Abstract
Purpose:
The purpose of this study was to identify and analyze the clinical and ocular surface risk factors influencing the progression of keratoconus (KC) using an artificial intelligence (AI) model.
Methods:
This was a prospective analysis in which 450 KC patients were included. We used the random forest (RF) classifier model from our previous study (which evaluated longitudinal changes in tomographic parameters to predict “progression” and “no progression”) to classify these patients. Clinical and ocular surface risk factors were determined through a questionnaire, which included presence of eye rubbing, duration of indoor activity, usage of lubricants and immunomodulator topical medications, duration of computer use, hormonal disturbances, use of hand sanitizers, immunoglobulin E (IgE), and vitamins D and B12 from blood investigations. An AI model was then built to assess whether these risk factors were linked to the future progression versus no progression of KC. The area under the curve (AUC) and other metrics were evaluated.
Results:
The tomographic AI model classified 322 eyes as progression and 128 eyes as no progression. Also, 76% of the cases that were classified as progression (from tomographic changes) were correctly predicted as progression and 67% of cases that were classified as no progression were predicted as no progression based on clinical risk factors at the first visit. IgE had the highest information gain, followed by presence of systemic allergies, vitamin D, and eye rubbing. The clinical risk factors AI model achieved an AUC of 0.812.
Conclusion:
This study demonstrated the importance of using AI for risk stratification and profiling of patients based on clinical risk factors, which could impact the progression in KC eyes and help manage them better.
Keywords: Artificial intelligence, demographics, keratoconus, progression, tomography
Keratoconus (KC) is an inflammatory disorder causing asymmetrical and progressive corneal ectasia that leads to visual impairment.[1] Tools to diagnose KC and evaluate its progression are predominantly imaging, such as corneal tomography and biomechanics.[2] Factors which can affect progression, such as eye rubbing, allergies, role of lubricants, and hormonal disturbances, are seldom studied. India has a much higher prevalence of KC,[3-5] with the prevalence of KC in the general population in excess of 2%. Therefore, early diagnosis of KC by combining advanced tomography and high-risk factors is also important.[3] Interestingly, no studies on assessment of KC progression linking presence of high-risk factors and demographics with artificial intelligence (AI) models have been done. It is particularly important to identify the risk of progression as early as possible as it has a bearing on the management of these cases, including planning and outcomes of crosslinking.[6]
Considering the disease burden in India, an adjunct approach of evaluating factors which put a patient at higher risk of progression is vital to effectively prioritize patients who may require corneal collagen cross-linking (CXL) earlier than others. The coronavirus disease 2019 (COVID-19) pandemic has necessitated changes in lifestyle, including mask usage, prolonged indoor stay, and usage of hand sanitizers.[7-9] Mask usage and prolonged indoor stay can alter the ocular surface, which, in turn, could lead to eye rubbing and progression of KC.[7-9] These factors may also significantly alter the chance of progression of KC. While individual parameters and cutoffs for some are known, a single multivariate model to assess progression of KC using all these parameters simultaneously is lacking. Therefore, the objective of this study was to develop an AI model to assess whether various clinical risk factors can distinguish between “progression” and “no progression.” Information pertaining to high-risk factors from patients was collected via a questionnaire.[10] The initial classification of progression and no progression was built using random forest (RF) classifier model from our previous study (that evaluated longitudinal changes in tomographic parameters) to determine progression and no progression in KC eyes using tomography only.[11]
Methods
This was a prospective analysis of 450 eyes of 450 KC patients who visited the outpatient department of a tertiary care eye hospital in Bengaluru, India, between July 2020 and June 2021. The study was approved by the institutional ethics committee. The study followed the tenets of the Declaration of Helsinki.
Inclusion and exclusion criteria
All the eyes were diagnosed cases of KC. One of the eyes of each patient was selected by randomization. The change in maximum curvature of the anterior surface (Kmax) between two visits (at least 6 months apart) was observed. Exclusion criteria were other ectatic conditions such as keratoglobus, pellucid marginal degeneration, post-refractive surgery ectasia, autoimmune disorders, any ocular or corneal surgery before the first visit of the patient or during the course of disease follow-up, patients on topical drops other than antiallergy and lubricants, patients with corneal scarring, and patients using contact lenses.
