Abstract
BACKGROUND:
Adults with cerebral palsy (CP) have increased risk for developing various secondary chronic diseases, especially when they have other neurodevelopmental disabilities (NDDs). Multiple medications are likely prescribed to manage the greater morbidity-related burden for adults with CP; however, because health care delivery and care coordination is suboptimal for this population, adults with CP may have an increased risk for polypharmacy. To date, very little is known about the prescribing practices and extent of polypharmacy for adults with CP.
OBJECTIVE:
To determine the prevalence and adjusted odds of polypharmacy among adults with CP only and those with CP+NDDs, compared with adults without CP.
METHODS:
Data from 2017 Optum Clinformatics Data Mart, a U.S. private administrative database, was used for this retrospective cohort study. Diagnosis codes were used to identify adults (aged ≥ 18 years) with CP, NDDs (e.g., intellectual disabilities, epilepsy, and autism spectrum disorders), and 24 relevant morbidities. Polypharmacy was examined as 0-4 versus ≥ 5, 0-9 versus ≥ 10, and 0-14 versus ≥ 15 medications. Logistic regression estimated the OR and 95% CI of polypharmacy before and after adjusting for age, sex, region of residence, and multimorbidity (as 0, 1, 2, 3, 4-5, and ≥ 6 morbidities). Exploratory analyses were conducted to compare polypharmacy among young (18-40 years) and middle-aged (41-64 years) adults with CP only and CP + NDDs with elderly (≥ 65 years) adults without CP.
RESULTS:
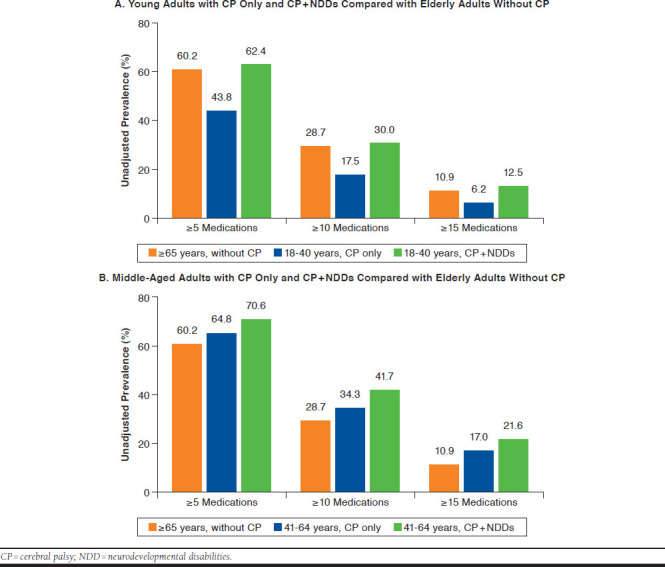
Adults with CP only (n = 5,603) and CP + NDDs (n = 2,474) had higher unadjusted prevalence and adjusted OR for each polypharmacy definition compared with adults without CP (n = 9.0 million; e.g., ≥ 5 medications: adjusted OR for CP only = 1.38, 95% CI = 1.30-1.47; CP + NDDs: OR = 2.42, 95% CI = 2.20-2.67). Adults with CP+NDDs had higher unadjusted prevalence and adjusted OR of each polypharmacy definition compared with CP only. Compared with elderly without CP, the unadjusted prevalence of polypharmacy was lower for young adults with CP only (e.g., ≥ 5 medications: 60.2%, 43.8%), similar for young adults with CP+NDDs (e.g., ≥ 15 medications: 10.9%, 12.5%), and elevated for middle-aged CP only and CP + NDDs (e.g., ≥ 10 medications: 28.7%, 34.3%, 41.7%).
CONCLUSIONS:
Privately insured adults with CP only and CP + NDDs have an elevated prevalence of polypharmacy compared with adults without CP, even after accounting for multimorbidity. Importantly, adults aged 18-40 years with CP have a similar (CP + NDDs) prevalence of polypharmacy compared with the general geriatric population, with the prevalence increasing further for CP by middle age.
What is already known about this subject
Adults with cerebral palsy (CP) have an early development of several chronic diseases compared with the general population.
Health care delivery and care coordination is suboptimal to meet the greater medical needs of adults with CP, which increases risk for mismanagement of medications to regulate the heightened morbidity-related burden.
What this study adds
Privately insured adults with CP only and CP with other neurodevelopmental disabilities (CP + NDDs) have an elevated prevalence of polypharmacy compared with the general population, even after adjusting for demographic variables and multimorbidities.
Adults with CP + NDDs had an elevated polypharmacy prevalence compared with adults with CP only.
Adults aged 18-40 years with CP had a similar (CP + NDDs) prevalence of polypharmacy compared with elderly adults (aged ≥ 65 years) without CP, which increased further for CP by middle age (41-64 years).
