Abstract
In 1988 Robert Rescorla published an article in the Annual Review of Neuroscience that addressed the circumstances under which learning occurs, key methodological issues, and what constitutes an example of learning. The paper has inspired a generation of neuroscientists, opening the door to a wider range of learning phenomena. After reviewing the historical context for his article, its key points are briefly reviewed. The perspective outlined enabled the study of learning in simpler preparations, such as the spinal cord. The period after 1988 revealed that pain (nociceptive) stimuli can induce a lasting sensitization of spinal cord circuits, laying down a kind of memory mediated by signal pathways analogous to those implicated in brain dependent learning and memory. Evidence suggests that the spinal cord is sensitive to instrumental response-outcome (R-O) relations, that learning can induce a peripheral modification (muscle memory) that helps maintain the learned response, and that learning can promote adaptive plasticity (a form of metaplasticity). Conversely, exposure to uncontrollable stimulation disables the capacity to learn. Spinal cord neurons can also abstract that stimuli occur in a regular (predictable) manner, a capacity that appears linked to a neural oscillator (central pattern generator). Disrupting communication with the brain has been shown to transform how GABA affects neuronal function (an example of ionic plasticity), releasing a brake that enables plasticity. We conclude by presenting a framework for understanding these findings and the implications for the broader study of learning.
Keywords: spinal cord, learning, pain, metaplasticity, ionic plasticity, BDNF, instrumental conditioning, GABA, timing, central pattern generator
Introduction
In 1988 Robert Rescorla published two review articles, one in the Annual Review of Neuroscience (“Behavioral studies of Pavlovian conditioning”) and another in the American Psychologist (“Pavlovian conditioning: It’s not what you think it is”) (Rescorla, 1988a, 1988b), that are among his most cited (6th and 3rd, respectively; Web of Science, Citation report, January, 2022). While each referred to his work, the papers went well beyond, acknowledging alternative findings that had fueled debates. We see the papers as perspective pieces, outlining his view of what researchers had discovered from the study of animal learning, how to study the process of learning, and why the area remains relevant. While we will reference both papers, our focus will be on his remarks in the Annual Review of Neuroscience (ARN), a paper on which the lead author has leaned heavily over the last 30 years. The presumed influence goes beyond an intuition, with Semantic Scholar (Janaury, 2022) listing the 2014 review by Grau (“Learning from the spinal cord: How the study of spinal cord plasticity influences our view of learning”) as one of the most highly influenced by Rescorla’s 1988 article. Rescorla’s paper is impactful because it provides neuroscientists with a framework for studying learning, outlines some important methodological pitfalls, introduces a host of new findings, and offers a broader view of what constitutes learning. The article remains a “must read” for those wishing to study the neurobiology of learning and a required reading in Grau’s graduate course on learning.
We will provide a personal of account, describing how Rescorla’s ARN article has provided a cornerstone for studies examining whether learning requires a brain. After providing a brief overview of the historical context, we will unpack how the 1988 article enabled the study of spinally-mediated learning and some of what we have learned, focusing on findings that we hope will change the way you think about the process of learning.
The review will provide an overview of the methodological issues that we have had to address in our study of spinal learning, what the observations suggest about how the system learns, and some surprising results that imply a form of peripheral memory. We will also discuss how engaging plasticity impacts future plastic potential (metaplasticity) and how a shift in the inhibitory effect of the neurotransmitter GABA (ionic plasticity) modulates learning. We will conclude with a descriptive model that attempts to integrate key observations. Because our aim is to cover a wide range of observations, details will be limited. Other recent reviews unpack the implications of our work for learning (Grau, 2014), the concepts of metaplasticity and ionic plasticity (Grau & Huang, 2018; Grau et al., 2014), potential implications for neurorehabilitation (Grau et al., 2020), and how pain input after spinal cord injury (SCI) affects tissue loss and recovery (Grau et al., 2017).
State of the field prior to 1988
To bring out the importance of Rescorla’s 1988 ARN paper, we begin with a brief overview of the historical context, introducing some common perspectives in the field of learning. To help the reader understand why we began to study the regulation of pain (nociceptive) signals within the spinal cord, we also provide an overview of how researchers characterized these processes 35 years ago.
Learning as a simple associative process
The foundation for the study of animal learning was, of course, built in the early and mid 1900’s, with the aim of detailing the principles of associative learning. At its root was a process capable of binding representations of events (nodes, ideas) that occurred contiguously in time and space. Pavlovian conditioning was seen as an attractive model for studying this process, demonstrating that animals can link a neutral cue [the conditioned stimulus (CS)] with a biologically significant event [the unconditioned stimulus (US)], yielding a conditioned response (CR) to the CS. A concern at this juncture was that other processes might transform how the animal behaves to the CS, causing it to drive a CR-like behavior. One potential problem stemmed from the observation that an intense US often has a sensitizing effect that alters how an animal responds to a CS independent of the CS-US pairing (pseudoconditioning) (Domjan, 2015). For example, exposure to a shock US can enhance startle to an auditory CS independent of whether the two events are paired. To address this issue, researchers routinely compare a CS that has been paired with the US (the CS+) to one that was presented in an unpaired manner (the CS-). The second and more insidious issue concerned the possibility that the CS was not really “neutral”; that it had some (likely, biologically prepared) potential to generate the target response prior to training. If the amplitude of this pre-existing (alpha) response is modulated by the CS-US relation, either because a paired US slows habituation to the CS (protection from habituation) or differentially amplifies (sensitizes) behavioral reactivity to the CS (pairing specific enhanced sensitization), a CS+/CS− difference could emerge without a new (de novo) association. Because the amplification of the CR stemmed from non-associative processes, researchers argued that such behavioral modifications do not reflect true learning which, by fiat, was assumed to be associative in nature. Noting that pre-existing CS-elicited responses tend to occur soon after stimulus onset (e.g., within 100 msec), and declined with repeated exposure (habituation), researchers designed their experiments to avoid this contaminating effect (Kimble, 1961; Mackintosh, 1974); ignoring the behavioral response observed soon after CS onset (on the assumption that training does not impact the timing of the CS-elicited response) and habituating animals to a tone prior to fear conditioning (on the assumption that the presentation of the US per se will not re-instate the CS-elicited response). Experimental procedures that failed these criteria were disparaged as examples of alpha conditioning (Gormezano & Kehoe, 1975), not true learning (which was assumed to be associative in nature).
When Rescorla began his work on conditioning, learning was couched in associative terms, with the strength of the CS-US link being determined by the number of times the events were paired. Rescorla re-defined our view of animal learning, providing evidence that learning does not depend upon contiguity alone and, with Wagner, detailed a model that described how events compete for association with the US and its effectiveness varies (Rescorla & Wagner, 1972). Along the way he detailed key methodological issues and unveiled how organisms can learn more than simple associations—how cues can interact and gain a form of hierarchical control (Rescorla, 1988b). But by the late 1980’s, much of Psychology had moved on from animal learning, enamored with the study of cognitive processing in humans. Those outside of animal learning generally maintained perspectives shaped by work conducted 20 to 30 years earlier.
Advances in the study of neurobiology of learning
The period before 1988 was also marked by advances in the study of the neurobiology of learning and memory (Dudai, 1989): Thompson and his colleagues had deciphered the neural processes that underlie delayed eyeblink conditioning (Thompson, 1986); LeDoux and Fanselow had started to unveil the processes that underlie fear conditioning, with a focus on the amygdala (Fanselow, 1986; LeDoux, 1987); evidence had emerged that the hippocampus provides a spatial map and supports a form of plasticity known as long-term potentiation (LTP) (Bliss & Lomos, 1973; O’Keefe & Nadel, 1978); and researchers exploring the neurobiology of learning in invertebrates had begun to detail the underlying physiological and cellular processes (Alkon, 1983; Kandel & Schwartz, 1982). Of particular note was the work being done with the invertebrate Aplysia. In this preparation, a gill withdrawal response can be elicited by touching the siphon or mantle shelf. If a noxious stimulus is applied to the tail, the gill withdrawal response is sensitized. In the early 1980’s, Kandel and his colleagues identified one of the signal pathways underlying this learning. Rescorla was surprisingly well versed in this line of work, having served on the dissertation committee of one of Kandel’s students—E. Terry Walters. Walters & Bryrne (1983) recognized that a Pavlovian-like conditioned response might be established using noxious stimulation to the tail as the US and electrophysiological stimulation of the sensory nerves innervating the siphon or mantle shelf as CSs (Walters & Byrne, 1983). In a parallel set of experiments, Carew et al. (1983) pursued the same question using mechanical stimulation of the siphon or mantle as CSs (Carew, Hawkins, & Kandel, 1983). In both cases, pairing one cue (the CS+) with the US enhanced the magnitude of the sensitization that developed, relative to the unpaired cue (CS-), a phenomenon known as pairing specific enhanced sensitization. In his paper, Walters acknowledged Rescorla. However, not all were impressed with this line of work, discounting the paradigm as an example of alpha conditioning.
Learning and the regulation of incoming pain signals
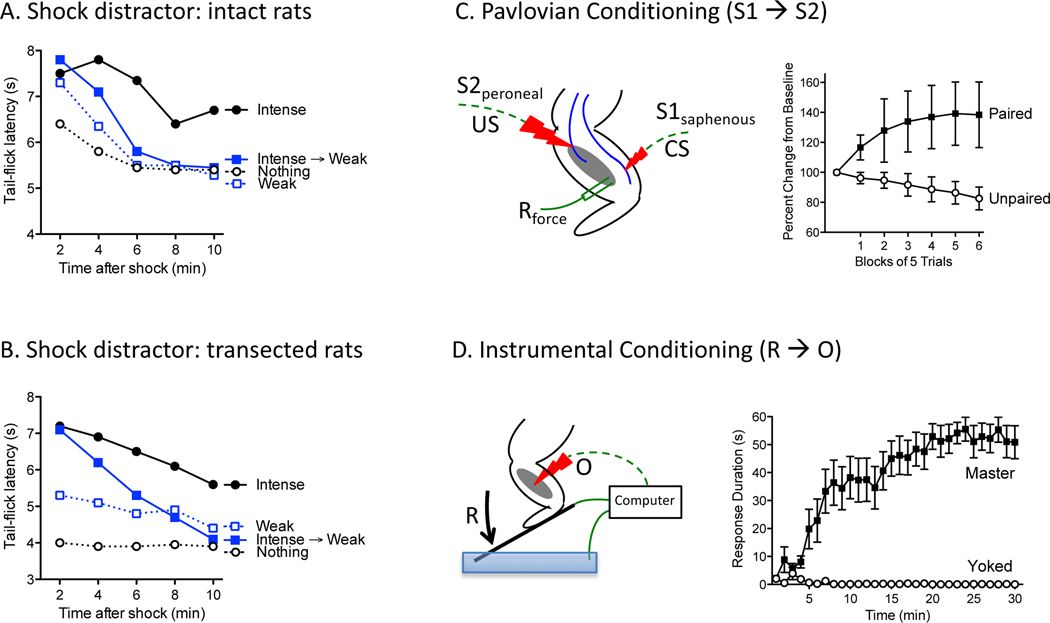
Early work by the lead author was informed by studies in the area of pain modulation and the recent discovery that the body makes opiate-like proteins (endogenous opioids) (Akil et al., 1984). The focus at the time was on the inhibition of nociceptive signaling (antinociception), with behavioral and physiological work showing that the brain can quell nociceptive activity soon after it enters the central nervous system (CNS) by means of descending serotonergic fibers (Basbaum & Fields, 1984). Because the antinociception was thought to be spinally mediated, it was frequently assessed using a spinal reflex—tail withdrawal from a noxious thermal stimulus (tail-flick test). A key question at the time concerned the circumstances under which environmental events engage this antinociceptive process, with Fanselow suggesting that this was linked to the acquisition of conditioned fear and defensive behavior (Fanselow, 1986). Grau took a different approach, suggesting instead that the memory of an aversive event elicits an opponent-like antinociceptive response (Grau, 1987a, 1987b). A key prediction from this memory-oriented perspective was that the presentation of a distracting stimulus after animals have experienced an aversive event should cause the antinociception to decay more rapidly. An initial test of the hypothesis used a visual distractor and lent some support for this cognitive approach. Grau discussed these data in a laboratory meeting with Rescorla, who suggested a stronger test, examining whether a weak shock could serve as a distractor. If the memory-oriented view has merit, the weak shock stimulus should displace the memory of intense shock and cause the antinociception to decay more rapidly. The novel prediction was that exposure to more aversive stimulation should lead to a weaker antinociceptive effect. The results were just as Rescorla had predicted (Figure 1A) (Grau, 1987a). The procedure was later extended to humans by Kahneman who showed that following a painful event with one that is less painful (a “better end”) attenuates the rated painfulness of the first event (Kahneman, Fredrickson, & Schreiber, 1993).
Figure 1.
(A) Uninjured rats exposed to a few moderately intense (1 mA) shocks to the tail (Intense) exhibit reduced reactivity (antinociception) to a noxious thermal stimulus applied to the tail, relative to a group that was treated the same but did not receive shock (Nothing). Presenting a weak (0.1 mA) shock distractor causes the antinociception to decay more rapidly (Intense→Weak). (B) In rats that have received a high thoracic (T2) transection, a very intense shock (3 mA) to the tail elicits antinociception. Following the intense shock with a weaker (1 mA) shock (“distractor”) causes the antinociception to decay more rapidly. (C) In spinally transected rats, pairing moderate electrical stimulation of the saphenous nerve (the CS) with more intense stimulation of the peroneal (the US) endows the CS with the capacity to elicit a stronger muscle response (the CR), relative to a CS that was presented in an unpaired manner. (D) In spinally transected rats, animals given shock to the tibialis anterior muscle of one hind leg whenever the limb is extended (Master) exhibit a progressive increase in flexion duration relative to animals given the same amount of stimulation independent of leg position (Yoked). Adapted from (Grau, 1987a, 1987b; Grau et al., 2020; Grau et al., 1990).
While Fanselow and Grau described the processes that drive antinociception in different psychological/behavioral terms, their views shared a focus on learning. Like Schull (Schull, 1979), they assumed a conditioned opioid antinociception could modulate the aversiveness of a US, helping to explain why a pretrained cue blocks learning about an added cue. At the time, it was recognized that antinociceptive processes could also be engaged by incoming nociceptive signals through connections within the spinal cord and brainstem/midbrain (Watkins, Cobelli, & Mayer, 1982; Watkins, Kinscheck, & Mayer, 1983; Watkins & Mayer, 1982). It was assumed, though, that the antinociception generated by these lower-level pathways reflected an unlearned (unconditioned) response to noxious stimulation (Grau, 1987b).
Prevailing views of spinal cord function
In the mid-1980’s, most viewed the adult spinal cord as a hard-wired system, capable of organizing some simple reflexes, but incapable of true learning. The prevailing view of this portion of the CNS emphasized its role in relaying incoming (afferent) and outgoing (efferent) neural commands between the brain and the periphery, a conduit-like function tied to the band of axons (white matter) that forms the outer rim. It was recognized that these fibers project to/from an inner region of neurons (the central gray) of the spinal cord, with peripheral sensory input innervating the upper region (dorsal horn) while the lower portion (ventral horn) drives motor behavior (Grau et al., 2006). It was also known that the spinal cord is organized into cellular layers (laminae) that subserve distinct functions and that the size of the central gray varies as you move down the spinal cord, from the cervical, thoracic, lumbar, to sacral region. Within each region, the vertebrae are numbered from top to bottom. The central gray is larger within the cervical and lumbar-sacral (lumbosacral) regions, as it contains the neurons needed to integrate sensory signals and motor commands for the upper and lower limbs, respectively.
