Abstract
The most common cause of corneal graft failure is corneal graft rejection (CGR). Although cornea is one of the immune-privileged sites, it can still get a rejection episode due to a breach in its natural protective mechanism. Both anatomical and structural properties of cornea and anterior chamber contribute toward its immune tolerance. Clinically, every layer of the transplanted cornea can get a rejection episode. A proper understanding of immunopathogenesis will help in understanding the various mechanism of CGR and the development of newer strategies for the prevention and management of such cases.
Keywords: Corneal graft rejection, endothelial rejection, graft failure, immune privilege, keratoplasty
Graft rejection is the most important complication of any organ transplantation. Although the cornea is considered immune-privileged, a corneal graft can still have an episode of rejection. An episode of corneal graft rejection (CGR) can lead to a significant amount of endothelial loss and rarely to graft failure. A few decades back, penetrating keratoplasty (PKP) used to be the treatment of choice for most corneal pathology. Over the years, endothelial transplant procedure has replaced PKP as the procedure of choice to manage corneal endothelial (CE) disorders. The mechanism and management of an episode of CGR are mostly well-defined irrespective of the nature of surgery. Nevertheless, the clinical spectrum varies significantly depending on the type of transplant performed. In this review, we will discuss the various immunological aspects of CGR following corneal transplantation.
Method of Literature Search
Articles related to CGR were searched using Medline, PubMed, Cochrane Library Database, EMBASE, and Scopus. The search was conducted using the following terms: CGR, endothelial rejection, epithelial rejection, stromal rejection, corneal immune privilege, high-risk keratoplasty, corneal neovascularization, PKP, EK, and lamellar keratoplasty. The abstracts of all the articles were screened, and relevant articles were included in this review. Reference lists from the selected articles were further checked to obtain further relevant articles not included in the electronic database. The emphasis was primarily to include randomized clinical trials and prospective studies; however, small case series and retrospective studies were included if found significant.
Mechanisms of CGR
Corneal immune privilege
Cornea transplantation, in contrast to other organ transplantation, has an excellent success rate primarily due to its immune privilege.[1,2] Immune-privileged sites are those where a transplanted graft survives for an extended period compared to a nonprivileged site.[3,4] Similarly, immune-privileged tissues are the one that survive for an extended period when placed at conventional body sites compared to nonprivileged tissues that often get rejected.[4] The cornea exhibits the property of both an immunoprivileged site and tissue. The anterior chamber of the eye exhibits the property of an immunoprivileged site.[1]
The molecular mechanisms contributing to immune privilege in corneal transplantation have been described in detail by several recent reviews.[1,5,6] Most of the evidence and theories of corneal immune privilege have been from experimental studies. The primary mechanism responsible for the corneal immune privilege is discussed below [Figs. 1 and 2].
Figure 1.
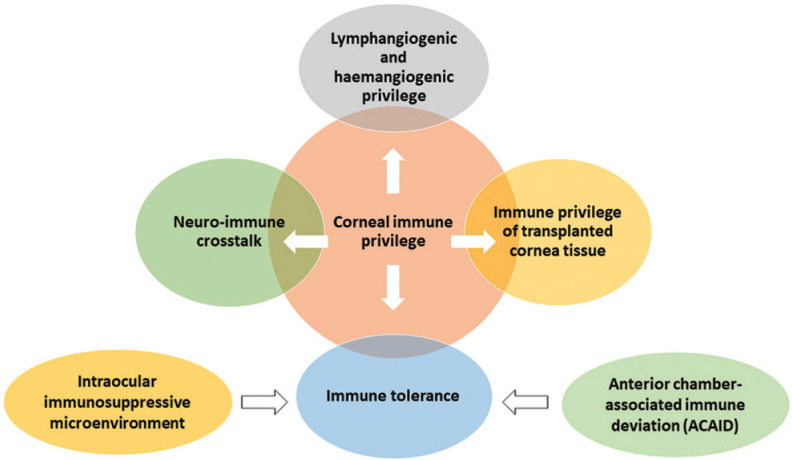
Flowchart depicting the mechanisms responsible for corneal immune privilege
Figure 2.
