Abstract
BACKGROUND:
Hepatic encephalopathy (HE) is a complication of cirrhosis of the liver causing neuropsychiatric abnormalities. Clinical manifestations of overt HE result in increased health care resource utilization and effects on patient quality of life. While lactulose has historically been the mainstay of treatment for acute HE and maintenance of remission, there is an unmet need for additional therapeutic options with a favorable adverse event profile. Compared with lactulose alone, rifaximin has demonstrated proven efficacy in complete reversal of HE and reduction in the incidence of HE recurrence, mortality, and hospitalizations. Evidence suggests the benefit of long-term prophylactic therapy with rifaximin; however, there is a need to assess the economic impact of rifaximin treatment in patients with HE.
OBJECTIVE:
To assess the incremental cost-effectiveness of rifaximin ± lactulose versus lactulose monotherapy in patients with overt HE.
METHODS:
A Markov model was developed in Excel with 4 health states (remission, overt HE, liver transplantation, and death) to predict costs and outcomes of patients with HE after initiation of maintenance therapy with rifaximin ± lactulose to avoid recurrent HE episodes. Cost-effectiveness of rifaximin was evaluated through estimation of incremental cost per quality-adjusted life-year (QALY) or life-year (LY) gained. Analyses were conducted over a lifetime horizon. One-way deterministic and probabilistic sensitivity analyses were conducted to assess uncertainty in results.
RESULTS:
The rifaximin ± lactulose regimen provided added health benefits despite an additional cost versus lactulose monotherapy. Model results showed an incremental benefit of $29,161 per QALY gained and $27,762 per LY gained with rifaximin ± lactulose versus lactulose monotherapy. Probabilistic sensitivity analyses demonstrated that the rifaximin ± lactulose regimen was cost-effective ~99% of the time at a threshold of $50,000 per QALY/LY gained, which falls within the commonly accepted threshold for incremental cost-effectiveness.
CONCLUSIONS:
The clinical benefit of rifaximin, combined with an acceptable economic profile, demonstrates the advantages of rifaximin maintenance therapy as an important option to consider for patients at risk of recurrent HE.
What is already known about this subject
Hepatic encephalopathy (HE) is a complication of significant liver disease that causes neuropsychiatric abnormalities and results in increased health care resource utilization.
Lactulose has historically been the first-line treatment for acute episodes of HE and maintenance of remission but is limited by inadequate efficacy and safety.
Rifaximin + lactulose combination therapy has demonstrated greater benefit in reducing HE recurrence and length of hospital stay compared with lactulose alone.
What this study adds
This research addresses limitations from a previous economic analysis of rifaximin for the treatment of patients with HE.
The current analysis demonstrates that the clinical benefits of a rifaximin-based regimen may be obtained at reasonable cost to U.S. payers relative to lactulose monotherapy.
Hepatic encephalopathy (HE) is a complication of cirrhosis of the liver causing neuropsychiatric abnormalities.1 Approximately 30%-45% of patients with cirrhosis and 10%-50% of patients who require transjugular intrahepatic portosystemic shunt (TIPS) insertion for complications of liver disease experience episodes of overt HE.2-4 The establishment of a TIPS bypasses ammonia and other neurotoxins directly into systemic circulation, increasing the incidence of HE, which often occurs soon after (i.e., within 3 months) TIPS insertion; over time, there is cerebral adaptation to gut-derived neurotoxins. A major risk factor for post-TIPS development of HE is a previous history of HE.5 Clinical manifestations of overt HE include generalized psychomotor dysfunction with alterations in consciousness.6 Episodes of overt HE may result in increased health care resource utilization and can affect quality of life of both patients and families.7,8
After an initial episode of overt HE, secondary prophylaxis is recommended to decrease the risk of recurrence, which is considered preventable with proper prophylaxis.9-12 Lactulose has historically been used as first-line therapy for acute HE and for maintenance of remission.6,13,14 Use of lactulose as a maintenance medication may be limited because of a lack of randomized, placebo-controlled trials to determine efficacy.14,15 In addition, lactulose is associated with unpleasant or sweet taste, dehydration, and gastrointestinal adverse events (AEs), such as diarrhea, nausea, bloating, and gas,6,14 which may lead to medication nonadherence.16 Given these concerns, additional HE therapies with a more favorable AE profile have been investigated in the treatment of overt HE.