Spinal reflexes involve elicited responses that can be evoked without input from the brain. These reflexes can be studied in animals by cutting communication with the brain by means of a thoracic spinal cord transection and applying stimulation below (caudal to) the transection. With this preparation, application of a noxious stimulus to one hind leg, or electrical stimulation of the afferent nerve, can elicit a flexion response. It has been known since Sherrington that experience can impact the vigor of a spinal reflexes, with repeated stimulation causing response vigor to wane (habituate) while exposure to an intense noxious stimulus can sensitize behavioral reactivity (Sherrington, 1906). Groves and Thompson built on these early observations, detailing the circumstances under which these processes are engaged and the nature of the intervening neural pathway, providing the foundation for their dual process theory (Groves & Thompson, 1970). It was also known that the application of an irritant caudal to a spinal transection can engage rhythmic scratching, implying that the spinal cord has the machinery needed to generate rhythmic behavior (Sherrington, 1906). In addition, DiGiorgio (1929) had shown that cerebellar lesions can induce a persistent hindlimb flexion response (fixation) that survives a subsequent spinal transection, implying that brain systems can induce a kind of spinal memory (Patterson, 2001).
Given the simpler architecture of the spinal cord, and its capacity to exhibit some basic learning phenomenon, Thompson and his colleagues explored whether it was capable of Pavlovian conditioning (Patterson, 1976; Patterson, Cegavske, & Thompson, 1973). For their US, they applied electrical stimulation to a nerve (e.g., superficial peroneal) of one hind leg at an intensity that elicited a strong flexion response [(the unconditioned response (UR)]. For their CS, they used stimulation to a distinct nerve (e.g., saphenous) of the same leg at an intensity that elicited a weak response. In animals that had undergone a thoracic transection, pairing the CS with the US amplified the CS-elicited response (CR), relative to a cue that was presented in an unpaired manner (Figure 1C). This consequence of training lasted hours and was stronger when the CS occurred prior to the US (forward pairing). Further, presenting the CS alone caused the response to wane (extinguish). While these findings suggested that neurons within the spinal cord are sensitive to stimulus-stimulus (S-S) relations, the training involved the amplification of a pre-existing CS-elicited response, and so it too was viewed as an instance of alpha conditioning, not true learning.
The turning point—1988
The review articles that Rescorla published in 1988 were designed to update perspectives on animal learning, what is learned and when, what defines learning, and how this process should be studied (Rescorla, 1988a, 1988b). His American Psychologist paper focused more on the underlying processes, bringing out the complexity of what was learned and how simple contiguity alone does not govern when it occurs. Of particular import, he argued learning involves more than the acquisition of a simple CS-US association; in some cases learning appears to lay down an associative structure that allows a cue to regulate how a CS-US relation is processed, yielding a form of hierarchical control.
As noted above, we will focus on the material laid out in his ARN paper (Rescorla, 1988a), which we suggest came to define the field of learning in neuroscience—how it can be studied and what constitutes an example of learning. The article remains relevant 30+ years after its publication, a paper that we view as required reading for any investigator interested in exploring the neurobiology of learning. To see why we consider the paper essential, let’s consider some of its main theses.
Guidelines for the study of learning
Rescorla began by providing a framework for the study of learning, relating this process to the environmental conditions used to engender learning, noting that these can be typically classified as involving exposure to a stimulus (S) alone, learning about a S-S relation, or the consequences of arranging a relation between a behavioral response (R) and an environmental outcome (O; aka reinforcer). As he notes, if we could explain how the organism encodes these three types of events, we would have a good understanding of learning as a whole.
The next step involved a key methodological detail: that to demonstrate learning, we must show that an experience at time 1 (t1) has an effect at time 2 (t2). Here there is an implicit requirement that the earlier experience induced a kind of memory, that it has a lasting effect. The requirement cuts to a common problem faced by those seeking to develop simple preparations to study the neurobiology of learning. Consider the modification of leg position by the application of electrical stimulation (shock) to an insect (Church & Lerner, 1976). If shock is applied whenever the leg moves outside of a prescribed “window”, the animal seemingly learns to maintain the leg in a position where shock does not occur. On the surface, this might appear to be a kind of avoidance learning. But we also know that the likelihood of movement is far higher when shock is presented, and that a leg movement is unlikely when shock is absent. Given this, a mechanical system incapable of learning would gravitate towards holding the limb in a position where shock does not occur. To show that learning is involved, one must demonstrate that the experience has a lasting effect; that the behavioral modification is not simply driven by the current environmental contingencies. Tied to this is a requirement that animals are treated the same, except for the event/relation to be learned, and tested under common conditions at t2. Minus this, it would be a mistake to conclude that the behavioral differences observed during t1 training of the insect leg reflects a form of learning.
In some cases, differences in acquisition curves can be so clear during t1 training that it might seem that little remains to be done. For example, when Thompson and his colleagues examined how the rate of habituation varies as a function of stimulus intensity, it was clear that a greater decrement was observed during the pre-exposure period (t1) when animals were exposed to a weaker stimulus (Groves, Lee, & Thompson, 1969). The problem here is that our conclusion is based on how groups, with distinct training histories, respond to distinct stimuli (intense versus weak). The comparison confounds the training history with stimulus intensity. To unconfound our appraisal, animals must be tested under common conditions using the same stimulus intensities. When this was done, the findings supported the opposite conclusion—that exposure to an intense stimulus induces greater habituation (Davis & Wagner, 1968). Rescorla’s article shaped future work by clearly describing this methodological pitfall and how it can be avoided.
Expanding our view of learning
Embedded within the text of his 1988 article were a number of points that were so gracefully written that many readers may not have noticed that he overturned the house, explicitly rejecting dogmatic views held by his predecessors, opening the door to a much wider range of learning phenomena. A key step here lies in his acceptance that organisms are biologically prepared to learn some relations and that CSs may sometimes elicit a CR-like response prior to training. He then goes a step further, noting that CS-US contiguity can matter “not because it promotes an association between the US and the CS but rather because it gives the US the opportunity to modulate other learning about the CS”. For example, experience with a CS-US relation could act to maintain attention to the CS or slow the rate of habituation (protection from habituation). Later in the paper he takes explicit aim at those who would denigrate work on learning in simpler systems because it involves a form of alpha conditioning:
“In some instances, a major consequence of Palvovian conditioning may be to allow a CS to elicit more successfully its original response. Although such enhancement of the original response to the CS has sometimes been rejected as not being “true” conditioning, little basis exists for such an attitude (cf Farley and Alkon, 1985). If a CS comes to evoke its original response more adequately because of its pairing with the US, but fails to do so when an appropriate control condition is arranged, then the performance is demonstrably dependent upon arranging a CS/US relation. That the learning of that relation is exhibited as the enhancement of an original response may be informative about its nature, but it surely is not grounds for banishing it from the hallowed realm of “true” conditioning.”
Implications
Rescorla’s 1988 ARN article defined the field of learning to the neuroscience community, providing a framework that still stands. By moving the distinguishing features to environmental events and relations, he sidestepped a key problem. We may disagree about the mechanisms that underlie habituation, Pavlovian conditioning, or instrumental performance, but we can agree on what the organism experienced. By linking the process to the outcome obtained under common conditions (at t2), he clarified a criterion for learning. By explicitly acknowledging how “non-associative” processes can be engaged by environmental relations and contribute to behavioral change, he opened the door to a much wider set of phenomena, and in this way he enabled our work examining whether neurons within the spinal cord support learning.
At this junction, we will advance a conclusion that some may feel goes too far. In detailing the richness of what the organism can learn, Rescorla divorced the term “associative learning” from the simple linking of two representations/nodes/ideas. For him, associative theory appears to have provided more of a framework for describing how learning affects behavior. At a mechanistic level, it does not reference a single entity (e.g., CS-US link), but rather a family of potential interactions that allow researchers to represent the alternative ways in which the constituents of learning may interact (e.g., to facilitate or inhibit performance in a conditional manner; Rescorla, 1988b). Used in this way, associative theory seems more of a descriptive style than a detailed theory. Of course, we can address this limitation by proposing that associative learning underlies the process of conditioning. But this course backs us into the problematic dichotomy between true learning and alpha conditioning. The traditionalists would appear to have a point here; to discount a “non-associative” process based on mechanism such as pairing specific enhanced sensitization would seemingly require a truly neutral CS. If we follow this road, we would reject many of our most popular paradigms for studying conditioning. After all, the CSs used in fear conditioning typically have some capacity to elicit a freezing/fear-like response prior to being paired with an aversive US. Even the prototypic example of true learning, eyeblink conditioning, builds upon pre-existing synaptic connections. In this paradigm a CS may appear unprepared because it can support a multitude of distinct conditioned responses. But at a biological level, this is possible because the CS engages parallel fibers within the cerebellum that radiate across the cortical surface and connect with hundreds of US inputs (climbing fibers), which select the appropriate response (Mauk, Steinmetz, & Thompson, 1986; Steinmetz, 2001; Thompson, 1986). The system appears unconstrained because it is overly prepared. Beyond this, the methods used to discount a role for alpha conditioning can themselves be discounted. To claim a CR does not build upon a pre-existing CS elicited response because these are limited to the early portion (first 100 msec) of the CS assumes that the behavior cannot be tuned by the temporal characteristics of the CS-US relation. Likewise, arguing that alpha conditioning plays no role because animals were habituated to the CS prior to training assumes that the presentation of the US does not reinstate the behavioral response. To our knowledge, neither assumption rests on a solid foundation. Further complexities arise when it is recognized that, after the CS and US are paired just enough to support an intermediate level of conditioned responding, omitting the CS may have little effect on the continued development of the CR (Kimble et al., 1955). This observation led Kimble to suggest “pseudoconditioning may be a part of all conditioning in which a noxious stimulus is employed” (Kimble, 1961). His conclusion, over 60 years ago, was that “the seemingly simple conditioned reflex experiment actually contains features of great complexity”, a mechanistic nuance that is lost in attempts to link the consequences of conditioning to a singular associative process.
Many may acknowledge the complexity of conditioning, that a variety of processes are at work, but retain a core belief in the central utility of associative learning—that it is the bedrock on which conditioning is built. Doubts, though, still remain. The key outcome of learning is a memory, a modification that preserves the act of learning over time. As Gallistel (2021) notes, memories are useful because they store “facts”, such as what, when, and where an event occurred. Much of the power of Rescorla’s work stems from his use of conditioning procedures to discover how organisms learn such facts. Yet, an association in and of itself contains no factual content, it simply indicates that two events have in some way been linked. To flesh out how a link provides an increment in factual knowledges requires additional mechanisms and it is not at all clear that the concept of “associative” learning will help us solve this puzzle (or uniquely do so).
The concept of association is so ingrained in our views of learning, we reflexively add it as a descriptor and begin our explanation of the underlying processes in associative terms. As we will describe below, we believe the latter tendency has impeded our understanding of the processes that underlie spinal learning. If conditioning builds upon a variety of processes, and there are reasons to question the explanatory value of simple associations, there would seem little benefit to adding the term as a descriptor of what we study. In most instances, it would seem more accurate to say we study “learning”, rather than “associative learning”, because we do not know whether an associative process underlies the learning. Minus a detailed theory of how links enable the storage of facts, referencing an association brings no additional understanding. If one wishes to convey additional content, reference the environmental experience under study (e.g., “relational” learning). Such a view, while seemingly heretical, is consistent with Rescorla’s push to reference alternative forms of learning in terms of the environmental experience that drives the process.
Revising our views of learning and the spinal cord
The period after 1988 was marked by advances in the field of learning and memory, with researchers describing the neural systems involved in fear conditioning and learned helplessness (LeDoux, 2000; Maier & Watkins, 2010). Studies related LTP to a gated channel, the NMDA receptor (NMDAR), which requires a form of neural contiguity (presynaptic glutamate release and a strong postsynaptic depolarization) to be engaged, providing a cellular process for Hebbian plasticity (Bliss & Collingridge, 1993). Research exploring the role of the hippocampus linked this structure to configural learning and trace conditioning, with the latter capacity depending upon a form of cognitive mediation (Clark & Squire, 1999; Sutherland, 1989). Building on earlier behavioral strategies, the hedonic experience generated by a drug (liking) was distinguished from drug craving (wanting), with the latter related to a form of conditioned incentive and dopaminergic drive (Berridge & Robinson, 2003). Of particular interest in the current context, Schultz showed that dopamine activity was governed by the discrepancy between the physical and expected reward (prediction error), providing a biological instantiation of the Rescorla-Wagner model (Schultz & Dickinson, 2000). Likewise Kim uncovered a form of negative feedback within the cerebellum that allowed a learned CS-US relation to lessen the effective of the US (Kim, Krupa, & Thompson, 1998). Similarly, further work on pain modulation showed how descending fibers can inhibit nociceptive processing within the spinal cord, reducing the painfulness of an expected US (McNally, Johansen, & Blair, 2011).
Learning to regulate pain without a brain
In the early 1990’s, we began to collect data that challenged some of our earlier claims (Grau, Salinas, Illich, & Meagher, 1990). As noted above, it was recognized that antinociceptive processes can be engaged by afferent signals at the level of the spinal cord and brainstem (Watkins & Mayer, 1982; Watkins et al., 1983), bypassing the forebrain-dependent processes that we argued underlie learning-related changes in pain reactivity. From this perspective, it was assumed that lower-level pathways are engaged in an unconditioned manner and serve as a kind of “safety switch” that is only activated when the organism is exposed to very intense noxious stimulus (Grau, 1987b). Parametric studies examining the induction of antinociception in animals that had the forebrain removed (decerebration) or high thoracic (T2) transection (spinalization) provided some support for this proposal (Meagher, Chen, Salinas, & Grau, 1993; Meagher, Grau, & King, 1989, 1990). If lower-level pathways abide by simpler rules, determined solely be the additive effect of noxious stimulation, presenting a weak shock “distractor” after an intense shock should not cause the antinociception to decay faster. If anything, increasing shock exposure should augment the magnitude and duration of the antinociceptive response. But when we tested this prediction, we obtained a pattern of results identical to that observed in intact animals (Figure 1B)—in rats that had undergone a T2 transection, presenting a weaker tail shock after (but not before) an intense one caused the antinociception to decay more rapidly (Grau et al., 1990). An experimental manipulation used to implicate a working memory-like mechanism in animals yielded identical results under conditions that precluded brain processing.
We then reconsidered our claim that conditioned antinociception was necessarily brain-dependent. To explore this issue, we used a differential conditioning paradigm analogous to that employed to establish a conditioned response in Aplysia. Spinally transected animals received an intense tail shock, which served as the US (Grau et al., 1990). For our CSs we used a weaker shock applied to the left or right hind leg. Stimulation to one hind leg was paired with the US (CS+), while the other was unpaired (CS-). After 60 min of training (30 pairings), we compared nociceptive reactivity during each CS using the tail-flick test and found that animals exhibited longer latencies during the CS+ and that the magnitude of this difference declined (extinguished) over trials. Further work showed that conditioning was weakened if the CS was presented alone prior to training (latent inhibition) and that learning about a new cue was blocked when it was presented in compound with a previously trained CS (Illich, Salinas, & Grau, 1994).