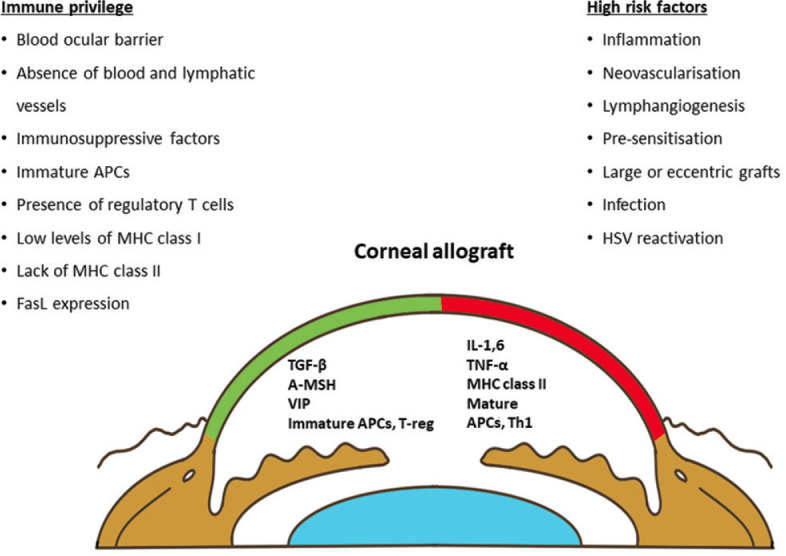
Schematic diagram demonstrates local factors contributing to immune privilege, factors maintaining immune privilege (green color) and risk factors (red color). Footnotes - α-MSH: α -melanocyte-stimulating hormone; ACAID: anterior chamber-associated immune deviation; APCs: antigen-presenting cells; FasL: Fas ligand; IL: interleukin; MHC: major histocompatibility complex; TGF-β: tumor growth factor-β; TNF-α: tumor necrosis factor-α; T-reg: regulatory T cells; VIP: vasoactive intestinal peptide
i. Lymphangiogenic and hemangiogenic privilege: Cornea lacks blood vessels and lymphatics. The absence of both elements allows the cornea to be transparent and an immune-privileged site. The mechanism involved in the absence of blood vessels involves both anatomical and cellular mechanisms.
Typically, blood vessels form a loop surrounding the cornea at the limbal area, located 1–5 mm outside of the corneal limbus.[7] The limbus has natural barriers that do not allow the limbal vessels to sprout into the adjacent cornea. The exact components of this barrier are not clearly known. Bowman’s membrane is one of these natural barriers. The collagens I and V present in the corneal stroma are regulatory and probably inhibit vessels’ growth in the corneal stroma. Normal blood vessels contain smooth muscle cells or pericytes that cover the vascular endothelium. Smooth muscle cells are absent in the limbal vessels close to the cornea, while the limbal arcade vessels do possess the pericytes. The endothelial cells and pericytes are attached by several factors including actin filaments. These factors are important in maintaining the barrier function of the limbus, and disruption of these factors or anatomical barriers could lead to corneal angiogenesis and lymphangiogenesis.[1,6]
The cellular factors that prevent blood vessels and lymphatics from entering the cornea primarily involve angiostatic factors present in the cornea and aqueous humor. Various components of the cornea express these factors. Corneal epithelium secrets several factors such as thrombospondin-1 (TSP-1), soluble FLT-1 (sFLT-1), VEGF receptor3 (VEGFR-3), pigment epithelium-derived factor (PEDF), and TNF-related apoptosis inducing ligand (TRAIL). The epithelial basement membrane expresses endostatin. The corneal endothelium expresses TSP-1. Aqueous humor contains several factors including TGF- β2, α-melanocyte-stimulating hormone (α-MSH), and vasoactive intestinal peptide (VIP). The corneal limbus has melanocytes that express tryrosinase.[1,2]
TSP-1 is a negative regulator of corneal angiogenesis and acts by binding with TGF-β and interacting with vascular endothelial cells to induce apoptosis.[8-10] sFLT-1 is normally found in the cornea, and any deficiency of it can lead to vessel growth from the limbus due to an increase in VEGF-A.[11] VEGFR-3 acts by behaving as a decoy receptor for angiogenic growth factor VEGF-C.[8] PEDF inhibits corneal angiogenesis induced by basic fibroblast growth factor. TRAIL inhibits both lymphangiogenesis and angiogenesis.[1] Tyrosinase is a copper-containing glycoprotein, and its deficiency has been shown to induce lymphatic growth in the corneal limbus in animal studies. VIP receptor-1 is present in endothelial cells of corneal blood vessels and lymphatic channels. Expression of VIP has been shown to suppress lymphatic endothelial cell (LEC) proliferation in in vitro studies.[12] The α-MSH receptor (MSHR)-1 is expressed on LEC, and α-MSH expression inhibits LEC proliferation. TGF-β acts as a negative regulator for lymphatic vessels, and its inhibition has demonstrated accelerated lymphangiogenesis in studies involving the mouse model.[13]