Data collection
A comprehensive ocular examination and corneal tomography was done on Pentacam HR (OCULUS Optikgerate GmbH, Wetzlar, Germany). Only those Pentacam scans were used which did not have any blinking or motion artifacts. These scans are automatically classified as “OK” by the Pentacam software. Further, the detected anterior and posterior edges of the corneal scans were manually confirmed, so that no missing portions of the detected edges confounded the tomography of the cornea.[12] A detailed questionnaire was given to assess the various clinical and ocular surface risk factors pertaining to KC evaluation. It also included basic demographic data of age, gender, occupation, educational status. The questionnaire parameters and their respective responses which were specifically included in the AI model are as follows:
Eye rubbing – presence or absence
Systemic allergy/asthma/eczema – presence or absence
Serum immunoglobulin E (IgE) in IU/ml, vitamin D in ng/ml, and vitamin B12 levels in pg/ml – obtained from blood reports and their respective values were noted
Usage of immunomodulators/lubricants – present or absent
Usage of mask >4 h/day – present or absent
Usage of computers >6 h/day – present or absent
Use of micronutrients – present or absent
Hormonal disturbances/polycystic ovarian disease (PCOD) – present or absent
Hours spent indoors >10 hours/day – present or absent
Usage of hand sanitizers more than four times per day – present or absent
All the above parameters were hypothesized to play a role in the progression of KC. The clinical risk factors obtained at the first visit were evaluated in an AI model using the initial classification of progression and no progression using tomography alone.[11]
AI design approach
Two AI models were constructed [Fig. 1], which are discussed below.
Figure 1.
Flow chart showing the two AI models used – tomographic model and clinical risk factors model. AI = artificial intelligence
a.Initial classification based on tomographic changes AI
For each eye, the tomographic parameters from two visits were extracted from Pentacam and differences between the two visits were used in the first AI model. A total of 37 tomographic parameters were exported from Pentacam. We used the RF classifier model from our previous study (that evaluated longitudinal changes in tomographic parameters to predict progression and no progression)[11] to classify the new dataset collected during the study period. We chose the model of Kmax change equal to 1 D.[11]
-
b.Initial classification used as target classification in a new AI based on clinical risk factors presented at the first visit of the patient
The subjective responses from the questionnaire were used as categorical variables, and quantitative values for serum IgE, vitamin D, and vitamin B12 from pathological reports were used as inputs in this model. The initial classification (progression or no progression) from the tomographic changes AI were used as target classification for the clinical risk factors AI. This allowed us to assess whether the clinical risk factors at the first visit were able to predict the progression of a given KC eye with equal efficacy as determined from changes in various tomography parameters.
Statistical analyses
Among the clinical risk factors, the mean and standard deviation were calculated for quantitative variables like IgE, vitamin D, and vitamin B12, after assessing for normality of distribution with the Kolmogorov–Smirnov test. If normality was not met, then the median and its 95% confidence interval (CI) were calculated. The categorical variables from questionnaire responses were represented using frequency (contingency) tables. The performance of the AI model was evaluated using several parameters including area under the curve (AUC), classification accuracy (CA), precision (Pr), recall (Rec), and F1-score. The Orange3 version 3.25.0 data mining package (University of Ljubljana, Slovenia) was used for building the AI models, and MedCalc v19 (MedCalc Inc., Belgium) was used for further statistical analyses.
Results
A total of 450 eyes of 450 patients, which included 239 males and 211 females with an average age of 26 ± 3.6 years, were analyzed. After assessing the quality of the scans, a total of 900 Pentacam scans of 450 eyes were included in the study. For the first AI model, differences between two visits for each eye were calculated. Three hundred and twenty-two eyes were initially classified as progression (target Kmax change >1 D) and the remaining as no progression. Table 1-A shows the confusion matrix from testing these tomographic changes on the RF model from our previous work.[11] The RF model successfully classified 95.5% of the actual (Kmax change <1 D) no progression cases as no progression with an AUC of 0.92. Similarly, about 75% of the actual progression cases were classified as progression by the AI. Interestingly, nearly 25% of the actual progression cases were classified as no progression. This indicated that there was some disagreement between local increase in Kmax and corresponding increase in other tomographic parameters in these eyes.[11] Table 1 also shows the mean ± standard deviation of RF probability scores (ranging from 0 to 1) for each of the predicted groups.
