Abstract
BACKGROUND:
Although direct-acting antivirals (DAA) have revolutionized the treatment of chronic hepatitis C virus (HCV), many state Medicaid programs have limited coverage because of their expense. In 2015, the Centers for Medicare & Medicaid Services (CMS) notified states about the legality of Medicaid coverage limitations, particularly within managed care programs.
OBJECTIVES:
To (1) examine how relaxation and alignment of hepatitis C policies within the Oregon Medicaid program affected DAA utilization and (2) describe changes in DAA coverage policies and patient characteristics of treated individuals over time.
METHODS:
We manually collected DAA Medicaid drug policies in the state of Oregon before and after the CMS notification was released. After categorizing DAA policies into 2 groups based on baseline prior authorization criteria (restrictive and permissive), we evaluated how changes in these DAA policies affected utilization over 3 time periods (pre-CMS period, post-CMS period, and fibrosis policy alignment). Immediate and gradual changes in trend were assessed using an interrupted time series regression model. Finally, we examined patient characteristics and liver disease complications over time as policy restrictions were removed and aligned with one another.
RESULTS:
From 2014 to 2018, Oregon’s coordinated care organizations and fee-for-service drug policies relaxed liver fibrosis and substance abstinence coverage criteria leading to immediate increases in DAA use in 2016 (0.62 prescriptions per 10,000 enrollees per month; 95% CI = 0.17 to 1.08) and 2018 (1.07 prescriptions per 10,000 enrollees per month; 95% CI = 0.63 to 1.51) among more restrictive coordinated care organizations at baseline. This was followed by a decrease in trend after the 2016 and 2018 impact (−0.05; 95% CI = −0.11 to −0.001 and −0.07; 95% CI = −0.13 to −0.02, respectively). Over the 3 periods, there was a decrease in treated individuals with liver-related complications (P < 0.0001) and an increase in those with a substance use diagnosis (P = 0.0013).
CONCLUSIONS:
Reducing coverage limitations resulted in treatment of patients with fewer liver-related complications and more substance use disorders. Expanding access to treatment did not result in sustained increases in utilization, and additional interventions may be necessary to meet HCV elimination goals.
What is already known about this subject
State Medicaid programs implemented prior authorization policies and clinical restrictions on direct-acting antivirals (DAAs) for chronic hepatitis C that resulted in a review and coverage notice from the Centers for Medicare & Medicaid Services.
Previous studies have concluded that relaxation of these policies resulted in increased use of DAAs in state Medicaid programs.
What this study adds
This study found changes in hepatitis C drug utilization trends that differed according to initial policy restrictiveness, and increases in use following policy changes were not sustained over time, suggesting depletion of initially “warehoused” patients.
In addition to an increase in use, this study described the changes in patient characteristics over time as a result of removing policy restrictions for the treatment of hepatitis C virus.
Study findings showed that harmonizing drug policies between fee-for-service and managed care programs lead to alignments in hepatitis C drug access across programs.
