Abstract
Background
Lung cancer is the most common cause of death among all types of cancer in Germany, with an annual death rate of 45 000 patients. Over the past 15 years, innovations in diagnosis and treatment have prolonged the survival of patients with non-small-cell lung cancer in all tumor stages.
Methods
This review of the diagnosis and treatment of lung cancer is based on current national and international guidelines, and on prospective trials with the highest possible level of evidence that were retrieved by a selective search of the literature.
Results
Improved outcomes in patients with non-small-cell lung cancer (85% of new diagnoses) were achieved with the aid of precise diagnostic techniques, including functional imaging and endobronchial procedures for localized disease stage. Contemporary surgical and radio-oncological technologies reduce the morbidity and expand the boundaries of local therapy. Molecular pathology, including the assessment of predictive biomarkers, is an integral part of the diagnostic evaluation of non-small-cell lung cancer in all tumor stages; it enables stratified cytotoxic/molecularly targeted treatments and immunotherapies and improves patient-reported outcomes. The percentage of long-term survivors in the metastatic stage has doubled by the introduction of immunotherapy. In contrast, there has been no major improvement in the survival of patients with small-cell lung cancer (15% of new diagnoses).
Conclusion
In addition to the implementation of lung cancer screening in high-risk populations, the further development and consistent implementation of personalized diagnosis and treatment in certified lung cancer centers can be expected to prolong survival and improve the patients’ quality of life.
Lung cancer is the most common cause of cancer death. The dominant risk factor is smoking. Additional risks arise from exposure in the workplace, and environmental influences are also under debate. In Germany, deaths from lung cancer are falling, especially among men, and this can be explained by a decrease in cigarette smoking. Notwithstanding, with a predicted 60 000 new diagnoses and 45 000 deaths per year, lung cancer is by a long way the leading cause of cancer death and of years of life lost (1, 2). Several studies on early detection of lung cancer by means of low-dose computed tomography have demonstrated a reduction in mortality in defined risk populations (3, 4).
Prevalence.
Lung cancer is by far the most common cause of cancer death in Germany, with a predicted 60 000 new diagnoses and 45 000 deaths per year, and is the leading cause of years of life lost.
Prognosis.
Advances in diagnosis and treatment have measurably improved the prognosis of patients with all stages of non–small-cell lung cancer.
Medical advances over the past 15 years have markedly improved survival rates for patients with all stages of non-small-cell lung cancer (NSCLC, about 85% of primary diagnoses). In contrast, over the same period the prognosis for those with small-cell lung cancer (SCLC, about 15%) has not changed significantly (5). In the treatment of NSCLC, personalized medicine is front and center. This approach involves combining a comprehensive diagnostic characterization of the individual patient and their tumor with targeted treatment procedures. A more precise diagnostic workup, optimized surgical and perioperative procedures, and advances in radiotherapy combined with drug therapies are key reasons for improved survival rates in patients with localized and locally advanced stages of NSCLC. More than half the cases of NSCLC in Germany are diagnosed at a metastatic stage (1). In addition, some patients with localized or locally advanced tumors are not eligible for curative treatment because of severe comorbidities. Such patients derive benefit from biomarker-guided targeted drug therapies and from the activation of immunologic tumor defense by immune checkpoint inhibitors (ICI), antibodies against the immunoregulatory surface molecules PD-1 (programmed cell death protein-1), PD-L1 (programmed cell death 1 ligand-1) and CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) (6).
This article is based on an evaluation of national guidelines (S3 Guideline on Lung Cancer, Onkopedia) and international guidelines (American Society for Radiation Oncology, ASTRO; European Society for Medical Oncology, ESMO; European Society of Thoracic Surgeons, ESTS; National Comprehensive Cancer Network, NCCN). High-evidence-level studies (phase III, phase II, systematic meta-analyses) up to the time of manuscript submission were also included. Due to space limitations, the presentation of results focuses on the primary endpoints. In addition to treatment effect, absolute survival times are significantly influenced by the make-up of the study population concerned. To facilitate comparison of the effect sizes of treatments being studied in separate trials, in addition to median survival times, the relative risk reduction (low number corresponds to strong effect) for the survival endpoint is also given.
