Abstract
Purpose:
To evaluate the postoperative visual outcomes, that is, corneal higher-order aberrations (HOAs) and visual quality, of patients with an angle kappa greater than 0.30 mm who underwent angle kappa adjustment during small-incision lenticule extraction (SMILE) 2 years after surgery compared to eyes with an angle kappa less than 0.30 mm.
Methods:
This was a retrospective study and included 12 patients from October 2019 to December 2019 who underwent the SMILE procedure for correction of myopia and myopic astigmatism and had one eye with a large kappa angle and another eye with a small kappa angle. Twenty-four months after surgery, an optical quality analysis system (OQAS II; Visiometrics, Terrassa, Spain) was used to measure the modulation transfer function cutoff frequency (MTFcutoff), Strehl2D ratio, and objective scatter index (OSI). HOAs were measured with a Tracey iTrace Visual Function Analyzer (Tracey version 6.1.0; Tracey Technologies, Houston, TX, USA). Assessment of subjective visual quality was achieved using the quality of vision (QOV) questionnaire.
Results:
At 24 months postoperatively, the mean spherical equivalent (SE) refraction was − 0.32 ± 0.40 and − 0.31 ± 0.35 in the S-kappa group (kappa <0.3 mm) and the L-kappa group (kappa ≥0.3 mm), respectively (P > 0.05). The mean OSI was 0.73 ± 0.32 and 0.81 ± 0.47, respectively (P > 0.05). There was no significant difference in MTFcutoff and Strehl2D ratio between the two groups (P > 0.05). Total HOA, coma, spherical, trefoil, and secondary astigmatism were not significantly different (P > 0.05) between the two groups.
Conclusion:
Adjustment of angle kappa during SMILE helps reduce the decentration, results in less HOAs, and promotes visual quality. It provides a reliable method to optimize the treatment concentration in SMILE.
Keywords: Ablation center, angle of kappa, HOAs, small-incision lenticule extraction (SMILE), visual quality, wavefront aberration
Femtosecond laser assisted small incision lenticule extraction (SMILE) technique has become a commonly accepted alternative to excimer laser-based techniques, such as femtosecond laser in situ keratomileusis (FS-LASIK) and photorefractive keratectomy (PRK), for the surgical correction of myopia and myopic astigmatism.[1] It is confirmed that SMILE produces promising refractive outcomes in terms of predictability, efficacy, stability, and safety in the correction of myopia and myopic astigmatism,[1,2] with high patient satisfaction.[3] In refractive surgery, the choice of treatment center is very important. Moving the ablation center from the center of the entrance pupil to the center of the visual axis results in less induction of higher-order aberrations (HOAs) and an equal or better visual when compared to laser ablation centered on the entrance pupil.[4,5] The exact definition of angle kappa is the angular distance between the visual and pupillary axes.[6] The angle of kappa is a necessary consideration in refractive surgery to avoid eccentricity of the treatment zone and thus obtain a better visual outcome.[7] SMILE does not have an eye-tracking and positioning system relative to excimer lasers; therefore, an accurate center is crucial in SMILE, especially in the case of a large angle kappa. SMILE cannot achieve personalized treatment, such as aberration or topographic map guidance, so manual cutting center location is adopted. Compared to SMILE, one potential advantage of excimer laser-based photoablation is the ability to deliberately modulate corneal HOAs. In keratorefractive surgery, an excess of threshold value–inducing HOAs is well known to convey an increased risk for bothersome visual symptoms such as haloes, starburst, and glare.[1,8] Previous studies have shown that patients with large-angle kappa are more likely to undergo eccentric ablation, resulting in postoperative HOAs and decreased visual quality.[9-12] Adjustment of the angle kappa during SMILE resulted in fewer HOAs.[9] Shao et al. described that intraoperative adjustment of kappa angle can reduce postoperative HOA when the kappa angle is 0.19 ± 0.09 mm. However, when the kappa angle is greater than 0.3 mm, it will inevitably bring about a larger displacement from the center of the pupil to the visual axis; so, it is not clear whether this adjustment is still feasible, as Shao et al.[9] reported. Our retrospective study aimed to analyze whether adjusting the ablation center according to the size and direction of the large kappa angle during SMILE reduces eccentric ablation and the resulting HOAs and poor visual quality.