ii. Immune privilege of transplanted cornea tissue: The transplanted tissue’s immune privilege partly contributes to the high success rate of cornea transplant compared to other solid organ transplants. Experimental studies suggest that the immunogenicity of different layers of the cornea may vary. It appears that epithelium, followed by stroma, is less immune-privileged compared to the endothelium. This is probably reflected clinically by a lower rate of graft rejection following endothelial keratoplasty (EK) compared to a deep lamellar keratoplasty or a full-thickness corneal graft.[14-16] However, the clinical implication of such a finding is not clear. The expression of CD95L by CE cells has been proposed to contribute to the immune-privileged status of cornea.[1]
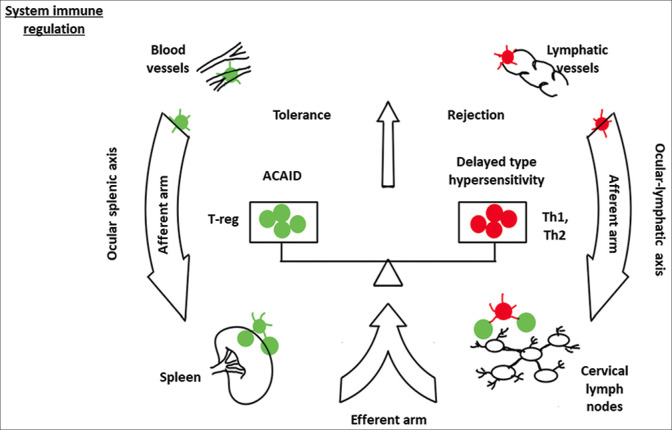
iii. Immune tolerance: Ocular immune privilege is achieved by two active mechanisms. The first mechanism involves anterior chamber-associated immune deviation (ACAID). The second mechanism is provided by an intraocular immunosuppressive microenvironment [Figs. 2 and 3].[6]
Figure 3.
Schematic diagram demonstrates the balance between immune tolerance and immune rejection. Footnotes - α-MSH: α-melanocyte-stimulating hormone; ACAID: anterior chamber-associated immune deviation; APCs: antigen-presenting cells; FasL: Fas ligand; IL: interleukin; MHC: major histocompatibility complex; TGF-β: tumor growth factor-β; TNF-α: tumor necrosis factor-α; T-reg: regulatory T cells; VIP: vasoactive intestinal peptide
ACAID
ACAID refers to systemic tolerance to alloantigens when placed in the anterior chamber of the eye.[1,17] Whenever an antigen enters the eye’s anterior chamber, it is captured by the antigen-presenting cells (APCs) of the host. These APCs enter the bloodstream through the trabecular meshwork and migrate preferentially to the spleen. At the spleen’s marginal zone, these cells induce the production of TGF-β, TSP-1, and MIP-2. MIP-2 attracts natural killer T cells (NKT) through chemotaxis. These NKT cells produce several factors including IL-10, TGF-β, and CCL-5. Besides, these NKT cells attract marginal zone B cells expressing Qa-1. APCs, NKT, and marginal zone B cells and mediators create an environment under which the responding T cells differentiate into T regulatory cells (Tregs). CD4 + ACAID-Tregs (known as “afferent regulators”) inhibit the differentiation of naive T cells into Th1 effector cells in the local lymph nodes. These Tregs also inhibit the function of effector Th1 and Th2 cells in the local site. Both these actions lead to an impaired alloantigen-specific delayed-type hypersensitivity (DTH) and increased graft survival.[1,6,17]
Regulatory T cells (Tregs): Tregs are generated by ACAID as well as by ocular resident cells, which include CE cells, ocular pigment epithelial cells, and aqueous humor.[18-20] Tregs act by various mechanisms and contribute to ACAID. Tregs release inhibitory cytokines and suppress the conventional T cells. Besides, Tregs can directly kill the conventional T cells by cytolysis through granzyme A/B. Tregs can consume the local IL-2 and cause metabolic disruption in conventional T cells. Tregs can lead to the downregulation of conventional T cells by promoting the local production of adenosine. Lastly, Tregs can lead to modulation of APCs by interacting with CTLA-4 and CD80/86, or LAG-3 and MHC-class II [Fig. 3].[1,5]
Intraocular immunosuppressive microenvironment: The immune privilege of the cornea is not absolute. Immune cells and molecules can still access the eye. The cornea and anterior segment have an immune suppressive microenvironment that protects the eye from such an attack. This immunosuppressive microenvironment is created by several cells and soluble factors present in the anterior segment. The various factors, their source, and the target immune cells are summarized in Table 1. A detailed discussion on these factors and their role can be accessed in the review article by Hori et al.[1]
Table 1.