Cerebral palsy (CP) results from damage to or malformation of the infant brain. CP is a neurological syndrome representing an array of movement disorders and is the most common pediatric physical disability.1 Children with CP experience many health and functional complications, including, but not limited to, low physical activity, musculoskeletal issues, excess body fat, communication impairments, and mental health disorders.2-5 As a result of health complications present early in life, individuals with CP have an elevated risk for early development of chronic diseases and mental health disorders as they age into and throughout their adult years.6-10
Multimorbidity is more than 3-fold higher for young adults (aged 18-30 years) with CP, compared with those without CP.6 Multiple medications, or polypharmacy, may be prescribed in order to manage the greater morbidity-related burden associated with CP. Unfortunately, the U.S. health care system is not well designed for care coordination among health care providers treating adults with CP,11-13 which can lead to excess prescription of or inappropriate grouping of medications, resulting in adverse medication reactions and medication-medication interactions.14,15 In the general population, it is well established that polypharmacy and multimorbidity are risk factors for adverse health outcomes, such as premature mortality.16,17 Intellectual disabilities are another population that also has an elevated risk of multimorbidity and is commonly comorbid with CP.18 A recent study that examined adults aged ≥ 50 years with intellectual disabilities found that polypharmacy was an independent risk factor for mortality beyond the effect of multimorbidity and other confounding factors.19 To date, very little is known about the prevalence and associated burden of polypharmacy for adults with CP.
The association between CP and polypharmacy may be confounded by comorbid neurodevelopmental disabilities (NDDs). The prevalence of intellectual disabilities, epilepsy, and autism spectrum disorders among individuals with CP has been reported as 45%,18 41%, and 6.9%,1 respectively, and these conditions (e.g., epilepsy) also can be associated with high medication use. Thus, the primary objective of this study was to determine the prevalence of polypharmacy among privately insured adults with CP only, CP with NDDs (CP + NDDs), and without CP. We hypothesized that adults with CP would have a higher prevalence of polypharmacy compared with adults without CP even after adjusting for sociodemographic variables and multimorbidity, which would be more pronounced among adults with CP + NDDs.
Methods
Data Source
Data from 2017 were extracted from the Clinformatics Data Mart Database (OptumInsight, Eden Prairie, MN), which is a nationwide deidentified single private payer administrative claims database in the United States. This database contains information on beneficiaries who have either commercial or Medicare Advantage health plans and includes all the inpatient, outpatient, pharmacy, emergency visit, office visit, and other ancillary service utilization throughout their enrollment in the insurance health plan.20 Individuals covered by Medicare may additionally enroll in a private Medicare Advantage health plan. To be enrolled in a private payer health plan, individuals of any age, income, or disability status pay for coverage or are covered through their employers. Administrative claims data are mainly used for billing reimbursement purposes, and health conditions are identified using specific codes attached to individual claims. Since data are deidentified, the University Institutional Review Board approved this study as nonregulated.
Sample Selection
All individuals aged 18 years and older who had 12 months of continuous enrollment during the calendar year 2017 and had at least 1 health care service utilization during the calendar year 2017 (to limit detection bias) were eligible for this cross-sectional study.
Identification of CP, NDDs, and Morbidities
All medical conditions (e.g., CP, NDDs, and morbidities) were identified using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Individuals with CP and NDDs (i.e., intellectual disabilities, epilepsy, and autism spectrum disorders) were identified on the basis of a single medical claim (e.g., inpatient, emergency department) that included 1 of the pertinent ICD-10-CM codes, which covered all diagnostic subtypes (e.g., CP: spasticity, quadriplegic, athetoid; intellectual disabilities: mild, moderate, profound), as previously described.21 Unfortunately, information regarding the severity of CP using common clinical measures (e.g., gross motor function classification system) are not available in claims data, and more than 70% of the cohort with CP and 60% of the cohort with NDDs had “other” or “unspecified” conditions.22 This limits the current study, since stratification or statistical adjustment for the clinical subtypes of CP and NDDs is not possible. The single claim-based definition has good accuracy for identifying pediatric-onset conditions with 99% sensitivity and a positive predictive value of 79%.23 We created a binary variable to indicate the presence or absence of NDDs. The final sample was categorized as adults with CP only, CP + NDDs, and without CP.
Morbidities were selected with guidance from the clinic and from the literature on CP, disease, and mortality among adults to identify relevant medical conditions that may explain polypharmacy attributes (e.g., prevalence). Morbidities were identified on the basis of a single claim that included 1 of the pertinent ICD-10-CM codes: hypertension; cardiovascular disease (i.e., ischemic heart disease, heart failure, and cerebrovascular disease); type 2 diabetes; respiratory disease (i.e., chronic obstructive pulmonary disease, acute respiratory infection [minus the “common cold”], pneumonia, other respiratory disease [e.g., respiratory failure]); acute kidney failure; chronic kidney disease (stages I-V); skeletal fragility (i.e., all-cause fracture, osteoporosis); arthritis (i.e., rheumatoid arthritis, osteoarthritis); mood affective disorders; dementia; and substance abuse (e.g., alcohol/drug dependence) including history of tobacco use, gastrointestinal problems (i.e., constipation, irritable bowel syndrome, inflammatory bowel syndrome, neurogenic bowel), neurogenic bladder, and dysphagia. We created a multimorbidity score as the sum of individual morbidities ranging from 0 to 24, and categorized as 0, 1, 2, 3, 4-5, and ≥ 6 morbidities, which was based on the data distribution.
Medications
Prescribed medications, not including vaccinations, dietary supplements, or vitamins, were identified on the basis of a single claim from outpatient pharmacy claims in 2017. Medications were differentiated based on the unique generic name in the database. The total number of medications was determined as the number of unique medications prescribed to each individual over the 12-month period, which was not based on the number of fills or dosage (e.g., 5 mg or 10 mg of the same prescribed medication was counted as 1 medication) or concurrent medication use. For example, if an individual was prescribed 6 medications in 2017, but only 4 overlapped at 1 time, then that individual’s total number of medications was 6. Pharmacy claims have been shown to reliably predict prescription medication exposure.24,25 For example, the sensitivity, specificity, and positive predictive value to identify statins, among other medications, was shown to be 95% to 99%.24
To examine the extent of polypharmacy, we categorized the total number of medications into 3 mutually exclusive variable groups, based on numerical cut-off definitions of polypharmacy from the geriatric population and intellectual and developmental disabilities population26: (1) 0-4 and ≥ 5 medications; (2) 0-9 and ≥ 10 medications; and (3) 0-14 and ≥ 15 medications.
