Abstract
Severe blunt ocular trauma may result in immediate and delayed complications requiring appropriate management algorithms. We hereby report a case of globe rupture, aphakia, traumatic aniridia, and secondary glaucoma in a 33-year-old male following road traffic accident. He was treated initially by primary repair followed by novel combined approach of aniridia IOL with Ahmed glaucoma valve implantation. Delayed corneal decompensation required deferred penetrating keratoplasty. After a follow-up of 3.5 years after last surgery, patient maintains good functional vision with stable IOL, clear corneal graft and controlled intraocular pressure. A meticulously planned and staged management approach appears better suited in complex ocular trauma in such scenarios giving a good structural and functional outcome.
Keywords: Complex trauma, corneal decompensation, traumatic aniridia - aphakia, traumatic glaucoma
Blunt ocular trauma resulting in open globe injury can lead to a variety of complications, some of which present later in course of disease. Loss of crystalline lens and partial aniridia further add to post-operative discomfort and visual loss. Secondary glaucoma can present with time. A timely management perspective is essential for post-operative visual recovery. It also includes a stepwise management regimen due to multiple factors involved. We report a case of managing post-traumatic corneal rupture with lens extrusion, traumatic aniridia, and secondary glaucoma with late corneal decompensation.
Surgical Technique
A 33-year-old male presented with trauma to the left eye in a road traffic accident by fall on the roadside when hit from behind by a vehicle. He was clinically stable with no other associated injuries. Visual acuity was 6/6 in right eye and perception of light, projection of rays accurate in left eye. There was no relative afferent pupillary defect. On presentation, he had a large triradiate limbus to limbus corneal tear with uveal tissue and vitreous prolapse with blood clots behind cornea in left eye. There was a probable loss of crystalline lens in its anatomical site. Corneal edema was diffused, and more superiorly no posterior details of fundus were seen. There was no clinical injury to bony adnexa. Right eye examination was within normal limits. X-ray orbits and CT scan (brain) were done to rule out associated injuries/intraocular foreign bodies (IOFBs) and were noted negative.
The patient underwent corneal tear repair by 10-0 interrupted nylon sutures, partial iris abscission, and limited anterior vitrectomy. The following day globe was formed, and corneal sutures were intact with an infero-temporal iridodialysis and near complete loss of iris tissue. Remnants of iris tissue were seen in 3 clock hours. B-scan ultrasound showed few vitreous opacities suggestive of vitreous hemorrhage but did not show presence crystalline lens in posterior segment which was thought to be spontaneously extruded. Retina was attached.
Two weeks later, the left eye had best corrected vision of 1/60 with well-formed globe, reducing corneal edema and stable retina on ultrasound, and intraocular pressure (IOP) was 17 mm Hg. After 1 month, IOP was noted as 27 mm Hg and patient was started on timolol eyedrops twice daily.
Three months later, left eye improved to 6/18 with correction. Slit-lamp examination showed relatively preserved corneal clarity except at suture line, near total iris absence, and formed anterior chamber. IOP was 18 mm Hg on timolol. Right eye was stable with vision 6/6. Fundus was normal both eyes.
Six months later, IOP control required three medications. At 9 months, IOP was not under control despite maximum topical therapy and oral acetazolamide, and patient was explained possible need for glaucoma surgery along with intraocular lens (IOL) implantation. He also complained of glare and photophobia.
A comprehensive risk assessment was done, and patient’s informed consent was taken to proceed for combined aniridia IOL with Ahmed glaucoma valve (AGV). Option of penetrating keratoplasty along with combined aniridia IOL and AGV was deferred at this stage due to high risk of graft failure.[1] The valve plate was placed supero-temporally by conjunctival dissection 3 mm from limbus, and valve plate was secured with two 8-0 nylon sutures. Due to paucity of surgical space, the large aniridia IOL was placed via a separate10 mm superior scleral section with back pockets after opening conjunctiva at limbus. We took care not to join the two conjunctival incisions. Limited anterior vitrectomy and removal of iris stump were done. Inferior partial thickness scleral section and back pocket were created to secure the inferior haptic, and similarly, the superior haptic was secured to the superior section and knot embedded in scleral pocket using 9-0 prolene. After securing the aniridia lens, the superior conjunctiva was closed and the tube of AGV was placed in anterior chamber, anterior to the IOL, and tube was covered by corneal patch graft; conjunctival closure was done with 8-0 Vicryl sutures in two layers.
One month post-surgery, he improved to 6/24 P left eye with -5.0 D Cyl@160 deg. IOP was 15 mm Hg with timolol eyedrops in left eye.