Methods
The surgical technique was the same following the classic SMILE scanning steps and the lenticule extraction as the VisuMax Femtosecond Laser System (Carl Zeiss, Oberkochen, Germany) required and the literature introduced.[13] Patients who underwent SMILE surgery, both male and female, aged 18–40 years, with kappa angle <0.3 mm in one eye and ≥0.3 mm in the other eye, and had complete 2-year follow-up data after surgery, were selected for the research. Twelve eligible patients underwent SMILE surgery in both eyes, and both eyes were included in the analysis. All visual data, HOA, and visual quality information were systematically recorded in structured patients’ medical records, and all information was comprehensively assessed through electronic records.
Ethics statement
The study protocol was approved by the West China Hospital and was in accordance with the tenets of the Declaration of Helsinki. Informed consent for surgery was obtained before the surgery.
Patient examination
The examinations conducted included slit-lamp microscope, uncorrected distance visual acuity (UDVA), intraocular pressure (IOP), refractions, and corneal topography with the Pentacam tomography system (Oculus Optikgeräte GmbH). We used the Pentacam to measure the displacement of the visual axis from the pupil center (chord distance), which essentially equates the angle kappa, and we used this displacement for the intraoperative angle kappa adjustments. The coefficients of vertical coma, horizontal coma, and spherical aberration were analyzed for a standardized diameter of 3 mm using an iTrace aberrometer because they are clinically significant in visual quality.[14,15] An optical quality analysis system (OQAS II; Visiometrics, Terrassa, Spain), based on the double-pass technique, was used to measure modulation transfer function (MTF) cutoff frequency (MTFcutoff, cycle per degree [cpd]), Strehl2D ratio (SR), OQAS values (OV) at different contrasts (100%, 20%, and 9%), and the objective scatter index (OSI) of each eye. All measurements were conducted in mesopic conditions with a 4.0-mm artificial pupil. The same experienced optometrists performed these examinations, and the average value from three good-quality images was used for analysis. In addition to objective examination, we assessed patients’ subjective visual quality by means of the quality of vision (QOV) questionnaire.[16] The QOV questionnaire consists of 10 items, including three subscales, and the QOV scores are given according to the symptom frequency, severity, and bothersome. Each item has four self-evaluation options. The first seven items have an associated picture to simulate visual symptoms, which ensures patient understanding. The QOV scores range from 0 to 100; the higher the score, the worse the QOV.[16,17] All patients were suggested to have a routine ophthalmic examination at 1 day, 1 week, 1 month, 3 months, 6 months, 12 months, and 24 months postoperatively.
Surgical procedure and perioperative medication
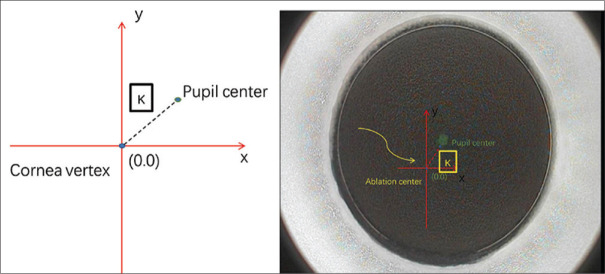
The SMILE procedures were completed by an experienced surgeon (DYP), who has more than 10 years of experience in refractive surgery. Briefly, the lenticule was extracted following these steps: fixate the light to centralize the corneal peak, suction on, create the posterior surface, make the border, create the anterior surface, make the incision, suction off, separate the lenticule, and extract it from the incision. The laser scan system (Visumax, Carl Zeiss Meditec AG) was set at a 500 kHz repetition rate. The diameter of the cap was 8 mm, and the thickness ranged from 100 to 110 μm. The thickness and diameter of the lenticules were dependent on pupil diameters, corneal thickness, and equivalent spherical quantity, while the diameter ranged from 6.0 to 6.5 mm. The incision was 2 mm, located at 11 o’clock in the right eye and 12 o’clock in the left eye to make the operation more convenient. After the lenticule was extracted, the corneal surface around the incision was flushed with a balanced salt solution. It is worth mentioning that the intraoperative adjustment of angle kappa was based on the results of the Pentacam scan, the relative position of the corneal vertex, and the pupil center (angle kappa size) obtained before SMILE surgery. Then, according to the size and direction of the angle kappa, the center of the suction ring was adjusted before suction, similar to excimer laser alignment[9] [Fig. 1].