It was clear from the start that our CSs generated a weak antinociception prior to training, implying that the learning involved a form of alpha conditioning. We recognized that pairing could matter because it disrupted habituation to the CS (protection from habituation) or sensitized the CS-elicited antinociception (pairing specific enhanced sensitization). To dissociate these alternatives, we compared the response elicited by a trained cue (CS+) to a novel stimulus (Joynes & Grau, 1996). If training amplifies the CS-elicited response, the CS+ should generate an antinociception stronger than a novel cue. It did not, implicating protection from habituation. We further reasoned that pairing the CS with a US might slow, but not prevent, habituation of the antinociceptive response. The novel prediction is that the CS+/CS− difference should fade with continued training, which it did. Because increasing the interval between trials weakens spinally mediated habituation, increasing the inter-trial interval should lessen habituation to the CS- and attenuate the CS+/CS− difference. Again, the results implicated protection from habituation. Critics naturally challenged our results because our example of learning built upon a pre-existing response. Following Rescorla (Rescorla, 1988a), we suggested otherwise, arguing that the key issue is whether or not the system is sensitive to a S-S relation (Grau & Joynes, 2005a, 2005b).
Advancing our views of spinal cord function
Around this same time, there were host of other discoveries that shifted our view of spinal cord function. In the pain literature, researchers discovered that the application of a peripheral irritant (e.g., capsaicin, the active ingredient in chili peppers) induces an alteration within the spinal cord that causes animals to exhibit a pain-like withdrawal response to non-noxious mechanical stimulation (Willis, 2001). This phenomenon is of interest because it mirrors a symptom of neuropathic pain: the emergence of pain sensations to light touch, a phenomenon known as allodynia (Simone, Baumann, & Lamotte, 1989). At the level of the spinal cord, the development of enhanced mechanical reactivity has been related to nociceptive sensitization within the dorsal horn (Latremoliere & Woolf, 2009), a modification that brings about a lasting change in how the organism responds to non-noxious sensory stimulation. Interestingly, the induction of this modification depends upon signal pathways analogous to those implicated in brain-dependent learning and memory, including the NMDAR (Ji, Kohno, Moore, & Woolf, 2003). Further parallels were provided by Sandkühler and his colleagues who showed that noxious stimulation can induce a form of LTP within the spinal cord and that this effect too is NMDAR dependent (Sandkuhler, 2000; Sandkuhler & Liu, 1998). These observations led Durkovic to explore the role of the NMDAR in spinal conditioning. Using electrophysiological procedures, he showed that pretreatment with a NMDAR antagonist blocked the development of a conditioned response (Durkovic & Prokowich, 1998).
Additional challenges to the traditional view of spinal cord function were provided by Rossignol and Edgerton, who in the 1980s attempted the seemingly impossible—to train animals that had undergone a complete thoracic transection to step with their hindlegs while suspended over a treadmill (Edgerton et al., 1992; Forssberg, Grillner, Halbertsma, & Rossignol, 1980; Lovely, Gregor, Roy, & Edgerton, 1986; Pearson & Rossignol, 1991). As would be expected, the paraplegic animals initially exhibited little capacity to step, with their paws dragged behind by the treadmill belt. But with some support, combined with rhythmic stimulation of the perineum, the animals slowly recovered the capacity to step.
From prior work it was known that noxious stimulus applied to the dorsal region of a hind paw engages different responses depending upon the phase of the step cycle, generating a flexion as the leg is brought forward (swing phase) and an extension when the leg is extending rearward (Forssberg, Grillner, & Rossignol, 1977). Building upon this observation, de Leon examined how transected step trained animals respond to an obstacle (a bar) positioned so that one hind paw hit it during the swing phase (Edgerton, Roy, DeLeon, Tillakaratne, & Hodgson, 1997). With experience, the animal learned to lift the leg higher, minimizing contact the bar. More importantly, this behavioral modification persisted after the bar was removed, implying some capacity to learn about a R-O (instrumental) relation.
Concurrent research by Wolpaw and his colleagues explored whether animals could learn to modify a spinal reflex (Wolpaw & Carp, 1990; Wolpaw & Lee, 1989). To study this, they used an electrical analog of the stretch reflex, known as the Hoffman (H) reflex. Animals received an appetitive reward for exhibiting either an increase or decrease in H-reflex magnitude. As expected, this operant training brought about a behavioral change. What was surprising is that the change in reflex magnitude persisted after the spinal cord was transected, implying that brain-dependent learning can induce a lasting modification (memory) within the spinal cord.
Spinally-mediated instrumental learning
The findings reviewed above set the stage for our work examining whether spinal neurons are sensitive to R-O relations—can this portion of the CNS support a form of instrumental conditioning without input from the brain? We were intrigued by this question, in part, because we saw this form of learning as especially relevant to rehabilitation after SCI. Indeed, as we have noted elsewhere (Grau et al., 2020), rehabilitation and learning have the same aim—to bring about a lasting change in neural function. We also recognized that work was needed in this area because prior studies (Buerger & Chopin, 1976; Chopin & Buerger, 1976) had been discounted on methodological grounds (Church, 1989; Church & Lerner, 1976). Key criticisms included a lack of essential controls, non-uniform training conditions, and a failure to test under common conditions. In addition, the work was faulted because it relied on just one procedure, the master-yoke paradigm, to demonstrate the R-O relation mattered. In the sections that follow, we describe how we addressed these criticisms and what we have learned from the study of spinally-mediated instrumental learning. Our focus here will be on new findings that we believe challenge and inform the way we think about the process of learning more broadly.
Learning about a R-O relation without a brain
To examine whether spinal neurons are sensitive to R-O relations, we cut the thoracic (T2) spinal cord in rats, verifying that no fibers remain. The next day, rats are secured in Plexiglas tubes that are notched in the rear, providing openings that allow the hind legs to hang freely below. Electrodes are inserted into the tibialis anterior muscle and electrical stimulation (shock) is applied to elicit a flexion response of equivalent magnitude across animals. Leg position is monitored by taping an insulated 7-cm rod (contact electrode) to the animals paw. A container of salt water is placed below the animal and the uninsulated tip of the electrode is submerged a set amount (4 mm). Whenever the contact electrode touches the salt solution, it completes a circuit that is monitored by a computer. A R-O relation can then be established by applying shock to the leg whenever it is extended. To show that the R-O relation matters, one group of animals (master) is given response-contingent stimulation while another (yoke) is given shock independent of leg position. To equate shock exposure across groups, animals given non-contingent (uncontrollable) shock are experimentally coupled (yoked) to rats in the master group. Using this procedure (Figure 1D), we have shown that master animals quickly learn to exhibit an increase in flexion duration that minimizes net shock exposure (Grau, Barstow, & Joynes, 1998). Yoked animals, given the same amount of shock independent of leg position, typically perform a flexion response when shock is presented, but do not exhibit an increase in flexion duration— they fail to learn. Importantly, if stimulation is turned off and animals are tested under common conditions, only master animals continue to maintain a flexion response (Tarbet, Hudson, Lout, & Grau, 2019). Just six minutes of training can induce an increase in flexion duration that persists for over an hour. Interestingly, this behavioral modification does not appear to reflect an increase in the force of the shock-elicited flexion response; both master and yoked animals exhibit a habituation-like decline in flexion force after training, with the magnitude of this effect determined by the duration and intensity of stimulation (Grau et al., 1998). What distinguishes the performance of master and yoked animals is the duration of the elicited response.
Further work was conducted to verify the learning depends upon neurons within the spinal cord. Supporting this, we showed that cutting the afferent input near the dorsal root entry zone, or inactivating spinal neurons by slowly infusing the anesthetic lidocaine over the lumbosacral spinal cord, blocked learning (Crown, Ferguson, Joynes, & Grau, 2002a). Using scalpel cuts to completely transect the lumbosacral spinal cord at particular levels, we were able to show that learning depended upon neurons that lie between the mid-lumbar (L3) and upper sacral (S2) region (Liu et al., 2005). We also showed that the pretreatment with an NMDAR antagonist (APV or MK-801) blocks acquisition (Ferguson, Crown, & Grau, 2006; Joynes, Janjua, & Grau, 2004). Interestingly, treatments that disrupt learning (as indexed by an increase in flexion duration) do not typically prevent the shock elicited response, implying that stimulation also triggers some transmitter release at the neuromuscular junction (NMJ) (Hoy et al., 2020). To address past criticisms of the master-yoke procedure, we also assessed the effect of experimentally manipulating response-outcome contiguity, by delaying either the onset or offset of shock (Grau et al., 1998). Delaying the onset of shock by just 100 msec prevented learning, whereas delaying the offset by this same duration had no effect. These observations suggest that the behavioral modification does not reflect a kind of escape learning. Learning to escape may require a brain. At the level of the spinal cord, the learning appears to be reinforced by shock onset.
From these initial observations, we can conclude that instituting a response-outcome relation brings about a rapid shift in flexion duration. Indeed, we subsequently showed that a master-yoke differences emerges within the first minute of training (Hoy et al., 2020). The rapidity of the learning led us to suggest that this behavioral modification depends upon a biologically prepared circuit (Grau et al., 2012). Our preliminary data also provides some guidance regarding the factors that support learning. The fact that shock onset induces an increase in flexion duration in master but not yoked animals implies that when the event occurs is critical. What distinguishes master and yoked animals is leg position at the time of shock onset; for master animals it occurs as the leg falls from a flexed position, whereas yoked rats will receive many shocks while the leg is down. Distinguishing these states requires an index of leg position, which could be provided by sensory (e.g., proprioceptive) cues. For master animals, the essential cue could be linked to a static stimulus (leg position at the time of shock onset), a vector that describes the leg movement that preceded shock onset, or a sensory cue engaged when the contact electrode touches the underlying solution (which would, due to increased resistance, slow the fall rate). If learning is tied to the static position, administering shock while the leg is held above the solution should be sufficient to drive an increase in flexion duration. It is not, which suggests that the key feature is tied to a sensory cue related to the movement of the leg at the time of shock onset. Given the speed with which this sensory relation drives an increase in flexion duration, we have suggested that it builds upon a pre-existing architecture, one that implicitly assumes that the onset of a noxious stimulus is tied to a predictive cue. In this sense, the system seems biased to favor the detection of R-O relations, a relation that then drives the appropriate behavioral response. From this perspective, the R-O relation acts to select the appropriate response (e.g., extension vs. flexion) which is sensitized by the onset of noxious stimulation. R-O contiguity matters because delaying the sensory cue tied to the leg movement at the time of shock onset disrupts the sensory integration needed to select the appropriate behavioral response. Within this framework, detecting the R-O relation, and selecting the appropriate motoric output, are assumed to depend upon pre-existing structures; learning impacts behavior by sensitizing the efferent (motor) output.
Training with controllable stimulation enables learning
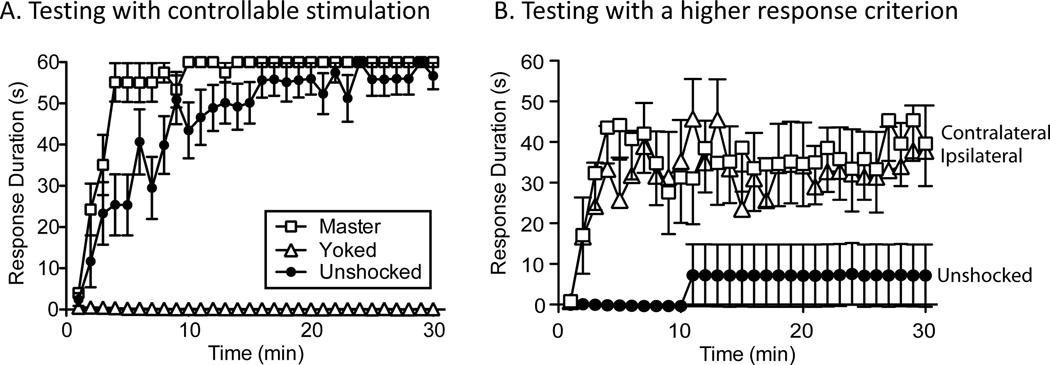
Behavioral training does more than induce an increase in flexion duration—it enhances the capacity to learn when animals are tested under common conditions with a higher criterion (Figure 2B). We showed this by testing rats with a higher (8 mm) response criterion after they had received 30 min of training (Crown et al., 2002a). At this height, naïve animals fail to learn, but pretrained animals can learn and this is true independent of whether they are tested on the same or the opposite leg. Interestingly, the development of this effect requires 30 min of training; 6 min of training, which induces a lasting increase in flexion duration, is not sufficient to enable learning when animals are tested with a higher criterion. Because this training effect involves a learning-induced modification in the capacity to learn, a kind of plasticity of plasticity, we have suggested that it reflects a form of metaplasticity (Abraham, 2008; Abraham & Bear, 1996; Grau et al., 2014). Subsequent work linked this metaplastic effect to the expression of brain-derived neurotrophic factor (BDNF) (Gomez-Pinilla et al., 2007; Huie, Garraway, et al., 2012). Supporting this, pretreatment with a BDNF inhibitor (TrkB-IgG) blocks the facilitation effect. Conversely, pretreatment with BDNF can substitute for training to enable learning (Figure 3).
Figure 2.
Testing pretrained animals under common conditions. (A) Spinally transected rats were trained with controllable (Master) or uncontrollable (Yoked) stimulation. A third group was set up in the same manner, but received no stimulation (Unshocked). Flexion force and contact electrode depth (response criterion) were then re-equated and animals were tested for 30 min with controllable stimulation applied to the pretrained leg. Prior training with controllable stimulation fostered learning whereas exposure to uncontrollable shock impaired learning. (B) Spinally transected animals received 30 min of training with controllable stimulation and a moderate response criterion (a contact electrode depth of 4 mm). All rats were then tested for 30 min with a higher (8 mm) response criterion. At this higher criterion, previously untrained (Unshocked) rats were unable to learn. Pretained animals were able to learn and this was true independent of whether they were tested on the pretrained (Ipsilateral) or opposite (Contralateral) leg. Adapted from (Grau, 2012).
Figure 3.
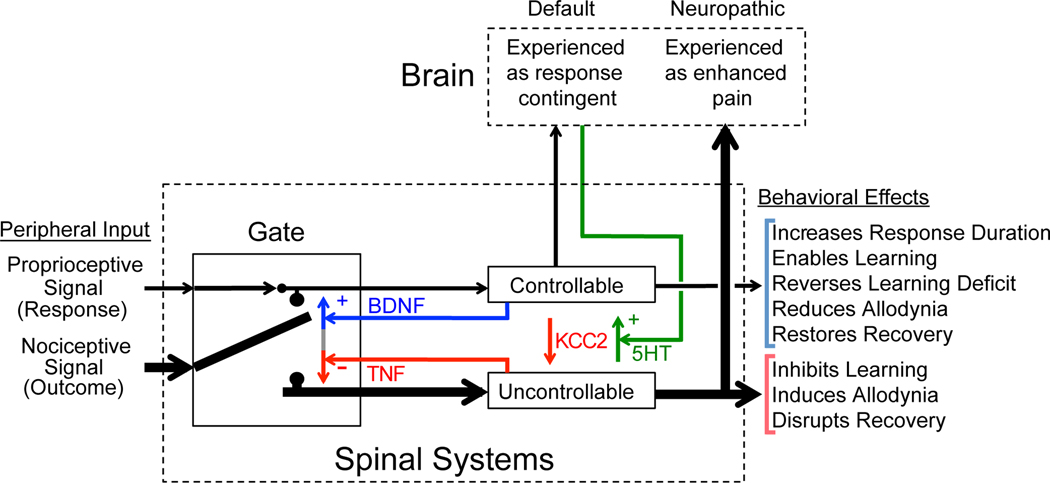
A schematic model illustrating how response contingent (controllable) and non-contingent (uncontrollable) noxious stimulation affect spinal cord function. It is assumed that proprioceptive/sensory cues provide an index of leg position and that the system is prepared to detect the relation between leg position and the onset of a noxious stimulus. Controllable stimulation brings an increase in flexion duration that reduces exposure to noxious stimulation. The early integration of sensory cues related to leg position (the R) and the onset of shock (the O) allows brain systems to directly perceive the underlying R-O relation. With extended training, BDNF is expressed, which has a restorative effect that counters the development of nociceptive sensitization and the consequent learning impairment and allodynia. If no R-O relation is detected after 180 stimuli, a state of over-excitation emerges (coupled to the expression of TNF) that impairs the capacity to learn and enhances mechanical reactivity. This nociceptive sensitization would amplify pain signals relayed to the brain, driving neuropathic pain. Exposure to uncontrollable, but not controllable, stimulation also impairs recovery after a contusion injury of the thoracic spinal cord. Adapted from (Grau, 2014).