Table 1.
Confusion matrices showing the proportion of actual states reclassified by the AI models and represented by the predicted states
| A.Testing tomographic changes AI | ||
|---|---|---|
|
| ||
| Model A | Predicted | |
|
| ||
| Progression | No progression | |
| Actual | ||
| Progression | 75% (0.73±0.13) | 25% (0.34±0.15) |
| No progression | 4.5% (0.98±0) | 95.5% (0.13±0.13) |
|
| ||
| B. Clinical risk factors AI | ||
|
| ||
| Model B | Predicted | |
|
| ||
| Progression | No progression | |
|
| ||
| Predictions from Model A | ||
| Progression | 76.4% (0.77±0.15) | 23.6% (0.32±0.12) |
| No progression | 32.9% (0.64±0.12) | 67.1% (0.3±0.12) |
AI=artificial intelligence, RF=random forest. Model-A: testing changes in tomographic parameters on previously reported RF model.[11] Model-B: predicted results from Model-A used to evaluate clinical risk factors. The mean±standard deviation of the respective RF classifier probability scores is mentioned in brackets
Table 1-B shows the final confusion matrix from clinical risk factors AI. About 76% of the cases that were classified as progression (from tomographic changes) were correctly predicted as progression based on clinical risk factors at the first visit. Similarly, about 67% of cases that were classified as no progression (based on tomographic changes) were predicted as no progression based on clinical risk factors at the first visit. This indicated that tomographic changes determining progression and no progression[11] were in agreement with clinical risk factors. However, 33% of cases that were classified as no progression (based on tomographic changes) were classified as progression based on clinical risk factors at the first visit. This indicated some discordance between changes in tomographic parameters determining progression and clinical risk factors (increased eye rubbing, presence of systemic allergies, IgE levels, vitamins D and B12 levels).
Table 2A shows the various clinical factors ranked in decreasing order of importance. IgE had the highest rank, followed by presence of eye rubbing, vitamin D levels, and systemic allergy. Other factors from the questionnaire, like hours spent indoors, presence of hormonal disturbances/PCOD, hand sanitizers, hours of usage of computer, and intake of micronutrients, were not the top ranked parameters. This indicates that these factors had little to no intrinsic information to determine the risk of progression. We also evaluated the performance of the clinical risk factors AI [Table 2B]. Fig. 2 shows the plot of receiver operating characteristic curve of the RF model predictions. The AUC was 0.812 (P < 0.001) with a sensitivity of ~73% for classifying progression and a specificity of ~86% toward no progression. Further, we performed post hoc analyses of the four predicted groups from the final confusion matrix [Table 1-B]. The mean ± standard deviation of clinical risk factors is summarized in Table 3. IgE levels were reasonably the lowest in group-4 (no progression according to both tomographic changes and clinical risk factors AI). Similarly, group-1 (progression according to both tomographic changes and clinical risk factors AI) showed the highest IgE levels. IgE also had the highest rank in discriminating progression and no progression. However, group-3 (no progression based on tomographic changes, but progression based on changes in clinical risk factors) also had higher IgE levels. This may have contributed to ~33% of misclassifications [Table 1-B]. In summary, a higher number of such misclassified cases (no progression on tomographic changes classified as progression based on clinical changes) showed the following interesting trends [Table 3, third column]:
Table 2A.
Top seven clinical risk factors ranked in decreasing order of importance
| Feature importance | Type |
|---|---|
| IgE | Continuous |
| Eye rubbing | Binary |
| VIT-D | Continuous |
| Systemic allergy | Binary |
| VIT-B12 | Continuous |
| Usage/nonusage of topical immunomodulators | Binary |
| Mask usage >4 h/day | Binary |
IgE=Immunoglobulin E (IU/ml), VIT-B12=Vitamin B12 (pg/ml), VIT-D=vitamin D (ng/ml)
Table 2B.
