Abstract
BACKGROUND:
Prostaglandin analogs are the most effective treatment for glaucoma, a common condition among older adults. Despite the availability of generic drugs, the costs associated with these prescription drugs are rising.
OBJECTIVE:
To characterize Medicare prescription drug plan (PDP) formulary coverage and beneficiary out-of-pocket cost for prostaglandin analogs from 2009 to 2017 and Medicare spending on prostaglandin analogs from 2013 to 2017.
METHODS:
This study was a retrospective analysis. We used 2009, 2013, and 2017 Medicare PDP formulary, beneficiary cost, and pricing files to determine beneficiary first-prescription out-of-pocket costs and plan coverage (unrestricted, restricted, or not covered) of branded latanoprost 0.005%, travoprost 0.004%, bimatoprost 0.03% and 0.01%, and tafluprost 0.0015% and of generic latanoprost 0.005% and generic bimatoprost 0.03%. We also used Medicare Part D spending data to determine aggregate spend in 2013 and 2017.
RESULTS:
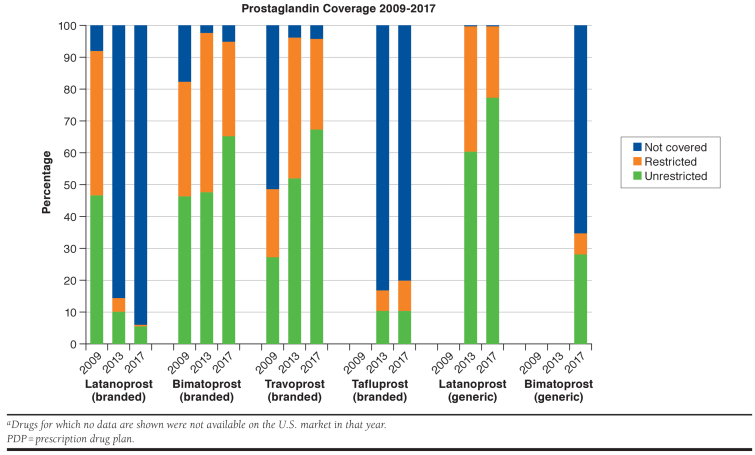
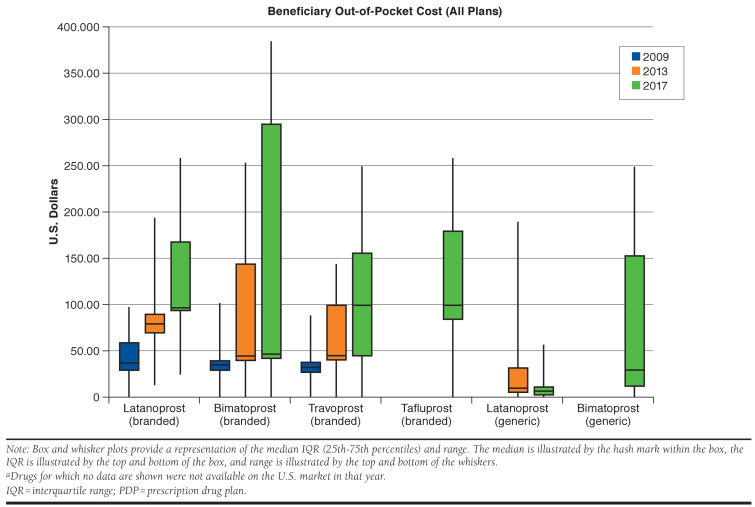
In 2009, 92% of plans covered branded latanoprost, 83% covered branded bimatoprost; and 49% covered branded travoprost, whereas in 2017, 6% of plans covered branded latanoprost; 95% covered branded bimatoprost; and 96% covered branded travoprost. Although generic latanoprost was universally covered, generic bimatoprost was only covered by 35% of plans in 2017. Median out-of-pocket cost of branded prostaglandins without generic equivalents was $35 (IQR = $29-$40) in 2009, $45 (IQR = $42-$101) in 2013, and $90 (IQR = $45-$159) in 2017. Median out-of-pocket cost of all available generic prostaglandins was $10 (IQR = $5-$33) in 2013 and $10 (IQR = $4-$15) in 2017. In 2013, Medicare spent $733 million on prostaglandin analogs; in 2017, this increased to $1.09 billion, with $943 million (86%) spent on branded prostaglandins and $148 million (14%) spent on generics.