Extended training establishes a peripheral memory
Our index of learning in spinally transected rats is tied to an increase in flexion duration, a behavioral modification we assumed is driven by efferent motor activity. If spinal neurons maintain the behavioral modification, inactivating the spinal cord with lidocaine or cutting the efferent (sciatic) nerve that contains the motor fibers should eliminate the learned response. Surprisingly, when Kevin Hoy performed these manipulations, neither affected the maintenance of the behavioral response (Hoy et al., 2020). The treatments did prevent the new learning required when the response criterion is raised (by increasing the contact electrode depth from 4 to 8 mm). But if the criterion is kept the same (at 4 mm), the previously established flexion response persisted without motor output from the spinal cord. Given these observations, we became concerned that the learning might reflect a modification in the muscle, a latch-like effect that preserves the flexion response. To explore this possibility, Hoy blocked acetylcholine receptors (AChR) at the NMJ by injecting the AChR antagonist curare into the tibialis anterior muscle in pretrained animals. He found that this caused the learned response to quickly fade, implying that it was driven by neurotransmitter release at the NMJ. Further work showed that the learning brought about an increase in the evoked electrical [electromyography (EMG)] response within the muscle and that this effect too survived a sciatic cut. Confocal microscopy showed that training increased fluorescent labeling of the AChR, implying an up-regulation that would amplify the elicited response.
Another intriguing discovery stemmed from an incidental observation. Immunohistochemistry showed labeling for the NMDAR, and the enzymes needed to manufacture glutamate, in the vicinity of the NMJ (Hoy et al., 2020; Malomouzh, Nurullin, Arkhipova, & Nikolsky, 2011). Glutamate release at the NMJ appears to play a functional role because injecting an NMDAR antagonist (MK-801) into the muscle blocked acquisition and the maintenance of the learned response. Given these observations, we suggested that engaging the NMDAR may enable Ca++ entry and initiate processes that act to strengthen the elicited response, allowing a flexion response to be maintained (a kind of memory) given minimal transmitter release.
As might be expected, inducing a modification at the NMJ takes some time to develop (consolidate). When communication with the spinal cord is disrupted by cutting the sciatic nerve immediately after training, animals that have received 30 min of training continue to maintain a flexion response whereas those trained for just 6 min do not. But if animals are given 6 min of training, which is sufficient to induce a lasting behavioral change, and the sciatic nerve is cut 24 min later, the flexion response persists. It appears that maintaining the behavioral response for 30 min is sufficient to drive a change at the NMJ.
Uncontrollable noxious stimulation disables the capacity to learn
Another surprising feature of spinally-mediated instrumental learning concerns the consequences of exposure to uncontrollable stimulation, which disables the capacity to learn (Grau et al., 1998). This was first observed when master and yoked animals were tested under common conditions with response-contingent shock (Figure 2A). As expected, animals that were pretrained (Master) exhibited a savings effect and re-acquired the response more rapidly. What was surprising is that prior exposure to uncontrollable stimulation (Yoked) blocked learning. This was not due to a performance deficit. Yoked animals repeatedly experienced the R-O relation, exhibiting a flexion response at shock onset, but this failed to produce an increase in flexion duration. Importantly, this learning impairment is observed independent of whether animals are tested on the same or contralateral leg (Joynes, Ferguson, Crown, Patton, & Grau, 2003). Indeed, even intermittent shock to the tail, applied on a variable schedule that emulates the pattern of stimulation produced by a typical master animal during the early portion of training, produces a learning impairment. Just 6 min of stimulation (180 shocks) disables the capacity to learn for up to 48 hrs (Crown, Ferguson, Joynes, & Grau, 2002b). Importantly, cutting the afferent input from the stimulated leg, or inactivating the spinal cord with lidocaine during the period of stimulation, blocks the induction of the learning impairment when animals are tested 24 hrs later with controllable stimulation applied to the contralateral leg, which confirms that the learning impairment is spinally mediated (Joynes et al., 2003). Because this phenomenon involves a kind of plasticity that impacts future adaptive plasticity, we have suggested that it too reflects an example of metaplasticity (Grau et al., 2014). Of course, those well versed in the learning literature will notice parallels to learned helplessness (Maier & Seligman, 2016; Maier & Seligman, 1976).
We have shown that controllable and uncontrollable stimulation have opposing effects on the capacity to learn, with the former enabling learning while the latter disables instrumental conditioning. Behavioral control also broadly impacts how animals respond to non-noxious mechanical stimulation applied to the paw, with controllable stimulation reducing reactivity whereas uncontrollable stimulation has a sensitizing effect (Hook, Huie, & Grau, 2008). Because application of a peripheral irritant (e.g., capsaicin) also sensitizes behavioral reactivity, we tested whether this treatment affects the capacity to learn. We found that capsaicin, as well as a number of other irritants known to induce nociceptive sensitization (formalin, carrageenan), produces a lasting learning impairment (Ferguson et al., 2006; Ferguson, Huie, Crown, & Grau, 2012). These observations led us to hypothesize that exposure to uncontrollable stimulation interferes with the capacity to modify a particular response because it induces a state of over-excitation within the spinal cord that saturates NMDAR-mediated plasticity. If this is true, pretreatment with a NMDAR antagonist (MK-801) should block the induction of the learning impairment. We found that it does (Ferguson et al., 2006). Further work related the induction of this state to the expression of a cytokine, tumor necrosis factor (TNF), and the up-regulation of Ca++ permeable AMPA receptors (Huie, Baumbauer, et al., 2012; Huie et al., 2015).
Other studies showed that training with controllable stimulation induces a protective effect that blocks the induction of a learning impairment when animals are exposed to uncontrollable stimulation or treated with capsaicin (Crown & Grau, 2001; Hook et al., 2008). Controllable stimulation also countered the enhanced mechanical reactivity induced by capsaicin treatment (Hook et al., 2008). The converse is true too—training with controllable stimulation, given in compound with a drug (naltrexone) that temporarily blocks the expression of the learning impairment (Joynes & Grau, 2004), restores the capacity to learn. More recently, Hudson showed that extended training (30 min) is required to induce this protective/restorative effect (Hudson, Tarbet, & Grau, 2021).
Leg position gates whether nociceptive stimulation induce a learning impairment
We posited that uncontrollable stimulation induces a learning impairment because the stimulation is provided in a non-relational manner, independent of leg position. Recent work by Hudson and Tarbet has forced us to revise our working model. In the course of exploring the circumstances that could weaken (extinguish) a pretrained response, Tarbet exposed animals to a long series of variable intermittent shock given independent of leg position. This stimulation had surprisingly little effect—pretrained animals continued to maintain a flexion response both during and after the period of uncontrollable stimulation (Tarbet et al., 2019). They posited that the period of non-contingent shock has little effect because it occurred while the leg was up. Indeed, we had previously suggested that this relation drives learning (Grau, 2014; Grau et al., 2012). Given this, Tarbet and Hudson hypothesized that leg position modulates how noxious stimulation impacts spinal cord function—that intermittent shock only induces a learning impairment if it occurs while the leg is down. To explore this possibility, they administered stimulation to one hind leg while it was either extended or held above the solution in a flexed position. As noted earlier, the latter did not induce an increase in flexion duration (or, we presume, the metaplastic effects linked to instrumental learning), which suggests that adaptive learning involves more than the simple pairing of a static index of leg position with shock onset. They then tested animals under common conditions with controllable stimulation applied to the contralateral leg. Prior exposure to uncontrollable shock only induced a learning impairment if it was given while the leg was down. It seems that leg position gates how nociceptive stimulation impacts spinal cord plasticity. Hudson then asked whether the same relation holds when animals are given intermittent stimulation to the tail, which engages some reflexive escape-like hind leg movements in spinally transected rats. When both hind legs were held in a flexed position, uncontrollable tail shock failed to induce a learning impairment (Hudson, Tarbet, & Grau, 2022). Finally, Hudson assessed whether leg position gaits the effect of a peripheral irritant. Animals were treated with capsaicin and the leg was, or was not, secured in a flexed position. She then assessed the capacity to learn on the contralateral leg. Again, maintaining the leg in a flexed position blocked the induction of the learning impairment. These findings suggest that the consequences of “uncontrollable” stimulation are modulated by leg position. Casually speaking, it is as if noxious stimulation given while an animal maintains an appropriate, escape-like, adaptive response does not induce a state of nociceptive sensitization.
Earlier we noted how cerebellar lesions can induce a persistent hindlimb flexion response (fixation). Interestingly, holding the animal’s legs in an extended position prevents the development of this effect (Patterson, 2001). Further work is needed to elucidate whether leg position modulates other spinal processes, such as the capacity to derive temporal regularity (see below). Another intriguing aspect of spinal fixation concerns the role of descending neural projections from the brain, which were thought to mediate the alteration in spinal function. Contrary to this view, cutting communication with the brain prior to brain injury does not affect the development of spinal fixation (Lukoyanov et al., 2021). This implies that the brain can alter spinal function through non-neuronal processes (e.g., endocrine signaling). Perhaps more surprising, offspring from pregnant rats that have experienced a brain injury also exhibit a spinally-mediated postural asymmetry (Carvalho et al., 2021).
Promoting the performance of adaptive behavior
In our spinal learning paradigm, training appears to promote the performance of a pre-existing response tendency. Instrumental training after SCI may involve a form of tuning that enables prepared behaviors. Indeed, this is how researchers typically interpret the effects of locomotor training; not that this teaches the animals how to step in a coordinated manner but rather that it reawakens the circuits that enables this behavior. Likewise, exposure to a R-O relation may enable learning because it helps to unveil pre-existing response tendencies that promote adaptive behavior.
Such an enabling effect could potentially alter (bias) performance to a noxious stimulus independent of whether the event occurred in a response-contingent manner. To evaluate this possibility, Hudson and Tarbet trained animals for 6 or 30 min and then tested animals with stimulation applied to the contralateral leg, given in either a controllable (master) or uncontrollable (yoked) manner. They found 30 min of training modified how the yoked animals responded to non-contingent stimulation; these animals exhibited a learning-like increase in flexion duration over the 30 min period of testing (Hudson et al., 2021). Six min of training was not sufficient to induce this effect. These observations suggest that prolonged behavioral training can induce a cellular process that promotes behavioral performance. Given our past work (Grau et al., 2012; Huie et al., 2012), we have hypothesized that this depends upon the expression of BDNF. From this perspective, training enables animals to learn when tested with a higher (8 mm) response criterion because they effectively have a “head-start”, a performance bump that augments the elicited flexion response.
Evidence the spinal cord has a sense of time
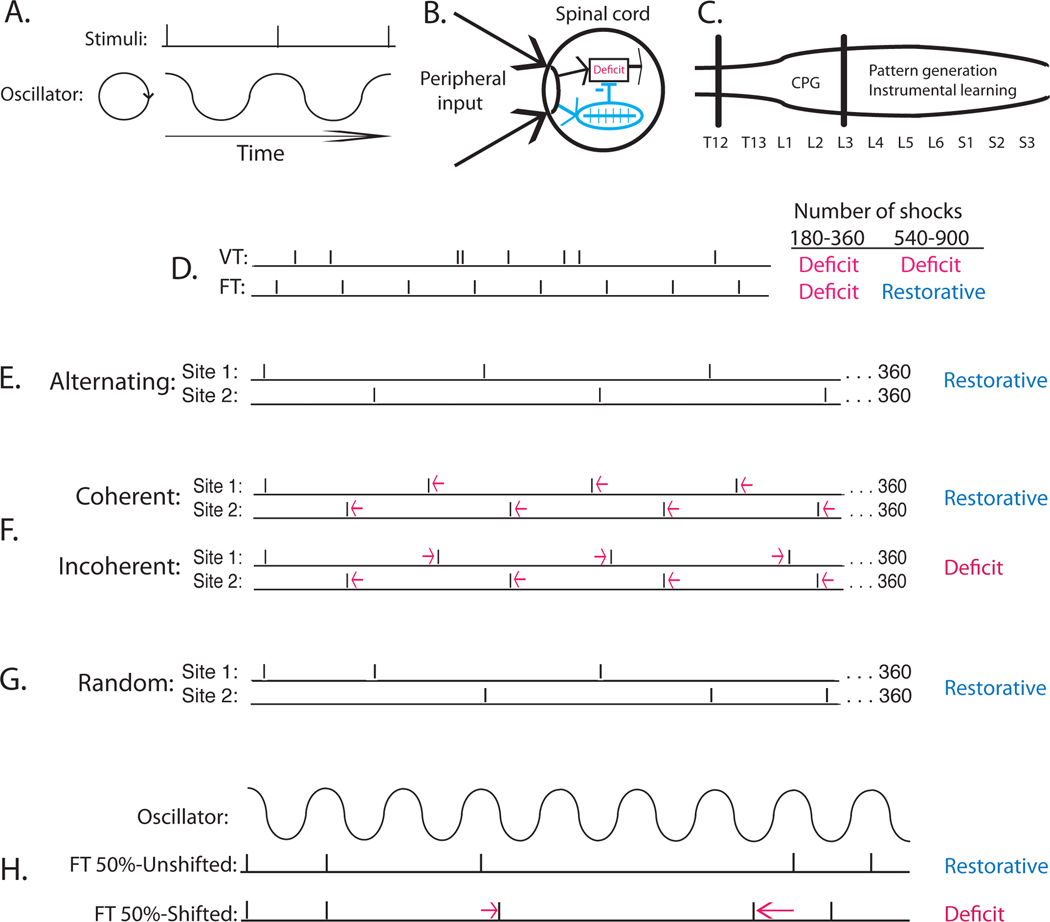
Further analyses of the stimulus conditions that lead to a learning impairment uncovered another surprising outcome—that the spinal cord has a sense of time. This line of work built upon an earlier observation, demonstrating that removing the temporal gap between shocks (continuous stimulation) eliminates its adverse effect on learning (Crown et al., 2002b). This suggests that the adverse effect of stimulation depends upon its intermittent character and the repetitive engagement of nociceptive fibers. Given this, we set out to define the frequency range over which intermittent shock affects spinal cord plasticity. Recognizing that the variable schedule used in prior studies would complicate our manipulation of stimulus frequency, we modified the program used to apply intermittent stimulation so that the brief (100 ms) shocks occurred in a regular [fixed time (FT)], rather than a variable [variable time (VT)], manner (Figure 4D). We assumed that this would not matter because the capacity to time is routinely linked to brain-dependent processes (Mauk & Buonomano, 2004). In fact, when animals are given 180 shocks over a 6 min period, both fixed time (FT) and variable time (VT) stimulation induce a learning impairment (Baumbauer et al., 2008). But when stimulus frequency is kept constant and shock number is increased 5-fold (to 30 min of stimulation; 900 shocks), only VT shock induces a learning impairment. The fact that 180 FT shocks induce a learning impairment, but 900 do not, implies that continued exposure to FT stimulation has a restorative effect (Figure 4B). Indeed, we showed that the capacity to learn could be restored after animals received 180 VT shocks by exposing them to an additional 720 shocks given on a FT schedule (Baumbauer, Huie, Hughes, & Grau, 2009). Conversely, exposure to FT stimulation has a lasting protective effect that prevents the induction of a learning impairment when animals are exposed to VT shock the next day. The induction of this protective effect is blocked by pretreating animals with either a protein synthesis inhibitor (cycloheximide) or a NMDAR antagonist (MK-801). We noted earlier that the protective effect of training with controllable stimulation is linked to the expression of BDNF. Likewise, the protective effect of FT stimulation is blocked by a BDNF inhibitor (TrkB-IgG). FT and VT stimulation also have divergent effects on mechanical reactivity; the former reduces it while the latter enhances reactivity (Baumbauer et al., 2012). Likewise, FT stimulation counters the EMR and learning impairment induced by capsaicin (Baumbauer & Grau, 2011).