AUC, sensitivity, specificity, accuracy, F1-score, precision, and recall of the clinical risk factors AI
| Parameter | Value | 95% confidence Interval |
|---|---|---|
| AUC | 0.812 | 0.711-0.897 |
| Sensitivity (%) | 72.92 | 64.1-82.9 |
| Specificity (%) | 85.62 | 76.3-95 |
| Classification accuracy | 0.823 | |
| Precision | 0.831 | |
| Recall | 0.865 | |
| F1-score | 0.847 |
AI=Artificial intelligence, AUC=Area under the curve
Figure 2.
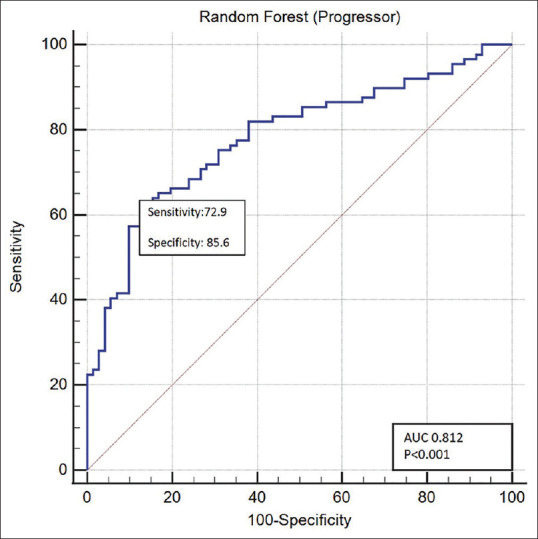
ROC curves for the clinical risk factors AI model. AI = artificial intelligence, ROC = receiver operating characteristic
Table 3.
Mean and standard deviation of clinical risk parameters
| Group | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Based on tomographic changes | Progression | Progression | No progression | No progression |
| Based on clinical risk factors | Progression | No progression | Progression | No progression |
| Number of cases | 246 | 76 | 42 | 86 |
| VIT-B12 (pg/ml) | 330.25±158.06 | 388.1±157.47 | 346.96±154.47 | 372.22±110.22 |
| VIT-D (ng/ml) | 12.65±9.54 | 26.33±6.63 | 20.24±8.63 | 35.48±3.95 |
| IgE (IU/ml) | 825.59±685.85 | 71.29±49.67 | 486.96±670.46 | 48.11±44.46 |
| Systemic allergies | ||||
| Yes | 186 | 30 | 28 | 11 |
| No | 60 | 46 | 14 | 75 |
| Eye rubbing | ||||
| Yes | 221 | 27 | 31 | 15 |
| No | 25 | 49 | 11 | 71 |
| Usage of topical immunomodulators | ||||
| Yes | 91 | 43 | 13 | 69 |
| No | 155 | 33 | 29 | 17 |
IgE=immunoglobulin E, VIT-B12=vitamin B12, VIT-D=vitamin D (ng/ml). Categorical variables are represented by their count in each group
Increased eye rubbing
Presence of systemic allergies
Less usage of topical immunomodulators
Table 4 shows the mean and standard deviation (difference between two visits) of tomographic parameters of the four predicted groups from the final confusion matrix [Table 1-B]. Evidently, group-1 (progression based on tomographic changes and progression based on clinical risk factors) shows the highest change in Kmax. Similar trends were observed in derived indices and anterior surface Zernike coefficients too. These results are in accordance with our previous study.[11]
Table 4.