Sociodemographic Variables
Age, sex, race, and U.S. region of residence (West, Midwest, South, Northeast) were available from the database and considered for statistical adjustment.
Statistical Analysis
Descriptive characteristics of all measures were summarized for each group. We constructed logistic regression models to estimate the odds ratio (OR) of polypharmacy for each definition (e.g., ≥ 5 medications) separately, with group as the primary exposure variable before and after adjusting for age (as continuous), sex, U.S. region of residence, and multimorbidity. Possible interactions between exposure status (group) with age or sex were assessed by conducting separate analyses for age or sex strata (to estimate group effects) and by including the product terms in the models (to test for interactions). The main effect of group was interpreted.
Sensitivity Analysis
Logistic regression models did not adjust for race because of the extent of missing/unknown data. We therefore conducted 2 related sensitivity analyses to assess possible confounding and selection bias. Sensitivity analysis 1 involved the restricted study population with complete data on race but not adjusting for race; sensitivity analysis 2 involved the same study population in 1 but adjusted for race. Results were compared between sensitivity analyses 1 and 2 to assess possible confounding by race. Results were also compared between sensitivity analysis 1 and the main analysis (full study population not adjusting for race) to assess possible selection bias from exclusion of adults without race data.
Because of the observational design, results are subject to bias from unmeasured confounding. In order to estimate the extent of unmeasured confounding for the main analysis, we computed e-values, which measure the minimum strength of association needed to explain away a specific exposure-outcome association, conditional on the set of covariates.27 We used the fully adjusted logistic regression model for each analysis.
Exploratory Analysis
Individuals with CP develop sarcopenia, frailty, altered body composition, and chronic diseases at a younger age than expected,2,6,7,28 with the chronic disease burden in adults with CP possibly being similar to the geriatric population without CP, although this has not been formally tested. Many of these factors (e.g., sarcopenia, body composition) affect medication metabolism. To determine if young and middle-aged adults with CP exhibit polypharmacy profiles similar to the geriatric population without CP, we compared the prevalence and (un) adjusted odds of polypharmacy between young (18-40 years) and middle-aged (41-64 years) adults with CP with elderly (≥ 65 years) adults without CP using the same procedures previously described but did not include age for statistical adjustment given the intent of the analysis.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). Effect estimates were reported as ORs with 95% confidence intervals (CIs). The P value of < 0.05, two-tailed, was considered statistically significant.
Results
Descriptive characteristics and prevalence of individual morbidities among adults with CP only (n = 5,603), CP + NDDs (n = 2,474), and without CP (n = 9,012,889) are presented in Table 1. The mean (standard deviation [SD]) and median (interquartile range [IQR]) for the number of medications was 7.6 (6.8) and 6.0 (2.0-11.0) for CP only, 8.7 (7.1) and 7.0 (3.0-12.0) for CP + NDDs, and 5.6 (5.5) and 4.0 (1.0-8.0) for without CP.
TABLE 1.
Descriptive Characteristics for Adults with CP Only, CP+NDDs, and Without CP
| CP Only (n = 5,603) | CP + NDDs (n = 2,474) | Without CP (n = 9,012,889) | |
|---|---|---|---|
| % (n) | % (n) | % | |
| Age, mean (SD) | 53.7 (18.0) | 44.2 (17.3) | 56.9 (18.8) |
| 18-40 years | 26.5 (1,484) | 46.3 (1,145) | 23.4 |
| 41-64 years | 40.9 (2,289) | 38.2 (945) | 34.8 |
| ≥ 65 years | 32.7 (1,830) | 15.5 (384) | 41.8 |
| Sex | |||
| Women | 49.8 (2,790) | 49.1 (1,215) | 55.2 |
| Men | 50.2 (2,813) | 50.9 (1,259) | 44.8 |
| Race | |||
| White | 61.4 (3,442) | 59.5 (1,473) | 59.8 |
| Black | 11.4 (639) | 13.0 (321) | 8.4 |
| Hispanic | 7.8 (437) | 8.9 (221) | 9.9 |
| Asian | 1.5 (84) | 1.5 (36) | 3.7 |
| Unknown/missing | 17.9 (1,001) | 17.1 (423) | 18.1 |
| U.S. region of residence | |||