Figure 1.
The method to offset the angle of kappa during SMILE. SMILE = small-incision lenticule extraction
Regularly, the operative management included preantibiotic (0.5% levofloxacin; Santen Pharmaceutical Co,Osaka, Japan) and preservative-free artificial tear drops (0.1% sodium hyaluronate; Ursapharm Arzneimittel GmbH, Saarbrücken, Germany) unsegmenting 12 times in 3 days. Postoperatively, the artificial tear 0.1% sodium hyaluronate was topically used four times daily for 3 months, while the steroid was started four times a day and reduced once weekly until 1 month. Meanwhile, the antibiotics were continued for 1 week, and IOP-lowering drops (0.2% brimonidine tartrate eye drops; Allergan Pharmaceuticals, Dublin, Ireland) was also used after surgery, which was beneficial to the stability of corneal biomechanics.
Statistical analysis
Statistical analysis for visual acuity (VA) was based on the logarithm of the minimum angle of resolution (logMAR) units. Continuous variables are expressed as the mean ± standard deviation, and categorical variables are expressed as a frequency (percentage). The Kolmogorov–Smirnov test was used to confirm data normality. Independent t-tests were used to compare clinical variables, induced corneal aberration, and visual quality between the two groups. When parameter analysis was not normal, the Wilcoxon test was used. When P values were less than 0.05, the difference was considered statistically significant. Data analysis was performed using IBM SPSS 26.0 software (IBM, Inc., Chicago, IL, USA).
Results
Patients
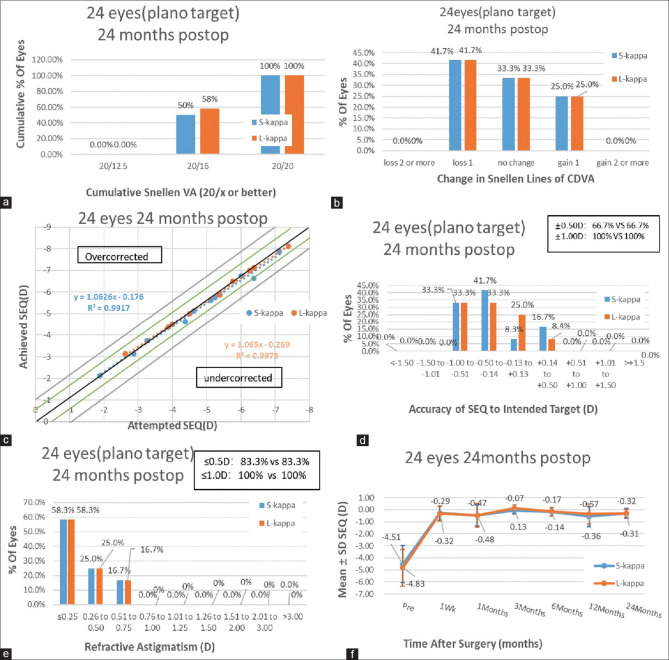
A total of 12 patients (24 eyes) were included in the analyses. The basic patient data are presented in Table 1. All surgical procedures were successful, and no postoperative complications affecting vision were observed throughout the follow-up period. At the postoperative 24-month visit, 100% of treated eyes in the S-kappa (kappa <0.3 mm) and L-kappa (kappa ≥0.3 mm) groups achieved a UDVA of 20/20 or better [Fig. 2a]. Similarly, 58.3% of the S-kappa group and 58.3% of the L-kappa group exhibited unchanged or better corrected distance visual acuity (CDVA) [Fig. 2b]. A scatter plot of the attempted versus the achieved spherical equivalent correction is presented in Fig. 2c. After surgery, the spherical equivalent in 66.7% of handled eyes in the S-kappa group and 66.7% of handled eyes in the L-kappa group were within ± 0.50 D. They were 100% and 100%, respectively, within ± 1.0 D [Fig. 2d]. Regarding astigmatism correction, 83.3% of treated eyes in the S-kappa group and 83.3% of treated eyes in the L-kappa group had postoperative astigmatism within ± 0.50 D cylinder, and both groups had postoperative astigmatism within ± 1.0 D [Fig. 2e]. The change in the manifest spherical equivalent is shown in Fig. 2f.