Direct-acting antivirals (DAAs) have revolutionized treatment of chronic hepatitis C virus (HCV). They are vastly more effective than the previous standard of care (interferon-based treatments such as pegylated interferon and ribavirin) in producing sustained virologic response (SVR) of ≥ 90%, have significantly shortened treatment durations (8-12 weeks), and cause few side effects.1 Since 2015, the American Association of the Study of Liver Diseases and the Infectious Diseases Society of America guidelines recommend treatment for all patients with chronic HCV infection, except for those with short life expectancies that cannot be remediated by treating HCV, by transplantation, or by other directed therapy.1 Earlier versions of the guidelines included prioritization of patients for treatment based on disease severity. Although data from randomized clinical trials are limited to short-term studies that primarily examine viral response, observational data suggest that SVR is associated with a decreased progression of liver disease, hepatocellular carcinoma, and mortality.2,3 In light of this evidence, the United States Preventive Services Task Force now recommends universal HCV screening for all adults aged 18-79 years.4 In 2016, the World Health Organization pledged to eliminate viral hepatitis by 2030.5
Despite their clinical advantages, DAA treatments are costly, and affordability has been a major concern for payers.6 Absorbing the costs of new DAAs has been particularly challenging for state Medicaid programs, which finance the care for a large number of individuals with HCV.7 A study by Barua et al found that many state Medicaid programs had limited access to DAAs for those with the most advanced liver disease and two-thirds limited prescribing based on prescriber type in the early DAA treatment era.8 A large majority of states (88%) also restricted treatment based on drug or alcohol use.8 Amid concerns that state Medicaid programs were imposing policies that restricted access beyond what is statutorily permitted by the Social Security Act, the Centers for Medicare & Medicaid Services (CMS) issued a notice in November 2015 to states clarifying the extent to which they are legally permitted to restrict DAA use in fee-for-service (FFS) and managed care programs.9 Further, the notice sought to remind states that Medicaid managed care organizations are required to provide a pharmacy benefit no more restrictive than the state FFS program. Early reports suggest that subsequent relaxation of hepatitis C coverage policies has resulted in increased DAA use within state Medicaid programs.7,10
The burden of hepatitis C is particularly high in Oregon, which has the third highest prevalence of hepatitis C in the United States and the second highest mortality rate.11 As in other states, the Medicaid program assumes a disproportionate amount of this burden. Oregon’s Medicaid program is administered through 16 distinct regional coordinated care organizations (CCOs). Each CCO operates within a capitated global budget and develops its own pharmacy benefit. The majority of the roughly 1,000,000 Medicaid patients in Oregon (approximately 80%) are enrolled in a CCO and the remaining are in the FFS program.12 Similar to the reported trends in other state Medicaid programs, Oregon’s CCOs originally implemented a diverse set of prior authorization (PA) policies to help prioritize treatment and stretch resources. However, in response to the CMS DAA coverage notice in late 2015, the Oregon CCOs were directed to reduce policy barriers, expand coverage, and harmonize DAA policies with the FFS program. While the FFS policy was changing frequently as more data became available, the policy in 2015 prioritized treatment for persons with advanced liver disease (fibrosis stage 3 or 4 [F3 or F4]) and for those at greatest risk for developing complications (liver transplantation, extrahepatic manifestations, and HIV coinfection). As more treatment options drove prices down and the community standard evolved, Oregon continued to expand treatment in a stepwise fashion to patients with less severe disease (Figure 1).
FIGURE 1.
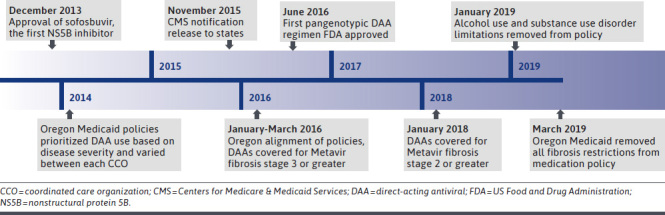
Timeline of Major Policy and Treatment Events
The primary aim of this study was to examine how relaxation and alignment of hepatitis C policies within the Oregon Medicaid program affected DAA utilization. The secondary aim was to describe changes in patient characteristics of treated individuals over time.
Methods
POLICY ASSESSMENT
Using archived web pages and communication with CCO pharmacy or medical directors, we collected Oregon CCO and FFS drug policies before (2015) and after (2016) the November 2015 CMS notification and subsequent Oregon Health Authority (OHA) directive. We compiled data for 16 CCOs and the FFS program (n = 17 policies) into an Excel spreadsheet (version 2013, Microsoft) and categorized coverage criteria into the following categories: disease severity (fibrosis stage); diagnostic requirements (ie, biopsy); prescriber limitations (requiring specialist prescribing or not); abstinence of drug, tobacco, and/or alcohol use (eg, no restrictions, 6 or 12 months of abstinence); and other factors used to restrict access to HCV treatment (Supplementary Table 1 (144.9KB, pdf) , available in online article).