CONCLUSIONS:
Medicare PDP coverage of branded prostaglandins remained stable from 2009 to 2017. While median beneficiary out-of-pocket costs associated with generic prostaglandins remained stable, those associated with branded prostaglandins increased nearly 3-fold.
What is already known about this subject
Prostaglandin analogs are the most effective treatment for glaucoma.
Adherence to prostaglandin analogs is suboptimal, which may partly be attributable to their high cost.
Previous studies have been limited to characterizing the costs of branded prostaglandin analogs in aggregate or in specific subpopulations of patients.
What this study adds
This study uses Medicare Part D formulary coverage data from all stand-alone and Medicare Advantage prescription drug plans in 2009, 2013, and 2017 to offer a comprehensive view of Medicare beneficiary access to glaucoma drugs over 8 years.
We determined that while generic latanoprost was universally covered, generic bimatoprost was not, and coverage of branded bimatoprost and travoprost increased, while coverage of branded latanoprost declined.
Median out-of-pocket cost of branded prostaglandins increased from $35 in 2009 to $90 in 2017 but was unchanged at $10 for generic prostaglandins.
Glaucoma is the second leading cause of irreversible blindness in the United States and is most common among older adults; approximately 12% of Medicare beneficiaries have been diagnosed with glaucoma.1 Lower intraocular pressure is correlated with slower progression of visual field deficits and optic disc deterioration, so the mainstays of therapy are aimed at decreasing intraocular pressure.2 Prostaglandin analogs are the most effective first-line treatment for lowering intraocular pressure in glaucoma.3,4
Adherence is suboptimal for prostaglandin analogs—studies of prescription records for ocular hypotensive agents in patients with glaucoma and ocular hypertension found that only 56% of days could have been dosed with the medication supply dispensed over the first year of therapy.5 High prescription cost is an important factor that increases the likelihood of nonadherence to topical ocular hypotensive medications in Medicare beneficiaries.6
Although some previous studies have characterized the high cost of branded prostaglandin analogs, they have focused on limited subsets of the affected population, such as beneficiaries of a single Medicare Advantage plan or a single pharmaceutical benefits manager.6,7 Further, no study has examined both coverage and beneficiary out-of-pocket costs of prostaglandin analogs at an individual drug level.8 Such an analysis may offer insight into how plans use formulary design to incentivize the use of certain prostaglandin analogs over another and may elucidate areas of opportunity for future cost savings.
Accordingly, our research objective was to use Medicare prescription drug plan (PDP) and spending data to characterize the coverage of, beneficiary out-of-pocket cost associated with, and total Medicare Part D spending on prostaglandins between 2009 and 2017. By using data from all Medicare PDPs, including stand-alone Part D plans and Medicare Advantage plans, we aimed to characterize how the coverage and pricing of prostaglandins throughout this period may have contributed to difficult patient access to these drugs. We combined this with an analysis of total Medicare spending on prostaglandin analogs, which may help identify areas of potential cost savings through incentivizing the use of generics over branded products.
Methods
We conducted a retrospective analysis of quarter (Q) 2 2009, Q2 2013, and Q2 2017 Medicare PDP formulary, beneficiary cost, and pricing files, which are available from Centers for Medicare & Medicaid Services (CMS). These files provide information on all PDPs that submitted complete and accurate information to CMS, including stand-alone Part D and Medicare Advantage plans operating in the United States. We excluded special needs PDPs and plans outside of the United States (e.g., Puerto Rico).
We characterized beneficiary coverage (including utilization management restrictions) and out-of-pocket costs for each prostaglandin analog used for glaucoma treatment: Xalatan (latanoprost) 0.005%, Travatan (travoprost) 0.004%, Lumigan (bimatoprost) 0.03% and 0.01% (2013, 2017), Zioptan (tafluprost) 0.0015%, generic latanoprost 0.005%, and generic bimatoprost 0.03%. By examining data from 2009, 2013, and 2017, our study includes points in time after which generic versions of certain prostaglandin analogs first became available, including latanoprost in 2011 and bimatoprost in 2015, in order to examine the effect that these launches may have had on coverage and cost of other prostaglandin analogs.