Figure 4.
Schematic illustrating the processes involved in spinal timing and the environmental relations used to explore how the system works. (A) Empirical work suggests that the capacity to time is linked to an internal oscillator that can become entrained to stimuli presented in a regular manner. (B) The oscillator (CPG) can abstract regularity across dermatomes. The abstraction of regularity inhibits the development of the learning impairment induced by uncontrollable stimulation. (C) Stepping and instrumental learning are organized by neurons contained within a portion of the lumbosacral spinal cord between L3 and S2 (Liu et al., 2005). The CPG that drives stepping has been localized to a rostral region (L1–2) (Magnuson et al., 1999). (D) Exposure to variable (VT) shock for 6–30 min (180–900 shocks) induces a learning impairment (Deficit). A brief exposure (180–360 shocks) to shock given in a regular manner (FT) also induces a learning deficit. Continued exposure to FT shock (540 or more shocks) engages a restorative effect that counters the learning impairment. (E) The spinal cord can abstract regularity when stimuli are given across dermatomes (tail and one leg) in a regular (alternating) manner. (F) Increasing the temporal gap between shocks applied to one dermatome, while the gap is reduced for stimuli applied to the other site, yields an irregular (incoherent) pattern of stimulation across dermatomes and results in a learning impairment. When the interval between shocks to each dermatome is shifted by the same amount, the alternating pattern is preserved (coherent) and the restorative effect develops. (G) The spinal cord can also abstract regularity when the site of stimulation is randomly varied over times. (H) The spinal cord can abstract regularity when half the shocks applied to one dermatome are randomly omitted (Unshifted). If the first shock after an omitted one is displaced (Shifted), so that its occurrence does not align with what would be expected given an oscillator, a deficit develops, implying that the internal oscillator was not entrained. Adapted from (Lee et al., 2016).
The fact that the FT effect depends upon protein synthesis and the NMDAR suggests that it may evoke a kind of learning, wherein animals encode that the stimuli occur in a regular (predictable) manner. If this is true, some savings may develop across days. We explored this possibility by exposing spinally transected rats to two bouts of 360 FT shocks, separated by 24 hr (Lee et al., 2015). We knew that the emergence of the FT effect requires exposure to 540 or more shocks. In line with this, animals given just one bout of 360 FT shocks exhibited a learning impairment. But if the rats received two bouts of 360 FT shocks, applied a day apart, learning was restored. Interestingly, what mattered across days is that each bout of stimuli occurred in a regular manner, not the locus of stimulation or particular frequency experienced. This suggests that an index of regularity is abstracted and underlies the additive effect of FT stimulation.
Further work revealed that the spinal cord can abstract regularity when the site of stimulation is randomly varied across dermatomes (leg and tail; Figure 4E) (Lee, Huang, & Grau, 2016). The system can also abstract regularity when half of the shocks are randomly omitted (Figure 4G). These observations led us to hypothesize that the abstraction of regularity may be related to an internal oscillator (Figure 4A), driven by a central pattern generator (CPG) (Grillner & Wallen, 1985; Rossignol & Frigon, 2011). If an internal oscillator is involved, the abstraction of regularity should be disrupted by presenting regular shock to distinct dermatomes, but at a slightly different interval (e.g., 1.9 versus 2.1 sec; Figure 4F). In this scenario, each site receives regular stimulation, but the relation between the two shock trains varies (slowly rotates). As predicted, applying regular shock to each dermatome at slightly different intervals disrupted the FT effect, implying reference to an internal oscillator. Likewise, when shocks are randomly omitted, the next stimulus must remain in phase with earlier stimuli (Figure 4H), an observation that again implies reference to an oscillator [and that discounts timing based on how fast a physiological process decays (hourglass); Karmarkar & Buonomano, 2007].
Interestingly, other work has identified a CPG in the rostral lumbar (L1-L2) spinal cord that functions to drive regular hind leg stepping (Grillner & Zangger, 1979; Kiehn, 2006; Kiehn & Kjaerulff, 1998; Magnuson et al., 1999) (Figure 4C). We hypothesized that regular stimulation has a unique effect because it entrains this oscillator. Because we had previously shown that learning depends upon a more caudal region of the spinal cord (L3-S2) (Liu et al., 2005), Lee was able to surgically disconnect this region from the L1–2 oscillator without disrupting the capacity to learn an instrumental response (Lee, Huang, & Grau, 2016). When the spinal cord was cut at L3, an extended exposure to FT stimulation induced a learning impairment, which suggests that the restorative effect of regular stimulation depends upon access to the rostral CPG.
Another observation consistent with the entrainment of a CPG was made by Dr. M. Strain, who was examining the consequences of exposure to FT stimulation applied to the tail in animals treated with a drug cocktail of serotonin and NMDA (Strain et al., 2014). One day she noticed something very odd that she was able to videotape (see https://youtu.be/imvcsR4vaV4). After FT stimulation to the tail ended, and the electrodes were removed, the animal exhibited cyclic tail swinging (“pendulate tail”). Detailed analysis of the video recording revealed that the tail swings occurred at the same frequency as the FT stimulation. Further, when the tail was manually held for a brief period, and then let go, the pendulum-like effect resumed, in phase with the earlier period. It is difficult to account for this effect without reference to an internal oscillator.
We have suggested that regular stimulation has a distinct effect because it engages the CPG that drives hind limb stepping. Indirect support for this claim comes from the frequency range that yields a restorative effect of FT stimulation, which corresponds to the frequency of stepping (Baumbauer, Turtle, & Grau, 2017; Cha et al., 2007). The observation is also consistent with work demonstrating that entraining the CPG with step training, combined with regular stimulation of the perineum, promotes adaptive behavior (Alluin et al., 2011; Rossignol, 2017). Interestingly, spinally transected animals trained to step on a treadmill at one speed exhibit superior performance across a range of step frequencies (Edgerton et al., 2001). This again implies that training does not promote the processing of a particular frequency but rather augments the capacity to abstract regularity and drive rhythmic behavior. Other data suggests that exposure to noxious stimulation can disrupt CPG function (Bouffard, Bouyer, Roy, & Mercier, 2014; Caudle et al., 2015). Stand training also impairs stepping behavior and interferes with instrumental learning (Bigbee et al., 2007; Edgerton et al., 1997, 2001). Interestingly, there appears to be a second CPG with the spinal cord that drives rhythmic swimming behavior (Pocratsky et al., 2017).
SCI enables plasticity by attenuating the inhibitory effect of GABA
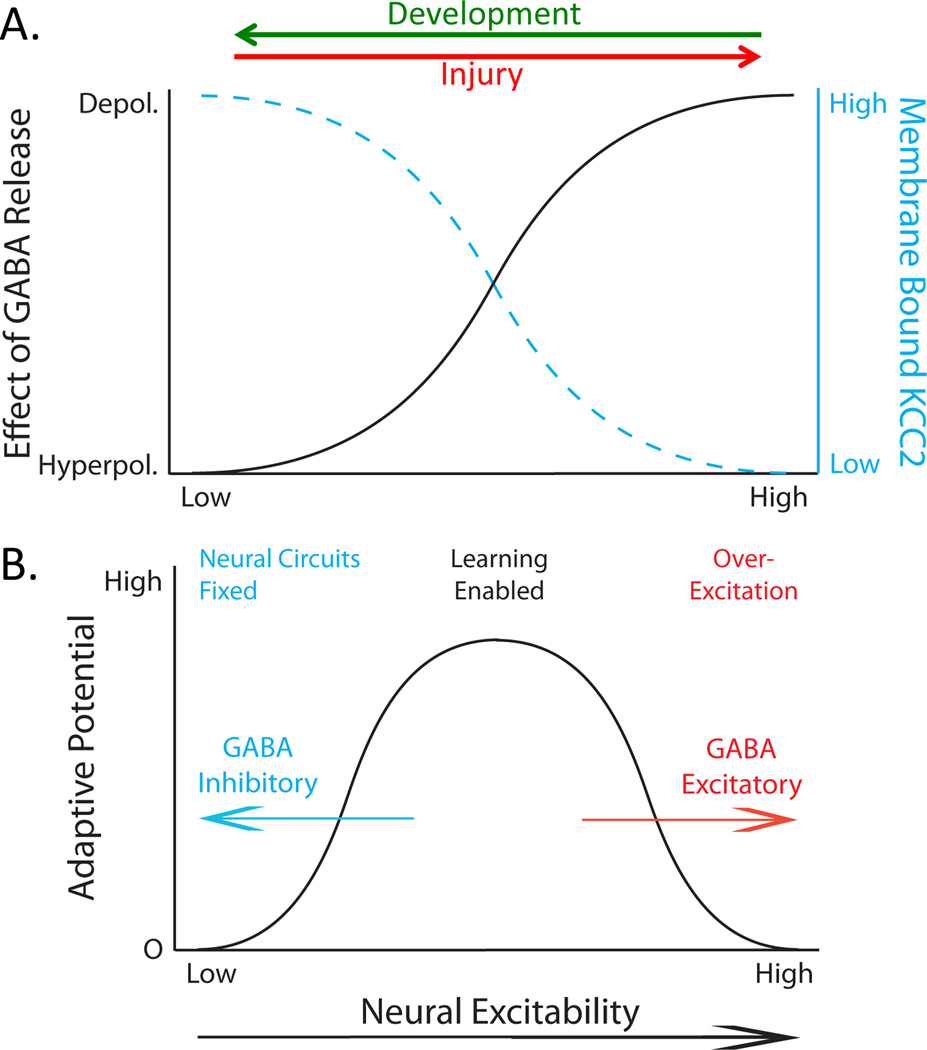
The last aspect of our work that we will highlight concerns a surprising shift in how the neurotransmitter GABA affects neural function (Kaila, Price, Payne, Puskarjov, & Voipio, 2014; Medina et al., 2014). GABA is classically considered an inhibitory neurotransmitter that limits neural excitability and plasticity. What is not widely appreciated is that the inhibitory effect of GABA varies (Figure 5A), a phenomenon known as ionic plasticity (Rivera, Voipio, & Kaila, 2005). This is observed early in development, when the release of GABA acts to excite rather than inhibit neurons (Ben-Ari, 2002, 2014). This shift in GABA function has been related to the GABA-A receptor and alterations in the intracellular concentration of the anion Cl−. Engaging the GABA-A receptor allows Cl− to flow across the cell membrane. The direction of ion flow is determined by the intracellular concentration of Cl−, which is regulated by membrane bound co-transporters, the most important of which is K+-Cl−-cotransporter 2 (KCC2). KCC2 transports Cl− out of the cell, which lowers its intracellular concentration. KCC2 is not expressed early in development, which curtails the extrusion of Cl−, causing its intracellular concentration to increase. In this state, engaging the GABA-A receptor allows Cl− to flow out of the cell. This reduces the negative charge within the cell, a depolarizing effect that is thought to promote plasticity and the formation of neural circuits. Later in development, KCC2 is expressed, which lowers the intracellular concentration of Cl−. Activating the GABA-A receptor now allows Cl− to enter the cell, a hyperpolarizing effect that inhibits neural excitability and plasticity. With the development of GABAergic inhibition, circuits laid down early in development would appear immutable (“hard wired”) (Figure 5B).
Figure 5.
(A) Intracellular Cl− is regulated by KCC2, which transports the anion out of the cell. In the uninjured adult CNS, membrane bound KCC2 maintains a low intracellular concentration of Cl−. Under these conditions, engaging the GABA-A receptor allows Cl− to enter the cell, which has an inhibitory (hyperpolarizing) effect. After SCI, there is a reduction in membrane-bound KCC2 below the injury, recapitulating an earlier developmental state. This leads to a rise in intracellular Cl−, which reduces the inward flow of Cl− and the inhibitory effect of engaging the GABA-A receptor. At the extreme, engaging this receptor can allow Cl− to exit the cell, which would have an excitatory (depolarizing) effect. (B) GABAergic inhibition will normally reduce neural excitability and plasticity in the adult CNS, causing the system to appear immutable (hard wired). The reduction in GABA inhibition (ionic plasticity) can enable learning. A further shift can foster pathological over-excitation. The resultant function is reminiscent of the non-monotonic relation between learning and arousal/stress (Yerkes-Dodson law). Adapted from (Grau & Huang, 2018).
Recent work has shown that cutting communication with the brain transforms how GABA acts caudal to injury, a modification that has been linked to the down-regulation of KCC2 and a weakening of GABA-dependent inhibition (Grau & Huang, 2018; Huang & Grau, 2018; Huang, Lee, & Grau, 2017; Huang, Lee, Murphy, Garraway, & Grau, 2016). This shift enables afferent nociceptive stimulation to induce a state of over-excitation in the dorsal horn, a nociceptive sensitization that may fuel chronic pain (Cramer et al., 2008). The loss of GABAergic inhibition also helps to explain why those with SCI exhibit bouts of uncontrollable muscle activity (spasticity) (Boulenguez et al., 2010). Locomotor training appears to benefit function, attenuating spasticity and behavioral signs of chronic pain, by increasing the expression of KCC2, which re-establishes the inhibitory effect of GABA (Cote, Gandhi, Zambrotta, & Houle, 2014). By increasing GABA-dependent inhibition and curtailing motor over-excitation, training may re-awaken dormant circuits. Finally, the injury-induced change in GABA function helps to address an old debate regarding the relative plasticity of spinal cord circuits. In the adult nervous system, the spinal cord may appear hardwired because GABAergic inhibition limits plasticity. After injury, this brake is removed, enabling the plasticity that underlies learning in spinally transected animals. The transformation also helps to explain why noxious stimulation does not have an adverse effect on spinal cord function in the absence of SCI (Washburn, Patton, Ferguson, Hudson, & Grau, 2007).
Interestingly, BDNF appears to have a restorative effect after SCI, in part, because it increases the expression of KCC2 (Huang et al., 2017), which would reduce the excitatory effect of GABA. Further, behavioral treatments that promote BDNF expression [instrumental training, regular stimulation, locomotor training (Baumbauer et al., 2009; Boyce & Mendell, 2014; Gomez-Pinilla et al., 2007)] may promote adaptive plasticity and attenuate nociceptive reactivity because they too drive KCC2 expression. Likewise, this view suggests that pharmacological treatments (e.g., bumetanide, CLP-290) that reduce the intracellular concentration of Cl− should have a therapeutic effect and limit plasticity. As predicted these drugs prevent spinal learning, nociceptive sensitization, and spasticity (Boulenguez et al., 2010; Huang et al., 2016; Hudson, Tarbet, & Grau, 2020).
We have sometimes characterized spinally mediated instrumental learning as a kind of adaptive plasticity, that selectively promotes the performance of a target response. Conversely, the over-excitation that arises when animals are given uncontrollable/unpredictable noxious stimulation has been viewed as a form of maladaptive plasticity that enhances behavioral reactivity and promotes a state of neural excitation that interferes with learning about specific relations (Ferguson, Huie, Crown, Baumbauer, et al., 2012). One could characterize these differences as a kind of tuning, with GABA-dependent inhibition limiting the scope of neural activity while the removal of this brake broadens the response profile. This broadening effect is evident from the transformation in reactivity to non-noxious mechanical stimulation, which gains the capacity to elicit a behavioral response. It is as if, minus a behavioral solution, the system casts a broader net, fostering a hyper-aroused state that enables defensive behavior. In the absence of control or prediction, such an outcome may be adaptive.