Rifaximin (Xifaxan 550 mg tablets) is a minimally absorbed, oral antimicrobial agent that was approved for reduction in the risk of overt HE recurrence in adults by the U.S. Food and Drug Administration in 2010.17 This therapy is often used concomitantly with lactulose.15 In a randomized controlled trial over a 6-month period, rifaximin maintained remission from HE and reduced the risk of HE-related hospitalizations more effectively than placebo in a cohort of patient with decompensated cirrhosis and previous episodes of overt HE, with approximately 90% in both arms receiving concomitant lactulose.18 The rates of AEs and hospitalizations were similar in a 24-month open-label follow-up study.19
Additional research indicates that rifaximin may be beneficial in reducing incidence of HE recurrence,20-24 mortality,20,21,23,24 and rate of hospitalizations by up to 58%,21,22 which may translate to a decrease in the health care cost burden attributed to HE.
This study aimed to assess the incremental cost-effectiveness of rifaximin ± lactulose versus lactulose monotherapy in patients with overt HE. For this purpose, a cost-effectiveness model of patients who were in remission from recurrent HE was developed.
Methods
Using Microsoft Excel, a cost-effectiveness model was created to predict costs and outcomes of patients with HE after initiation of maintenance therapy with rifaximin ± lactulose to avoid recurrent HE episodes. Rifaximin ± lactulose refers to the use of rifaximin 550 mg twice daily ± lactulose, reflecting a population consistent with the rifaximin phase 3 maintenance of remission trial in which 91.4% of patients received both medications.18
Poor compliance and adherence to HE therapy contributes to increased disease burden.25 Because of a lack of available data to explicitly model these factors, the potential effect of poor compliance with, or adherence to, therapy was captured indirectly via observed risks of overt HE. Quality-adjusted life-years (QALYs) and life-years (LY) were evaluated for both cohorts in the context of the direct costs associated with each treatment regimen. The analysis was conducted from a third-party U.S. payer perspective.
Model Structure
A Markov model was used to predict the course of HE following initiation of maintenance therapy to avoid recurrent HE episodes. Patients are assumed to begin in the remission state, where they are at risk for an overt HE episode with or without hospitalization, death, or liver transplant in each 2-week cycle over a lifetime horizon (Figure 1). Patients in the overt HE state (with or without a hospitalization) can transition back to the remission state, die, or receive a liver transplant. Patients transitioning to the death state exit the model after accruing appropriate costs and outcomes. The liver transplantation state is also an absorbing or exit state. Patients accrue the cost of transplantation, the average life expectancy after transplantation is applied, and QALYs/LYs are accrued.
FIGURE 1.
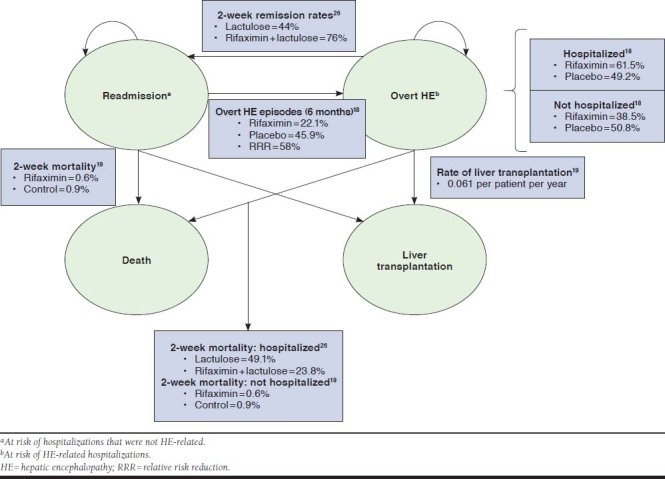
Model Structure
Clinical Inputs
Clinical inputs and data sources for the model are provided in Table 1. The risk of an initial HE episode was based on clinical trial data.18 The risk of a subsequent episode of overt HE was based on the initial 2-week hazard function estimate and was assumed to be invariable over time. The effect of varying risk (excess risk with each additional episode) was tested in the sensitivity analysis, since this excess risk is not reported in the literature.