We categorized the DAA policies (CCOs and FFS) into 2 groups based on their baseline criteria and the changes enacted in response to the CMS/OHA directive. We considered CCOs “restrictive” at baseline if they relaxed policy criteria related to liver fibrosis and/or abstinence requirements (n = 12 policies) in order to comply with the directive. These policies at baseline included requirements for severe fibrosis (F4) and/or required abstinence from drugs and alcohol for at least 12 months. We considered CCOs “permissive” at baseline if they permitted treatment for individuals with fibrosis stage 3 and 6 months or less drug and alcohol abstinence and therefore made no changes in either liver fibrosis or abstinence criteria (n = 5 policies).
We selected these 2 criteria domains (fibrosis and abstinence) to categorize policies because they were the most consistently used criteria by state Medicaid programs.13 Although we labeled groups as permissive, all policies had some level of restrictions, and none had completely unfettered access.
ANALYSIS OF DAA UTILIZATION
After categorizing CCO and FFS policies into the 2 groups, we used interrupted time series (ITS) regression models to determine how changes in DAA policies affected overall Medicaid utilization over time. We used Medicaid claims data to identify any paid claim for any DAA that was approved at that time. For the restrictive and permissive policy groupings, we aggregated all paid DAA claims in each month and divided by the aggregated number of Medicaid enrollees in that grouping to generate the final unit of analysis of claims per 10,000 enrollees per month. We excluded 2014 because utilization was changing rapidly since the introduction of sofosbuvir (2013, quarter 4) and ledipasvir/sofosbuvir (2014, quarter 4). Thus, our study period was from January 2015 to December 2018.
Using ITS regression models, we examined changes in DAA utilization across 3 policy periods: period 1, pre-CMS directive period (January 2015-December 2015); period 2, post-CMS directive period (April 2016-December 2017); and period 3, final DAA policy alignment period (January 2018-December 2018). We removed the first quarter of 2016 from the analysis because CCOs gradually modified their DAA policies in response to the CMS directive over this period. Period 3 (final DAA policy alignment) captured the period where all CCOs changed fibrosis restrictions from F3 to F2 on January 2018 (Figure 1).
The general form of our ITS regression model was:
In this model, the dependent variable (Yt) was the number of paid DAA claims per 10,000 enrolled Medicaid beneficiary per month. Period1Trendt was a continuous variable from 0 to 47, indicating time in months from the beginning of the study period. Period2t was a dummy variable set at 0 if the month was before April 2016 and 1 if the month was after that date. Period2Trendt was a continuous variable indicating the number of months after April 2016; this variable was set at 0 if before. Period3t was a dummy variable set at 0 if the month was before January 2018 and 1 otherwise. Period3Trendt was a continuous variable indicating the number of months after December 2017; this variable was set at 0 if before January 2018.
The estimate β0 is a measure of utilization at time 0 (intercept); β1 estimates the monthly change in utilization over time (baseline trend) before January 2016; β2 estimates the immediate change in utilization (level change) occurring after March 2016; β3 estimates the change in the monthly utilization trend (trend change) after March 2016; β4 estimates the immediate change in utilization (level change) occurring after the final fibrosis policy alignment on January 2018; and β5 estimates the change in the monthly utilization trend (trend change) after the policy alignment on January 2018.
All regressions were performed using PROC AUTOREG within SAS version 9.3 (SAS Institute) assuming first-degree auto correlated errors.
ANALYSIS OF PATIENT CHARACTERISTICS
We used pharmacy and medical encounter claims data to examine changes in characteristics of treated individuals across similar time periods: period 1, pre-CMS directive period (January 2014-December 2015); period 2, post-CMS directive period (January 2016-December 2017); and period 3, DAA policy alignment period (January 2018-December 2018). All patients with a paid claim for any DAA were included. Unlike in the ITS regression analysis, the first 3 months of 2014 and 2016 were not excluded from the patient analysis to allow for a complete preview of changes in characteristics over time. We assigned patients into these groups based on their first DAA prescription (any drug). We characterized age, sex, race, liver disease complications, and other comorbidities.