Table 1.
Basic information of S-kappa groups and L-kappa group of patients
| Characteristic | S-kappa | L-kappa | P |
|---|---|---|---|
| Angle of kappa | 0.19±0.07 (0.05-0.27) | 0.34±0.27 (0.30-0.38) | <0.001 |
| Sex (% women) | 58% | 58% | - |
| Eyes (n) | 12 | 12 | - |
| Age (years) | 28.9±6.5 (18-39) | 28.9±6.5 (18-39) | |
| Refractive errors (D) | |||
| Spherical | −4.29±1.53 (−7.0-−1.75) | −4.56±1.50 (−6.0-−1.75) | 0.667 |
| Cylindrical | −0.44±0.28 (−0.75- 0) | −0.54±0.50 (−1.75-0) | 0.536 |
| MRSE | −4.51±1.55 (−7.12-−2.88) | −4.83±1.50 (−7.38-−2.63) | 0.609 |
MRSE=Manifest refraction spherical equivalent. Values are presented as the mean±standard deviation (range)
Figure 2.
Visual outcomes with angle kappa adjustment during SMILE:(a)Uncorrected distance visual acuity (UDVA) outcomes;(b)change in corrected distance visual acuity (CDVA);(c)distribution of achieved spherical equivalent outcomes;(d)spherical equivalent refractive accuracy;(e)refractive astigmatism, and (f) stability of spherical equivalent refraction at 24 months postoperatively; D = diopters
Wavefront aberrations
At 24 months postoperatively, induced changes in total HOA, vertical coma, horizontal coma, spherical aberration, trefoil, and secondary astigmatism were not significantly different between the S-kappa and L-kappa groups (P > 0.05) [Table 2]. At the same time, there were no significant differences in postoperative eccentricity between the two groups (P > 0.05) [Table 2].
Table 2.
Aberrations and eccentricity of the cornea postoperatively
| Characteristic | S-kappa | L-kappa | P |
|---|---|---|---|
| HOAs | 0.113±0.05 | 0.125±0.09 | 0.701 |
| Eccentricity | 0.584±0.23 | 0.625±0.15 | 0.611 |
| C6 vertical trefoil (Z−33) | −0.030±0.05 | −0.026±0.04 | 0.796 |
| C7 vertical coma (Z−13) | −0.035±0.07 | −0.044±0.08 | 0.757 |
| C8 horizontal coma (Z+13) | 0.025±0.07 | 0.014±0.10 | 0.759 |
| C9 horizontal trefoil (Z+33) | −0.000±0.05 | −0.001±0.04 | 0.986 |
| C11 secondary astigmatism (Z−24) | 0.002±0.01 | −0.003±0.01 | 0.799 |
| C12 spherical aberration (Z04) | 0.001±0.02 | −0.009±0.03 | 0.336 |
| C13 secondary astigmatism (Z+24) | −0.010±0.02 | −0.001±0.02 | 0.308 |
HOAs=Higher-order aberrations
Optical quality and intraocular scattering measurement
As shown in Table 3, no significant differences were found in MTFcutoff, SR, OV100%, OV20%, and OV9% between the two groups after 24 months of SMILE.
Table 3.
Visual quality of S-kappa groups and L-kappa group of patients two year after SMILE
| Characteristic | S-Kappa | L-Kappa | P |
|---|---|---|---|
| OSI | 0.73±0.32 | 0.81±0.47 | 0.569 |
| MTFcutoff | 39.41±4.77 | 37.95±7.06 | 0.557 |
| SR | 0.22±0.04 | 0.21±0.04 | 0.910 |
| OV100% | 1.32±0.16 | 1.28±0.23 | 0.686 |
| OV20% | 0.91±0.18 | 0.92±0.21 | 0.834 |
| OV9% | 0.54±0.14 | 0.55±0.12 | 0.878 |
MTFcutoff=Modulation transfer function cutoff frequency, OSI=Objective scatter index, OV=Optical quality analysis system values, SR=Strehl2D ratio in two dimensions
QOV questionnaire
None of the 24 eyes experienced distortion or depth perception. The most frequent visual symptoms were blurred vision (50%), glare (50%), halo (40.0%), and focusing difficulties (30.0%), and the incidence of other symptoms was less than 30% [Fig. 3]. In all 12 patients with symptoms, severity was assessed as “not at all”or “mild”, and bothersome was assessed as “not at all”.