Cells and soluble factors creating an immune suppressive microenvironment in the anterior segment of the eye
| Site | Factor | Target immune component |
|---|---|---|
| Soluble factors in the anterior chamber | Alpha-Melanocyte-stimulating hormone (α-MSH) | T cells, macrophages, neutrophils |
| Vasoactive intestinal peptide (VIP) | T cells | |
| Somatostatin | T cells | |
| Calcitonin gene-related peptide (CGRP) | Macrophages | |
| Transforming growth factor-beta 2 (TGF-β2) | T cells, macrophages, NK cells | |
| Thrombospondin-1 (TSP-1) | Macrophages | |
| Macrophage migrating inhibitory factor (MIF) | NK cells | |
| interleukin 1 receptor antagonist (IL-1Ra) | IL-1 | |
| Soluble Fas ligand (sFas L) | T cells, neutrophils | |
| CD46, CD55, CD59, C3ib | Complement | |
| Cell surface molecules of the cornea and iris–ciliary body | PD-L1 (B7-H1) | T cells |
| Inducible costimulatory molecule ligand (ICOSL) | T cells | |
| Glucocorticoid-induced tumor necrosis factor receptor family-related protein (GITR) ligand | T cells | |
| Galectin-9 | T cells | |
| Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) | T cells | |
| Fas L (CD95 L) | T cells, neutrophils | |
| Cytotoxic T lymphocyte-associated antigen-2 alpha CTLA-2α) | T cells | |
| MHC class Ib | T cells, NK cells | |
| CD46, CD55, CD59 | Complement |
iv. Neuroimmune crosstalk: Neural networks can influence the innate immune system through cholinergic anti-inflammatory pathways. It acts by inhibiting the excessive proinflammatory cytokine responses and thereby prevents immune-mediated organ damage.[21,22] Cornea is one of the most highly innervated tissue, any inflammatory reaction within it could be influenced by the neural network.[23] Various neurotransmitters such as substance P, VIP, calcitonin gene-related peptide, galanin, catecholamines, acetylcholine, and α-MSH in the Peripheral Nervous System (PNS) could influence the inflammatory response in the event of a rejection episode.[24]
Experimental studies suggest that APCs are present in both central and peripheral cornea; however, during steady state, the central APCs remain in a uniformly MHC class II-negative state and the peripheral APCs remains in an MHC class II-positive state.[25] Shortly after inflammation is induced, either by thermal burn or surgical injury, a subset of resident corneal dendritic cells (cDCs) in the central cornea enter a “mature” state by expressing MHC class II and costimulatory markers.[25] Within 24 h after corneal transplantation, resident cDCs in the donor button migrated out to the recipient’s peripheral cornea and into draining cervical lymph nodes, suggesting the presentation of donor-derived antigen to T-cells and induction of an allogeneic immune reaction.[26]
The corneal innervation status influences the motility of these cDCs. When a corneal graft is transplanted onto a denervated host bed, DCs acquire motility with high directionality to the cervical lymph node and prime alloreactive T cells. In contrast, following transplantation on an innervated host bed, fewer DCs migrate to delphian lymph node (dLN), leading to less sensitization. Thus, corneal innervation may be playing some role in the immune privilege of the cornea. However, most of this evidence is from experimental studies, and the exact role of the neuroimmune interaction is still to be established.[1]
Immunology of CGR
Corneal immune privilege is not absolute. Any breach in it could lead to the host immune system’s activation against the antigens present in the donor corneal button, leading to an immune reaction that would lead to donor tissue destruction mediated by the cells and mediators of the innate and adaptive immune responses. The host immune response has two arms, an afferent arm and an effector arm.