Liver disease complications and comorbidities were quantified using International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification diagnosis codes occurring within the entire study period. Liver disease complications included diagnoses of HCV infection, cirrhosis (decompensated and compensated), hepatocellular carcinoma, and liver transplant. We used diagnosis codes from the Elixhauser Comorbidity Index to quantify nonliver-related comorbidity.14-16
For categorical variables, we tested the statistical significance between the 3 periods using the Mantel-Haenszel chi-square test for linear trend. Differences continuous variables (age) were tested using analysis of variance.
Results
QUALITATIVE POLICY REVIEW
Before the CMS/OHA directed policy alignment, CCO and FFS policies varied considerably with respect to liver fibrosis stage, abstinence from substance use, and consultation requirements with a specialist medical provider (Supplementary Table 1 (144.9KB, pdf) , available in online article). Eight of 17 policies required patients to have cirrhosis (fibrosis stage 4) to qualify for DAA therapy, and all but 2 policies required some level of abstinence from illicit drugs and/or alcohol for DAA approval. Some policies also included criteria related to prescriber specialty (eg, hepatologist), liver biopsy for diagnosis, history of noncompliance, tobacco and marijuana use, houselessness or high-risk living situations, uncontrolled comorbidities, and motivation for successful treatment completion.
By the second quarter of 2016, all policies adopted uniform criteria that approved DAA therapy for all individuals with stage 3 fibrosis or higher. They also approved treatment for patients with one or more of the recognized HCV-related extrahepatic manifestations (type 2 or 3 cryoglobulinemia with end-organ manifestations, proteinuria, nephrotic syndrome, membranoproliferative glomerulonephritis, or porphyria cutanea tarda), peri-transplant, or stage 2 fibrosis in those with an HIV coinfection. CCOs retained flexibility in determining prescriptive authority and abstinence requirements, but the policy variability generally declined (Supplementary Table 2 (144.9KB, pdf) , available in online article).
In January 2018, all policies simultaneously moved from approving DAAs for fibrosis stage 3 or greater to those with chronic hepatitis C with fibrosis stage 2 or greater and aligned other restrictions (Figure 1).
QUANTITATIVE UTILIZATION ANALYSIS
Figure 2 summarizes observed and ITS-modeled DAA utilization for CCO and FFS programs grouped by baseline restrictiveness. ITS regression estimates are summarized in Table 1. In the year preceding the CMS notification letter (period 1), both policy groups had significant positive trends in utilization. The monthly trend in utilization for permissive CCOs (0.10 prescriptions per 10,000 enrollees per month; 95% CI = 0.03 to 0.18) was about twice the monthly trend of the restrictive CCOs (0.06 prescriptions per 10,000 enrollees per month; 95% CI = 0.01 to 0.11). In the year following the CMS directive (period 2), there was a significant immediate increase (0.62 prescriptions per 10,000 enrollees; 95% CI = 0.17 to 1.08) in utilization for CCOs with restrictive policies that was not observed in the policies that were more permissive at baseline. There were also significant declines in the monthly utilization trend for CCOs with both permissive policies (−0.19 prescriptions per 10,000 enrollees per month; 95% CI = −0.28 to −0.09) and restrictive policies (−0.05 prescriptions per 10,000 enrollees per month; 95% CI = −0.11 to −0.001).
FIGURE 2.
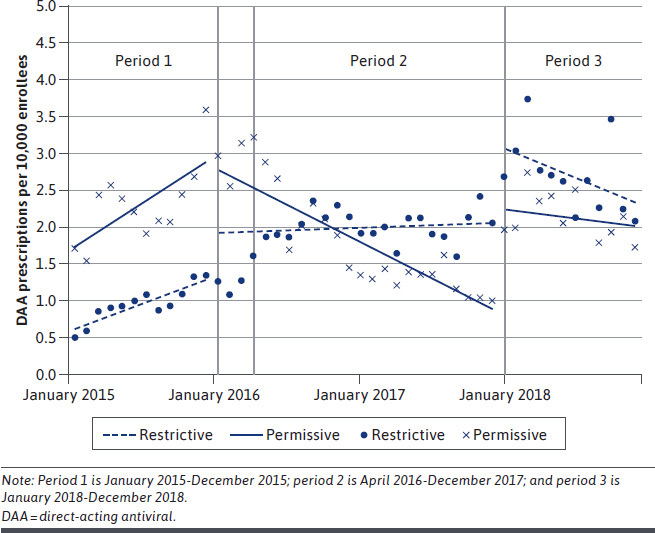
Observed Utilization and Interrupted Time Series Regression Modeled Estimates
TABLE 1.