Mean and standard deviation of tomographic parameters
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Based on tomographic changes | Progression | Progression | No progression | No progression |
| Based on clinical risk factors | Progression | No progression | Progression | No progression |
| Number of cases | 246 | 76 | 42 | 86 |
| Anterior keratometry indices | ||||
| Kmax (D) | 2.64±2.39 | 1.86±0.61 | 0.84±0.59 | 0.68±0.69 |
| CCT (mm) | −11.4±16.49 | −9.05±18.61 | −4.16±2.84 | −4.04±3.04 |
| TCT (mm) | −12.05±16.63 | −10.22±20.88 | −3.44±16.64 | −8.07±22.29 |
| K1 F (D) | 1.26±1.12 | 0.94±0.55 | 0.27±1.1 | 0.26±1.08 |
| K2 F (D) | 2.06±1.83 | 1.51±1.23 | 0.12±1.28 | 0.44±1.18 |
| Pentacam-derived indices | ||||
| BAD D | 0.22±3.18 | 0.54±4 | −0.72±3 | −0.15±2.15 |
| IHA | 4.08±24.3 | 2.4±21.92 | 8±25.89 | −3.64±24.08 |
| IHD | 0.02±0.03 | 0.02±0.02 | 0±0.02 | 0±0.03 |
| ISV | 13.74±14.12 | 11±8.57 | 0.52±10.63 | −0.83±12.11 |
| IVA | 0.11±0.19 | 0.09±0.1 | −0.01±0.14 | −0.04±0.18 |
| KI | 0.04±0.03 | 0.04±0.03 | 0±0.03 | 0±0.04 |
| CKI | 0.02±0.02 | 0.01±0.01 | −0.01±0.01 | 0±0.02 |
| Anterior Zernike coefficients | ||||
| Defocus (mm) | −1.08±1.67 | −0.81±0.92 | 0.38±1.09 | 0.51±1.42 |
| RMS COMA (mm) | 0.41±0.52 | 0.33±0.26 | 0.02±0.39 | −0.05±0.45 |
| RMS LOA (mm) | 2.06±2.1 | 1.67±0.98 | −0.11±1.89 | −0.39±1.91 |
| RMS HOA (mm) | 0.48±0.47 | 0.41±0.25 | 0±0.4 | −0.11±0.49 |
| PSA (mm) | −0.26±0.32 | −0.19±0.2 | 0.05±0.2 | 0.05±0.24 |
CCT=central corneal thickness, TCT=thinnest corneal thickness, BAD-D=Belin-Ambrósio enhanced ectasia display total deviation value, IHA=Index of height asymmetry, IHD=Index of height decentration, ISV=Index of surface variance, IVA=Index of vertical asymmetry, KI=Keratoconus index, CKI - Central keratoconus index, PSA=Primary Spherical aberration, RMS COMA=Root mean square of coma, RMS LOA=Root mean square of lower order aberrations, RMS HOA=Root means square of higher order aberrations
Discussion
Although a number of risk factors for progression of KC have been identified, there are a number of as yet unidentified factors which can play a role. To the best of our knowledge, a comprehensive review of the clinical and ocular surface risk factors and their profiling using AI has not been attempted earlier. The AI models looked at various clinical risk factors and combinations of which were significantly altered between progression and no progression groups. Serum IgE was the most important parameter to evaluate the risk of progression as determined by the AI analyses. The association of IgE and allergy is well known. IgE triggers release of mediators such as histamine and interleukin-13 (IL-13), which activate transient receptor potential channels on sensory nerve endings to trigger an itch response.[13] IgE is a common mediator in major risk factors for progression of KC, such as allergy, atopy, and eye rubbing. Serum IgE is significantly elevated in a number of KC patients who have allergy, compared to those without clinical signs and symptoms of allergy.[14-16] Interestingly, elevated serum IgE is also associated with a subset of KC patients without signs of ocular allergy. Hence, the measurement of IgE should be considered irrespective of whether a patient has systemic allergy or not.[14-16] An important aspect was that the IgE mean value was significantly higher (825.59 ± 685.85 IU/ml) in the group progression classified based on tomography and clinical risk factors compared to the value of 48.11 ± 44.46 IU/ml seen in the no progression group.