For each drug, we determined the number and median proportion of plans that did not provide coverage; provided coverage with utilization management restrictions (prior authorization, step therapy, and/or quantity limits); and provided coverage without restrictions. We also determined the number of branded prostaglandins that each plan covered (with or without restrictions); determined the tier of coverage for each drug in each plan; and using this information, determined how many plans had fewer restrictions for a branded drug compared with its generic and how many covered a branded drug under the same cost-sharing tier or under a lower (more favorable) cost-sharing tier than its generic.9
Next, we determined the median out-of-pocket cost for a first-prescription, 30-day supply of each drug across all plans. While some Medicare beneficiaries obtain 90-day drug supplies, these are not offered by all Medicare PDPs. For consistency of comparisons, our analyses considered only the out-of-pocket costs associated with 30-day supplies. Because we focused on first-prescription cost, we assumed that the deductible, where applicable, had not been met when calculating out-of-pocket cost.
We also used Medicare Part D drug spending data (available from CMS) to look at aggregate Medicare Part D spending for each prostaglandin analog for 2013 and 2017 (these data are not available for 2009). These costs are based on the gross drug cost, which represents total spending for the prescription claim, including Medicare, plan, and beneficiary payments. The Part D spending metrics do not reflect any manufacturers’ rebates or other price concessions, so we performed a sensitivity analysis to estimate the effect of rebates on spending using a previously described method.10 All analyses were performed using Stata, version 15.1 (StataCorp, College Station, TX), and Excel, version 14.1.3 (Microsoft, Redmond, WA).
Results
Data were available on 3,607 Medicare PDPs in 2009, 2,609 in 2013, and 2,668 in 2017. In 2009, 92% of plans covered branded latanoprost; 83% covered branded bimatoprost; and 49% covered branded travoprost (Figure 1). In 2013, 14% of plans covered branded latanoprost; 98% covered branded bimatoprost; and 96% covered branded travoprost. In 2017, only 6% of plans covered branded latanoprost, whereas 95% covered branded bimatoprost; and 96% covered branded travoprost. Branded tafluprost coverage stayed relatively steady, with 17% of plans covering the drug in 2013 and 20% covering it in 2017. In 2013, 2 years after market availability, all plans covered generic latanoprost (61% without restrictions in 2013, 77% in 2017); in contrast, in 2017, 2 years after market availability, only 35% of plans covered generic bimatoprost (28% without restrictions). Quantity limits were used by more plans than other types of coverage restrictions in all years (Table 1). Of the 883 plans that covered both branded and generic bimatoprost in 2017, 2% (16 plans) had fewer restrictions for branded bimatoprost than for generic bimatoprost; 28% (243 plans) covered branded bimatoprost under the same cost-sharing tier as they did generic bimatoprost; and 15% (129 plans) covered branded bimatoprost under a lower (more favorable) cost-sharing tier than they did generic bimatoprost. Thus, only 58% of these plans placed generic bimatoprost in a lower (more favorable) cost-sharing tier than branded bimatoprost.
FIGURE 1.
Medicare PDP Coverage of Branded and Generic Prostaglandin Analogs, 2009, 2013, and 2017 a
TABLE 1.
Number of Medicare PDPs with Coverage Restrictions for Each Prostaglandin Analog as a Percentage of All Plans in that Year
| Plans Requiring Prior Authorization n (%) | Plans Requiring Step Therapy n (%) | Plans Requiring Quantity Limits n (%) | |
|---|---|---|---|
| 2017 | |||
| Latanoprost (branded) | 0 (0.0) | 6 (0.2) | 4 (0.1) |
| Bimatoprost (branded) | 0 (0.0) | 97 (3.6) | 719 (26.9) |
| Travoprost (branded) | 0 (0.0) | 69 (2.6) | 712 (26.7) |
| Tafluprost (branded) | 10 (0.4) | 123 (4.6) | 146 (5.5) |
| Latanoprost (generic) | 0 (0.0) | 0 (0.0) | 607 (22.8) |
| Bimatoprost (generic) | 1 (0.0) | 39 (1.5) | 145 (5.4) |
| 2013 | |||
| Latanoprost (branded) | 2 (0.1) | 13 (0.5) | 100 (3.8) |
| Bimatoprost (branded) | 13 (0.5) | 107 (4.1) | 1,214 (46.5) |
| Travoprost (branded) | 0 (0.0) | 85 (3.3) | 1,076 (41.2) |
| Tafluprost (branded) | 13 (0.5) | 71 (2.7) | 138 (5.3) |
| Latanoprost (generic) | 0 (0.0) | 0 (0.0) | 1,028 (39.4) |
| 2009 | |||
| Latanoprost (branded) | 0 (0.0) | 110 (3.0) | 1,630 (45.2) |
| Bimatoprost (branded) | 0 (0.0) | 49 (1.4) | 1,270 (35.2) |
| Travoprost (branded) | 0 (0.0) | 8 (0.2) | 1,534 (42.5) |
PDP = prescription drug plan.