Interrupted Time Series Regression Estimates
| Variable | Permissive policies | Restrictive policies | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate a | P value | 95% CI | Estimate a | P value | 95% CI | |||
| Intercept | 1.62 | < 0.0001 | 1.01 | 2.23 | 0.56 | 0.003 | 0.20 | 0.93 |
| Trend | 0.10 | 0.01 | 0.03 | 0.18 | 0.06 | 0.02 | 0.01 | 0.11 |
| Period 2: CMS directive | ||||||||
| Level change 1b | −0.02 | 0.95 | −0.71 | 0.67 | 0.62 | 0.01 | 0.17 | 1.08 |
| Trend change 1c | −0.19 | < 0.0001 | −0.28 | −0.09 | −0.05 | 0.047 | −0.11 | −0.001 |
| Period 3: F3 to F2 fibrosis policy alignment | ||||||||
| Level change 2b | 1.37 | < 0.0001 | 0.71 | 2.03 | 1.07 | < 0.0001 | 0.63 | 1.51 |
| Trend change 2c | 0.06 | 0.19 | −0.03 | 0.15 | −0.07 | 0.01 | −0.13 | −0.02 |
a Estimates listed are DAA prescriptions per 10,000 enrollees.
b Level change indicates the immediate changes in DAA prescriptions per 10,000 enrollees.
c Trend change indicates the change in DAA prescriptions per 10,000 enrollees per month.
CMS = Centers for Medicare & Medicaid Services; DAA = direct-acting antiviral.
In January 2018, all CCOs reduced fibrosis-related restrictions from F3 to F2. Following that change (period 3), there were immediate increases in utilization for both permissive (1.37 prescriptions per 10,000 enrollees; 95% CI = 0.71 to 2.03) and restrictive (1.07 prescriptions per 10,000 enrollees; 95% CI = 0.63 to 1.51) policies. As shown in Figure 2, increased DAA utilization was not continued over time. Among restrictive CCOs, the immediate increase was followed by a significant decline in monthly trend (−0.07 prescriptions per 10,000 enrollees per month; 95% CI = −0.13 to −0.02).
QUANTITATIVE EVALUATION OF DAA USE
Table 2 summarizes patient demographics and comorbidities in 2,930 Medicaid beneficiaries treated with a DAA in the pre-CMS directive period (period 1; n = 581), post-CMS directive period (period 2; n = 1,206), and F3 to F2 fibrosis policy alignment (period 3; n = 1,143) period. Over this 5-year period, the proportion of individuals aged 19-40 years increased significantly (P < 0.0001) from 19% to 29%, with a corresponding decline in older individuals (aged 50-70 years). There was a significant decline in the number of patients with any liver-related complication treated over time (59%, 52.9%, and 31.5%; P < 0.0001). This trend was also mirrored for all of the individual liver-related diagnoses.
TABLE 2.