Learning objectives
Non–small-cell lung cancer.
More than half the cases of non–small-cell lung cancer in Germany are diagnosed at a metastatic stage.
After reading this article, the reader should:
Know the diagnostic pathway according to guidelines for the histological classification of lung cancer, clinical tumor staging, and determination of the biomarkers relevant to treatment;
Know the indications for surgery, radiotherapy, and drug therapy with curative or palliative intent for the different tumor stages;
Understand immunologic treatments and genomic biomarker-guided targeted drug therapy for patients with lung cancer.
Diagnosing the tumor and targeting treatment
A suspected diagnosis of lung cancer is usually made when a patient has been referred for diagnostic imaging. In addition, there may be incidental findings detected during the diagnosis or treatment of other morbidities. Anatomic assessment of thoracic foci, lung parenchyma, mediastinum, and other thoracic organs is primarily by means of contrast-enhanced computed tomography. 2-Fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (FDG-PET/CT) defines N and M stage and is therefore set in the guidelines as a mandatory staging study. Diagnostic imaging is completed with contrast-enhanced magnetic resonance imaging (or, if that is contraindicated, computed tomography) of the brain (7).
Diagnosis.
A suspected diagnosis of lung cancer is usually made when a patient has been referred for diagnostic imaging. In addition, there may be incidental findings detected during the diagnosis or treatment of other morbidities.
Histological confirmation of early-stage thoracic tumors can be performed by navigation-guided bronchoscopy. For locally advanced tumors, endobronchial ultrasound (EBUS)-guided needle biopsy is usually used (e1), which in 94% of cases leads to diagnosis. Various biopsy techniques are available, such as forceps biopsy, needle biopsy, and, especially, cryobiopsy, the advantages of which over cytology are clear given the need for further histopathologic and molecular pathologic diagnostic procedures (e2). Cytology remains useful for the detection of malignant effusion, for example in pleura or pericardium, or of cerebrospinal fluid involvement. In addition to inspection of the central airways, bronchoscopy facilitates interventional management of hemorrhage or central airway stenosis and retrieval of secretions for microbiologic analysis, including systematic assessment of the mediastinal, hilar, paratracheobronchial, and paraesophageal lymph node stations . EBUS is used for this, supplemented by EBUS-guided transbronchial needle aspiration (EBUS-TBNA) of sonographically abnormal lymph nodes or those larger than 8 mm (8). In contrast, diagnostic mediastinoscopy is less to the fore in the staging of lung cancer. If pulmonary foci suggestive of cancer are not accessible to bronchoscopic biopsy retrieval, histologic confirmation can be achieved by wedge resection and frozen section pathology, with the resection extended to oncologic resection if NSCLC is detected—and, if metastatic disease is present, by CT-guided puncture. If this procedure is contraindicated due to severe comorbidities, stereotactic ablative radiotherapy of lung tumors that fulfill clear clinical and imaging criteria of malignancy can be performed even without histologic confirmation so long as the clinical decision for it is multidisciplinary (e3).