The next most important parameter detected by the AI model was eye rubbing. Eye rubbing is a common habit that can occur in response to an itchy eye and also involuntarily as a response to ocular irritation fatigue and emotional stress.[17,18] In a survey that included 240 KC patients, nearly 66% of them had a history of eye rubbing.[19] Other studies reported eye rubbing in KC patients to range from 50% to 83%.[20,21] One study reported eye rubbing as the most common risk factor in progression of KC in 100% of the diagnosed KC subjects.[22] Several mechanisms have been suggested for the onset of KC secondary to eye rubbing. The frequency and force of rubbing are the factors that influence corneal eye rubbing-related changes since these changes occur due to the viscoelastic nature of human cornea, which makes the corneal shape susceptible to changes in force in addition to intraocular pressure (IOP).[23,24] The keratocyte density in human corneas was reduced significantly by slight eye rubbing for 10 s repeated over 30 times for over 30 min.[25] Further, the changes in IOP due to eye rubbing can lead to the development of KC due to indirect traumatization of the keratocytes.[25]
Serum vitamin D level was an important parameter detected by the AI model to play a role in progression. Low vitamin D levels may contribute to the development and progression of KC.[26] Individuals with both progressive and nonprogressive KC have significantly lower serum 25-hydroxy vitamin D levels than those without KC.[26] A comparison showed that decreased vitamin D levels increased the probability of progressive KC by 1.29 times compared to the control group.[27] In our study, the group classified as progression (from tomographic and clinical risk factors) had a mean vitamin D of 18.65 ± 9.54, which was significantly lower than the no progression group, which had a mean of 25.48 ± 3.95. Another factor which was detected and is often related to IgE and eye rubbing was the presence of systemic allergy. Since Bereston and Baer[28] initially demonstrated the association between atopic dermatitis and KC in 1942, allergic disease has been continuously suggested to be one of the important risk factors for onset and progression of KC. Unlike with ocular allergy, there has been some debate around whether other allergic diseases might promote the development or progression of KC. In addition, although some medical conditions have been suggested to be related to KC, no conclusions have been established because of the lack of large-scale studies. Recently, a large-scale study involving 16,053 patients with KC was conducted in the USA in which multivariate logistic regression was used to determine the risk factors of KC based on data from a national healthcare claims database.[29] In that study, sleep apnea and asthma showed increased odds for the development of KC. Allergic rhinitis is another consistent risk factor noted for the onset and progression of KC in other studies.[29,30]
A study found no significant differences in the levels of vitamin D, B12, and folate between progressive and nonprogressive KC.[27] However, it may have a role in ocular surface regeneration and this may help to stabilize and strengthen cornea in inflammatory conditions.[27]
The use of immunomodulators and certain lubricants was also interestingly picked up by the AI model as possible modifiers of KC. This is similar to other studies which showed that immunomodulators, such as topical cyclosporine 0.05% and trehalose, could be useful in management of KC and preventing its progression.[31] The elevated levels of proinflammatory cytokines and matrix metalloproteinases (MMPs) in the tears and corneal epithelial cells of patients with KC could play an important role in KC pathogenesis.[31,32] Treatment with topical cyclosporine reduced MMP-9 levels in KC patients, and therefore, it can be useful in possibly preventing progression of KC.[33] Trehalose-based lubricants are known to protect human corneal cells from tumor necrosis factor-alpha (TNF-α) and desiccation stress-mediated inflammation through autophagy activation and p38MAPK inhibition, which may play a role in KC management and preventing its progression.[34] The COVID-19 pandemic has forced prolonged usage of face masks, which has been shown in some studies to have a deleterious effect on the ocular surface.[35] Altered tear film metrics, such as tear film break-up time (T-BUT), ocular surface staining, and increased ocular surface inflammation, were noted in these subjects.[35] As discomfort from dry eyes may also increase eye rubbing and face touching behaviors,[7] these could indirectly increase the risk of progression of KC. However, this did not achieve a high rank in our AI model.
A limitation of the present study was that it was done entirely on Asian (Indian) population. Another limitation was that the study did not quantify the changes in the levels of IgE, vitamin D, and vitamin B12 at follow-ups.
Conclusion
The complex interplay among all of the clinical and ocular surface risk factors could lead to progression of KC, but they are difficult to identify routinely just by visual examination. While the more common topographical and clinical risk factors are intuitive for most clinicians treating KC, the less-frequently noted risk factors may get missed. Here, our AI analyses seek to simplify this task of the clinicians. This study is unique since it has used two AI models to link tomographically determined progression[11] with clinical risk factors at the first visit. Identifying and stratifying these high-risk factors with AI could help us manage KC better and predict the need for surgical treatment in the near future.