In 2009, 12% of plans covered only 1 branded prostaglandin; 52% of plans covered 2 branded prostaglandins; and 35% of plans covered 3 branded prostaglandins. In 2013, 6% of plans covered only 1 branded prostaglandin; 73% of plans covered 2 branded prostaglandins; 10% of plans covered 3 branded prostaglandins; and 11% of plans covered 4 branded prostaglandins. In 2017, 2% of plans covered no branded prostaglandins; 6% of plans covered 1 branded prostaglandin; 72% of plans covered 2 branded prostaglandins; 15% of plans covered 3 branded prostaglandins; and 5% covered 4 branded prostaglandins. Notably, of the 2,531 plans that covered branded bimatoprost in 2017, 1,648 (65%) did not also cover generic bimatoprost.
The median Medicare beneficiary out-of-pocket cost of branded prostaglandins without generic equivalents was $35 (interquartile range [IQR] = $29-$40) in 2009, $45 (IQR = $42-$101) in 2013, and $90 (IQR = $45-$159) in 2017. Median out-of-pocket costs in each year of branded latanoprost, bimatoprost, travoprost, and tafluprost were broadly similar. In contrast, the median out-of-pocket cost of generic latanoprost was $10 (IQR = $5-$33) in 2013 and $7 (IQR = $2-$12) in 2017, whereas generic bimatoprost was $30 (IQR = $12-$154) in 2017 (Figure 2). Although branded tafluprost was covered by a small number of plans in 2013, there was no associated Medicare spending in 2013, so it is not included in our 2013 out-of-pocket cost analysis.
FIGURE 2.
Median Medicare Beneficiary Out-of-Pocket Costs for Branded and Generic Prostaglandin Analogs Covered by Medicare PDPs, 2009, 2013, and 2017 a
In 2013, Medicare spent $733 million on all prostaglandins—$122 million (17% of total) on generic prostaglandins and $611 million (83% of total) on branded prostaglandins. Generic latanoprost, the generic glaucoma drug for which Medicare had the highest spend, accounted for $120 million (16%) of all spending on prostaglandins. Branded bimatoprost, the branded glaucoma drug for which Medicare had the highest spend, accounted for $313 million (43%) of all spending on prostaglandins. Assuming 17.5% manufacturer rebates (the average rebate reported by Medicare in 2014), total spending would have been $626 million; branded spending would have been $504 million (81% of total); and branded bimatoprost spending would have been $259 million (41% of total). Assuming 26.3% manufacturer rebates (the highest reported rebate by Medicare in 2014), total spending would have been $572 million; branded spending would have been $451 million (79% of total); and branded bimatoprost spending would have been $231 million (40% of total).
In 2017, Medicare spent $1.09 billion on prostaglandins, a 49% increase over the 2013 spend—$148 million (14% of total) was spent on generics, while $943 million (86% of total) was spent on branded drugs. Branded latanoprost accounted for $145 million (13% of total spend), while branded bimatoprost accounted for $517 million (47%) of all spending on prostaglandin analogs ($517 million). Only $3.6 million, or 0.33% of total spending, was for generic bimatoprost. Assuming 17.5% manufacturer rebates, total spending would have been $926 million; branded spending would have been $778 million (84% of total); and branded bimatoprost spending would have been $427 million (46% of total). Assuming 26.3% manufacturer rebates, total spending would have been $843 million, branded spending would have been $695 million (82% of total), and branded bimatoprost spending would have been $381 million (45% of total).