These insights regarding the regulation of GABAergic inhibition (ionic plasticity) may have broad implications that extend to other regions of the CNS. Pathological neural activity in epilepsy and drug addiction has been linked to a reduction in KCC2 and a depolarizing shift in GABA function (Kaila et al., 2014; Ostroumov & Dani, 2018; Ostroumov et al., 2016). It is also of interest to note that LTP is frequently studied in vitro under conditions that limit GABA inhibition (e.g., by adding the GABA-A antagonist bicuculline to the medium). Much of the CNS, not just the spinal cord, likely appears hard wired in adult animals because neural plasticity is limited by GABAergic inhibition. Learning may require removing this brake, just enough to foster adaptive plasticity without enabling pathological over-excitation.
Interestingly, in the adult uninjured CNS, the down-regulation in KCC2 can be triggered by the expression of BDNF, which has opposing effects on the co-transporter depending upon the biological state of the system [and the presence/absence of phospholipase C gamma (PLC) (Ferrini & De Koninck, 2013; Rivera et al., 2005; Rivera et al., 2004). After SCI, BDNF helps to re-establish GABAergic inhibition and counter the development of nociceptive sensitization by up-regulating KCC2 (Huang et al., 2017). In the absence of injury, BDNF has the opposite effect, which fuels the development of chronic pain (Coull et al., 2005; Merighi et al., 2008).
Summary and Implications
Over the last three decades we have explored whether neurons within the spinal cord can learn. To assure that the changes observed do not reflect the action/influence of brain systems, we cut communication with the brain by means of a rostral transection. We have since learned that this procedure removes a GABA-dependent brake on plasticity that enables learning (Grau & Huang, 2018). Under these conditions, noxious stimulation can induce LTP and LTD by means of biochemical processes analogous to those discovered in the hippocampus (Sandkuhler, 2000). An advantage of the spinal cord as a model system is that these physiological alterations can be readily related to behavioral modifications, providing biological correlates to sensitization and habituation.
We have explored the capacity for learning relying on the criteria outlined by Rescorla (Rescorla, 1988a). Research suggests that spinal systems are sensitive to S-S relations and that the behavioral change induced reflects the action of non-associative processes (Grau, 2014). Following Rescorla, we propose that this does not constitute a good reason to reject the phenomena as learning, arguing instead that learning is for the most part biologically prepared. We went a step beyond Rescorla to ask whether the concept of “association” has out-lived its usefulness.
We focused the bulk of our review on data examining whether the spinal cord is sensitive to R-O relations, providing evidence that instituting a contingency between leg position and the application of a noxious shock brings about an increase in flexion duration that minimizes net shock exposure (Figure 6A). Given the speed with which this learning occurs, we suggested that it is biologically prepared. Here we should acknowledge that it remains unclear whether the trained response builds upon a genetic program, a feature of physiological design, or a circuit shaped early in development. The only real claim is that the response tendency, in the adult animal, existed prior to SCI (i.e., was pre-existing).
Figure 6.
Illustrations of how training affects spinal cord function and the underlying processes. (A) A schematic illustrating the sequential induction of learning and metaplastic processes by alternative forms of stimulation. When noxious intermittent stimulation is given in a response-contingent manner (predicted by cues related to limb position/response criterion), the R-O relation drives an increase in flexion duration. If stimulation is given in the absence of an adaptive behavioral response for six minutes (180 shocks), while the leg is down, a learning impairment is induced that inhibits the capacity to learn. The consequent state of over-excitation enhances mechanical reactivity and blocks learning (the change in response duration normally induced by response-contingent stimulation). Because the induction of this process has a lasting effect (involves a form of plasticity) that interferes with the capacity to learn, it reflects an example of metaplasticity. Because animals given an equivalent amount of controllable stimulation do not develop this effect, it is assumed that processes engaged by a R-O relation inhibit its development. Continued training with controllable stimulation for additional 24 min, or the presentation of regular (FT) shocks (540 or more), induces a restorative effect that counters the adverse consequences of uncontrollable stimulation. Because this restorative effect has a lasting impact on plasticity, it too reflects a form of metaplasticity. Behaviorally, it enables (amplifies) learning, reduces mechanical reactivity, and counters the development of nociceptive sensitization. Adapted from (Baumbauer et al., 2017). (B) The mechanisms thought to underlie spinally-mediated instrumental conditioning, timing, and the consequences of uncontrollable stimulation. It is assumed that the spinal cord contains processes that support sensory-motor integration which can detect the relationship between cues indicative of limb position (and reaching the response criterion) and the onset of noxious stimulation. Our results suggest that the effect of noxious stimulation is initially gated on the basis of leg position; if the leg(s) is flexed, stimulation has no impact on spinal function. If the legs are not flexed, pre-existing circuitry enables the rapid detection of a relationship between the onset of noxious stimulation and sensory cues related to the current leg position (and/or contacting the underlying solution). In this model, the R-O relation is encoded by sensory cues. If there is a clear relation, the stimulation is classified as controllable and the appropriate behavioral response is selected. In addition, with continued training, a process is engaged that facilitates sensory-motor integration. Exposure to regular stimulation can also activate this faciliatory effect through the engagement of a rostral central pattern generator (CPG). If noxious stimulation is given in an uncontrollable/unpredictable manner, it induces a process that impairs sensory-motor integration. Noxious stimulation can also interfere with CPG function and the generation of rhythmic behavior (Bouffard et al., 2014; Caudle et al., 2015). The consequences of training that have a lasting effect are enclosed with dashed ovals. Training with controllable stimulation sensitizes the motor output and, with continued training, induces a modification at the neuromuscular junction that fosters the performance of a flexion response. Exposure to controllable stimulation also has a metaplastic effect that enables the abstraction of R-O relations. Conversely, uncontrollable stimulation has a metaplastic effect that interferes with this process. These metaplastic effects have a general impact that modulates the capacity to detect sensory relations when stimuli are applied to the contralateral limb. Further work is needed to determine whether exposure to irregular stimulation (independent of the R-O relation) interferes with CPG function (indicated with a “?”) or impacts sensory-motor integration. Likewise, we do not know whether controllable stimulation fosters CPG function. Note that in panels A and B, we indicate that uncontrollable stimulation impairs sensory motor integration using a minus sign. While this accurately describes the functional operation of the system, at a neural level uncontrollable stimulation may impair processing by inducing a state of over-excitation (not inhibition; Ferguson et al., 2006). Conversely, the facilitory effects noted in panel B with a plus may reflect a BDNF-dependent reduction in over-excitation after SCI (Cote et al., 2014; Huang et al., 2017; Tashiro et al., 2015).
Our work suggests that spinally-mediated instrumental learning is driven (reinforced) by the onset of the noxious stimulus, which occurs when the falling leg reaches the response criterion (Grau, 2014; Grau et al., 1998). For shock to have an effect that depends upon when it occurs requires a cue tied to leg position and/or contact with the underlying solution. In either case, it is assumed here that the R-O relation is given by sensory events, which foster the performance of the appropriate response—an increase in flexion duration (Figure 6B). As noted earlier, we presume that these sensory cues are integrated by a pre-wired system that enables the rapid, and automatic, execution of the appropriate defensive (flexion) response. Elsewhere (Grau, 2014), we have suggested that this sensory processing could also enable higher neural systems in the brain to directly perceive a R-O relation. When one taps a finger against a hard surface, the change in finger position and the outcome (hitting the hard surface) could be integrated within the spinal cord; further brain processing may not be needed to derive the relation. This is, of course, analogous to a Gibsonian interpretation of depth in vision (Gibson, 1979), which focuses on cues inherent in the sensory stimulus (e.g., texture gradients) in lieu of subsequent computations (e.g., binocular disparity).
At the level of the spinal cord, the immediate consequence of detecting a relation between shock onset and cues tied to reaching the response criterion is an increase in motoric (efferent) drive. The long-term consequence of this learning is a peripheral modification at the NMJ, a muscle memory that helps preserve the behavioral response over time (Hoy et al., 2020). Notice here that we are assuming a pre-wired system detects the R-O relation. We know that this relation (the criterion for instrumental conditioning) is critical because disrupting it (by presenting shock independent of leg position or delaying its onset) disrupts acquisition. While this processing allows the selection and modification of the appropriate behavioral response, it is not in of itself an integral component of what is retained over time (the memory). From this view, what is retained is the augmentation of the efferent output. An intriguing aspect of this model is that it implies that, after a behavioral modification is acquired, processes that impact sensory-motor integration (e.g., exposure to uncontrollable stimulation) should have little effect (Hudson et al., 2021). From this perspective, undoing (extinguishing) a learned response would require acquisition of a new behavior that interferes with the performance of the previously acquired response (Hull, 1943).
If no relation is detected, noxious stimulation appears to engage a secondary process that drives a form of over-excitation that undermines the capacity to learn (Ferguson et al., 2006; Ferguson et al., 2012). Our assumption is that it does so by disrupting the sensory-motor integration that allows the selection, and amplification, of an adaptive response. Because this process has a lasting effect, and depends upon a protein synthesis (implying a structural modification; Patton et al., 2004), it too represents a kind of memory. Conversely, detecting that stimulation occurs in a controllable, or predictable, manner appears to engage a process that enables learning, by countering the over-excitation (and thereby restoring the capacity for sensory-motor integration) and augmenting behavioral performance. At a neurochemical level, the former process has been related to the release of pro-inflammatory cytokines and a reduction in GABA-dependent inhibition. The restorative effect appears tied to the expression of BDNF (Grau & Huang, 2018).
A potential source of confusion stems from our use of the term “response”. On the one hand, we say that shock is response contingent because it occurs whenever the leg is extended. On the other, we say that the outcome of learning is an increase in the duration of our target response—flexion. In our scheme, we envision this adaptive response to stimulation as being gated by an index of leg position at the time of shock onset. From this view, leg position at the time of shock onset (i.e., the R-O relation) acts as a kind of hierarchical cue that determines how noxious stimulation impacts motor systems (Figure 6B).
Our work on spinal learning builds upon decades of research detailing its processing capacity, demonstrating that spinal neurons can support rhythmic stepping, that performance of this behavior can be modified by experience, and that the effect of a noxious stimulus is gated by leg position/step cycle. Further, the rhythmicity of stepping implies the existence of a central pattern generator, an oscillator, that we have argued enables spinal cord systems to detect stimulus regularity. In this framework, sensory-motor integration allows for the rapid selection and modification of adaptive responses. Our persepctive is consistent with approaches that build upon evolutionary/organismic biology, that assume learning occurs within a pre-organized behavior system (Gallistel, 2000; Timberlake, 1990; Timberlake & Lucas, 1989). From this perspective, “learning provides the fine tuning necessary for successful adaptation” (Garcia & Garcia Y Robertson, 1989). In the system outlined above, pre-existing structures abstract the R-O relation, how this is translated to motor performance, and the capacity to detect regularity over time. Neither the particulars of the R-O relation nor the temporal interval between stimuli appear to be stored. Rather, what is altered (remembered) is the performance of the adaptive response and the capacity for sensory motor integration.
An interesting question at this juncture is whether some examples of brain-dependent learning and memory can be described in analogous terms. Applying the model outlined in Figure 6B more broadly, we would suggest that organisms have sensory/perceptual processes that allow the detection/abstraction of regularity and behavioral control—that these abilities are prewired, not acquired. In our view, the detection of behavioral control selects the appropriate behavioral response and fosters its performance. When the latter has a lasting effect, it would count as an example of learning. It is further assumed that the detection of behavioral control/regularity has a metaplastic effect that enhances sensory-motor integration. In the absence of behavioral control, a brake is removed from sensory-motor integration, yielding a state of over-excitation that would generally enhance motor reactivity. In spinally transected animals, this is evident from an enhancement in reactivity to light touch; in intact animals, a diffuse state akin to free-floating anxiety can enhance startle and defensive behavior. In the absence of a behavioral solution, vigilance is enhanced. To restore normal function, the model we have outlined suggests the implementation of procedures that allow the organism to detect behavioral control, which we have suggested will drive a form of positive feedback that counters the state of over-excitation. These relations are, of course, in line with decades of research in the area of learned helplessness (Maier & Seligman, 2016; Rosen & Schulkin, 1998).
While we acknowledge the import of pre-existing architectures, we have not given up on the idea that common rules may help us to understand how systems learn. By analogy, consider perceptual systems which by design are biologically prepared to detect distinct forms of stimulations. Despite qualitative differences in the underlying biology, principles have been derived that illuminate how the systems operate across modalities. Likewise, common rules may describe how behavior systems are tuned. One can argue for the import of evolutionary biology and propose that aspects of how a system is modified can be described by a general principle (e.g., time-scale invariance; Gallistel, 2000).
The approach that Rescorla outlined in his 1988 ARN paper has helped us convince skeptics that the spinal cord can learn. Our procedures have been linked to environmental relations (S-S and R-O) and care has been taken to test animals under common conditions. Trained in the associative tradition, we have been prone to describe how the system learns in those terms, suggesting at times that instrumental learning fosters the development of a kind of S-R habit, wherein cues tied to leg position at the time of shock onset gain the capacity to drive the behavioral response (Grau, 2014). While we have not eliminated this possibility, we now recognize that no such link may be needed to account for our results. This is particularly true after extended training, where we have shown that disconnecting the central nervous system from the efferent neurons has no effect on behavioral performance (Hoy et al., 2020). The outcome of learning appears attributable to the sensitization of the motoric output alone. Likewise, we have identified modulatory (metaplastic) effects that appear to impact the capacity for sensory motor integration and motor performance. But sensory-motor integration, or the capacity to time, in of themselves are not (we assume) learned. Both capacities depend upon pre-existing structures. Here, associations play no role. Our stronger claim is that this may be true for much of conditioning.
Acknowledgments:
The authors wish to thank Jacob Davis, Grace Giddings, Travis Johnston, and Sienna Partipilo for their help and input.
Funding:
Work on this project was supported by the Mary Tucker Currie Professorship to JWG and the National Institute of Neurological Disorders and Stroke (NS104422)
Footnotes
Author Disclosure Statement : The authors declare no competing financial interests.
