.437 (−0.495, −0.379) | <.001 |
| South | 3.94 ± 0.97 | 4.21 ± 0.80 | −0.272 (−0.464, −0.081) | 0.006 | |
| Total | 3.80 ± 0.85 | 4.21 ± 0.70 | −0.417 (−0.473, −0.361) | <0.001 | |
| 4. I fully understand the benefits and risks of the choice of muscle relaxant reversal agent [Risk-benefit ratio]. | North | 3.88 ± 0.84 | 4.23 ± 0.69 | −0.348 (−0.406, −0.289) | <0.001 |
| South | 3.94 ± 0.93 | 4.23 ± 0.77 | −0.293 (−0.478, −0.108) | 0.002 | |
| Total | 3.89 ± 0.85 | 4.23 ± 0.69 | −0.341 (−0.396, −0.285) | <0.001 | |
| 5. My choice of muscle relaxant reversal agent feels right to me [Sure of myself]. | North | 4.07 ± 0.86 | 4.23 ± 0.84 | −0.159 (−0.224, −0.094) | <0.001 |
| South | 4.30 ± 0.71 | 4.41 ± 0.68 | −0.108 (−0.258, 0.041) | 0.160 | |
| Total | 4.10 ± 0.85 | 4.25 ± 0.83 | −0.148 (−0.208, −0.088) | <0.001 | |
| 6. I received necessary support and suggestions while deciding on the muscle relaxant reversal agent [Encouragement]. | North | 4.00 ± 1.22 | 4.06 ± 1.16 | −0.060 (−0.151, 0.030) | 0.190 |
| South | 4.40 ± 0.69 | 4.53 ± 0.59 | −0.131 (−0.269, 0.008) | 0.060 | |
| Total | 4.05 ± 1.17 | 4.11 ± 1.13 | −0.057 (−0.140, 0.025) | 0.170 |
The questions represent the 4-item SURE (sure of myself; understand information; risk-benefit ratio; encouragement) scale. Values are presented as mean ± SD. PtDAs: patient decision aids. The mean differences between groups were tested using t-tests and presented as two-sided P values.
The subgroup analysis of the northern and southern regions revealed a significant difference in decisional conflicts in the PtDA group over the classical group for most of our questions, except for questions 1 and 6, as an overall analysis. Both regions had similar results, except item 5 regarding feeling confident in the choice of muscle relaxant reversal agent, suggesting no significant difference between the classical and the PtDA groups in the southern region (4.30 ± 0.71 vs. 4.41 ± 0.68, MD: −0.108, 95% CI [−0.258, 0.041], P = 0.160).
Individual variables attributable to the choice of medication: univariate logistic regression
The choice of reversal agent in this study was not manipulated by any means prior to the surgery. We evaluated the effect the two methods of introducing medication options (PtDA vs. classical) had on patients’ choice of reversal agent.
We used univariate logistic regression to evaluate the contribution of demographic factors (age, sex, ASA status, education of the decision-maker, and hospital location) and clinical variables (surgical specialty and experimental group) on the choice of reversal agents. A significant tendency to choose sugammadex over neostigmine was associated with the PtDA group (OR: 1.90, 95% CI [1.63, 2.21], P < 0.001), age (per 1 year, OR: 1.01, 95% CI [1.01, 1.02], P < 0.001), female sex (OR: 1.34, 95% CI [1.15, 1.56], P < 0.001), southern region (OR: 1.43, 95% CI [1.12, 1.83], P = 0.004), and the decision-maker having a university or college education (OR: 1.49, 95% CI [1.23, 1.80], P < 0.001). However, patients in the urology (OR: 0.65, 95% CI [0.49, 0.85], P = 0.002) and plastic surgery (OR: 0.50, 95% CI [0.32, 0.78], P = 0.002) specialties tended not to choose sugammadex (Table 3).
Table 3.