TABLE 1.
Cost-Effectiveness Model Clinical Inputs
| Input | Rifaximin ± Lactulose | Placebo ± Lactulose | Source |
|---|---|---|---|
| Population inputs | |||
| On concomitant lactulose, % | 91.4 | 91.2 | Bass 201018 |
| Remission state inputs | |||
| With overt episodes by 6 months, %a | 22.1 | 45.9 | Bass 201018 |
| Hospitalizations per person-years of exposure | 0.24b | 0.58c | Mullen 201419 |
| Mortality at year 5, %d | 52.8 | 69.9 | Mullen 201419 |
| Health utility | 0.937e | Guest 201428 | |
| Overt state inputs | |||
| Hospitalized among those with an overt episode, % | 61.5 | 49.2 | Bass 201018 |
| Reversed after 2 weeks among hospitalized patients, % | 76.0 | 44.0 | Sharma 201326 |
| In-hospital 2-week mortality, % | 23.8 | 49.1 | Sharma 201326 |
| Two-week mortality after hospitalization, %d | 0.6 | 0.9 | Mullen 201419 |
| Nonhospitalized 2-week mortality, %d | 0.6 | 0.9 | Mullen 201419 |
| Health utility for HE | 0.783f | Bass 201018; Guest 201428 | |
| Liver transplantation inputs | |||
| Number of liver transplantations per patient per year | 0.061 | 0.061 | Mullen 201419 |
| Life expectancy after liver transplantationg | 29.3 years | OPTN and SRTR 2016 Annual Data Report (Kim 201827) | |
| Health utility after liver transplantation | 0.962h | Guest 201428 | |
a Time to first breakthrough HE episode Kaplan-Meier curves from Bass 201018 were fit to follow Weibull distributions with γ and λ parameters. The γ parameter is fixed in the analysis, and percentage at 6 months from Bass 201018 is used to calibrate the λ parameter (rifaximin group γ = 0.6376, λ = 0.2466; lactulose group γ = 0.7716, λ = 1.1555).
b Based on all-rifaximin group.
c Based on historical placebo group.
d Constant hazard assumed based on events per person-years of exposure.
e Average of reported health utilities for Conn grades 0 and 1.
f Weighted average of health utilities reported for Conn grades 2, 3, and 428 was taken based on distribution of Conn scores during most recent HE episode before the rifaximin study.18
g Five-year survival curve extrapolated to estimate median life expectancy.18
h Health utility reported for Conn grade 0 assumed to reflect posttransplant health utility.
HE = hepatic encephalopathy; OPTN = Organ Procurement and Transplantation Network; SRTR = Scientific Registry of Transplant Recipients.
Upon transition to an overt HE episode, the proportion of patients requiring an HE-related hospitalization was applied to the rifaximin + lactulose alone patients separately using estimates from the literature.18 Patients with an overt episode requiring hospitalization remained in the overt state until remission, death, or liver transplantation. Rates of reversal for patients hospitalized with overt HE and initial 2-week inpatient mortality were obtained from a randomized controlled trial comparing treatment with a rifaximin regimen with lactulose alone.26 Mortality after discharge was estimated from a 24-month open-label study of rifaximin from which biweekly rates were calculated and applied.19 For these patients, the average cost of a hospitalization was applied once during the cycle corresponding to the time an overt episode occurred.
The remaining proportion of patients transitioning to overt HE was assumed not to be hospitalized. Mortality during this period was assumed to be the same as the postdischarge mortality for hospitalized patients.19 All nonhospitalized patients were assumed to recover within 2 weeks and revert completely to the remission state.