Disclosures
Dr. Rohit Shetty and Dr. Abhijit Sinha Roy have intellectual rights over use of artificial intelligence diagnostics in corneal tomography.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shetty R, Deshmukh R, Ghosh A, Sethu S, Jayadev C. Altered tear inflammatory profile in Indian keratoconus patients-The 2015 Col Rangachari Award paper. Indian J Ophthalmol. 2017;65:1105–8. doi: 10.4103/ijo.IJO_233_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masiwa LE, Moodley V. A review of corneal imaging methods for the early diagnosis of pre-clinical Keratoconus. J Optom. 2020;13:269–75. doi: 10.1016/j.optom.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gokhale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61:382–3. doi: 10.4103/0301-4738.116054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas JB, Nangia V, Matin A, Kulkarni M, Bhojwani K. Prevalence and associations of keratoconus in rural maharashtra in central India:The central India eye and medical study. Am JOphthalmol. 2009;148:760–5. doi: 10.1016/j.ajo.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Das AV, Donthineni PR, Sai Prashanthi G, Basu S. Allergic eye disease in children and adolescents seeking eye care in India:Electronic medical records driven big data analytics report II. Ocul Surf. 2019;17:683–9. doi: 10.1016/j.jtos.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Jeng BH, Farid M, Patel SV, Schwab IR. Corneal cross-linking for keratoconus:A look at the data, the Food and Drug Administration, and the future. Ophthalmology. 2016;123:2270–2. doi: 10.1016/j.ophtha.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarino AI, Steptoe A, Hamer M, Michie S. Covid-19:Important potential side effects of wearing face masks that we should bear in mind. BMJ. 2020;369:m2003. doi: 10.1136/bmj.m2003. [DOI] [PubMed] [Google Scholar]
- 8.Khan MH, Yadav H. Sanitization during and after COVID-19 pandemic:A short review. Trans Indian Natl AcadEng. 2020;5:617–27. [Google Scholar]
- 9.Idarraga MA, Guerrero JS, Mosle SG, Miralles F, Galor A, Kumar N. Relationships between short-term exposure to an indoor environment and dry eye (DE) symptoms. J Clin Med. 2020;9:1316. doi: 10.3390/jcm9051316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boparai JK, Singh S, Kathuria P. How to design and validate a questionnaire:A guide. Curr Clin Pharmacol. 2018;13:210–5. doi: 10.2174/1574884713666180807151328. [DOI] [PubMed] [Google Scholar]
- 11.Shetty R, Kundu G, Narasimhan R, Khamar P, Gupta K, Singh N, et al. Artificial intelligence efficiently identifies regional differences in the progression of tomographic parameters of keratoconic corneas. J Refract Surg. 2021;37:240–8. doi: 10.3928/1081597X-20210120-01. [DOI] [PubMed] [Google Scholar]
- 12.Matalia H, Narasimhan R, Chinnappaiah N, Kumar V, Sinha Roy A. An interesting case of data gaps in measurement of corneal curvature with Scheimpflug tomography. J Refract Surg. 2020;36:350–1. doi: 10.3928/1081597X-20200325-02. [DOI] [PubMed] [Google Scholar]
- 13.Kittaka H, Tominaga M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol Int. 2017;66:22–30. doi: 10.1016/j.alit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rahi A, Davies P, Ruben M, Lobascher D, Menon J. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761–4. doi: 10.1136/bjo.61.12.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp EG, Lewis CJ. Immunoglobulin patterns in keratoconus with particular reference to total and specific IgE levels. Br J Ophthalmol. 1982;66:717–20. doi: 10.1136/bjo.66.11.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cingu AK, Cinar Y, Turkcu FM, Sahin A, Ari S, Yuksel H, et al. Effects of vernal and allergic conjunctivitis on severity of keratoconus. Int J Ophthalmol. 2013;6:370–4. doi: 10.3980/j.issn.2222-3959.2013.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shetty R, Sureka S, Kusumgar P, Sethu S, Sainani K. Allergen-specific exposure associated with high immunoglobulin E and eye rubbing predisposes to progression of keratoconus. Indian JOphthalmol. 2017;65:399–402. doi: 10.4103/ijo.IJO_217_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shneor E, Millodot M, Blumberg S, Ortenberg I, Behrman S, Gordon-Shaag A. Characteristics of 244 patients with keratoconus seen in an optometric contact lens practice. Clin Exp Optom. 2013;96:219–24. doi: 10.1111/cxo.12005. [DOI] [PubMed] [Google Scholar]