Characteristics of Individuals Treated with a DAA for Chronic Hepatitis C Infection
| Period 1 Jan 2014-Dec 2015 | Period 2 Jan 2016-Dec 2017 | Period 3 Jan 2018-Dec 2018 | P value | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| 581 | 1,206 | 1,143 | |||||
| Age, years | < 0.0001 | ||||||
| ≤ 18 | 0 | 0.0 | 1 | 0.1 | 2 | 0.2 | |
| 19-49 | 108 | 18.6 | 210 | 17.4 | 334 | 29.2 | |
| 50-70 | 472 | 81.2 | 994 | 82.4 | 806 | 70.5 | |
| ≥ 71 | 1 | 0.2 | 1 | 0.1 | 1 | 0.1 | |
| Sex | 0.065 | ||||||
| Female | 227 | 39.1 | 452 | 37.5 | 487 | 42.6 | |
| Male | 354 | 60.9 | 754 | 62.5 | 656 | 57.4 | |
| Liver-related diagnoses | |||||||
| Compensated cirrhosis | 328 | 56.5 | 615 | 51.0 | 333 | 29.1 | < 0.0001 |
| Decompensated cirrhosis | 164 | 28.2 | 252 | 20.9 | 130 | 11.4 | < 0.0001 |
| Hepatocellular carcinoma | 28 | 4.8 | 31 | 2.6 | 17 | 1.5 | < 0.0001 |
| Liver transplant | 10 | 1.7 | 9 | 0.7 | 1 | 0.1 | 0.0001 |
| Any liver-related complication (any of above) | 343 | 59.0 | 638 | 52.9 | 360 | 31.5 | < 0.0001 |
| Elixhauser comorbiditiesa | |||||||
| Liver disease | 557 | 95.9 | 1180 | 97.8 | 1109 | 97.0 | 0.36 |
| Hypertension | 220 | 37.9 | 476 | 39.5 | 402 | 35.2 | 0.14 |
| Substance use disorder (not alcohol) | 126 | 21.7 | 267 | 22.1 | 318 | 27.8 | 0.0013 |
| Depression | 110 | 18.9 | 242 | 20.1 | 252 | 22.0 | 0.11 |
| Psychoses | 106 | 18.2 | 153 | 12.7 | 166 | 14.5 | 0.13 |
| Chronic pulmonary disease | 98 | 16.9 | 237 | 19.7 | 232 | 20.3 | 0.11 |
| Diabetes | 82 | 14.1 | 99 | 8.2 | 70 | 6.1 | < 0.0001 |
| Coagulopathy | 79 | 13.6 | 119 | 9.9 | 68 | 5.9 | < 0.0001 |
| Alcohol use disorder | 73 | 12.6 | 191 | 15.8 | 185 | 16.2 | 0.075 |
| Obesity | 66 | 11.4 | 148 | 12.3 | 179 | 15.7 | 0.0061 |
| Deficiency anemia | 58 | 10.0 | 88 | 7.3 | 84 | 7.3 | 0.092 |
| Hypothyroidism | 56 | 9.6 | 92 | 7.6 | 76 | 6.6 | 0.031 |
| Fluid and electrolyte disorders | 56 | 9.6 | 98 | 8.1 | 82 | 7.2 | 0.077 |
| Diabetes, complicated | 39 | 6.7 | 134 | 11.1 | 128 | 11.2 | 0.011 |
| Other neurological disorders | 37 | 6.4 | 92 | 7.6 | 95 | 8.3 | 0.16 |
| AIDS/HIV | 37 | 6.4 | 42 | 3.5 | 27 | 2.4 | < 0.0001 |
| Solid tumor without metastasis | 35 | 6.0 | 66 | 5.5 | 31 | 2.7 | 0.0004 |
aOnly diagnoses over 5% listed, DAA = direct-acting antiviral.
There was a statistically significant decrease in the number of patients with noncomplicated diabetes (14.1%, 8.2%, and 6.1%; P < 0.0001), and a significant increase in patients with complicated diabetes treated over time (6.7%, 11.1%, and 11.2%; P = 0.011). There was a nonsignificant increase in the patients with a diagnosis of alcohol abuse (12.6%, 15.8%, and 16.2%; P = 0.075) and a statistically significant increase in patients with a diagnosis of substance use disorder (21.7%, 22.1%, and 27.8%; P = 0.0013). There was also a significant increase in the number of patients with a diagnosis of obesity and significant declines in the number of individuals with HIV or cancer (without metastasis).