Rates of liver transplantation were assumed to be equal for patients receiving rifaximin ± lactulose, regardless of HE status. Probability of transplantation was obtained from the open-label rifaximin study,19 and survival among transplant recipients was adjusted according to published life expectancy estimates.27
Health Utility Inputs
All health utility values for patients with overt HE (0.783), patients with decompensated cirrhosis in remission/no overt HE (0.937), and patients receiving liver transplant (0.962) were obtained from published literature.18,28
Costs
Wholesale drug acquisition costs, hospitalization costs, and costs related to liver transplant were included in the model (Table 2).29-32 The full cost of therapy was included in the model, since there were insufficient data to adjust for compliance and adherence in the base analysis. Indirect costs were not included because the analysis was from the U.S. payer perspective. Costs were estimated and reported in 2018 U.S. dollars. If charges from previous years were used, they were inflated to current values using the medical care component of the Consumer Price Index and adjusted to private payer payments.33-35
TABLE 2.
Cost-Effectiveness Model Cost Inputs
| Costsa ($) | Rifaximin ± Lactulose | Placebo ± Lactulose | Source |
|---|---|---|---|
| Cost of HE-related hospitalizationb | 19,710 | 24,527 | Leevy 200730; BLS 201833; CMS FY 201835; AHA 201634 |
| Cost of non-HE-related hospitalizationc | 15,892 | AHRQ HCUP 201831; AHA 201634; BLS 201833 | |
| Cost of liver transplantationd,21,23,25 | 183,132 | Wai 201432; BLS 201833; AHA 201634 | |
a Drug costs per day for rifaximin and lactulose were $73.17 and $1.30, respectively.29
b Per-hospitalization charge from Leevy 200730 converted to 2014 private payer payment using the medical care component of Consumer Price Index 2018,33 cost-to-charge ratios from the CMS,35 and the 2016 payment-to-cost ratio from the AHA report.34
c HCUP 2014 cost31 (ICD-9-CM diagnosis codes 571.2, 571.3, 571.5, 571.6, 571.8, 571.9, 572.4, 584.9, 567.23, 456.0) inflated to 2018 cost and converted to private payer cost using 2016 payment-to-cost ratio from the AHA report.34
d One-time liver transplant cost from Wai 201432 converted to 2018 private payer payment using medical care component of Consumer Price Index33 and 2016 payment-to-cost ratio from the AHA report.34
AHA = American Hospital Association; AHRQ = Agency for Healthcare Research and Quality; BLS = Bureau of Labor Statistics; CMS = Centers for Medicare & Medicaid Services; FY = fiscal year; HCUP = Healthcare Cost and Utilization Project; HE = hepatic encephalopathy; ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification.
Analysis
Total costs associated with each treatment were reported in aggregate and by component (drug, other direct, hospitalization, and liver transplantation costs). LYs, QALYs, and costs were discounted at an annual rate of 3.0%. Cost-effectiveness of rifaximin was assessed through estimation of the incremental cost per QALY gained and the cost per LY gained. Analyses were conducted over a lifetime horizon. Threshold analyses were conducted to estimate the wholesale acquisition cost (WAC) at which rifaximin would need to be set to achieve a cost per QALY of $50,000, $100,000, and $150,000.
One-way deterministic sensitivity analyses were conducted on individual parameters to estimate the potential impact of variability in these estimates defined by the boundaries of the standard error for each input (Table 3). Probabilistic sensitivity analyses, where all parameters are varied simultaneously in a simulation by randomly sampling values based on predefined distributions (Table 3),36 were also conducted to estimate the robustness of the results over the range of potential input values defined by the standard error and shape of the distribution.
TABLE 3.