Discussion
In our study of Medicare prescription drug coverage of prostaglandins used for the treatment and management of glaucoma, we found that the number of branded prostaglandins covered by plans did not increase from 2009 to 2017, but the patterns of formulary coverage shifted from predominantly covering branded latanoprost to branded travoprost, with consistent coverage of branded bimatoprost. However, during this time frame, median beneficiary out-of-pocket costs associated with branded prostaglandins increased nearly 3-fold, to $90 for a 30-day supply.
After 2009, generic versions of 2 prostaglandin analogs became available: latanoprost in 2011, which is now universally covered, and bimatoprost in 2015, which has limited coverage. Moreover, 65% of plans covering branded bimatoprost did not also cover generic bimatoprost in 2017. Of the plans that did cover both, only 58% incentivized use of generic bimatoprost over the branded version by placing generic bimatoprost in a lower (more favorable) cost-sharing tier than branded bimatoprost. There are 4 possible reasons that may account for this more limited coverage. First, bimatoprost is priced higher, perhaps making latanoprost a more competitive selection for plans. Second, the branded product’s manufacturer, Allergan, may have dampened coverage through a “forced switch” strategy, whereby branded bimatoprost was reformulated from a 0.03% to a 0.01% dosage in 2010 in anticipation of generic competition, a strategy previously employed by pharmaceutical manufacturers.11 Third, Allergan’s rebate contracts may have explicitly stipulated the exclusion of generic bimatoprost from the formularies of the pharmacy benefit managers in exchange for manufacturer rebates. Finally, plans may have offered more limited coverage because the results of an Allergan-funded trial demonstrated better safety and equivalent efficacy for the 0.01% compared with the 0.03% dosage.12 However, the clinical importance of the safety differences is less clear and may not fully explain the large differences in coverage between branded bimatoprost and generic bimatoprost because at the end of the 12-month study period, 80% of bimatoprost 0.01% users and 77% of 0.03% users reported being very or extremely willing to continue using the study medication.12
We are also likely observing the effects of pharmaceutical manufacturer marketing and promotion strategies in the Medicare Part D spending trends from 2013 to 2017. Despite the entry of generic bimatoprost as a new drug between the 2 years, the percentage of total spend that branded drugs accounted for increased from 83% to 86%. Also, in both years, more was spent on branded bimatoprost than on any other prostaglandin analog, and the percentage of total prostaglandin analog spend it accounted for increased from 43% to 47%. At the same time, after market entry of generic bimatoprost in 2015, as of 2017 it only accounted for 0.33% of total spend on prostaglandin analogs. Even assuming maximum manufacturer rebates of 26.3%, the percentages of total spend accounted for by branded bimatoprost individually, and branded drugs as a whole, increased. Further, branded bimatoprost was still the prostaglandin analog with the highest spend in both years.
This large amount of spending suggests that there remains huge potential for cost savings to Medicare and its beneficiaries through increased coverage and use of generic bimatoprost and generic latanoprost. This conversation becomes even more important as new branded prostaglandin analogs are released, for example, Vyzulta (latanoprostene) in 2018, since trends from 2009 to 2017 show that increased medication options do not necessarily lead to improved coverage or lower prices for Medicare beneficiaries.
Our research highlights that Medicare PDPs overall are not adequately incentivizing the use of lower-cost generic prostaglandin analogs through utilization management strategies. Inadequate PDP coverage enables branded drugs that already have generic competition, such as branded bimatoprost, to continue to secure large amounts of revenue simply through a dosage reformulation, while leaving unrealized the potential cost savings for Medicare and its beneficiaries through generic drug use. Despite the launch of several new drugs in the past decade, this lack of coverage by Medicare PDP formularies and the tripling of out-of-pocket costs associated with branded prostaglandins increasingly puts patients at risk for cost-related nonadherence. Since these drugs are used chronically, the financial burden on patients is multiplied over time, increasing the risk of nonadherence that can lead to significant morbidity.