He remained stable for close to 3 years with routine follow-ups. The cornea gradually showed decompensation, and vision dropped to 6/60 left eye. Option of penetrating keratoplasty (PK) was given, and the same was done with trimming of AGV tube through an open sky technique after corneal button was removed. A 8.5-mm donor graft was secured to 8-mm recipient cornea using sixteen 10-0 nylon interrupted sutures. One month following surgery, the vision improved to 6/18 in left eye with IOP of 16 mm Hg on timolol eyedrops [Fig. 1{a-f}].
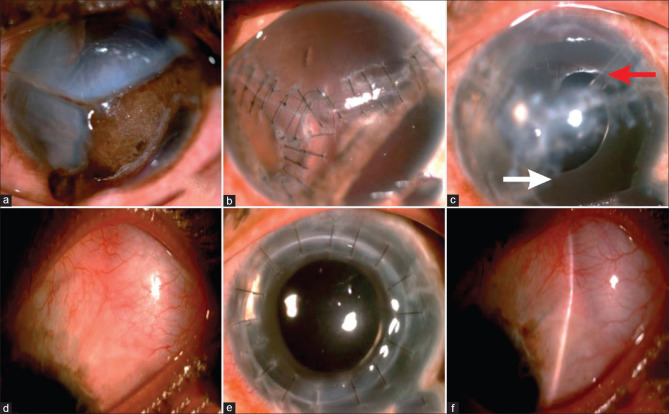
Figure 1.
(a-f) Pre- and Post-operative images (a): Left eye rupture with triradiate tear of cornea extending to limbus, uveal tissue prolapse. (b) Sealed globe with 10-0 nylon interrupted sutures, reduced corneal edema, iris remnants, and aphakia at 2 months post-primary repair. (c) Aniridia SFIOL with Ahmed glaucoma valve (AGV tube marked with red arrow, aniridia IOL with white arrow). (d) Diffuse bleb seen supero-temporally. (e) Post-penetrating keratoplasty, with a clear graft. (f) Well-functioning diffused bleb with controlled intraocular pressure
Two years after PK, on his last visit, the patient was stable with best corrected vision of 6/6 right eye with -1.0 DCyl at 170 deg and 6/9 left eye with -0.50 DSph/-1.0 DCyl at 10 deg. Left eye was on timolol eyedrops twice daily. Left eye showed clear compact graft, tube in situ, and aniridia SFIOL in place. IOP was 16 mm hg in right and 14 mm Hg left eye. Fundus was normal with healthy optic disks for both eyes.
Discussion
Cases of post-traumatic aniridia and spontaneous extrusion of lens have been reported.[1] The primary aim of our initial surgery was to close the open globe via a good and meticulous closure. This was fundamental to further surgical planning. We did not plan any immediate further surgery for visual rehabilitation for couple of months for the complex traumatized eye to stabilize. However, there were two main concerns—a raised IOP despite medication and loss of crystalline lens nearly 10 months from trauma. The IOP control mandated the need for glaucoma surgery; we prefer the Ahmed Glaucoma Valve (AGV) in this scenario as conventional surgery was likely to fail. The other option at this point to control IOP could be cycloablative procedures; however, we preferred surgery in view of a good visual potential and planned further surgery for IOL. However, there was no ideal place to put the AGV tube due to single chamber eye, loss of iris and crystalline lens, and presence of vitreous. The patient was also concerned about functional and cosmetic issues due to near total aniridia which would need an aniridia IOL. So we planned a combined approach of aniridia IOL with AGV, placing the tube of valve anterior to the IOL [Fig. 2{a-f}]. We did comprehend the possibility of tube block by vitreous tags and so preferred a relatively longer tube over the aniridia IOL. The aniridia IOL used was single-piece PMMA (Model ANI5; Caregroup Sight Solution Private Limited, Gujarat, India) having an optic diameter of 5.95 mm and total length of 12.75 mm. It has a central clear zone and peripheral opaque zone with A constant of 118.2. Haptics are PMMA with acute angulations having eyelet for passing proline sutures.