- 20.McGhee CN, Kim BZ, Wilson PJ. Contemporary treatment paradigms in keratoconus. Cornea. 2015;34((Suppl 10)):S16–23. doi: 10.1097/ICO.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 21.Rabinowitz YS. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16:607–20. doi: 10.1016/s0896-1549(03)00099-3. vii. [DOI] [PubMed] [Google Scholar]
- 22.AlShammari Z, AlShammari R, AlOrf S, AlShammari R, AlShammari W, ALShammari W. Prevalence, clinical features and associated factors of keratoconus patients attending Ophthalmology Department, King Khalid Hospital, Hail City, Saudi Arabia. EC Ophthalmology. 2016;3:388–400. [Google Scholar]
- 23.McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28:607–15. doi: 10.1097/ICO.0b013e318198384f. [DOI] [PubMed] [Google Scholar]
- 24.Hawkes E, Nanavaty MA. Eye rubbing and keratoconus:A literature review. Int J Kerat Ect Cor Dis. 2014;3:118–21. [Google Scholar]
- 25.Kallinikos P, Efron N. On the etiology of keratocyte loss during contact lens wear. Invest Ophthalmol Vis Sci. 2004;45:3011–20. doi: 10.1167/iovs.04-0129. [DOI] [PubMed] [Google Scholar]
- 26.Akkaya S, Ulusoy DM. Serum Vitamin D Levels in Patients with Keratoconus. Ocul ImmunolInflamm. 2020;28:348–53. doi: 10.1080/09273948.2019.1604002. [DOI] [PubMed] [Google Scholar]
- 27.Aslan MG, Fındık H, Okutucu M, Aydın E, OruçY, Arpa M, et al. Serum 25-Hydroxy Vitamin D, Vitamin B12, and folic acid levels in progressive and nonprogressive keratoconus. Cornea. 2021;40:334–41. doi: 10.1097/ICO.0000000000002475. [DOI] [PubMed] [Google Scholar]
- 28.Bereston ES, Baer RL. Keratoconus associated with atopic dermatitis:Report of two cases. Arch Dermat Syph. 1942;46:358–61. [Google Scholar]
- 29.Woodward MA, Blachley TS, Stein JD. The association between sociodemographic factors, common systemic diseases, and keratoconus:An analysis of a nationwide heath care claims database. Ophthalmology. 2016;123:457–65. doi: 10.1016/j.ophtha.2015.10.035. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merdler I, Hassidim A, Sorkin N, Shapira S, Gronovich Y, Korach Z. Keratoconus and allergic diseases among Israeli adolescents between 2005 and 2013. Cornea. 2015;34:525–9. doi: 10.1097/ICO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 31.Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 32.Lema I, Sobrino T, Durán JA, Brea D, Díez-Feijoo E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93:820–4. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 33.Shetty R, Ghosh A, Lim RR, Subramani M, Mihir K, Reshma AR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56:738–50. doi: 10.1167/iovs.14-14831. [DOI] [PubMed] [Google Scholar]
- 34.Panigrahi T, Shivakumar S, Shetty R, D'souza S, Nelson EJR, Sethu S, et al. Trehalose augments autophagy to mitigate stress induced inflammation in human corneal cells. Ocul Surf. 2019;17:699–713. doi: 10.1016/j.jtos.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Mastropasqua L, Lanzini M, Brescia L, D'Aloisio R, Nubile M, Ciancaglini M, et al. Face mask-related ocular surface modifications during COVID-19 pandemic:A clinical, in vivo confocal microscopy, and immune-cytology study. Transl Vis Sci Technol. 2021;10:22. doi: 10.1167/tvst.10.3.22. [DOI] [PMC free article] [PubMed] [Google Scholar]