Figure 3.
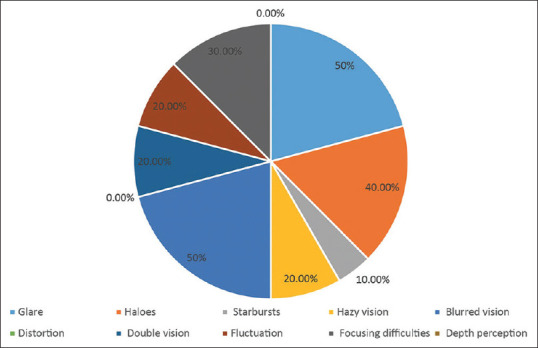
Subjective visual quality of patients 2 years after surgery
Discussion
Previous studies reported that SMILE induced significant amounts of corneal HOAs.[18,19] Decentered photoablation may lead to overcorrection or undercorrection, induction of HOAs, especially coma,[20] reduced visual acuity (both corrected and uncorrected), induced astigmatism, and reduced contrast sensitivity and night vision disturbances (such as glare).[21] When laser ablation is performed using existing pupil-tracking systems, any eye with a large angle of kappa, regardless of its refractive status, is more susceptible to eccentric treatment and may face symptomatic eccentricity, unless compensation of angle kappa is considered intraoperatively.[4] Also, because the Pupil center is dynamic, especially when light conditions change during surgery[22]; The results of Okamoto et al.[23] also suggested that using the pupillary center as the ablation center does not show the best safety, effectiveness, and contrast sensitivity, although some studies have shown that when the pupil is used as the ablation center, satisfactory visual results are obtained.[24,25] One explanation is that most patients have small kappa angles, thus the average outcome at the center of the incident pupil does not differ much from that of the visual axis center. Wong et al.[10] confirmed that using the pupil as the ablation center even affected the visual outcomes when the kappa angle was greater than 0.6 mm. Park et al.[4] also found that the larger the kappa angle, the greater the eccentricity in patients who underwent SMILE with the pupil center as the cutting center. When the kappa angle is large, even a small amount of eccentric ablation can cause glare, which affects visual quality.[26] In our study, we adjusted the relative position of the corneal surface from the contact ring based on the magnitude and direction of the angle kappa before applying suction, just as Shao et al.[9] did in their study, thereby moving the treatment center closer to the visual axis, which helped in a reduction in the magnitude of induced coma.[9]
It has been confirmed that the large angle kappa needs to be adjusted to obtain better visual quality. In the case of large angle kappa, eccentric ablation is more likely due to the increased distance between the pupil center and the visual axis. Miao et al.[19] reported that coma is a well-known cause of HOAs and has been shown to be associated with treatment eccentricity.[14] Eccentric ablation can cause many visual symptoms, including glare, decreased visual acuity, and diplopia. In fact, the small angle kappa in SMILE surgery may not need to be adjusted. When the kappa angle is small, the cutting center centered on the pupil center or the visual axis is not enough to cause significant off-center cutting.[9,11] Therefore, we assumed that the visual quality and HOA data of patients in the S-kappa group after SMILE without eccentric ablation. Our data showed that there was no significant difference between the two groups in terms of coma, spherical, or total corneal HOAs and also confirmed that there was no eccentric ablation in the L-kappa group. Therefore, it is reasonable to believe that our compensation for the large kappa angle in SMILE is beneficial.