| West | 21.4 (1,201) | 17.1 (424) | 21.7 |
| Midwest | 21.1 (1,183) | 25.3 (626) | 24.3 |
| South | 42.3 (2,367) | 45.1 (1,115) | 42.4 |
| Northeast | 15.2 (852) | 12.5 (309) | 11.7 |
| NDDs | |||
| Intellectual disabilities | 0 | 42.1 (1,042) | 0.1 |
| Epilepsy | 0 | 77.1 (1,908) | 1.2 |
| Autism spectrum disorders | 0 | 8.1 (200) | 0.1 |
| Morbidities | |||
| Multimorbidity | |||
| 0 morbidities | 24.5 (1,370) | 28.9 (716) | 37.9 |
| 1 morbidity | 23.6 (1,321) | 22.9 (566) | 23.8 |
| 2 morbidities | 17.4 (973) | 15.2 (375) | 15.7 |
| 3 morbidities | 11.7 (658) | 12.0 (297) | 9.4 |
| 4-5 morbidities | 12.7 (712) | 11.4 (282) | 8.5 |
| ≥ 6 morbidities | 10.2 (569) | 9.6 (238) | 4.7 |
| Hypertension | 46.4 (2,602) | 30.3 (750) | 39.7 |
| Cardiovascular disease | 17.1 (960) | 14.2 (352) | 12.9 |
| Ischemic heart disease | 9.4 (525) | 4.7 (116) | 9.1 |
| Heart failure | 6.7 (376) | 4.8 (118) | 4.1 |
| Cerebrovascular disease | 7.3 (407) | 8.5 (209) | 3.7 |
| Type 2 diabetes mellitus | 16.9 (949) | 11.2 (276) | 17.2 |
| Respiratory disease | 28.9 (1,617) | 33.1 (820) | 21.5 |
| Chronic obstructive pulmonary disease | 15.9 (892) | 15.3 (378) | 9.8 |
| Acute respiratory infection | 11.9 (669) | 14.8 (365) | 12.0 |
| Pneumonia | 5.4 (302) | 9.9 (245) | 2.1 |
| Other respiratory disease | 8.0 (449) | 12.6 (312) | 3.7 |
| Acute kidney failure | 3.8 (213) | 4.1 (101) | 2.0 |
| Chronic kidney disease | 6.4 (361) | 3.2 (79) | 6.0 |
| Skeletal fragility | 10.6 (593) | 12.1 (299) | 6.3 |
| Fracture | 6.0 (338) | 7.0 (174) | 3.2 |
| Osteoporosis | 5.8 (327) | 6.8 (168) | 3.6 |
| Arthritis | 17.8 (996) | 10.4 (256) | 12.2 |
| Rheumatoid arthritis | 1.9 (105) | 1.1 (27) | 1.5 |
| Osteoarthritis | 16.8 (942) | 9.9 (244) | 11.4 |
| Mood affective disorders | 20.0 (1,119) | 21.0 (520) | 9.1 |
| Dementia | 3.6 (202) | 4.5 (110) | 2.0 |
| Substance abuse/tobacco use | 12.5 (700) | 6.8 (167) | 9.3 |
| Gastrointestinal problems | 10.1 (564) | 16.3 (403) | 3.7 |
| Constipation | 8.4 (469) | 15.1 (374) | 2.6 |
| Irritable bowel syndrome | 1.6 (90) | 0.7 (18) | 0.9 |
| Inflammatory bowel disease | 0.4 (21) | 0.7 (16) | 0.4 |
| Neurogenic bowel | 0.5 (28) | 0.4 (11) | 0.0 |
| Neurogenic bladder | 3.7 (207) | 4.2 (105) | 0.3 |
| Dysphagia | 8.1 (455) | 15.4 (380) | 1.9 |
CP = cerebral palsy; NDD = neurodevelopmental disabilities; SD = standard deviation.
Polypharmacy Prevalence
The unadjusted prevalence of polypharmacy by various numerical cut-off definitions is presented in Figure 1. The OR of polypharmacy by various numerical cut-off definitions is presented in Table 2. Compared with adults without CP, the unadjusted OR was higher for each polypharmacy definition for adults with CP only and CP + NDDs and was larger for each subsequent cut-off definition. When the 2 CP groups were compared, the unadjusted OR was higher for each polypharmacy definition for adults with CP + NDDs versus CP only. After adjusting for age, sex, U.S. region of residence, and multimorbidity, the OR remained significantly elevated for each polypharmacy definition for adults with CP only and CP + NDDs compared with adults without CP and for adults with CP + NDDs versus CP only.
FIGURE 1.

Unadjusted Prevalence of Polypharmacy by Various Numerical Cut-Off Definitions for Adults with CP Only, CP + NDDs, and Without CP
TABLE 2.
Odds Ratio of Polypharmacy for Adults with CP Only, CP+NDDs, and Without CPa
| > 5 Medications | > 10 Medications | >15 Medications | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| CP only vs. without CP | |||
| Unadjusted | 1.71 (1.62-1.80) | 1.89 (1.79-2.00) | 2.19 (2.03-2.36) |
| Model 1 | 1.38 (1.30-1.47) | 1.38 (1.29-1.47) | 1.34 (1.23-1.46) |
| CP+NDDs vs. without CP | |||
| Unadjusted | 2.34 (2.15-2.54) | 2.42 (2.23-2.62) | 2.65 (2.39-2.93) |
| Model 1 | 2.42 (2.20-2.67) | 2.12 (1.92-2.35) | 1.59 (1.40-1.81) |
| CP+NDDs vs. CP only | |||
| Unadjusted | 1.37 (1.24-1.52) | 1.28 (1.16-1.41) | 1.21 (1.07-1.37) |
| Model 1 | 1.75 (1.56-1.96) | 1.54 (1.37-1.74) | 1.19 (1.02-1.39) |
Note: Model 1: age (as continuous), sex, U.S. region of residence, and multimorbidity.
a The without CP group served as the reference group.
CI = confidence interval; CP = cerebral palsy; NDD = neurodevelopmental disabilities; OR = odds ratio.