Crude Odds Ratio and Adjusted Odds Ratio of Choosing Sugammadex
| Variable | Crude odds ratio | Adjusted odds ratio* | ||||
|---|---|---|---|---|---|---|
| Crude OR | 95% CI | P value | Adjusted OR | 95% CI | P value | |
| Group | ||||||
| Classic | 1.00 | 1.00 | ||||
| PtDA | 1.90 | (1.63, 2.21) | <0.001 | 1.93 | (1.65, 2.25) | <0.001 |
| Age, per 1 year | 1.01 | (1.01, 1.02) | <0.001 | 1.02 | (1.01, 1.03) | <0.001 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.34 | (1.15, 1.56) | <0.001 | 1.33 | (1.03, 1.72) | 0.032 |
| ASA | 0.310 | |||||
| I | 1.00 | |||||
| II | 1.00 | (0.81, 1.22) | 0.960 | |||
| III | 1.25 | (0.90, 1.74) | 0.180 | |||
| IV | 0.28 | (0.03, 3.14) | 0.300 | |||
| Surgical specialty | 0.003 | 0.015 | ||||
| General surgery | 1.00 | 1.00 | ||||
| Thoracic surgery | 0.94 | (0.60, 1.47) | 0.770 | 0.98 | (0.62, 1.57) | 0.950 |
| Colorectal surgery | 0.89 | (0.58, 1.39) | 0.620 | 0.95 | (0.60, 1.50) | 0.810 |
| Orthopedic surgery | 0.76 | (0.61, 0.95) | 0.016 | 0.81 | (0.64, 1.02) | 0.070 |
| Otolaryngology | 0.65 | (0.48, 0.87) | 0.004 | 0.74 | (0.54, 1.00) | 0.050 |
| Gynecological surgery | 0.74 | (0.54, 1.02) | 0.060 | 0.69 | (0.50, 0.97) | 0.030 |
| Neurosurgery | 1.05 | (0.78, 1.43) | 0.740 | 1.09 | (0.79, 1.49) | 0.610 |
| Urology | 0.65 | (0.49, 0.85) | 0.002 | 0.66 | (0.50, 0.88) | 0.005 |
| Plastic surgery | 0.50 | (0.32, 0.78) | 0.002 | 0.57 | (0.36, 0.91) | 0.018 |
| Others | 0.87 | (0.58, 1.31) | 0.510 | 1.02 | (0.67, 1.55) | 0.930 |
| Decision-maker | ||||||
| Age, per 1 year | 1.01 | (1.00, 1.01) | 0.028 | 1.00 | (0.99, 1.01) | 0.480 |
| Sex | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.25 | (1.07, 1.45) | 0.004 | 1.03 | (0.80, 1.32) | 0.850 |
| Relationship with patient | ||||||
| Patient | 1.00 | |||||
| Others | 1.18 | (0.97, 1.43) | 0.100 | |||
| Education | <0.001 | |||||
| Junior high school or below | 1.00 | 1.00 | ||||
| Senior high or vocational school | 0.98 | (0.81, 1.17) | 0.790 | 1.16 | (0.95, 1.42) | 0.150 |
| University or college | 1.49 | (1.23, 1.80) | <0.001 | 2.04 | (1.62, 2.57) | <0.001 |
| Graduate school | 1.74 | (1.14, 2.67) | 0.011 | 2.48 | (1.57, 3.91) | <0.001 |
| Location of hospital | ||||||
| North | 1.00 | 1.00 | ||||
| South | 1.43 | (1.12, 1.83) | 0.004 | 1.53 | (1.18, 1.99) | 0.001 |
OR: odds ratio, PtDAs: patient decision aids, ASA: American Society of Anesthesiologists physical status. *Adjusted for variables with significant crude odds ratios.
Multiple variables attributable to the choice of medication: multiple logistic regression
Most variables showing a significant tendency toward choosing sugammadex in the univariate logistic regression analysis remained significant in further multivariate logistic regression models, including the PtDA group (OR: 1.93, 95% CI [1.65, 2.25], P < 0.001), age (per 1 year, OR: 1.02, 95% CI [1.01, 1.03], P < 0.001), female sex (OR: 1.33, 95% CI [1.03, 1.72], P = 0.032), southern hospital (OR: 1.53, 95% CI [1.18, 1.99], P = 0.001), and the decision-maker having a university or college education (OR: 2.04, 95% CI [1.62, 2.57], P < 0.001). Furthermore, the decision-maker having a graduate education was associated with a significantly greater tendency to choose sugammadex (OR: 2.48, 95% CI [1.57, 3.91], P < 0.001) in the multivariate logistic regression models. However, unlike the univariate logistic regression, no significant differences were observed in the surgical specialties of urology and plastic surgery (Table 3).