Sensitivity Analysis Inputs
| Input | Range of Inputs Used for Deterministic and Probabilistic Sensitivity Analyses | Probabilistic Sensitivity Analysis Statistical Distribution | |
|---|---|---|---|
| Rifaximin ± Lactulose | Placebo ± Lactulose | ||
| Population | |||
| On concomitant lactulose, % | 86.2-95.5 | 86.3-95.1 | Beta |
| Remission state | |||
| With overt episodes by 6 months, %a | 15.2-29.0 | 38.2-53.6 | Normal |
| Hospitalizations per person-years exposure | 0.18-0.30 | 0.44-0.73 | Gamma |
| Mortality at year 5, %a | 47.9-58.0 | 62.8-77.0 | Normal |
| Health utility | 0.92-0.95 | Beta | |
| Overt state | |||
| Hospitalization among those with an overt episode, % | 53.3-69.4 | 41.4-57.0 | Beta |
| Reversed after 2 weeks among hospitalized patients, % | 64.7-85.7 | 31.4-57.0 | Beta |
| In-hospital 2-week mortality, % | 14.1-35.1 | 36.2-62.1 | Beta |
| Two-week mortality after hospitalization, % | 0.1-1.6 | 0.1-2.8 | Beta |
| Nonhospitalized 2-week mortality, % | 0.1-1.6 | 0.1-2.8 | Beta |
| Health utility for HE | 0.74-0.82 | Beta | |
| Liver transplantation | |||
| Number of liver transplantations per patient per year | 0.05-0.08 | Gamma | |
| Life expectancy after liver transplantation | 24.0-35.0 years | Normal | |
| Health utility after liver transplantation | 0.95-0.97 | Beta | |
| Costs, $ | |||
| Lactulose daily cost | 1.16-1.45 | Gamma | |
| Cost of HE-related hospitalization | 15,093-24,934 | 18,782-31,026 | Gamma |
| Cost of non-HE-related hospitalization | 12,169-20,104 | Gamma | |
| Cost of liver transplantation | 169,574-197,204 | Gamma | |
| Other variables | |||
| Relative risk for subsequent overt episodesb | 0.25-2.00 | Lognormal | |
| Discount rate, costs, % | 0.0-6.0 | N/A | |
| Discount rate, benefits, % | 0.0-6.0 | N/A | |
Results
Predicted QALYs and LYs associated with rifaximin ± lactulose were twice as high compared with lactulose alone (Table 4). Because of this extended life expectancy, the number of patients receiving liver transplantation doubled with rifaximin ± lactulose, with an estimated 20 patients predicted to reach transplantation compared to 9 with lactulose alone (Table 4). The added health benefits with rifaximin ± lactulose were achieved at an additional cost of $96,375 over lactulose alone (Table 4), with an incremental cost per QALY gained of $29,161 and the incremental cost per LY gained of $27,762.
TABLE 4.
Clinical and Economic Outcomes
| Outcome | Rifaximin + Lactulose | Placebo + Lactulose | Difference |
|---|---|---|---|
| Clinical outcomes | |||
| QALYs per patient, discounted (undiscounted) | 6.4 (8.9) | 3.1 (4.1) | 3.3 (4.9) |
| LYs per patient, discounted (undiscounted) | 6.7 (9.4) | 3.2 (4.3) | 3.5 (5.1) |
| Number of liver transplantations (per 100) | 20 | 9 | 11 |
| Costs, $ | |||
| Drug costs | 84,521 | 664 | 83,857 |
| Other direct costs | 67,885 | 55,367 | 12,518 |
| Hospitalizations | 34,036 | 39,316 | –5,280 |
| HE-related | 22,469 | 26,257 | –3,789 |
| Non-HE-related | 11,568 | 13,059 | –1,491 |
| Liver transplantation | 33,849 | 16,051 | 17,798 |
| Total | 152,406 | 56,031 | 96,375 |
HE = hepatic encephalopathy; LY = life-year; QALY = quality-adjusted life-year.
The cost-effectiveness of a rifaximin ± lactulose regimen remained between ~$20,000 per QALY and ~$40,000 per QALY across univariate sensitivity analyses, irrespective of the parameter considered. At the time the model was developed, the WAC for rifaximin was 42% below the cost to exceed the $50,000 per QALY threshold and 71% and 81% below the cost to exceed the $100,000 per QALY and $150,000 per QALY thresholds, respectively. Outcomes were most sensitive to variation in the relative risk of subsequent overt HE episodes with lactulose monotherapy. Outcomes were most sensitive to variation in the relative risk of subsequent overt HE episodes with lactulose monotherapy.