In addition to aberration, the effects of diffraction and scattering should also be taken into account when objectively evaluating the factors affecting the imaging quality of human eyes, which cannot be achieved by ordinary wavefront aberration inspection equipment. The double-pass system objective visual therapy and analysis system OQAS II is based on double-pass technology. By recording the image results formed by the reflection of a point light source projected onto the retina, the visual quality, including HOAs and scattering, can be analyzed.[27,28] Because HOAs and intraocular scattering are two independent factors, both can affect the quality of retinal imaging. It is necessary to consider the effect of intraocular scattering to comprehensively evaluate visual quality after refractive surgery.[29] MTF refers to the ratio of the contrast between the imaging and the actual object on the retina at different spatial frequencies. The MTFcutoff of OQAS II is the cutoff frequency (cpd) at 1% of the maximum MTF.[30] The SR is the ratio of the central luminance of the point image of the measured eye to that of the ideal eye without refractive medium problems. The ideal value of 1 represents a perfect system that is only affected by diffraction.[31] The OSI is the ratio of light energy in the periphery to the center of a double-pass image. The OQAS takes the ratio of light energy at 12–20 arcminutes to that at the center 1 arcminute to represent OSI.[32] OV100%, OV20%, and OV9% represent the OQAS values of 100%, 20%, and 9% contrast, respectively, simulating contrast visual acuity values under different illuminations corresponding to day, dusk, and night, respectively.[32] In general, the higher the MTFcutoff, SR, and OV values, the lower the OSI and the better the visual quality. On analyzing the above indices, there was no significant difference between the S-kappa and L-kappa groups, and compared to other studies,[29] the examination data were also satisfactory.
Visual quality is a subjective entity based on an individual’s unique perception of his vision. This perception is multifactorial, including not only visual factors but also psychological factors. Although optical vision is easy to measure, none of these measurements can explain how the patient subjectively perceives his or her vision complexity.[16] Since this was a retrospective study, only postoperative data were obtained. In terms of visual symptoms, blurred vision and glare had the highest incidence, which is consistent with previous studies because coma was most commonly caused after SMILE.[20,21] The uncorrected visual acuity of all patients after surgery was not less than 20/20, as for 50% of patients’ consciously blurred vision, we suspect that it may be related to dry eye or the deviation caused by the small sample size. Further large sample studies are needed to confirm this. The objective and subjective visual quality data we collected after SMILE showed that the postoperative visual quality of these 12 patients was acceptable; in other words, the method of adjusting the treatment center during SMILE for patients with a large kappa angle can, indeed, reduce eccentric ablation.
Conclusion
In summary, this study showed that the adjustment of angle kappa and making the treatment center near the visual axis during the SMILE procedure will not lead to eccentric ablation in the eyes according to the direction and size of angle kappa. We believe that the current method will help optimize the ablation center and contribute to better visual quality, although in patients with angle kappa >0.3 mm only.
Authors’ contributions
Jing T and Yingping Deng conceived and designed the study. Mengzhen Xie, Chengshu Sun, and Leimei Qiu participated in information gathering and editing and they analyzed and interpreted all the data. Mengzhen Xie wrote the manuscript. Jing Tang reviewed and edited the manuscript. All authors read and approved the final manuscript.