Association between PtDAs and the operating room turnover rate
We calculated the duration of drug to extubation, drug to operating room discharge, drug to PACU entry and stay, total surgical duration, and anesthesia duration to analyze the effect of the different groups on the operating room turnover rate and efficacy. A nonsignificant trend toward a shorter duration in drug to extubation (MD: 0.31, 95% CI [−0.17, 0.78], P = 0.200), operating room discharge (MD: 0.28, 95% CI [−0.24, 0.80], P = 0.290), PACU entry (MD: 0.12, 95% CI [−0.37, 0.60], P = 0.640), and PACU stay (MD: 0.31, 95% CI [−0.96, 1.58], P = 0.630) was observed in the PtDA group compared with the classical group (Table 4).
Table 4.
Comparison of Differences in the Operating Room Turn-over Rate between the Classical and PtDA Groups
| Duration | Classical group (n =1,470) | PtDA group (n = 1,516) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Drug to extubation (min) | 7.03 ± 6.17 | 6.72 ± 6.75 | 0.31 (−0.17, 0.78) | 0.200 |
| Drug to OR discharge (min) | 9.49 ± 6.62 | 9.21 ± 7.61 | 0.28 (−0.24, 0.80) | 0.290 |
| Drug to PACU entry (min) | 18.47 ± 6.55 | 18.35 ± 6.91 | 0.12 (−0.37, 0.60) | 0.640 |
| Duration of PACU stay (min) | 50.16 ± 13.57 | 49.85 ± 20.63 | 0.31 (−0.96, 1.58) | 0.630 |
| Duration of surgery (min) | 116.83 ± 84.83 | 120.96 ± 85.79 | −4.13 (−10.25, 1.99) | 0.190 |
| Duration of anesthesia (min) | 170.39 ± 97.75 | 174.34 ± 95.42 | −3.95 (−10.87, 2.98) | 0.260 |
| Total time | 62.03 ± 31.58 | 62.84 ± 30.62 | −0.81 (−3.10, 1.48) | 0.490 |
Values are presented as mean ± SD. PtDAs: patient decision aids, OR: operating room, PACU: post-anesthesia care unit. The mean differences between groups were tested using t-tests and presented as two-sided P values.
Discussion
Communication with patients is often challenging, particularly in fast-paced clinical settings. Patients and their families frequently find it difficult to understand the medical terms and the benefits and risks of medical interventions. Our findings revealed that, compared with the classical group, the PtDA group had more confidence about receiving sufficient medical information (item 2), felt better informed about the advantages and disadvantages of the medication choice (item 3), had a superior understanding of the benefits and risks of the different medication options (item 4), and were surer of their choice (item 5). No significant between-group difference was found for the baseline knowledge question (item 1). These results suggest that PtDAs improve the quality of communication between physicians and patients, leading to a conclusion that is closest to the patient’s preference.
We also analyzed whether PtDAs affected patients’ choice of medication. Univariate logistic regression revealed a significant tendency to choose sugammadex over neostigmine in the PtDA group. A similar tendency was noted in women, older patients, and decision-makers with higher education levels (university or college). However, the use of PtDAs did not significantly change the operating room turnover rate compared with the classical group.
Sugammadex markedly reduces the incidence of postoperative residual paralysis [16,17] and may also decrease postoperative pulmonary complications and the associated medical costs [7,18]. However, the high price of sugammadex compared to that of neostigmine is its most obvious disadvantage in terms of patient selection. This weakness is compounded by Taiwan’s National Health Insurance system, which requires clinical practitioners to sign additional documents when using sugammadex.
PtDAs are significantly beneficial to patients for various medical decisions, such as the choice of type 2 diabetes mellitus medication [12], surgical intervention for breast cancer [19], pediatric vaccination for rotavirus [20], and clinical care for lumbar degenerative diseases [21]. The use of PtDAs in shared clinical decision-making encourages patients and decision-makers to ask questions, thereby making them better informed to make proper medical decisions for themselves or their families.