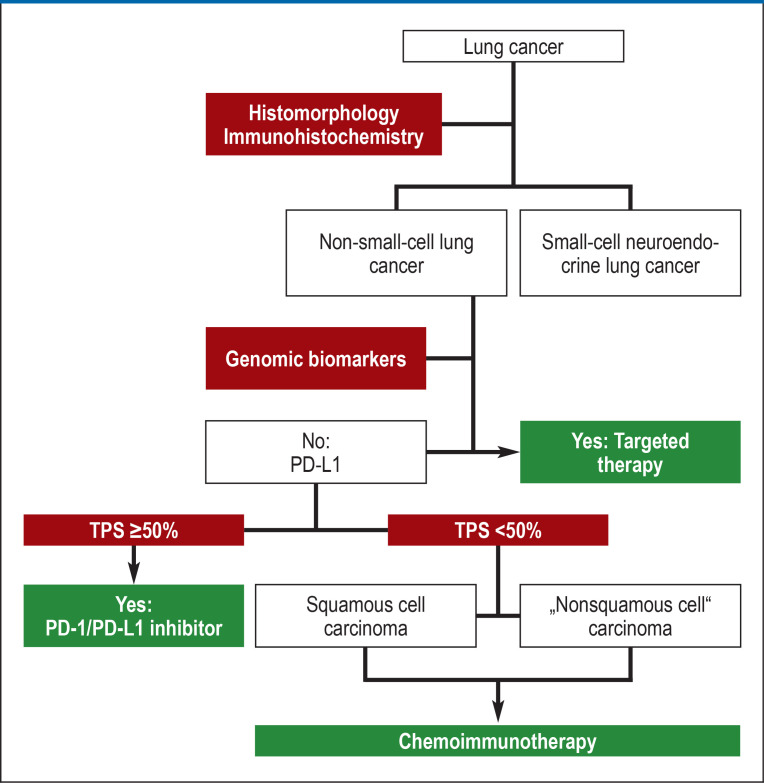
Histologic diagnosis (figure 1) includes classification according to histomorphologic criteria supported by immunohistochemical markers (table 1). In line with the linking of the approval of immunologic and targeted adjuvant therapies to biomarker studies, diagnostic biomarker studies are mandatory as part of the primary diagnostic workup for patients with NSCLCs undergoing primary surgery and for those given curative radiochemotherapy (table 2). For patients with stage IVA or IVB NSCLC, those who have received palliative systemic therapy at earlier stages because curative treatment approaches were contraindicated, and those with recurrence after primary curative therapy, comprehensive diagnostic biomarker studies are mandatory before the initiation of treatment (table 2). The exception is in situations where inducing remission is urgent, for example in a patient with compression of the superior vena cava; in this case, diagnostic studies are performed in parallel with the first cycle of chemotherapy and/or radiotherapy with biomarker-guided adjustment of treatment in subsequent cycles. Selected somatic genomic biomarkers can be detected on the basis of the primary tumor, metastases, and circulating nucleic acids (ctDNA) released by tumor cells into the blood. This “liquid biopsy” has been introduced for diagnostic purposes in patients with acquired resistance to tyrosine kinase inhibitors (TKIs) targeting the epidermal growth factor receptor (EGFR) (9). Any false-negative results of genomic biomarker diagnostic testing are primarily due to prediagnostic factors, such as inadequate biopsy, too low a tumor cell content in the biopsy, or a low ctDNA concentration in the blood. In Germany, comprehensive diagnostic biomarker studies for patients with NSCLC, coupled with a counseling service within the National Network Genomic Medicine Lung Cancer (Nationales Netzwerk Genomische Medizin Lungenkrebs, www.nngm.de), established with the support of German Cancer Aid and other molecular pathology institutions meeting the quality requirements, is available and is covered by the vast majority of German public health insurance funds.
Figure 1.
Algorithm for the diagnosis and treatment of lung cancer
PD-1, programmed cell death protein-1; PD-L1, programmed cell death 1 ligand-1;
TPS, tumor proportion score
Table 1. Supplementary histopathologic diagnostic investigations in lung cancer.
| Histomorphologic classification | Supplementary investigations required |
| Squamous cell carcinoma with intercellular bridges and/or keratin pearls | None |
| Non–small-cell carcinoma | PAS–diastase, p40, TTF1, CD56 |
| Neuroendocrine tumor or large-cell neuroendocrine carcinoma | Chromogranin A, synaptophysin, CD56, TTF1, Ki-67 |
| Small-cell neuroendocrine carcinoma | CD56, synaptophysin, TTF1, Ki-67 |
| Sarcomatoid carcinoma | Pan-CK, CD56, p40, TTF1, S100 protein, desmin, CD31 |
| Metastases | Depend on the primary tumor |
CK, cytokeratin; CD, cluster of differentiation; Ki67, Kiel antigen no. 67;
TTF1, thyroid transcription factor 1; PAS, periodic acid–Schiff reaction
Table 2. Predictive biomarkers with relevance for treatment in patients with non-small-cell lung cancer.