In probabilistic sensitivity analyses, when multiple parameters were varied simultaneously, the combination of rifaximin ± lactulose was estimated to be cost-effective over half the time at a willingness-to-pay threshold of ~$28,000 per QALY/LY gained or higher. This regimen was predicted to be cost-effective ~99% of the time at a threshold of $50,000 per QALY/LY gained. These estimates are within the commonly accepted threshold for incremental cost-effectiveness of $50,000.37
Discussion
Current guidelines recommend prophylactic treatment following an episode of overt HE to reduce the risk of recurrent HE.15 Considerable evidence suggests the benefit of long-term prophylactic therapy with rifaximin1,19-21,23,24,26,38,39; however, evidence evaluating the economic effect of rifaximin treatment is lacking. The few published resource utilization studies identified consider the costs associated with HE in general,25 in the acute inpatient setting,8 or in diagnostic screening in patients with minimal HE.39
A study by Huang et al. (2007) concluded that although rifaximin monotherapy is clinically safe and effective,40 when used as monotherapy, it is unlikely to be cost-effective under the majority of circumstances. Alternatively, a recent systematic review of studies reporting economic data for HE and rifaximin and/or lactulose found that rifaximin had a favorable economic profile.41 The current analysis demonstrates that the clinical benefits of a rifaximin-based regimen may be obtained at reasonable cost to U.S. payers relative to lactulose monotherapy and that the price of rifaximin provides good value relative to commonly accepted cost per QALY thresholds.
The present study improves on the identified limitations of the Huang et al. economic analysis,40 which served as a basis for this analysis. Differences in the original economic analysis and the present study may account for differences in overall findings. Studies were conducted over a decade apart, during which time significant contributions to the knowledge and understanding of rifaximin treatment for overt HE have been made.18-20,23,24,26,38,42 This economic analysis was able to include data from head-to-head comparison studies of rifaximin ± lactulose,18 including long-term follow-up data.19 The Huang et al. paper relied on studies of varying duration and design to inform the analysis.40 The current analysis also includes a rifaximin + lactulose treatment arm not considered in the 2007 Huang study, partially due to the refinement of treatment guidelines for HE after its publication.15,40 Finally, understanding of the potential effect of HE on patient-centered outcomes (e.g., quality of life) has evolved during the time period between these 2 economic analyses.28
Limitations
There are potential assumptions and limitations that must be considered when interpreting the results of this model. Simplifying assumptions were made to account for data shortcomings, with a goal of achieving balance between face validity of the model and the intricacies of clinical practice and disease progression. For example, the model did not explicitly take into account patient compliance to therapy because the available data were considered to be inadequate. The treatment effectiveness inputs should inherently reflect the underlying compliance of patients within the context of the included studies. Costs of drugs assumed full compliance, which should result in conservative estimates of the overall benefit of a rifaximin regimen by allocating the full cost of therapy whether or not a patient achieves the full clinical benefit of therapy.
While it is theoretically possible that HE treatment may have a disease-modifying effect (i.e., reversal of decompensation), the current model did not account for this potential. Therefore, the model may underestimate the actual benefits of long-term maintenance therapy with rifaximin.
In addition, patients still in the overt HE state at 2 weeks after hospital discharge were assumed to have the same risk of death as patients with overt HE who were not hospitalized. Estimates were based on observed deaths in an open-label extension study under the assumption of a constant hazard function.19 It may be assumed that patients who are hospitalized for an overt HE episode are more severe or at higher risk of death than those managed in the outpatient setting.
Patients may also be at risk of ongoing neurological complications following recurrent episodes after transplant.43-45 These aspects are captured in the elevated mortality risk during hospitalization. Risk of subsequent overt HE episodes was calculated based on the initial hazard function estimate. Variation in these parameters did not substantially affect the results.
Finally, health utility estimates used in the model were obtained from a study using the standard gamble and time trade-off techniques,28 and the estimates appear to be lower than what has previously been published for HE.46 Hence, a separate scenario where overt and remission state utilities were taken as 0.55 and 0.74, respectively, was implemented based on a survey of 114 patients with cirrhosis.46 This resulted in a slight increase in incremental cost-effectiveness of $32,182 per QALY gained for rifaximin.
Conclusions
This model expands on the work of Huang et al. by integrating current clinical evidence and guidelines.40 The clinical benefit of rifaximin, combined with an acceptable economic profile, demonstrates the advantages of rifaximin maintenance therapy as an important option to consider for patients at risk of recurrent HE.