Interactions of Groups with Age and Sex
We observed statistically significant interactions between groups with age when polypharmacy was defined as ≥ 5 and ≥ 10 medications (P for interactions < 0.05) and between groups with sex when polypharmacy was defined as ≥ 15 medications (P for interaction ≤ 0.05). To enhance model parsimony, we categorized age as young (18-40 years), middle age (41-64 years), and elderly (≥ 65 years) to reflect the general stages of adulthood. The age- and sex-stratified analysis using the fully adjusted model (age as continuous, sex not adjusted for in sex-stratified analyses) is presented in Table 3. Compared with adults without CP, adults with CP only and CP + NDDs had higher adjusted ORs for all definitions of polypharmacy for each age category and for each sex but was more pronounced for the 18-40 years age category and for women (CP only) or men (CP + NDDs) in their respective analyses.
TABLE 3.
Adjusted Odds Ratio of Polypharmacy for Adults with CP Only, CP + NDDs, and Without CP by Age or Sexa
| Aged 18-40 Years (n = 2,113,721) | Aged 41-64 Years (n = 3,138,023) | Aged > 65 Years (n = 3,769,222) | Women (n = 4,977,990) | Men (n = 4,042,976) | |
|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| ≥ 5 medications | |||||
| CP only vs. without CP | 1.61 (1.42-1.82) | 1.26 (1.13-1.39) | 1.19 (1.07-1.32) | ||
| CP + NDDs vs. without CP | 3.52 (3.06-4.05) | 1.66 (1.40-1.96) | 1.99 (1.53-2.59) | ||
| CP + NDDs vs. CP only | 2.19 (1.82-2.64) | 1.32 (1.08-1.61) | 1.68 (1.26-2.23) | ||
| ≥ 10 medications | |||||
| CP only vs. without CP | 1.88 (1.59-2.22) | 1.26 (1.13-1.41) | 1.20 (1.08-1.33) | ||
| CP + NDDs vs. without CP | 3.65 (3.10-4.30) | 1.69 (1.43-1.99) | 1.71 (1.35-2.16) | ||
| CP + NDDs vs. CP only | 1.94 (1.54-2.45) | 1.34 (1.10-1.63) | 1.43 (1.10-1.84) | ||
| ≥ 15 medications | |||||
| CP only vs. without CP | 1.46 (1.31-1.64) | 1.20 (1.05-1.38) | |||
| CP + NDDs vs. without CP | 1.36 (1.14-1.62) | 2.01 (1.67-2.42) | |||
| CP + NDDs vs. CP only | 0.93 (0.76-1.14) | 1.68 (1.33-2.11) | |||
Note: Adjusted for age (as continuous), sex (for age-stratified analysis), U.S. region of residence, and multimorbidity.
a The without CP group served as the reference group.
CI = confidence interval; CP = cerebral palsy; NDD = neurodevelopmental disabilities; OR = odds ratio.
Sensitivity Analysis
The adjusted OR for individuals with complete data on race (n = 7,392,429) is presented in Appendix A (available in online article). A comparison of OR estimates from sensitivity analyses 1 and 2 and sensitivity analysis 1 and the main analysis show similar results for all polypharmacy definitions, suggesting no evidence that race is a confounder in the main analysis and no evidence of selection bias.
The e-value (lower 95% CI) needed to fully explain away the effect for CP only versus without CP was 1.63 (1.54) for ≥ 5 medications, 1.63 (1.53) for ≥ 10 medications, and 1.58 (1.46) ≥ 15 medications. The e-value (lower 95% CI) needed to fully explain away the effect for CP + NDDs versus without CP was 2.49 (2.33) for ≥ 5 medications, 2.27 (2.12) for ≥ 10 medications, and 1.83 (1.65) ≥ 15 medications. Given the large e-values, it appears unlikely that unmeasured confounding largely biased effect estimates.
Exploratory Analysis
Compared with elderly adults without CP, the unadjusted prevalence of polypharmacy was lower for young adults with CP only, similar for young adults with CP only and CP + NDDs and higher for middle-aged adults with CP only and CP + NDDs (Appendix B, available in online article).
The OR of polypharmacy by various numerical cut-off definitions is presented in Table 4. After adjusting for sex, U.S. region of residence, and multimorbidity and compared with elderly adults without CP, the OR was elevated for young adults and middle-aged adults with CP only and CP + NDDs, except young adults with CP only for ≥ 5 medications, which was not different.
TABLE 4.
Odds Ratio of Polypharmacy for Young and Middle-Aged Adults with CP Only and CP + NDDs Compared with Elderly Adults Without CP
| > 5 Medications | > 10 Medications | >15 Medications | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Elderly, ≥ 65 years, without CP | Reference | Reference | Reference |
| Young, 18-40 years | |||
| CP only | |||
| Unadjusted | 0.51 (0.46-0.57) | 0.53 (0.46-0.61) | 0.54 (0.44-0.67) |
| Model 1 | 1.03 (0.93-1.15) | 1.46 (1.26-1.69) | 2.01 (1.59-2.54) |
| CP +NDDs | |||
| Unadjusted | 1.09 (0.97-1.23) | 1.06 (0.94-1.21) | 1.16 (0.98-1.39) |
| Model 1 | 2.09 (1.84-2.38) | 2.57 (2.22-2.97) | 3.27 (2.66-4.02) |
| Middle age, 41-64 years | |||
| CP only | |||
| Unadjusted | 1.21 (1.12-1.32) | 1.30 (1.19-1.41) | 1.66 (1.49-1.85) |
| Model 1 | 1.55 (1.41-1.70) | 1.85 (1.68-2.05) | 2.60 (2.29-2.96) |
| CP + NDDs | |||
| Unadjusted | 1.58 (1.37-1.82) | 1.78 (1.56-2.03) | 2.24 (1.92-2.62) |
| Model 1 | 1.99 (1.71-2.32) | 2.43 (2.09-2.83) | 3.11 (2.58-3.75) |
Note: Model 1: sex, U.S. region of residence, and multimorbidity.