The afferent arm, also known as the induction phase, consists of donor antigen presentation to the naive T cells in draining lymph nodes.[27] The donor antigen presentation is done by host APCs, namely, DCs, derived from bone marrow and residing in the corneal epithelium as well as stroma. These cells travel the cornea, capture donor antigens, and transport them to draining lymph nodes, where antigen presentation occurs by recognizing self MHC-II by naive T cells.[27,28] Some evidence suggests that the donor DCs can also present the antigens directly to the draining lymph nodes. This pathway is known as the direct pathway, and the former is the indirect pathway of antigen presentation. The direct pathway may play an essential role in high-risk corneal beds with higher immunogenicity and compromised immune privilege.[29-31] Following corneal transplantation, the quiescent environment of the cornea becomes inflammatory. This brings several changes in corneal DCs, such as the expression of MHC-II by central DCs, expression of costimulatory molecules like CD80, CD86, and CD40 by central as well as peripheral DCs, expression of differential adhesion molecules for activation of T cells, and release of cytokines such as IL-1, -6, and -12.[27]
The effector arm of the graft rejection is primarily dispensed through T cells. T cell allo-sensitization and activation occur in the draining lymph nodes. These alloreactive activated T cells migrate to the cornea, recognizing donor MHC antigens and inducing inflammation and tissue destruction.[27,31] The primary cellular mediators of graft rejection are CD8 + CTL and CD4 + T-helper (Th) lymphocytes, the mediators of DTH. Th cells play a central role in CGR. These cells secret IL-2, IFN-gamma, and lymphotoxin, leading to inflammation and attack on the donor antigen. These chemokines also recruit leukocytes and immune cells to the inflamed cornea. All these recruited cells release various cytokines, such as TNF-α, IL-1, that mediate the tissue damage.[27,32,33] While all these are going on, alloreactive T cell also induces the formation of memory T cells. These memory cells are responsible for the enhanced immune response seen on re-exposure to the same antigen as seen in cases of regraft.[27]
Although the above-said mechanism is the simplest way to describe CGR’s immunopathogenesis, a lot still needs to be explored. One such dilemma is even though antigen recognition occurs through MHC-II expression, MHC matching has no impact on CGR.[34] Recent experimental studies show that minor histocompatibility antigens (HA-1,2,3,4, and 5) and male-specific antigens (H-Y) may be playing an important role in antigen recognition following corneal transplantation.[35-37]
The above discussion on CGR is largely from the available literature on endothelial rejection; however, the graft rejection following lamellar keratoplasties follows a similar pathway. Following anterior lamellar keratoplasty, such as deep anterior lamellar keratoplasty, endothelial immune reactions cannot occur and the donor cornea may suffer epithelial, subepithelial, and stromal rejection only. The APCs may reach the donor stroma through intrastromal recognition or infiltrating vessels. Previous inflammation, as discussed above, significantly increases the risk of stromal rejection, and several studies have shown a prolonged steroid therapy often prevents an episode of stromal rejection. The other major difference is the lack of exposure of the donor antigens to the anterior chamber and the subsequent lack of activation of ACAID.[38] The lack of ACAID negates one significant immune tolerance mechanism inherent to cornea. Thus, the episode of stromal rejection following lamellar keratoplasty may be higher compared to a full-thickness graft.
The mechanism of endothelial rejection following EK is similar to that discussed above, with few differences. Since most of the APCs are in the anterior stroma and donor epithelium is not transplanted, the chances of rejection following EK are less. Besides, as discussed above, the donor antigens are exposed to the anterior chamber and subsequent mechanism of ACAID. This further reduces the chance of CGR following EK. Following Descemet stripping endothelial keratoplasty (DSEK), some stroma is transplanted, which probably explains the higher risk of graft rejection following DSEK compared to DMEK (Descemet membrane endothelial keratoplasty).
Although the mechanism of CGR following PKP is well defined, the mechanism following modern lamellar surgeries is still not clear and requires future research.