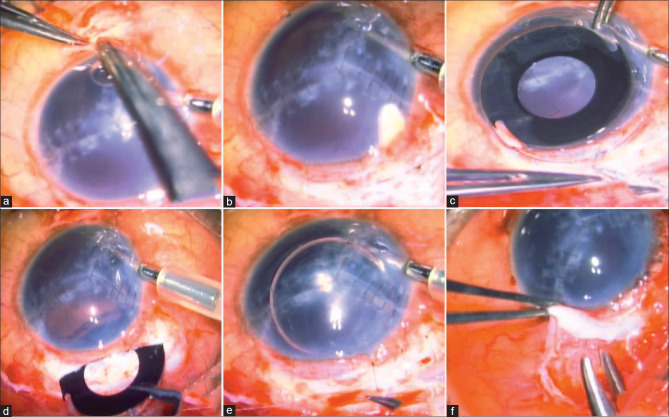
Figure 2.
(a-f) Surgical steps of combined aniridia IOL-Ahmed glaucoma valve surgery. Note the anterior chamber maintainer used during surgery (a) Inferior scleral flap. (b): Superior section for aniridia IOL. (c) Eyelets of IOL tied to 9-0 prolene. (d) Aniridia IOL insertion. (e) Superior haptic tied to scleral section made for IOL insertion. (f) AGV tube insertion via separate conjunctival dissection
Posterior chamber IOLs with opaque peripheral segments were developed by Morcher and have been described since 1994 by Sundmacher et al.[2] in patients with congenital aniridia.[3] Gradually, indications have increased to post-traumatic or surgical cases with partial or complete loss of iris tissue.[4] Aniridia tends to cause glare and light sensitivity along with decreased depth of field, reduced contrast, and cosmetic concerns.[1]
Chorągiewicz et al.[1] assessed long-term outcomes of black diaphragm IOL (BD IOL) in post-traumatic aniridia and aphakia due to eye rupture in 14 eyes. Six eyes (43%) developed glaucoma, of which three required Ahmed glaucoma valve implantation as a secondary procedure. Seven eyes each required anterior vitrectomy and pars plana vitrectomy with tamponade (for associated retinal detachment). Corneal decompensation occurred in six cases (43%). Simultaneous PK was required in two eyes which later developed graft opacities. PK as a secondary procedure was needed in two additional eyes. Mechanisms postulated for corneal decompensation include a heavier and larger lens and movements with postural changes and/or proximity of IOL edge with anterior chamber angle causing endothelial cell loss.[1] It is pertinent that in addition to glaucoma induced by trauma itself, a direct compression of haptics to trabecular meshwork may cause glaucoma after uncomplicated aniridia IOL implantations itself.[1,5] The large section needed to place the rigid aniridia IOL combined with haptic fixation sites and a separate site for the AGV compounded the surgical space availability issues. So we modified our surgical technique to an inferior scleral pocket, and the superior haptic was fixed to the scleral section itself used for the IOL placement. The valve was placed supero-temporally via a separate incision adjacent to limbus.
Since the residual cornea was compact and patient was comfortable with good vision, we deferred further corneal surgery till the time of further need. Nearly 3 years after the combined aniridia IOL-AGV surgery, patient required penetrating keratoplasty (PK) for corneal decompensation. We partially trimmed the AGV tube to avoid risk of decompensation to the donor cornea.
Conclusion
Complex ocular trauma management has to be planned meticulously on a case-to-case basis. Our case emphasizes the need for careful surgical planning and salient points of a novel surgical approach in select cases. To the best of our knowledge, no prior indexed published reports of combined aniridia IOL-AGV in a case of complex trauma requiring corneal transplant are available.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chorągiewicz T, Nowomiejska K, Haszcz D, Nowakowska D, Avitabile T, Reibaldi M, et al. Transscleral fixation of black diaphragm intraocular lens in complete aniridia and aphakia due to posttraumatic eye rupture:A pilot study. J Clin Med. 2019;8:46. doi: 10.3390/jcm8010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundmacher T, Reinhard T, Althaus C. Black diaphragm intraocular lens in congenital aniridia. Ger J Ophthalmol. 1994;3:197–201. [PubMed] [Google Scholar]
- 3.Sundmacher R, Reinhard T, Althaus C. Black-diaphragm intraocular lens for correction of aniridia. Ophthalmic Surg. 1994;25:180–5. [PubMed] [Google Scholar]
- 4.Wang H, Jung J, Lin SR, Olson MD, Miller KM. Safety and efficacy of colored iris reconstruction lens implantation. Am J Ophthalmol. 2020;216:174–85. doi: 10.1016/j.ajo.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Aslam SA, Wong SC, Ficker LA, MacLaren RE. Implantation of the black diaphragm intraocular lens in congenital and traumatic aniridia. Ophthalmology. 2008;115:1705–12. doi: 10.1016/j.ophtha.2008.03.025. [DOI] [PubMed] [Google Scholar]