CI = confidence interval; CP = cerebral palsy; NDD = neurodevelopmental disabilities; OR = odds ratio.
Discussion
This large cohort study of privately insured individuals from the United States found that adults with CP had an elevated prevalence of polypharmacy, defined in this study as the total number of unique medications in a recent 12-month period, compared with adults without CP, after accounting for their higher prevalence of multimorbidity. The polypharmacy prevalence was more pronounced for adults with CP who had comorbid NDDs compared with adults with CP only. Importantly, after accounting for demographics and multimorbidity, we found that, in general, the odds of polypharmacy was already elevated in young adults with CP and increased further by middle age.
It is important to note that higher medication prescription is anticipated for the CP population, given their elevated risk for secondary chronic diseases and other health complications. Nevertheless, because clinical care and care coordination is suboptimal for adults with CP, it is possible that responsible medication prescription is not occurring for this vulnerable population. However, this study examined polypharmacy from a quantitative manner rather than from a qualitative manner. Future studies are needed to determine if the elevated prevalence of polypharmacy observed in this study is associated with adverse outcomes (e.g., chronic diseases and premature mortality), which medication combinations are being prescribed (e.g., qualitative assessment of polypharmacy), and if certain medication combinations have negative health outcomes specific to adults with CP.
Using a U.S. private administrative database, we found the prevalence of polypharmacy (≥ 5 medications) among adults without CP in this study to be 47.7% and 60.2% for elderly participants, which is similar to other studies leveraging administrative databases. For example, Ellenbogen et al. (2020) reported that 56.7% of elderly Medicare beneficiaries from the United States had polypharmacy,29 while Moriarty et al. (2015) reported that 60.4% of elderly adults from Ireland had polypharmacy.30 In the current study, we found that the unadjusted odds of polypharmacy, using the traditional cut-off of 5 medications, was 71% higher for adults with CP only compared with adults without CP. The unadjusted odds were more than 2.2-fold higher for adults with CP + NDDs compared with adults without CP. The elevated prevalence of polypharmacy became larger with the higher cut-off definitions of polypharmacy, with the unadjusted odds of ≥ 15 medications being 2.4-fold higher for adults with CP only and nearly 3-fold higher for adults with CP + NDDs. Further, the unadjusted odds of polypharmacy (all definitions) was approximately 21%-37% higher for adults with CP + NDDs compared with CP only.
Because of the elevated risk of a variety of chronic diseases and other morbidities among adults with CP,6,7 we adjusted the models for demographics and multimorbidity to determine the effect of CP (± NDDs) on the odds of polypharmacy beyond the presence of multimorbidity. While the elevated odds were attenuated, the change was modest and the adjusted odds remained substantially elevated for all comparisons. After adjustments and compared with adults without CP, adults with CP only were 34%-38% more likely to have polypharmacy, while adults with CP + NDDs were 59%-142% more likely to have polypharmacy. Further, after adjustments, adults with CP + NDDs were 19%-75% more likely to have polypharmacy compared with adults with CP only. The increase in the unadjusted to adjusted group difference comparing CP + NDDs with CP only is likely because of the age adjustment, since CP + NDDs adults were on average 9.5 years younger than CP only adults.
In the exploratory analysis, we observed that after accounting for demographics and multimorbidity, adults aged 18-40 years with CP experienced a similar or elevated burden of polypharmacy compared with the general geriatric population. This is consistent with the notion that adults with CP experience “accelerated aging,”31,32 which was defined as chronic disease development occurring earlier than expected. While polypharmacy is typically studied in the geriatric population, it is associated with adverse health and fatal outcomes.16,17 With regards to the population with CP, it may be necessary to take multiple medications to clinically manage multiple health conditions and morbidities,6,7 such as antiseizure medication to manage seizures among individuals with epilepsy, especially when comorbid with NDDs. The consequence of not taking multiple medications may lead to negative health outcomes, such as unmanaged type 2 diabetes. However, polypharmacy and lack of medication coordination by health care providers increases the risk of inappropriate medication prescription, potentially leading to adverse medication reactions. While this study provides robust evidence that adults with CP have an elevated prevalence of polypharmacy, we did not examine the constituents of polypharmacy profiles, such as presence of medications known to negatively interact with one another. Future studies are needed to characterize the polypharmacy profiles and determine their association with adverse health outcomes specific to adults with CP.
It is important to note that since this study leveraged private insurance claims, the sample with CP and CP + NDDs likely represented a healthier sector of the CP population.33 The implication is that the elevated polypharmacy prevalence reported here may be underrepresenting the true extent of polypharmacy for the greater CP population. This underestimation extends to the CP + NDDs group, as comorbidity of NDDs for this CP sample, which increases the medical complexity and medical needs of the individual with CP, was lower than expected based on other population-based studies.18,34
Limitations
This study has some limitations to consider. Since specific medications were not examined in this study, it is unknown if the elevated polypharmacy prevalence contains medications that are associated with adverse outcomes (e.g., mortality and nephrotoxicity). While this study breaks new ground by providing empirical evidence of the elevated polypharmacy prevalence beyond the presence of multimorbidity, the polypharmacy profiles need to be fully characterized in future studies, such as identifying which medications are being prescribed, along with their dosage and chronicity, to assist improvements in clinical care and management for aging with CP. Given that the polypharmacy prevalence among young adults with CP is similar to or elevated compared with the general geriatric population, future studies guided by the American Geriatrics Society Beers Criteria for Potentially Inappropriate Medication Use in Older Adults may provide unique insight into the potential harmful effects of polypharmacy for adults with CP.35 These updated guidelines detail medications that have potential adverse effects and medication-medication interactions.