References
- Abraham WC (2008). Metaplasticity: tuning synapses and networks for plasticity. Nature Reviews Neuroscience, 9(5), 387–399. doi: 10.1038/nrn2356 [DOI] [PubMed] [Google Scholar]
- Abraham WC, & Bear MF (1996). Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci, 19(4), 126–130. [DOI] [PubMed] [Google Scholar]
- Akil H, Watson S, Young E, Lewis ME, Khachaturiam H, & Walker JM (1984). Endogenous opioids: Biology and function. Annual Review of Neuroscience, 7, 223–255. [DOI] [PubMed] [Google Scholar]
- Alkon DL (1983). Learning in a marine snail. Scientific American, 249(1), 70–74. [DOI] [PubMed] [Google Scholar]
- Alluin O, Karimi-Abdolrezaee S, Delivet-Mongrain H, Leblond H, Fehlings MG, & Rossignol S. (2011). Kinematic study of locomotor recovery after spinal cord clip compression injury in rats. Journal of Neurotrauma, 28(9), 1963–1981. doi: 10.1089/neu.2011.1840 [DOI] [PubMed] [Google Scholar]
- Basbaum AI, & Fields HL (1984). Endogenous pain control-systems - brain-stem spinal pathways and endorphin circuitry. Annual Review of Neuroscience, 7, 309–338. doi: 10.1146/annurev.ne.07.030184.001521 [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, & Grau JW (2011). Timing in the absence of supraspinal input iii: regularly spaced cutaneous stimulation prevents and reverses the spinal learning deficit produced by peripheral inflammation. Behavioral Neuroscience, 125(1), 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Hoy KC, Huie JR, Hughes AJ, Woller SA, Puga DA, … Grau JW (2008). Timing in the absence of supraspinal input I: Variable, but not fixed, spaced stimulation of the sciatic nerve undermines spinally-mediated instrumental learning. Neuroscience, 155(4), 1030–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Huie JR, Hughes AJ, & Grau JW (2009). Timing in the absence of supraspinal input ii: regularly spaced stimulation induces a lasting alteration in spinal function that depends on the nmda receptor, bdnf release, and protein synthesis. Journal of Neuroscience, 29(46), 14383–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Lee KH, Puga DA, Woller SA, Hughes AJ, & Grau JW (2012). Temporal regularity determines the impact of electrical stimulation on tactile reactivity and response to capsaicin in spinally transected rats. Neuroscience, 227, 119–133. doi: 10.1016/j.neuroscience.2012.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbauer KM, Turtle JD, & Grau JW (2017). Fixed spaced stimulation restores adaptive plasticity within the spinal cord: Identifying the eliciting conditions. Physiology & Behavior, 174, 1–9. doi: 10.1016/j.physbeh.2017.02.028 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. (2002). Excitatory actions of GABA during development: The nature of the nurture. Nature Reviews Neuroscience, 3(9), 728–739. doi: 10.1038/nrn920 [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. (2014). The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience, 279, 187–219. doi: 10.1016/j.neuroscience.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (2003). Parsing reward. Trends Neurosci, 26, 507–513. [DOI] [PubMed] [Google Scholar]
- Bigbee AJ, Crown ED, Ferguson AR, Roy RR, Tillakaratne NJK, Tobin AJ, Grau JW, & Edgerton VR (2007). Two chronic motor training paradigms differentially alter the potential to perform a novel motor learning task in spinally transected rats. Behavioural Brain Research, 180, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, & Collingridge GL (1993). A synaptic model of memory: long-term potentiation in the hippocampus. Nature, 361(6407), 31–39. [DOI] [PubMed] [Google Scholar]
- Bliss TV, & Lomos T. (1973). Long-lasting potentiation of synaptic transmission in the dentage area of the anaesthetized rabbit folllowing stimulation of the perforant path. Journal of Physiology, 232, 357–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouffard J, Bouyer LJ, Roy JS, Mercier C. (2014). Tonic pain experienced during locomotor training impairs retention despite normal performance during acquisition. Journal of Neuroscience, 34, 9190–9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, … Vinay L. (2010). Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med, 16(3), 302–307. doi: 10.1038/nm.2107 [DOI] [PubMed] [Google Scholar]
- Boyce VS, & Mendell LM (2014). Neurotrophins and spinal circuit function. Front Neural Circuits, 8, 59. doi: 10.3389/fncir.2014.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger AA, & Chopin SF (1976). Instrumental avoidance conditioning in spinal vertebrates. Advances in psychobiology, 3, 437–461. [PubMed] [Google Scholar]
- Carew TJ, Hawkins RD, & Kandel ER (1983). Differential classical conditioning of a defensive withdrawal reflex in Aplysia californica. Science, 219(4583), 397–400. [DOI] [PubMed] [Google Scholar]
- Carvalho LS, Brito HM, Lukoyanova EA, Maia GH, Sarkisyan D, Nosova O, … Bakalkin G. (2021). Unilateral brain injury to pregnant rats induces asymmetric neurological deficits in the offspring. European Journal of Neuroscience, 53, 3621–3633. [DOI] [PubMed] [Google Scholar]
- Caudle KL, Atkinson DA, Brown EH, Donaldson K, Seibt E, Chea T, Smith E, Chung K, Shum-Siu A, Cron CC, Magnuson DS (2015). Hindlimb stretching alters locomotor function after spinal cord injury in the adult rat. Neurorehabilitation and Neural Repair 29, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, & De Leon RD (2007). Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. Journal of Neurotrauma, 24(6), 1000–1012. [DOI] [PubMed] [Google Scholar]
- Chopin SF, & Buerger AA (1976). Instrumental avoidance-conditioning in spinal rat. Brain Research Bulletin, 1(2), 177–183. doi: 10.1016/0361-9230(76)90067-8 [DOI] [PubMed] [Google Scholar]
- Church RM (1989). The yoked control design. In Nilsson TAL (Ed.), Aversion, avoidance, and anxiety: Perspectives on aversively motivated behavior. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Church RM, & Lerner ND (1976). Does headless roach learn to avoid. Physiological Psychology, 4(4), 439–442. [Google Scholar]
- Clark RE, & Squire LR (1999). Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychological Science, 10. [Google Scholar]
- Cote MP, Gandhi S, Zambrotta M, & Houle JD (2014). Exercise modulates chloride homeostasis after spinal cord injury. Journal of Neuroscience, 34(27), 8976–8987. doi: 10.1523/jneurosci.0678-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, … De Koninck Y(2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature, 438(7070), 1017–1021. doi: 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, … Resnick D. (2008). The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Molecular Pain, 4, 36. doi: 10.1186/1744-8069-4-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, & Grau JW (2002a). Instrumental learning within the spinal cord II. Evidence for central mediation. Physiology & Behavior, 77(2–3), 259–267. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, & Grau JW (2002b). Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behavioral Neuroscience, 116(6), 1032–1051. [DOI] [PubMed] [Google Scholar]
- Crown ED, & Grau JW (2001). Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. Journal of Neurophysiology, 86(2), 845–855. [DOI] [PubMed] [Google Scholar]
- Davis M, & Wagner AR (1968). Startle responsiveness after habituation to different intensities of tone. Psychonomic Science(12), 337–338. [Google Scholar]
- DiGiorgio AM (1929). Persistenza nell’animale spinale, di asimmetrie osturali e motorie di origine cerebellare. Archivo de Fisiologia, 27: 518–580. [Google Scholar]
- Domjan M. (2015). Principles of Learning and Behavior. Stamford, CT: Cengage. [Google Scholar]
- Domjan M. (2016). Elicited versus emitted behavior: Time to abandon the distinction. Journal of Experimental Analysis of Behavior, 105, 231–245. [DOI] [PubMed] [Google Scholar]
- Dudai Y. (1989). The Neurobiology of Memory Concepts, Findings, Trends. Oxford: Oxford University Press. [Google Scholar]
- Durkovic RG, & Prokowich LJ (1998). D-2-Amino-5-phosphonovalerate, an NMDA receptor antagonist, blocks induction of associative long-term potentiation of the flexion reflex in spinal cat. Neuroscience Letters, 257(3), 162–164. doi: 10.1016/s0304-3940(98)00820-9 [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, et al. (2001). Retraining the injured spinal cord. Journal of Physiology, 533, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, DeLeon R, Tillakaratne N, & Hodgson JA (1997). Does motor learning occur in the spinal cord? Neuroscientist, 3(5), 287–294. [Google Scholar]
- Edgerton VR, Roy RR, Hodgson JA, Prober RJ, de Guzman CP, & de Leon R. (1992). Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. Journal of Neurotrauma, 9 Suppl 1, S119–128. [PubMed] [Google Scholar]
- Farely J, & Alkon DL (1985). Cellular mechanisms of learning, memory, and information storage. Annual Review of Neuroscience, 36, 419–494. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1986). Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Annals New York Academy of Sciences, 467, 40–54. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, & Grau JW (2006). Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience, 141(1), 421–431. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Huie JR, Crown ED, Baumbauer KM, Hook MA, Garraway SM, … Grau JW (2012). Maladaptive spinal plasticity opposes spinal learning and recovery in spinal cord injury. Front Physiol, 3. doi: 10.3389/fphys.2012.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Huie JR, Crown ED, & Grau JW (2012). Central nociceptive sensitization vs. spinal cord training: opposing forms of plasticity that dictate function after complete spinal cord injury. Front Physiol, 3. doi: 10.3389/fphys.2012.00396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrini F, & De Koninck Y. (2013). Microglia control neuronal network excitability via BDNF signalling. Neural Plast, 2013, 429815. doi: 10.1155/2013/429815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, & Rossignol S. (1980). The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiolica Scandinavica, 108(3), 283–295. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, & Rossignol S. (1977). Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Research, 132(1), 121–139. doi: 10.1016/0006-8993(77)90710-7 [DOI] [PubMed] [Google Scholar]
- Gallistel CR (2000). The replacement of general-purpose learning models with adaptively specialized learning modules. In Gazzaniga MS (Ed.), The Cognitive Neurosciences, 2d ed. (pp. 1179–1191). Cambridge, MA: MIT Press. [Google Scholar]
- Gallistel CR (2021). The physical basis of memory. Cognition, 213, 104533. [DOI] [PubMed] [Google Scholar]
- Garcia J, Brett LP, & Rusiniak KW (1989). Limits of Darwinian conditioning. In Klein SB, & Mowrer RR (Ed.), Contemporary learning theory: Instrumental conditioning and the impact of biological constraints on learning. (pp. 237–275). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Gibson JJ (1979). The ecological approach to visual perception. Boston, MA: Houghton Mifflin. [Google Scholar]
- Gomez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, … Grau JW (2007). BDNF and learning: Evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience, 148(4), 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormezano I, & Kehoe EJ (1975). Classical conditioning: Some methodological- conceptual issues Handbook of learning and cognitive processes (Vol. 2, pp. 143–179). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Grau JW (1987a). Activation of the Opioid and Nonopioid Analgesic Systems - Evidence for a Memory Hypothesis and against the Coulometric Hypothesis. Journal of Experimental Psychology-Animal Behavior Processes, 13(3), 215–225. [PubMed] [Google Scholar]
- Grau JW (1987b). The central representation of an aversive event maintains the opioid and nonopioid forms of analgesia. Behavioral Neuroscience, 101(2), 272–288. [DOI] [PubMed] [Google Scholar]
- Grau JW (2014). Learning from the spinal cord: How the study of spinal cord plasticity informs our view of learning. Neurobiology of Learning and Memory, 108, 155–171. doi: 10.1016/j.nlm.2013.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Baine RE, Bean PA, Davis JA, Fauss GNK, Henwood MK, … Strain MM (2020). Learning to promote recovery after spinal cord injury. Experimental Neurology, 330(113334), 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, & Joynes RL (1998). Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral Neuroscience, 112(6), 1366–1386. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, & Miranda RC (2006). Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavior Cognition & Neuroscience Review, 5(4), 191–239. doi: 10.1177/1534582306289738 [DOI] [PubMed] [Google Scholar]
- Grau JW, & Huang YJ (2018). Metaplasticity within the spinal cord: Evidence brain-derived neurotrophic factor (BDNF), tumor necrosis factor (TNF), and alterations in GABA function (ionic plasticity) modulate pain and the capacity to learn. Neurobiology of Learning & Memory, 154, 121–135. doi: 10.1016/j.nlm.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huang YJ, Turtle JD, Strain MM, Miranda RC, Garraway SM, & Hook MA (2017). When pain hurts: nociceptive stimulation induces a state of maladaptive plasticity and impairs recovery after spinal cord injury. Journal of Neurotrauma, 34(10), 1873–1890. doi: 10.1089/neu.2016.4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Garraway SM, Hook MA, Crown ED, Baumbauer KM, … Ferguson AR (2012). Impact of behavioral control on the processing of nociceptive stimulation. Front Physiol, 3. doi: 10.3389/fphys.2012.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Lee KH, Hoy KC, Huang YJ, Turtle JD, … Garraway SM (2014). Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front Neural Circuits, 8, 100. doi: 10.3389/fncir.2014.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, & Joynes RL (2005a). A neural-functionalist approach to learning. International Journal of Comparative Psychology, 18, 1–22. [Google Scholar]
- Grau JW, & Joynes RL (2005b). Neurofunctionalism revisited: Learning is more than you think it is. International Journal of Comparative Psychology, 18, 46–59. [Google Scholar]
- Grau JW, Salinas JA, Illich PA, & Meagher MW (1990). Associative learning and memory for an antinociceptive response in the spinalized rat. Behavioral Neuroscience, 104(3), 489–494. [DOI] [PubMed] [Google Scholar]
- Grillner S, & Wallen P. (1985). Central pattern generators for locomotion, with special reference to vertebrates. Annual Review of Neuroscience, 8, 233–261. doi: 10.1146/annurev.ne.08.030185.001313 [DOI] [PubMed] [Google Scholar]
- Grillner S, & Zangger P. (1979). Central generation of locomotion in the low spinal cat. Experimental Brain Research, 34(2), 241–261. [DOI] [PubMed] [Google Scholar]
- Groves PM, Lee D, & Thompson RF (1969). Effects of stimulus frequency and intensity on habituation and sensitization in acute spinal cat. Physiology & Behavior, 4(3), 383-&. doi: 10.1016/0031-9384(69)90194-2 [DOI] [Google Scholar]
- Groves PM, & Thompson RF (1970). Habituation a dual process theory. Psychological Review, 77(5), 419–450. doi: 10.1037/h0029810 [DOI] [PubMed] [Google Scholar]
- Hook MA, Huie JR, & Grau JW (2008). Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behavioral Neuroscience, 122(1), 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy KC, Strain MM, Turtle JD, Lee KH, Huie JR, Hartman JJ, … Grau JW(2020). Evidence that the central nervous system can induce a modification at the neuromuscular junction that contributes to the maintenance of a behavioral response. Journal of Neuroscience, 40, 9186–9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, & Grau JW (2018). Ionic plasticity and pain: The loss of descending serotonergic fibers after spinal cord injury transforms how GABA affects pain. Experimental Neurology, 306, 105–116. doi: 10.1016/j.expneurol.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Lee KH, & Grau JW (2017). Complete spinal cord injury (SCI) transforms how brain derived neurotrophic factor (BDNF) affects nociceptive sensitization. Experimental Neurology, 288, 38–50. doi: 10.1016/j.expneurol.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Huang YJ, Lee KH, Murphy L, Garraway SM, & Grau JW (2016). Acute spinal cord injury (SCI) transforms how GABA affects nociceptive sensitization. Experimental Neurology, 285(Pt A), 82–95. doi: 10.1016/j.expneurol.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson KE, Tarbet MM, & Grau JW (2020). Instrumental learning in the spinal cord: The role of ionic plasticity. Paper presented at the International Symposium on Neural Regeneration, Pacific Grove, CA. [Google Scholar]
- Hudson KE, Tarbet MM, & Grau JW (2021). Positivity in the spinal cord: Learning promotes adaptive responding in the face of uncontrollable stimulation. Paper presented at the Society for Neuroscience, Chicago, IL. [Google Scholar]