Financial support and sponsorship
This work was supported by the Science and Technology Department of Sichuan Province (China) funding project (No. 2021YFS0221) and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang Y, Shen Q, Jia Y, Zhou D, Zhou J. Clinical outcomes of SMILE and FS-LASIK used to treat Myopia:A meta-analysis. J Refract Surg. 2016;32:256–65. doi: 10.3928/1081597X-20151111-06. [DOI] [PubMed] [Google Scholar]
- 2.Ang M, Farook M, Htoon HM, Mehta JS. Randomized clinical trial comparing femtosecond LASIK and Small-Incision lenticule extraction. Ophthalmology. 2020;127:724–30. doi: 10.1016/j.ophtha.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Reinstein DZ, Archer TJ, Vida RS, Carp GI, Reinstein JFR, McChesney T, et al. Small incision lenticule extraction (SMILE) for the correction of high myopia with Astigmatism. J Refract Surg. 2022;38:262–71. doi: 10.3928/1081597X-20220314-01. [DOI] [PubMed] [Google Scholar]
- 4.Park CY, Oh SY, Chuck RS. Measurement of angle kappa and centration in refractive surgery. Curr Opin Ophthalmol. 2012;23:269–75. doi: 10.1097/ICU.0b013e3283543c41. [DOI] [PubMed] [Google Scholar]
- 5.Lazaridis A, Droutsas K, Sekundo W. Topographic analysis of the centration of the treatment zone after SMILE for myopia and comparison to FS-LASIK:Subjective versus objective alignment. J Refract Surg. 2014;30:680–6. doi: 10.3928/1081597X-20140903-04. [DOI] [PubMed] [Google Scholar]
- 6.Chang DH, Waring GO. The subject-fixated coaxially sighted corneal light reflex:A clinical marker for centration of refractive treatments and devices. Am J Ophthalmol. 2014;158:863–74. doi: 10.1016/j.ajo.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Prakash G, Agarwal A, Prakash DR, Kumar DA, Agarwal A, Jacob S. Role of angle kappa in patient dissatisfaction with refractive-design multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:1739–40. doi: 10.1016/j.jcrs.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Gyldenkerne A, Ivarsen A, Hjortdal J. Optical and visual quality after small-incision lenticule extraction. J Cataract Refract Surg. 2019;45:54–61. doi: 10.1016/j.jcrs.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 9.Shao T, Wang Y, Ng ALK, Chan TCY, Hao W, Zhang J, et al. The effect of intraoperative angle kappa adjustment on higher-order aberrations before and after small incision lenticule extraction. Cornea. 2020;39:609–14. doi: 10.1097/ICO.0000000000002274. [DOI] [PubMed] [Google Scholar]
- 10.Wong JX, Wong EP, Htoon HM, Mehta JS. Intraoperative centration during small incision lenticule extraction (SMILE) Medicine (Baltimore) 2017;96:e6076. doi: 10.1097/MD.0000000000006076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arba Mosquera S, Verma S, McAlinden C. Centration axis in refractive surgery. Eye Vis (Lond) 2015;2:4. doi: 10.1186/s40662-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y, Wang Y. Optical quality comparison between laser ablated myopic eyes with centration on coaxially sighted corneal light reflex and on entrance pupil center. J Opt Soc Am A Opt Image Sci Vis. 2019;36:B103–9. doi: 10.1364/JOSAA.36.00B103. [DOI] [PubMed] [Google Scholar]
- 13.Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism:Results of a 6 month prospective study. Br J Ophthalmol. 2011;95:335–9. doi: 10.1136/bjo.2009.174284. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Zhao J, Miao H, Shen Y, Sun L, Tian M, et al. Mild decentration measured by a Scheimpflug camera and its impact on visual quality following SMILE in the early learning curve. Invest Ophthalmol Vis Sci. 2014;55:3886–92. doi: 10.1167/iovs.13-13714. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Roberts CJ, Arba-Mosquera S, Kang DSY, Reinstein DZ, Kim T-I. Relationship between decentration and induced corneal higher-order aberrations following small-incision lenticule extraction procedure. Invest Ophthalmol Vis Sci. 2018;59:2316–24. doi: 10.1167/iovs.17-23451. [DOI] [PubMed] [Google Scholar]
- 16.McAlinden C, Pesudovs K, Moore JE. The development of an instrument to measure quality of vision:The Quality of Vision (QoV) questionnaire. Invest Ophthalmol Vis Sci. 2010;51:5537–45. doi: 10.1167/iovs.10-5341. [DOI] [PubMed] [Google Scholar]