ACKNOWLEDGMENTS
The authors acknowledge Minha Choi, PharmD, and Kylie Matthews, of Xcenda, Palm Harbor, FL, for medical writing and editorial support, which was funded by Salix Pharmaceuticals. The authors also acknowledge Howard Franklin, MD, MBA, an employee of Salix, for his clinical review of the manuscript.
REFERENCES
- 1.Bajaj JS, Wade JB, Sanyal AJ. Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014-21. [DOI] [PubMed] [Google Scholar]
- 2.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25(Suppl 1):3-9. [DOI] [PubMed] [Google Scholar]
- 3.Elwir S, Rahimi RS. Hepatic encephalopathy: an update on the pathophysiology and therapeutic options. J Clin Transl Hepatol. 2017;5(2):142-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz SJ. Hepatic encephalopathy. Med Clin North Am. 2008;92(4):795-812, viii. [DOI] [PubMed] [Google Scholar]
- 5.Copelan A, Kapoor B, Sands M. Transjugular intrahepatic portosystemic shunt: indications, contraindications, and patient work-up. Semin Intervent Radiol. 2014;31(3):235-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chacko KR, Sigal SH. Update on management of patients with overt hepatic encephalopathy. Hosp Pract (1995). 2013;41(3):48-59. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj JS, Wade JB, Gibson DP, et al. . The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanova M, Mishra A, Venkatesan C, Younossi ZM. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9): 1034-41.e1. [DOI] [PubMed] [Google Scholar]
- 9.Acharya C, Bajaj JS. Current management of hepatic encephalopathy. Am J Gastroenterol. 2018;113(11):1600-12. [DOI] [PubMed] [Google Scholar]
- 10.Kornerup LS, Gluud LL, Vilstrup H, Dam G. Update on the therapeutic management of hepatic encephalopathy. Curr Gastroenterol Rep. 2018;20(5):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saab S. Evaluation of the impact of rehospitalization in the management of hepatic encephalopathy. Int J Gen Med. 2015;8:165-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blei AT, Cordoba J, Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968-76. [DOI] [PubMed] [Google Scholar]
- 14.Thompson JR. Treatment guidelines for hepatic encephalopathy. Pharmacotherapy. 2010;30(5 Pt 2):4S-9S. [DOI] [PubMed] [Google Scholar]
- 15.Vilstrup H, Amodio P, Bajaj J, et al. . Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715-35. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj J, Sanyal A, Bell D, Gilles H, Hueuman D. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31(9):1012-17. [DOI] [PubMed] [Google Scholar]
- 17.Xifaxan (rifaximin) tablets, for oral use. Salix Pharmaceuticals. January 2019. Available at: https://shared.salix.com/shared/pi/xifaxan550-pi.pdf. Accessed April 28, 2020.
- 18.Bass NM, Mullen KD, Sanyal A, et al. . Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071-81. [DOI] [PubMed] [Google Scholar]
- 19.Mullen KD, Sanyal AJ, Bass NM, et al. . Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol. 2014;12(8):1390-97.e2. [DOI] [PubMed] [Google Scholar]
- 20.Bannister CA, Orr JG, Reynolds AV, et al. . Natural history of patients taking rifaximin-alpha for recurrent hepatic encephalopathy and risk of future overt episodes and mortality: a post-hoc analysis of clinical trials data. Clin Ther. 2016;38(5):1081-89.e4. [DOI] [PubMed] [Google Scholar]
- 21.Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31(4):434-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajaj JS, Barrett AC, Bortey E, Paterson C, Forbes WP. Prolonged remission from hepatic encephalopathy with rifaximin: results of a placebo crossover analysis. Aliment Pharmacol Ther. 2015;41(1):39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang SH, Lee YB, Lee JH, et al. . Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther. 2017;46(9):845-55. [DOI] [PubMed] [Google Scholar]
- 24.Ryan JD, Tsochatzis EA. Rifaximin treatment for encephalopathy reduces hospital resource use: real-world data don’t fail to IMPRESS. Frontline Gastroenterol. 2017;8(4):230-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neff G. Factors affecting compliance and persistence with treatment for hepatic encephalopathy. Pharmacotherapy. 2010;30(5 Pt 2):22S-27S. [DOI] [PubMed] [Google Scholar]
- 26.Sharma BC, Sharma P, Lunia MK, Srivastava S, Goyal R, Sarin SK. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458-63. [DOI] [PubMed] [Google Scholar]
- 27.Kim WR, Lake JR, Smith JM, et al. . OPTN/SRTR 2016 annual data report: Liver. Am J Transplantation. 2018;18(S1):172-253. [DOI] [PubMed] [Google Scholar]
- 28.Guest JF, Nanuwa K, Barden R. Utility values for specific hepatic encephalopathy health states elicited from the general public in the United Kingdom. Health Qual Life Outcomes. 2014;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.IBM Watson Health. IBM Micromedex RED BOOK. [Database]. 2018. Available at: https://www.micromedexsolutions.com/micromedex2/librarian/ssl/true. Accessed April 28, 2020.