Discussion
CMS officials released a memo in November 2015 notifying state Medicaid programs that managed care plans may not impose criteria that are more restrictive than the state’s FFS program, and it was unacceptable to place unreasonable restrictions on HCV treatments. In this study, we found that Oregon’s efforts to relax and harmonize DAA policy restrictions from across its CCOs were associated with significant increases and alignment of DAA use between initially restrictive and more permissive CCOs. Further, loosening of these criteria resulted in meaningful changes in the composition of treated patients.
Consistent with the intent of these policy changes, over time DAA-treated patients were younger, less likely to have cirrhosis and other liver-related complications, less likely to have HIV, and more likely to have a substance use disorder. These changes are important to consider, since treating HCV in those without cirrhosis also results in a significant decrease in mortality.17 Previous modeling studies have shown a clinical benefit when treatment is initiated earlier before significant fibrosis develops.18 Additionally, since injection drug use is the most common reason for HCV transmission in the United States, treating more individuals with substance use disorder will likely have a positive impact on reducing transmission in Oregon.19
Changes in DAA utilization were notable for several reasons. As the DAA treatment era had just begun in 2014, an increasing trend was observed in permissive and restrictive CCOs before the CMS notification. However, DAA utilization was increasing faster among CCOs with more permissive policies compared with CCOs with more restrictive policies. Relaxing restrictions in 2016 produced a significant jump in DAA utilization for CCOs that were initially more restrictive.
The trend in utilization among CCOs that were initially permissive declined after 2016 compared with period 1, possibly reflecting saturation in population demand. A similar pattern was observed after the final fibrosis policy alignment in January 2018. Following an initial immediate increase for permissive and restrictive CCOs, the monthly trend did not continue to increase, which suggests that major changes to drug policy may have resulted in an immediate surge in use as “warehoused” patients were rapidly treated. However, this increase was not sustained over time and was consistent with the known barriers and delay in the HCV screening, diagnosis, and linkage to care sequence.20 In addition, since over time most of the symptomatic HCV patients would have been treated, the majority left to treat would be asymptomatic and likely not identified without proactive case-finding approaches being used.
Finally, we found that alignment of CCOs policies resulted in a convergence of utilization between CCOs with initially restrictive and permissive policies. Thus, Oregon’s policy coordination also reflects a more careful consideration of the CMS requirement to ensure coverage comparability for Medicaid managed care.
Our findings are generally consistent with Kapadia et al, who found that relaxation of DAA use criteria was associated with increased use at the national level.10 Expanding on this, we also found that this expansion occurred in younger individuals with fewer liver-related complications and more likely to have substance use disorder, which directly reflected the intent of the policy changes. As shown in Figure 1, in March 2019, Oregon removed all fibrosis restrictions from the policy. It seems likely that this further relaxation of use criteria would elicit a similar immediate increase followed by a decline or stabilization of use.
LIMITATIONS
This study has some limitations to consider. First, our data represented Oregon’s Medicaid program. Since there is significant variation in how each state’s Medicaid program functions and the extent of managed care contracting, generalizability may be a concern. Despite this, state policymakers can learn from Oregon’s experience.
A second limitation is the likely impact that new drug approvals and market changes had on use over time that was not accounted for in our analysis, since there was no control group. Regardless, our findings remain valid because changes in drug availability was consistent among different CCOs in both groups.
Third, there was also variability in how quickly CCOs incorporated changes in their PA policies in response to the CMS directive. Although we excluded 3 months from the ITS to account for this, it was difficult to pinpoint exactly when each policy was fully implemented. Although we are not aware of any major co-occurring Medicaid policy changes, it is possible that our findings may have been confounded by unknown changes in policy or enrollment over time.
Finally, claims data may not have fully captured the complexity of patient health status; it was challenging to identify policy implementation dates, since they were constantly being updated and revised.
Conclusions
Relaxation and alignment of DAA policies in Oregon’s Medicaid program resulted in increased DAA use and notably expanded use among individuals without liver complications, who were most likely to benefit from viral clearance. We also showed that expanding access did not result in sustained increases in utilization, suggesting that proactive approaches to reach untreated patients may be required to reduce the burden of HCV-related morbidity and mortality.