Because claims data do not provide or reliably code for information regarding severity or type of CP, we were unable to account for relevant clinical phenotypes of the CP condition.
Finally, total number of medications in this study was defined as the total count of unique medications over the 12-month period and did not reflect exactly the number of medications that were concurrently prescribed. Claims data provide information on whether or not a prescription is filled, but not on whether the individual actually takes the medication and follows the dosage and timing correctly, making concurrent medication analysis challenging. Therefore, the term “polypharmacy” in this study may not be exact and should be considered a proxy for polypharmacy. Although, as previously noted, the prevalence of polypharmacy in the non-CP group was similar to other studies. Considering the extreme ends of the spectrum, the definition for the total number of medications encompassed individuals who could have been prescribed 6 nonconcurrent medications over the 12-month period or 6 concurrent medications. Importantly, each scenario could present different clinical situations and outcomes and requires further investigation, especially for adults with CP. Nevertheless, this study provides, for the first time, clinically meaningful findings of the number of medications adults with CP may be exposed to in a relatively short time span of 12 months, which can have latent or lasting physiological effects following cessation of some medications.
Conclusions
Privately insured adults with CP have an elevated prevalence of polypharmacy compared with adults without CP, even after accounting for multimorbidity. The polypharmacy prevalence is further elevated among adults with CP that have comorbid NDDs. Importantly, adults aged 18-40 years with CP have a similar (CP + NDDs) unadjusted prevalence of polypharmacy compared with the general population aged ≥ 65 years. Future studies are needed to determine if the polypharmacy profiles are appropriate for the elevated burden of adverse health associated with CP (e.g., chronic diseases), and if polypharmacy is associated with adverse health outcomes specific to adults with CP.
APPENDIX A. Odds Ratio of Polypharmacy for Adults with CP Only, CP + NDDs, and Without CPa with Complete Data on Race (N = 3,196,813)
| ≥ 5 Medications | ≥ 10 Medications | ≥ 15 Medications | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| CP only vs. without CP | |||
| Model 1 | 1.39 (1.30-1.49) | 1.36 (1.26-1.47) | 1.35 (1.23-1.49) |
| Model 1 + race | 1.38 (1.29-1.48) | 1.35 (1.26-1.46) | 1.35 (1.22-1.49) |
| CP + NDDs vs. without CP | |||
| Model 1 | 2.52 (2.26-2.80) | 2.23 (2.00-2.49) | 1.59 (1.38-1.83) |
| Model 1 + race | 2.50 (2.24-2.78) | 2.22 (1.99-2.48) | 1.58 (1.38-1.82) |
| CP + NDDs vs. CP only | |||
| Model 1 | 1.81 (1.60-2.06) | 1.64 (1.43-1.87) | 1.17 (0.99-1.39) |
| Model 1 + race | 1.81 (1.59-2.06) | 1.64 (1.43-1.87) | 1.17 (0.99-1.39) |
Note: Model 1: age (as continuous), sex, U.S. region of residence, and multimorbidity.
a The without CP group served as the reference group.
CI = confidence interval; CP = cerebral palsy; NDD = neurodevelopmental disabilities; OR = odds ratio.
APPENDIX B. Unadjusted Prevalence of Polypharmacy by Various Numerical Cut-Off Definitions
REFERENCES
- 1.Christensen D, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning – Autism and Developmental Disabilities Monitoring Network, USA, 2008. Dev Med Child Neurol. 2014;56(1):59-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitney DG, Singh H, Miller F, et al. Cortical bone deficit and fat infiltration of bone marrow and skeletal muscle in ambulatory children with mild spastic cerebral palsy. Bone. 2017;94:90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitney DG, Singh H, Zhang C, Miller F, Modlesky CM. Greater visceral fat but no difference in measures of total body fat in ambulatory children with spastic cerebral palsy compared to typically developing children. J Clin Densitom. September 22, 2018. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitney DG, Miller F, Pohlig RT, Modlesky CM. BMI does not capture the high fat mass index and low fat-free mass index in children with cerebral palsy and proposed statistical models that improve this accuracy. Int J Obes (Lond). 2019;43(1):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitney DG, Warschausky SA, Peterson MD. Mental health disorders and physical risk factors in children with cerebral palsy: a cross-sectional study. Dev Med Child Neurol. 2019;61(8):937-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitney DG, Hurvitz EA, Ryan JM, et al. Noncommunicable disease and multimorbidity in young adults with cerebral palsy. Clin Epidemiol. 2018;10:511-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitney DG, Kamdar NS, Ng S, Hurvitz EA, Peterson MD. Prevalence of high-burden medical conditions and health care resource utilization and costs among adults with cerebral palsy. Clin Epidemiol. 2019;11:469-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cremer N, Hurvitz EA, Peterson MD. Multimorbidity in middle-aged adults with cerebral palsy. Am J Med. 2017;130(6):744.e749-744.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitney DG, Warschausky SA, Ng S, Hurvitz EA, Kamdar NS, Peterson MD. Prevalence of mental health disorders among adults with cerebral palsy: a cross-sectional analysis. Ann Intern Med. 2019;171(5):328-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson MD, Ryan JM, Hurvitz EA, Mahmoudi E. Chronic conditions in adults with cerebral palsy. JAMA. 2015;314(21):2303-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berry JG, Berry SD. Caring for patients with neurological impairment: conversations between a pediatrician and geriatrician. JAMA Pediatr. 2018;172(9):795-96. [DOI] [PubMed] [Google Scholar]