Our results revealed that medication choice was significantly different between the PtDA and classical groups, with the PtDA group having a higher level of confidence in and understanding of their choices. However, multiple other factors influenced the choice of treatment, including the patient’s age, education level of the decision-maker, and location of the hospital. Older age may represent a key factor in decision-makers’ concerns about the operation and anesthetic method, and education level is likely correlated with the economic background of the family. Because of the cultural and historical differences between northern and southern Taiwan, and since patients from southern Taiwan are believed to show better medical advice compliance, we evaluated regional differences using the hospital location as a confounding factor. The tendency of Southern residents to choose self-paid medications likely reflects their strong belief in professionalism [22].
Our sequential analysis revealed that the PtDA intervention did not significantly increase the operating room turnover rate. This may be due to the high number of patients choosing sugammadex over neostigmine as the reversal agent in both the PtDA (72.32%) and classical groups (56.73%), diminishing the potential effect of sugammadex on the turnover rate.
This study was conducted in two urban teaching hospitals located in southern and northern Taiwan separately to ameliorate the limitations associated with location. The high follow-up rate makes this study statistically powerful, and the large amount of real world data makes it more persuasive to Eastern society. However, this study had some limitations. First, the different sample sizes from the two hospitals made it difficult to compare the impact of location on the findings. Second, we did not assess for long-term postoperative pulmonary complications. However, compared with neostigmine, sugammadex is associated with a much lower incidence of postoperative pulmonary complications, and following up patients for more than 30 days after their operation is challenging. Third, though patients’ economic status could play an important role in the decision-making process, we could not assess the patients’ specific economic status in this study.
PtDAs help with the most challenging part of the patient-healthcare professional relationship: communication. Our findings revealed that PtDAs enhance the shared decision-making process by helping patients obtain a better understanding of their choices, choose the treatment closer to their needs, and be surer about their choice. However, although sugammadex was the predominant choice in the PtDA group, the operating room turnover rate was not significantly higher in this group. This communication tool was confirmed to be effective with minimal effort in Asian countries as in Western countries, and should be routinely used in other departments and medical fields to enhance shared clinical decision-making.
Acknowledgments
We would like to thank Miss Tseng Hui Yun, a registered nurse in the Department of Anesthesiology, for her assistance in creating the PtDAs.
Appendix 1. Patient Decision Aids (English Version)
Appendix 2. Patient Decision Aids (Traditional Chinese Version)
Footnotes
Funding
The authors received no specific funding for this study. This work was supported by a research grant from the Chi Mei Medical Center and Taipei Medical University (Grant No. 107CM-TMU-12). The sponsoring organization was not involved in the study design, data analysis, or interpretation.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
Li-Kai Wang (Data curation; Formal analysis; Investigation)
Yao-Tsung Lin (Data curation; Formal analysis; Investigation)
Jui-Tai Chen (Data curation; Formal analysis; Investigation)
Winnie Lan (Formal analysis; Writing – original draft)
Kuo-Chuan Hung (Data curation; Investigation)
Jen-Yin Chen (Data curation; Investigation)
Kuei-Jung Liu (Data curation; Investigation)
Yu-Chun Yen (Formal analysis; Investigation)
Yun-Yun Chou (Formal analysis; Investigation)
Yih-Giun Cherng (Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing)
Ka-Wai Tam (Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing)
Supplementary Materials
Patient Decision Aids (English Version).
Patient Decision Aids (Traditional Chinese Version).