- 17.McAlinden C, Skiadaresi E, Gatinel D, Cabot F, Huang J, Pesudovs K. The quality of vision questionnaire:Subscale interchangeability. Optom Vis Sci. 2013;90:760–4. doi: 10.1097/OPX.0b013e3182993856. [DOI] [PubMed] [Google Scholar]
- 18.Siedlecki J, Schmelter V, Schworm B, Mayer WJ, Priglinger SG, Dirisamer M, et al. Corneal wavefront aberrations and subjective quality of vision after small incision lenticule extraction. Acta Ophthalmol. 2020;98:e907–13. doi: 10.1111/aos.14420. [DOI] [PubMed] [Google Scholar]
- 19.Miao H, Tian M, Xu Y, Chen Y, Zhou X. Visual outcomes and optical quality after femtosecond laser small incision lenticule extraction:An 18-month prospective study. J Refract Surg. 2015;31:726–31. doi: 10.3928/1081597X-20151021-01. [DOI] [PubMed] [Google Scholar]
- 20.Mrochen M, Kaemmerer M, Mierdel P, Seiler T. Increased higher-order optical aberrations after laser refractive surgery:A problem of subclinical decentration. J Cataract Refract Surg. 2001;27:362–9. doi: 10.1016/s0886-3350(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 21.Arba Mosquera S, Verma S. Numerical nonwavefront-guided algorithm for expansion or recentration of the optical zone. J Biomed Opt. 2014;19:088001. doi: 10.1117/1.JBO.19.8.088001. doi:10.1117/1. JBO.19.8.088001. [DOI] [PubMed] [Google Scholar]
- 22.Marcos S, Barbero S, Llorente L, Merayo-Lloves J. Optical response to LASIK surgery for myopia from total and corneal aberration measurements. Invest Ophthalmol Vis Sci. 2001;42:3349–56. [PubMed] [Google Scholar]
- 23.Okamoto S, Kimura K, Funakura M, Ikeda N, Hiramatsu H, Bains HS. Comparison of wavefront-guided aspheric laser in situ keratomileusis for myopia:Coaxially sighted corneal-light-reflex versus line-of-sight centration. J Cataract Refract Surg. 2011;37:1951–60. doi: 10.1016/j.jcrs.2011.05.040. [DOI] [PubMed] [Google Scholar]
- 24.Rabina G, Mimouni M, Slomovic J, Sorkin N, Nemet A, Kaiserman I. Centration of myopic refractive ablation:Should we center treatment on the pupil or the visual axis? Lasers Med Sci. 2021;36:1733–9. doi: 10.1007/s10103-021-03358-2. [DOI] [PubMed] [Google Scholar]
- 25.Kermani O, Oberheide U, Schmiedt K, Gerten G, Bains HS. Outcomes of hyperopic LASIK with the NIDEK NAVEX platform centered on the visual axis or line of sight. J Refract Surg. 2009;25:S98–103. doi: 10.3928/1081597X-20090115-04. [DOI] [PubMed] [Google Scholar]
- 26.Chan CCK, Boxer Wachler BS. Centration analysis of ablation over the coaxial corneal light reflex for hyperopic LASIK. J Refract Surg. 2006;22:467–71. doi: 10.3928/1081-597X-20060501-08. [DOI] [PubMed] [Google Scholar]
- 27.Díaz-Doutón F, Benito A, Pujol J, Arjona M, Güell JL, Artal P. Comparison of the retinal image quality with a Hartmann-Shack wavefront sensor and a double-pass instrument. Invest Ophthalmol Vis Sci. 2006;47:1710–6. doi: 10.1167/iovs.05-1049. [DOI] [PubMed] [Google Scholar]
- 28.van Bree MCJ, van Verre HP, Devreese MT, Larminier F, van den Berg TJTP. Straylight values after refractive surgery:Screening for ocular fitness in demanding professions. Ophthalmology. 2011;118:945–53. doi: 10.1016/j.ophtha.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Miao H, He L, Shen Y, Li M, Yu Y, Zhou X. Optical quality and intraocular scattering after femtosecond laser small incision lenticule extraction. J Refract Surg. 2014;30:296–302. doi: 10.3928/1081597X-20140415-02. [DOI] [PubMed] [Google Scholar]
- 30.Saad A, Saab M, Gatinel D. Repeatability of measurements with a double-pass system. J Cataract Refract Surg. 2010;36:28–33. doi: 10.1016/j.jcrs.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Vilaseca M, Arjona M, Pujol J, Issolio L, Güell JL. Optical quality of foldable monofocal intraocular lenses before and after injection:Comparative evaluation using a double-pass system. J Cataract Refract Surg. 2009;35:1415–23. doi: 10.1016/j.jcrs.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 32.Artal P, Benito A, Pérez GM, Alcón E, De Casas A, Pujol J, et al. An objective scatter index based on double-pass retinal images of a point source to classify cataracts. PLoS On. 6:e16823. doi: 10.1371/journal.pone.0016823. [DOI] [PMC free article] [PubMed] [Google Scholar]