Future challenges
CGR offers several challenges that need to be addressed in the coming future. Several unanswered questions related to donor selection, ideal transplant procedure, and choice of immunosuppressive regimens need to be addressed. The pathogenesis part needs more clarity. Whatever evidence available in literature is largely based on experimental models, global epidemiological surveys, and regional registries. Experimental models serve as excellent models for exploring the local and systemic pathophysiology of CGR and targetable pathogenic pathways, but the development of an ideal immunosuppression regimen, management of CGR, and the long-term outcomes of CGR requires robust, multicenter clinical trial initiatives might be able to address the questions such as why stromal rejection and epithelial rejections are less common than endothelial or could they be less reported? and how stromal rejection can occur independent of epithelial or endothelial rejection even in a PKP?
Conclusion
CGR is the most common cause accounting for most cases of graft failure. Despite being an immune-privileged site, the occurrence of graft rejection in cornea is a predicament, but it also provides an opportunity to explore the possibility of preventing an episode of rejection.
A knowledge of immunopathogenesis of CGR would help the clinician to understand various protective mechanisms and factors that compromise those inherent corneal properties that lead to an episode of rejection. Early diagnosis of rejection is essential to initiate the treatment before irreversible endothelial cell damage occurs. This article will help the readers to not only know the risk factors but also the factors that incite an episode of CGR. Therefore, we believe having knowledge about the immunopathology of CGR would help in the early diagnosis and management of CGR.
Over the decades, various interventions have been developed to prevent CGR, for example, diathermy/cauterization of blood vessels, ABO-HLA compatibility matching, gene therapy, anti-VEGF, and immunomodulators have been tried with variable success. A knowledge of immunopathogenesis would stimulate the physician not only to use these modalities judiciously but also to come out with new interventions to prevent an episode of CGR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hori J, Yamaguchi T, Keino H, Hamrah P, Maruyama K. Immune privilege in corneal transplantation. Prog Retin Eye Res. 2019;72:100758. doi: 10.1016/j.preteyeres.2019.04.002. doi:10.1016/j.preteyeres. 2019.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Niederkorn JY. See no evil, hear no evil, do no evil:The lessons of immune privilege. Nat Immunol. 2006;7:354–9. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 3.Streilein JW. New thoughts on the immunology of corneal transplantation. Eye (Lond) 2003;17:943–8. doi: 10.1038/sj.eye.6700615. [DOI] [PubMed] [Google Scholar]
- 4.Streilein JW. Ocular immune privilege:Therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 5.Keino H, Horie S, Sugita S. Immune privilege and eye-derived T-regulatory cells. J Immunol Res. 2018;2018:1679197. doi: 10.1155/2018/1679197. doi:10.1155/2018/1679197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor AW. Ocular immune privilege and transplantation. Front Immunol. 2016;7:37. doi: 10.3389/fimmu.2016.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer PA. The circulation of the human limbus. Eye (Lond) 1989;3:121–7. doi: 10.1038/eye.1989.19. [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Maruyama K, Bock F, Saban D, Sadrai Z, Lawler J, et al. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J Exp Med. 2011;208:1083–92. doi: 10.1084/jem.20092277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cursiefen C, Masli S, Ng TF, Dana MR, Bornstein P, Lawler J, et al. Roles of thrombospondin-1 and -2 in regulating corneal and iris angiogenesis. Invest Ophthalmol Vis Sci. 2004;45:1117–24. doi: 10.1167/iovs.03-0940. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–8. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 11.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–7. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bock F, Maruyama K, Regenfuss B, Hos D, Steven P, Heindl LM, et al. Novel anti (lymph) angiogenic treatment strategies for corneal and ocular surface diseases. Prog Retin Eye Res. 2013;34:89–124. doi: 10.1016/j.preteyeres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Oka M, Iwata C, Suzuki HI, Kiyono K, Morishita Y, Watabe T, et al. Inhibition of endogenous TGF-beta signaling enhances lymphangiogenesis. Blood. 2008;111:4571–9. doi: 10.1182/blood-2007-10-120337. [DOI] [PubMed] [Google Scholar]