ACKNOWLEDGMENTS
The authors acknowledge Luke Middleton, BS, for his contributions to data analysis.
REFERENCES
- 1.AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clinl Infect Dis. 2018;67(10):1477-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet. 2019;393(10179):1453-64. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsen JC, Nielsen EE, Feinberg J, et al. Direct-acting antivirals for chronic hepatitis C. Cochrane Database Syst Rev. 2017;6(6):CD012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Preventive Services Task Force, Owens DK, Davidson KW, et al. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2020;323(10):970-75. [DOI] [PubMed] [Google Scholar]
- 5.Waheed Y, Siddiq M, Jamil Z, Najmi MH. Hepatitis elimination by 2030: progress and challenges. World J Gastroenterol. 2018;24(44):4959-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry B. Drug pricing & challenges to hepatitis C treatment access. J Health Biomed Law. 2018;14:265-83. [PMC free article] [PubMed] [Google Scholar]
- 7.Liao JM, Fischer MA. Restrictions of hepatitis C treatment for substance-using medicaid patients: cost versus ethics. Am J Public Health. 2017;107(6):893-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid Reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3):215-23. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare & Medicaid Services. For state technical contacts: assuring Medicaid beneficiaries access to hepatitis C (HCV) Drugs. Medicaid drug rebate program notice. Release No. 172. November 5, 2015. Accessed June 7, 2018. https://www.medicaid.gov/medicaid-chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/state-releases/state-rel-172.pdf
- 10.Kapadia SN, Jeng PJ, Schackman BR, Bao Y. State Medicaid hepatitis C treatment eligibility criteria and use of direct-acting antivirals. Clin Infect Dis. 2018;66(10):1618-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oregon Health Authority Communicable Disease Control. Chronic heaptitis C. September 5, 2019. Accessed December 2019. https://www.oregon.gov/OHA/PH/ABOUT/Documents/indicators/hepatitisc.pdf
- 12.Medicaid.gov. Medicaid managed care enrollment report. 2017. Accessed June 7, 2018. https://www.medicaid.gov/medicaid/managed-care/enrollment/index.html
- 13.National Viral Hepatitis Roundtable, Harvard Law School Center for Health Law and Policy Innovation. Hepatitis C: the state of Medicaid access. 2017 national summary report. October 23. 2017. Accessed June 8, 2020. https://www.chlpi.org/wp-content/uploads/2013/12/State-of-HepC_2017_FINAL.pdf
- 14.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. [DOI] [PubMed] [Google Scholar]
- 15.Healthcare Cost and Utilization Project (HCUP). Elixhauser Comorbidity Software Refined for ICD-10-CM. Agency for Healthcare Research and Quality. October 2020. Accessed May 27, 2021. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comorbidity_icd10.jsp
- 16.Healthcare Cost and Utilization Project (HCUP). Elixhauser Comorbidity Software, version 3.7. Agency for Healthcare Research and Quality. June 2017. Accessed May 27, 2021. www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp
- 17.Kalidindi Y, Jung J, Feldman R, Riley T 3rd.. Association of direct-acting antiviral treatment with mortality among medicare beneficiaries with hepatitis C. JAMA Netw Open. 2020;3(7):e2011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda T, McCombs JS, Tonnu-Mihara I, McGinnis J, Fox DS. The impact of delayed hepatitis C viral load suppression on patient risk: historical evidence from the Veterans Administration. Forum Health Econ Policy. 2016;19(2):333-51. [DOI] [PubMed] [Google Scholar]
- 19.Trickey A, Fraser H, Lim AG, et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4(6):435-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calner P, Sperring H, Ruiz-Mercado G, et al. HCV screening, linkage to care, and treatment patterns at different sites across one academic medical center. PloS One. 2019;14(7):e0218388. [DOI] [PMC free article] [PubMed] [Google Scholar]