- 12.Vohra R, Madhavan S, Sambamoorthi U, St Peter C. Access to services, quality of care, and family impact for children with autism, other developmental disabilities, and other mental health conditions. Autism. 2014;18(7):815-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844-52. [DOI] [PubMed] [Google Scholar]
- 14.Sergi G, De Rui M, Sarti S, Manzato E. Polypharmacy in the elderly: can comprehensive geriatric assessment reduce inappropriate medication use? Drugs Aging. 2011;28(7):509-18. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi H, Hasanloei MA, Mahmoudi J. Polypharmacy-induced drug-drug interactions; threats to patient safety. Drug Res (Stuttg). 2014;64(12):633-37. [DOI] [PubMed] [Google Scholar]
- 16.Leelakanok N, Holcombe AL, Lund BC, Gu X, Schweizer ML. Association between polypharmacy and death: a systematic review and meta-analysis. J Am Pharm Assoc (2003). 2017;57(6):729-738.e10. [DOI] [PubMed] [Google Scholar]
- 17.Nunes BP, Flores TR, Mielke GI, Thume E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130-38. [DOI] [PubMed] [Google Scholar]
- 18.Reid SM, Meehan EM, Arnup SJ, Reddihough DS. Intellectual disability in cerebral palsy: a population-based retrospective study. Dev Med Child Neurol. 2018;60(7):687-94. [DOI] [PubMed] [Google Scholar]
- 19.Schoufour JD, Oppewal A, van der Maarl HJK, et al. Multimorbidity and polypharmacy are independently associated with mortality in older people with intellectual disabilities: a 5-year follow-up from the HA-ID Study. Am J Intellect Dev Disabil. 2018;123(1):72-82. [DOI] [PubMed] [Google Scholar]
- 20.Whitney D, Kamdar N, Hirth RA, Hurvitz EA, Peterson MD. Economic burden of paediatric-onset disabilities among young and middle-aged adults in the USA: a cohort study of privately insured beneficiaries. BMJ Open. 2019;9(9):e030490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitney DG, Whibley D, Jepsen KJ. The effect of low-trauma fracture on one-year mortality rate among privately insured adults with and without neurodevelopmental disabilities. Bone. 2019;129:115060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitney DG, Kamdar NS, Ng S, Hurvitz EA, Peterson MD. Prevalence of high-burden medical conditions and healthcare resource utilization and costs among adults with cerebral palsy. Clin Epidemiol. 2019;11:469-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves S, Garcia E, Kleyn M, et al. Identifying sickle cell disease cases using administrative claims. Acad Pediatr. 2014;14(5 Suppl):S61-S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drieling RL, LaCroix AZ, Beresford SA, Boudreau DM, Kooperberg C, Heckbert SR. Validity of self-reported medication use compared with pharmacy records in a cohort of older women: findings from the Women’s Health Initiative. Am J Epidemiol. 2016;184(3):233-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson K, Kenny RA, Peklar J, Bennett K. Agreement between patient interview data on prescription medication use and pharmacy records in those aged older than 50 years varied by therapeutic group and reporting of indicated health conditions. J Clin Epidemiol. 2013;66(11):1308-16. [DOI] [PubMed] [Google Scholar]
- 26.Stortz JN, Lake JK, Cobigo V, Ouellette-Kuntz HM, Lunsky Y. Lessons learned from our elders: how to study polypharmacy in populations with intellectual and developmental disabilities. Intellect Dev Disabil. 2014;52(1):60-77. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268-74. [DOI] [PubMed] [Google Scholar]
- 28.Whitney DG, Miller F, Pohlig RT, Modlesky CM. BMI does not capture the high fat mass index and low fat-free mass index in children with cerebral palsy and proposed statistical models that improve this accuracy. Int J Obes (Lond). 2019;43(1):82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellenbogen MI, Wang P, Overton HN, et al. Frequency and predictors of polypharmacy in U.S. Medicare patients: a cross-sectional analysis at the patient and physician levels. Drugs Aging. 2020;37(1):57-65. [DOI] [PubMed] [Google Scholar]
- 30.Moriarty F, Hardy C, Bennett K, Smith SM, Fahey T. Trends and interaction of polypharmacy and potentially inappropriate prescribing in primary care over 15 years in Ireland: a repeated cross-sectional study. BMJ Open. 2015;5(9):e008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson MD, Gordon PM, Hurvitz EA. Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obes Rev. 2013;14(2):171-82. [DOI] [PubMed] [Google Scholar]
- 32.Verschuren O, Smorenburg ARP, Luiking Y, Bell K, Barber L, Peterson MD. Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: a narrative review of the literature. J Cachexia Sarcopenia Muscle. 2018;9(3):453-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitney DG. Prevalence of high-burden medical conditions among young and middle-aged adults with pediatric-onset medical conditions: findings from U.S. private and public administrative claims data. Int J Health Policy Manag. 2019;8(11):629-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonnsen BL, Boan AD, Bradley CC, Charles J, Cohen A, Carpenter LA. Prevalence of autism spectrum disorders among children with intellectual disability. Am J Intellect Dev Disabil. 2016;121(6):487-500. [DOI] [PubMed] [Google Scholar]
- 35.2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674-94. [DOI] [PubMed] [Google Scholar]