- 14.Anshu A, Price MO, Price FW. Risk of corneal transplant rejection significantly reduced with Descemet's membrane endothelial keratoplasty. Ophthalmology. 2012;119:536–40. doi: 10.1016/j.ophtha.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez A, Price MO, Feng MT, Lee C, Arbelaez JG, Price FW. Immunologic rejection episodes after deep anterior lamellar keratoplasty:Incidence and risk factors. Cornea. 2017;36:1076–82. doi: 10.1097/ICO.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa A, Yamaguchi T, Mitamura H, Tomida D, Shimazaki-Den S, Murat D, et al. Aetiology-specific comparison of long-term outcome of deep anterior lamellar keratoplasty for corneal diseases. Br J Ophthalmol. 2016;100:1176–82. doi: 10.1136/bjophthalmol-2015-307427. [DOI] [PubMed] [Google Scholar]
- 17.Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:27–35. doi: 10.1159/000099251. [DOI] [PubMed] [Google Scholar]
- 18.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–23. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Okamura T, Sumitomo S, Morita K, Iwasaki Y, Inoue M, Nakachi S, et al. TGF-b3-expressing CD4+CD25(-) LAG3+regulatory T cells control humoral immune responses. Nat Commun. 2015;6:6329. doi: 10.1038/ncomms7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity. 2018;49:1004–19. doi: 10.1016/j.immuni.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–81. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 22.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 23.Müller LJ, Marfurt CF, Kruse F, Tervo TMT. Corneal nerves:Structure, contents and function. Exp Eye Res. 2003;76:521–42. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 25.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–8. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qazi Y, Hamrah P. Corneal allograft rejection:Immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013;2013((Suppl 9)):006. doi: 10.4172/2155-9899.S9-006. doi:10.4172/2155-9899.S9-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong EM, Dana MR. Graft failure IV. Immunologic mechanisms of corneal transplant rejection. Int Ophthalmol. 2008;28:209–22. doi: 10.1007/s10792-007-9099-9. [DOI] [PubMed] [Google Scholar]
- 29.Boisgérault F, Liu Y, Anosova N, Dana R, Benichou G. Differential roles of direct and indirect allorecognition pathways in the rejection of skin and corneal transplants. Transplantation. 2009;87:16–23. doi: 10.1097/TP.0b013e318191b38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol. 2004;173:4464–9. doi: 10.4049/jimmunol.173.7.4464. [DOI] [PubMed] [Google Scholar]
- 31.Yamagami S, Amano S. Role of resident corneal leukocytes and draining cervical lymph nodes in corneal allograft rejection. Cornea. 2003;22((7 Suppl)):S61–5. doi: 10.1097/00003226-200310001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Yamagami S, Hamrah P, Zhang Q, Liu Y, Huq S, Dana MR. Early ocular chemokine gene expression and leukocyte infiltration after high-risk corneal transplantation. Mol Vis. 2005;11:632–40. [PubMed] [Google Scholar]
- 33.Yamagami S, Isobe M, Tsuru T. Characterization of cytokine profiles in corneal allograft with anti-adhesion therapy. Transplantation. 2000;69:1655–9. doi: 10.1097/00007890-200004270-00022. [DOI] [PubMed] [Google Scholar]
- 34.The collaborative corneal transplantation studies (CCTS) Effectiveness of histocompatibility matching in high-risk corneal transplantation. The Collaborative Corneal Transplantation Studies Research Group. Arch Ophthalmol. 1992;110:1392–403. [PubMed] [Google Scholar]
- 35.Böhringer D, Spierings E, Enczmann J, Böhringer S, Sundmacher R, Goulmy E, et al. Matching of the minor histocompatibility antigen HLA-A1/H-Y may improve prognosis in corneal transplantation. Transplantation. 2006;82:1037–41. doi: 10.1097/01.tp.0000235908.54766.44. [DOI] [PubMed] [Google Scholar]
- 36.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14:419–25. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 37.Streilein JW, Arancibia-Caracamo C, Osawa H. The role of minor histocompatibility alloantigens in penetrating keratoplasty. Dev Ophthalmol. 2003;36:74–88. doi: 10.1159/000067655. [DOI] [PubMed] [Google Scholar]
- 38.Hos D, Matthaei M, Bock F, Maruyama K, Notara M, Clahsen T, et al. Immune reactions after modern lamellar (DALK, DSAEK, DMEK) versus conventional penetrating corneal transplantation. Prog Retin Eye Res. 2019;73:100768. doi: 10.1016/j.preteyeres.2019.07.001. doi:10.1016/j.preteyeres. 2019.07.001. [DOI] [PubMed] [Google Scholar]