References
- 1.Hristovska AM, Duch P, Allingstrup M, Afshari A. Efficacy and safety of sugammadex versus neostigmine in reversing neuromuscular blockade in adults. Cochrane Database Syst Rev. 2017;8:CD012763. doi: 10.1002/14651858.CD012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo J, Chen S, Min S, Peng L. Reevaluation and update on efficacy and safety of neostigmine for reversal of neuromuscular blockade. Ther Clin Risk Manag. 2018;14:2397–406. doi: 10.2147/TCRM.S179420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alday E, Muñoz M, Planas A, Mata E, Alvarez C. Effects of neuromuscular block reversal with sugammadex versus neostigmine on postoperative respiratory outcomes after major abdominal surgery: a randomized-controlled trial. Can J Anaesth. 2019;66:1328–37. doi: 10.1007/s12630-019-01419-3. [DOI] [PubMed] [Google Scholar]
- 4.Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–81. doi: 10.1213/01.ane.0000244594.63318.fc. [DOI] [PubMed] [Google Scholar]
- 5.Paton F, Paulden M, Chambers D, Heirs M, Duffy S, Hunter JM, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105:558–67. doi: 10.1093/bja/aeq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welliver M, McDonough J, Kalynych N, Redfern R. Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent. Drug Des Devel Ther. 2009;2:49–59. doi: 10.2147/dddt.s2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carron M, Baratto F, Zarantonello F, Ori C. Sugammadex for reversal of neuromuscular blockade: a retrospective analysis of clinical outcomes and cost-effectiveness in a single center. Clinicoecon Outcomes Res. 2016;8:43–52. doi: 10.2147/CEOR.S100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, et al. Toward the 'tipping point': decision aids and informed patient choice. Health Aff (Millwood) 2007;26:716–25. doi: 10.1377/hlthaff.26.3.716. [DOI] [PubMed] [Google Scholar]
- 9.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Soc Sci Med. 1997;44:681–92. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 10.Barry MJ, Edgman-Levitan S. Shared decision making--pinnacle of patient-centered care. N Engl J Med. 2012;366:780–1. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- 11.Sepucha K, Atlas SJ, Chang Y, Dorrwachter J, Freiberg A, Mangla M, et al. Patient decision aids improve decision quality and patient experience and reduce surgical rates in routine orthopaedic care: a prospective cohort study. J Bone Joint Surg Am. 2017;99:1253–60. doi: 10.2106/JBJS.16.01045. [DOI] [PubMed] [Google Scholar]
- 12.Stacey D, Menard P, Gaboury I, Jacobsen M, Sharif F, Ritchie L, et al. Decision-making needs of patients with depression: a descriptive study. J Psychiatr Ment Health Nurs. 2008;15:287–95. doi: 10.1111/j.1365-2850.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13 Suppl 2(Suppl 2):S2. doi: 10.1186/1472-6947-13-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Légaré F, Kearing S, Clay K, Gagnon S, D'Amours D, Rousseau M, O'Connor A. Are you SURE?: Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician. 2010;56:e308–14. [PMC free article] [PubMed] [Google Scholar]
- 15.Brehaut JC, O’Connor AM, Wood TJ, Hack TF, Siminoff L, Gordon E, et al. Validation of a decision regret scale. Med Decis Making. 2003;23:281–92. doi: 10.1177/0272989X03256005. [DOI] [PubMed] [Google Scholar]
- 16.Brueckmann B, Sasaki N, Grobara P, Li MK, Woo T, de Bie J, et al. Effects of sugammadex on incidence of postoperative residual neuromuscular blockade: a randomized, controlled study. Br J Anaesth. 2015;115:743–51. doi: 10.1093/bja/aev104. [DOI] [PubMed] [Google Scholar]
- 17.Ledowski T, Hillyard S, O’Dea B, Archer R, Vilas-Boas F, Kyle B. Introduction of sugammadex as standard reversal agent: impact on the incidence of residual neuromuscular blockade and postoperative patient outcome. Indian J Anaesth. 2013;57:46–51. doi: 10.4103/0019-5049.108562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ünal DY, Baran İ, Mutlu M, Ural G, Akkaya T, Özlü O. Comparison of sugammadex versus neostigmine costs and respiratory complications in patients with obstructive sleep apnoea. Turk J Anaesthesiol Reanim. 2015;43:387–95. doi: 10.5152/TJAR.2015.35682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ter Stege JA, Oldenburg HS, Woerdeman LA, Witkamp AJ, Kieffer JM, van Huizum MA, et al. Decisional conflict in breast cancer patients considering immediate breast reconstruction. Breast. 2021;55:91–7. doi: 10.1016/j.breast.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin SC, Tam KW, Yen JY, Lu MC, Chen EY, Kuo YT, et al. The impact of shared decision making with patient decision aids on the rotavirus vaccination rate in children: a randomized controlled trial. Prev Med. 2020;141:106244. doi: 10.1016/j.ypmed.2020.106244. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Kang YN, Chiu PY, Huang YJ, Elwyn G, Wu MH, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns. 2021;104:2498–504. doi: 10.1016/j.pec.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan RJ, Bolin J, Wang S, Zhu L, Kim M. Urban/rural differences in decision making and the use of advance directives among nursing home residents at admission. J Rural Health. 2004;20:131–5. doi: 10.1111/j.1748-0361.2004.tb00019.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Patient Decision Aids (English Version).
Patient Decision Aids (Traditional Chinese Version).