- Hudson KE, Tarbet MM, & Grau JW (2022). Leg position modulates the adverse effect nociceptive stimulation has on spinal cord function. Paper presented at the Neurotrauma Society, Atlanta, GA. [Google Scholar]
- Huie JR, Baumbauer KM, Lee KH, Bresnahan JC, Beattie MS, Ferguson AR, & Grau JW (2012). Glial tumor necrosis factor alpha (TNF alpha) generates metaplastic inhibition of spinal learning. Plos One, 7(6). doi: 10.1371/journal.pone.0039751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Garraway SM, Baumbauer KM, Hoy KC, Beas BS, Montgomery KS, … Grau JW (2012). Brain-derived neurotrophic factor promotes adaptive plasticity within the spinal cord and mediates the beneficial effects of controllable stimulation. Neuroscience, 200, 74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huie JR, Stuck ED, Lee KH, Irvine KA, Beattie MS, Bresnahan JC, … Ferguson AR (2015). AMPA receptor phosphorylation and synaptic co-localization on motor neurons drive maladaptive plasticity below complete spinal cord injury. eNeuro. doi: 10.1523/eneuro.0091-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CL (1943). Principles of behavior. New York: Appleton-Century-Crofts. [Google Scholar]
- Illich PA, Salinas JA, & Grau JW (1994). Latent inhibition, overshadowing, and blocking of a conditioned antinociceptive response in spinalized rats. Behavioral and Neural Biology, 62(2), 140–150. [DOI] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, & Woolf CJ (2003). Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci, 26(12), 696–705. doi: 10.1016/j.tins.2003.09.017 [DOI] [PubMed] [Google Scholar]
- Joynes RL, Ferguson AR, Crown ED, Patton BC, & Grau JW (2003). Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behavioural Brain Research, 141(2), 159–170. [DOI] [PubMed] [Google Scholar]
- Joynes RL, & Grau JW (1996). Mechanisms of pavlovian conditioning: Role of protection from habituation in spinal conditioning. Behavioral Neuroscience, 110(6), 1375–1387. [DOI] [PubMed] [Google Scholar]
- Joynes RL, & Grau JW (2004). Instrumental learning within the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiology of Learning and Memory, 82(1), 35–51. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, & Grau JW (2004). Instrumental learning within the spinal cord: VI The NMDA receptor antagonist, AP5, disrupts the acquisition and maintenance of an acquired flexion response. Behavioural Brain Research, 154(2), 431–438. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Fredrickson BL, & Schreiber CA (1993). When more pain is preferred to less: Adding a better end. Psychological Science, 4, 401–405. [Google Scholar]
- Kaila K, Price TJ, Payne JA, Puskarjov M, & Voipio J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nature Reviews Neuroscience, 15(10), 637–654. doi: 10.1038/nrn3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, & Schwartz JH (1982). Molecular-biology of learning - modulation of transmitter release. Science, 218(4571), 433–443. doi: 10.1126/science.6289442 [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, and Buonomano DV (2007). Timing in the absence of clocks: encoding time in neural network states. Neuron 53, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. (2006). Locomotor circuits in the mammalian spinal cord. Annual Review of Neuroscience, 29, 279–306. [DOI] [PubMed] [Google Scholar]
- Kiehn O, & Kjaerulff O. (1998). Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci, 860, 110–129. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, & Thompson RF (1998). Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science, 279(5350), 570–573. [DOI] [PubMed] [Google Scholar]
- Kimble GA (1961). Hilgard and Marquis’ conditioning and learning. Appleton-Century-Crofts: New York. [Google Scholar]
- Kimble GA, Mann LI, & Dufort RH (1955). Classical and instrumental eyelid conditioning. Journal of Experimental Psychology, 49, 407–417. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, & Woolf CJ (2009). Central sensitization: A generator of pain hypersensitivity by central neural plasticity. Journal of Pain, 10(9), 895–926. doi: 10.1016/j.jpain.2009.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (1987). Emotion. In Plum F. (Ed.), Handbook of Physiology (Vol. V, pp. 419–459). Bethesda, MD: American Physiological Society. [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–184. [DOI] [PubMed] [Google Scholar]
- Lee KH, Huang YJ, & Grau JW (2016). Learning about Time within the Spinal Cord II: Evidence that Temporal Regularity Is Encoded by a Spinal Oscillator. Front Behav Neurosci, 10, 14. doi: 10.3389/fnbeh.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Huang Y-J, & Grau JW (2016). Learning about time within the spinal cord II: Evidence that temporal regularity is encoded by a spinal oscillator. Frontiers in Behavioral Neuroscience, 10, 14. doi: 10.3389/fnbeh.2016.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Turtle JD, Strain MM, Huang Y-J, Baumbauer KM, & Grau JW (2015). Learning about time within the spinal cord: Evidence that spinal neurons can abstract and store an index of regularity. Frontiers in Behavioral Neuroscience, 9, 274. doi: 10.3389/fnbeh.2015.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GT, Ferguson AR, Crown ED, Bopp AC, Miranda RC, & Grau JW (2005). Instrumental learning within the rat spinal cord: Localization of the essential neural circuit. Behavioral Neuroscience, 119(2), 538–547. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, & Edgerton VR (1986). Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Experimental Neurology, 92(2), 421–435. [DOI] [PubMed] [Google Scholar]
- Lukoyanov N, Watanabe H, Carvalho LS, Kononenko O, Sarkisyan D, Zhang M, … Bakalkin G. (2021). Left-right side-specific endocrine signaling complements neural pathways to mediate acute asymmetric effects of brain injury. Elife, 10. doi: 10.7554/eLife.65247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ (1974). The psychology of animal learning. Academic Press: London. [Google Scholar]
- Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, & Shields CB (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Experimental Neurology, 156(1), 191–204. doi: 10.1006/exnr.1999.7016 [DOI] [PubMed] [Google Scholar]
- Maier SF, & Seligman ME (2016). Learned helplessness at fifty: Insights from neuroscience. Psychological Review, 123(4), 349–367. doi: 10.1037/rev0000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, & Seligman MEP (1976). Learned helplessness - theory and evidence. Journal of Experimental Psychology-General, 105(1), 3–46. doi: 10.1037/0096-3445.105.1.3 [DOI] [Google Scholar]
- Maier SF, & Watkins LR (2010). Role of the medial prefrontal cortex in coping and resilience. Brain Research, 1355, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malomouzh AI, Nurullin LF, Arkhipova SS, & Nikolsky EE (2011). NMDA receptors at the endplate of rat skeletal muscles: precise postsynaptic localization. Muscle Nerve, 44(6), 987–989. doi: 10.1002/mus.22250 [DOI] [PubMed] [Google Scholar]
- Mauk MD, & Buonomano DV (2004). The neural basis of temporal processing. Annual Review of Neuroscience, 27, 307–340. doi: 10.1146/annurev.neuro.27.070203.144247 [DOI] [PubMed] [Google Scholar]
- Mauk MD, Steinmetz JE, & Thompson RF (1986). Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proceedings of the National Academy of Sciences, 83, 5349–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Johansen JP, & Blair HT (2011). Placing prediction into the fear circuit. Trends Neurosci, 34(6), 283–292. doi: 10.1016/j.tins.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Chen PS, Salinas JA, & Grau JW (1993). Activation of the Opioid and Nonopioid Hypoalgesic Systems at the Level of the Brain-Stem and Spinal-Cord - Does a Coulometric Relation Predict the Emergence or Form of Environmentally Induced Hypoalgesia. Behavioral Neuroscience, 107(3), 493–505. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Grau JW, & King RA (1989). Frontal-Cortex Lesions Block the Opioid and Nonopioid Hypoalgesia Elicited by Brief Shocks but Not the Nonopioid Hypoalgesia Elicited by Long Shocks. Behavioral Neuroscience, 103(6), 1366–1371. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Grau JW, & King RA (1990). Role of supraspinal systems in environmentally induced antinociception - effect of spinalization and decerebration on brief shock-induced and long shock-induced antinociception. Behavioral Neuroscience, 104(2), 328–338. [DOI] [PubMed] [Google Scholar]
- Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, & Pellegrino C. (2014). Current view on the functional regulation of the neuronal K+-Cl-cotransporter KCC2. Frontiers in Cellular Neuroscience, 8, 18. doi: 10.3389/fncel.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, & Bardoni R. (2008). BDNF as a pain modulator. Progress in Neurobiology, 85(3), 297–317. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L. (1978). The hippocampus as a cognitive map. Oxford: Oxford University Press. [Google Scholar]
- Ostroumov A, & Dani JA (2018). Convergent Neuronal Plasticity and Metaplasticity Mechanisms of Stress, Nicotine, and Alcohol. Annual Review of Pharmacology & Toxicology, 58, 547–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, & Dani JA (2016). Stress Increases Ethanol Self-Administration via a Shift toward Excitatory GABA Signaling in the Ventral Tegmental Area. Neuron, 92, 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MM (1976). Mechanisms of classical conditioning and fixation in spinal mammals. Advances in psychobiology, 3, 381–436. [PubMed] [Google Scholar]
- Patterson MM (2001). Spinal fixation: Long-term alterations in spinal reflex excitability with altered or sustained sensory inputs. Spinal cord plasticity: Alterations in reflex function (pp. 77–100). Norwell, MA: Kluwer Academic Publishers. [Google Scholar]
- Patterson MM, Cegavske CF, & Thompson RF (1973). Effects of a classical-conditioning paradigm on hind-limb flexor nerve response in immobilized spinal cats. Journal of Comparative and Physiological Psychology, 84(1), 88–97. doi: 10.1037/h0035021 [DOI] [PubMed] [Google Scholar]
- Patton BC, Hook MA, Crown ED, Ferguson AR, & Grau JW (2004). The behavioral deficit observed following noncontingent shock in spinalized rats is prevented by the protein synthesis inhibitor cycloheximide. Behavioral Neuroscience, 118, 653–658. [DOI] [PubMed] [Google Scholar]
- Pearson KG, & Rossignol S. (1991). Fictive motor patterns in chronic spinal cats. J Neurophysiol, 66(6), 1874–1887. [DOI] [PubMed] [Google Scholar]
- Pocratsky AM, Burke DA, Morehouse JR, Beare JE, Riegler AS, Tsoulfas P, … Magnuson DSK (2017). Reversible silencing of lumbar spinal interneurons unmasks a task-specific network for securing hindlimb alternation. Nature Communications, 8, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA (1988a). Behavioral-studies of Pavlovian conditioning. Annual Review of Neuroscience, 11, 329–352. [DOI] [PubMed] [Google Scholar]
- Rescorla RA (1988b). Pavlovian conditioning: It’s not what you think it is. American Psychologist, 43, 151–160. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. Classical Conditioning 11 (pp. 64–99). New York: Appleton-Century-Crofts. [Google Scholar]
- Rivera C, Voipio J, & Kaila K. (2005). Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. Journal of Physiology, 562(Pt 1), 27–36. doi: 10.1113/jphysiol.2004.077495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, … Kaila, K. (2004). Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. Journal of Neuroscience, 24(19), 4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JB, Schulkin J. (1998). From normal fear to pathological anxiety. Psychological Review, 105, 325–350. [DOI] [PubMed] [Google Scholar]
- Rossignol S. (2017). Plasticity of stepping in rhythms in the intact and injured mammalian spinal cord. In Sherman M. (Ed.), Oxford Research Encyclopedia of Neuroscience. New York: Oxford University Press. [Google Scholar]
- Rossignol S, & Frigon A. (2011). Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annual Review of Neuroscience, 34, 413–440. doi: 10.1146/annurev-neuro-061010-113746 [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. (2000). Learning and memory in pain pathways. Pain, 88(2), 113–118. doi: 10.1016/s0304-3959(00)00424-3 [DOI] [PubMed] [Google Scholar]
- Sandkuhler J, & Liu XG (1998). Induction of long-term potentiation at spinal synapses by noxious stimulation or nerve injury. European Journal of Neuroscience, 10(7), 2476–2480. doi: 10.1046/j.1460-9568.1998.00278.x [DOI] [PubMed] [Google Scholar]
- Schull J. (1979). A conditioned opponent theory of Pavlovian conditioning and habituation. In Bower G. (Ed.), The Psychology of Learning and Motivation (Vol. 13). New York: Academic Press. [Google Scholar]
- Schultz W, & Dickinson A. (2000). Neuronal coding of prediction errors. Annual Review of Neuroscience, 23, 473–500. [DOI] [PubMed] [Google Scholar]
- Sherrington CS (1906). The integrative action of the nervous system. New Haven, CT: Yale University Press. [Google Scholar]
- Simone DA, Baumann TK, & Lamotte RH (1989). Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain, 38(1), 99–107. doi: 10.1016/0304-3959(89)90079-1 [DOI] [PubMed] [Google Scholar]
- Skinner BF (1938). The behavior of organisms. Englewood Cliffs, NJ: Prentice-Hall. [Google Scholar]
- teinmetz JE (2001). The cerebellum and classical eyeblink conditioning: Much ado about something. In Steinmetz JE, Gluck MA, & Solomon PR (Ed.), Model systems and the neurobiology of associative learning: A festschrift in honor of Richard F. Thompson Lawrence Elrbaum Associates: Mahwah, NJ. [Google Scholar]
- Strain MM, Pesek BS, Huang Y-J, Stump CR, Turtle JD, Lee KH, & Grau JW (2014). Acquired pendulate tail: Evidence for the activation of an oscillator following fixed spaced shock. 2014 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. [Google Scholar]
- Sutherland RJR, J. W. (1989). Configural association theory: The role of the hippocampus formation in learning, memory, and amnesia. Psychobiology, 17, 129–144. [Google Scholar]
- Tarbet MM, Hudson KL, Lout ET, & Grau JW (2019). Memories at the neuromuscular junction are resistant to extinction. Paper presented at the Society for Neuroscience, Chicago, IL. [Google Scholar]
- Tashiro S, Shinozaki M, Mukaino M, Renault-Mihara F, Toyama Y, Liu M, Nakamura M, Okano H. (2015). BDNF induced by treadmill training contributes to the suppression of spasticity and allodynia after spinal cord injury via upregulation of KCC2. Neurorehabilitation & Neural Repair, 29, 677–689. [DOI] [PubMed] [Google Scholar]
- Thompson RF (1986). The neurobiology of learning and memory. Science, 233(4767), 941–947. [DOI] [PubMed] [Google Scholar]
- Timberlake W. (1990). Biological behaviorism. In O’Donohuse W, & Kitchener R. (Ed.), Handbook of behaviorism (pp. 244–286). New York: Academic Press. [Google Scholar]
- Timberlake W, & Lucas GA (1989). Behavior systems and learning: From misbehavior to general principles. In Klein SB, & Mowrer RR (Ed.), Contemporary learning theory: Instrumental conditioning and the impact of biological constraints on learning. (pp. 237–275). Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Walters ET, & Byrne JH (1983). Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science, 219(4583), 405–408. doi: 10.1126/science.6294834 [DOI] [PubMed] [Google Scholar]
- Washburn SN, Patton BC, Ferguson AR, Hudson KL, & Grau JW (2007). Exposure to intermittent nociceptive stimulation under pentobarbital anesthesia disrupts spinal cord function in rats. Psychopharmacology, 192(2), 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Cobelli DA, & Mayer DJ (1982). Opiate vs non-opiate footshock induced analgesia (FSIA): descending and intraspinal components. Brain Research, 245(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Kinscheck IB, & Mayer DJ (1983). The neural basis of footshock analgesia: the effect of periaqueductal gray lesions and decerebration. Brain Research, 276(2), 317–324. [DOI] [PubMed] [Google Scholar]
- Watkins LR, & Mayer DJ (1982). Organization of endogenous opiate and nonopiate pain control systems. Science, 216(4551), 1185–1192. [DOI] [PubMed] [Google Scholar]
- Willis WD (2001). Mechanisms of central sensitization of nociceptive dorsal horn neurons. In Patterson MM & Grau JW (Eds.), Spinal Cord Plasticity: Alterations in Reflex Function (pp. 127–161). Boston: Kluwer Academic Publishers. [Google Scholar]
- Wolpaw JR, & Carp JS (1990). Memory traces in spinal-cord. Trends Neurosci, 13(4), 137–142. doi: 10.1016/0166-2236(90)90005-u [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, & Lee CL (1989). Memory traces in primate spinal cord produced by operant conditioning of H-reflex. Journal of Neurophysiology, 61(3), 563–572. doi: 10.1152/jn.1989.61.3.563 [DOI] [PubMed] [Google Scholar]