Limitations
There are important limitations to our study that deserve consideration. First, because we looked at first-prescription cost, our analysis may not be generalizable to all beneficiaries’ prescriptions throughout a coverage year, since our calculations assumed that a plan’s deductible had not yet been met, which may not be representative of cumulative annual prescription costs. For instance, our estimates did not take into account prescription costs during the coverage gap phase, which often require larger out-of-pocket costs than during the initial coverage phase.
Second, our analysis focused on median beneficiary out-of-pocket prescription costs and did not take into account insurance plan premiums, which may have changed over the study period. Third, Medicare PDP data do not include the number of beneficiaries enrolled in each plan, nor the volume of prescriptions filled, so our coverage and out-of-pocket cost estimates do not weight more heavily plans with more enrollees nor those that filled greater numbers of prescriptions for prostaglandin analogs.
Fourth, by focusing our analyses on the out-of-pocket costs associated with 30-day prescriptions, we may have slightly overestimated costs, since 90-day prescriptions may be available for lower prorated amounts.
Finally, although our analysis accounts for the potential effect of manufacturer rebates on the overall amount Medicare spent on these drugs, our data do not include rebates on an individual drug and plan level.
Conclusions
Medicare PDPs provided coverage for similar numbers of branded prostaglandin analogs in 2009 as in 2017, but beneficiary out-of-pocket costs were almost 3 times higher, despite the introduction of 2 generic versions over this time. All Medicare PDPs provided coverage of generic latanoprost, but not of generic bimatoprost, and median beneficiary out-of-pocket costs for generic prostaglandin analogs remained stable. Because the entry of generic bimatoprost has neither lowered the cost of nor diverted spend away from the more expensive branded version of the drug, our findings suggest that there are opportunities for Medicare PDPs to increase generic prostaglandin analog use, which may improve patient access to therapy by reducing out-of-pocket prescription drug costs and thereby decreasing glaucoma-associated disease morbidity.
REFERENCES
- 1.Cassard SD, Quigley HA, Gower EW, Friedman DS, Ramulu PY, Jampel HD.. Regional variations and trends in the prevalence of diagnosed glaucoma in the Medicare population. Ophthalmology. 2012;119(7):1342-51. [DOI] [PubMed] [Google Scholar]
- 2.Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT.. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ. 2005;331(7509):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart WC, Konstas AG, Nelson LA, Kruft B.. Meta-analysis of 24-hour intraocular pressure studies evaluating the efficacy of glaucoma medicines. Ophthalmology. 2008;115(7):1117-22.e1111. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Huang W, Zhang X.. Efficacy and safety of different regimens for primary open-angle glaucoma or ocular hypertension: a systematic review and network meta-analysis. Acta Ophthalmol. 2018;96(3):e277-e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reardon G, Kotak S, Schwartz GF.. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer Adherence. 2011;5:441-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheer R, Bunniran S, Uribe C, Fiscella RG, Patel VD, Chandwani HS.. Predictors of nonadherence to topical intraocular pressure reduction medications among Medicare members: a claims-based retrospective cohort study. J Manag Care Spec Pharm. 2016;22(7):808-17. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2016.22.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmier JK, Covert DW.. First-year treatment costs among new initiators of topical prostaglandin analogs: pooled results. Clin Ophthalmol. 2010;4:437-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman-Casey PA, Woodward MA, Niziol LM, Lee PP, De Lott LB.. Brand medications and Medicare Part D: how eye care providers’ prescribing patterns influence costs. Ophthalmology. 2018;125(3):332-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Socal MP, Bai G, Anderson GF.. Favorable formulary placement of branded drugs in Medicare prescription drug plans when generics are available. JAMA Intern Med. 2019;179(6):832-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacks CA, Lee CC, Kesselheim AS, Avorn J.. Medicare spending on brand-name combination medications vs their generic constituents. JAMA. 2018;320(7):650-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downing NS, Ross JS, Jackevicius CA, Krumholz HM.. Avoidance of generic competition by Abbott Laboratories’ fenofibrate franchise. Arch Intern Med. 2012;172(9):724-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz LJ, Cohen JS, Batoosingh AL, Felix C, Shu V, Schiffman RM.. Twelve-month, randomized, controlled trial of bimatoprost 0.01%, 0.0125%, and 0.03% in patients with glaucoma or ocular hypertension. Am J Ophthalmol. 2010;149(4):661-71.e661. [DOI] [PubMed] [Google Scholar]