- 30.Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737-41. [DOI] [PubMed] [Google Scholar]
- 31.HCUPnet (Healthcare Cost and Utilization Project). Free health care statistics. 2018. Agency for Healthcare Research and Quality, Rockville, MD. Available at: https://hcupnet.ahrq.gov/#setup. Accessed April 28, 2020. [Google Scholar]
- 32.Wai H, Stepanova M, Saab S, Erario M, Srishord M, Younossi ZM. Inpatient economic and mortality assessment for liver transplantation: a nationwide study of the United States data from 2005 to 2009. Transplantation. 2014;97(1):98-103. [DOI] [PubMed] [Google Scholar]
- 33.Bureau of Labor Statistics. Consumer Price Index-All Urban Consumers. 2018. Available at: http://data.bls.gov/cgi-bin/surveymost?cu. Accessed April 28, 2020.
- 34.American Hospital Association. Table 4.4. Aggregate hospital payment-to-cost ratios for private payers, Medicare, and Medicaid, 1995-2016. In: TrendWatch Chartbook 2018. Available at: https://www.aha.org/system/files/2018-05/2018-chartbook-table-4-4.pdf. Accessed April 28, 2020.
- 35.Centers for Medicare & Medicaid Services. Cost to charge ratios. Tables 8A, 8B, 8C. FY 2019 Final Rule, Correction Notice, and Notice Tables. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2019-IPPS-Final-Rule-Home-Page-Items/FY2019-IPPS-Final-Rule-Tables. Accessed May 14, 2020.
- 36.Briggs AH, Weinstein MC, Fenwick EA, et al. . Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force—6. Value Health. 2012;15(6):835-42. [DOI] [PubMed] [Google Scholar]
- 37.Grosse SD. Assessing cost-effectiveness in healthcare: history of the $50,000 per QALY threshold. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):165-78. [DOI] [PubMed] [Google Scholar]
- 38.Sanyal A, Younossi ZM, Bass NM, et al. . Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34(8):853-61. [DOI] [PubMed] [Google Scholar]
- 39.Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology. 2012;55(4):1164-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang E, Esrailian E, Spiegel BM. The cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy - a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147-61. [DOI] [PubMed] [Google Scholar]
- 41.Neff G, Zachry W 3rd.. Systematic review of the economic burden of overt hepatic encephalopathy and pharmacoeconomic impact of rifaximin. Pharmacoeconomics. 2018;36(7):809-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450-55. [DOI] [PubMed] [Google Scholar]
- 43.Bajaj J, Schubert C, Heuman D, et al. . Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fu K, DiNorcia J, Sher L, et al. . Predictive factors of neurological complications and one-month mortality after liver transplantation. Front Neurol. 2014;5:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stracciari A, Guarino M. Neuropsychiatric complications of liver transplantation. Metab Brain Dis. 2001;16(1-2):3-11. [DOI] [PubMed] [Google Scholar]
- 46.Wells CD, Murrill WB, Arguedas MR. Comparison of health-related quality of life preferences between physicians and cirrhotic patients: implications for cost-utility analyses in chronic liver disease. Dig Dis Sci. 2004;49(3):453-58. [DOI] [PubMed] [Google Scholar]