| UICC stage | Drugs with approval conditional on biomarkers | ||||||
| Biomarker | Method | IB | II | III(op) | III(crtx) | IV | |
| ALK | IHC, NGS, ISH | + | + | + | − | + | Alectinib, brigatinib, ceritinib, crizotinib, lorlatinib |
| BRAF | NGS | − | − | − | − | + | Dabrafenib/trametinib |
| EGFR | NGS | + | + | + | − | + | Afatinib, amivantamab, dacomitinib, erlotinib, gefitinib, mobocertinib*, osimertinib |
| HER2 | NGS | − | − | − | − | +* | Poziotinib*, trastuzumab-deruxtecan* |
| KRAS | NGS | − | − | − | − | + | Sotorasib, adagrasib* |
| MET | NGS | − | − | − | − | + | Capmatinib, tepotinib |
| NTRK1–3 | IHC, NGS, ISH | − | − | − | − | + | Entrectinib, larotrectinib |
| PD-L1 | IHC | + | + | + | + | + | Atezolizumab, cemiplimab, durvalumab, pembrolizumab |
| RET | NGS, ISH | − | − | − | − | + | Pralsetinib, selpercatinib |
| ROS1 | IHC, NGS, ISH | − | − | − | − | + | Crizotinib, entrectinib |
ALK, anaplastic lymphoma kinase; BRAF, B-rat fibrosarcoma; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; IHC, immunhistochemistry; ISH, in situ hybridization; KRAS, Kirsten rat sarcoma viral oncogene; MET, MET proto-oncogene/receptor tyrosine kinase; NGS, deoxyribonucleic acid or ribonucleic acid parallel sequencing (“next-generation sequencing”); NTRK1–3, neurotrophic tyrosine kinase 1–3; PD-L1, programmed cell death 1 ligand-1; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1; UICC, Union for International Cancer Control; III(op), stage III primary surgery; III(crtx), stage III definitive chemoradiotherapy
+* Not currently approved in Europe for this indication.
Surgical treatment
Diagnosis and staging of lung cancer.
Bronchoscopy-assisted tissue retrieval and functional imaging are key elements in the diagnosis and staging of lung cancer.
Histopathology and molecular pathology.
Histopathology and molecular pathology results are used to guide personalized drug therapy using curative and palliative treatment algorithms in patients with non–small-cell lung cancer.
Curative resection is the treatment of choice for stage I or II NSCLC (25% of new diagnoses), and for stage IIIA if there is interdisciplinary consensus. Because it carries lower morbidity and perioperative mortality, organ-preserving resection (primarily lobectomy) is the aim where possible, employing bronchoplasty and angioplasty resection procedures where anatomy or function require this. As a result, pneumonectomy is now indicated only in selected cases.
In patients with very small tumors with a radiologic tumor diameter of 2 cm or less, initial studies suggest that anatomic segmental resection, requiring selective removal of segments of arteries, veins, and bronchi, and lobectomy are equivalent in terms of overall survival (10).
Video-assisted thoracoscopic surgery (VATS) is the access of choice for curative surgery. The multicenter, randomized VIOLET trial (11) is comparing VATS with open surgery in relation to postoperative recovery, morbidity, safety, and oncologic efficacy. A conference presentation on the trial (e4) showed an advantage of VATS in terms of perioperative morbidity and postoperative recovery; the final results are still awaited.
In curative resection of the primary tumor, the addition of systematic lymph node dissection is mandatory. Based on evidence of superior results in lymph node dissection, robot-assisted surgical procedures are being introduced in lung cancer surgery (e5).
Organ-preserving lung surgery.
Minimally invasive thoracic surgery and extended resection techniques improve postoperative morbidity and have expanded the boundaries of organ-preserving lung surgery.
Stage III non-small-cell lung cancer (NSCLC).
For stage III NSCLC that is not primarily operable, curative resection can be performed in the context of multimodal therapy. Five-year survival rates of 40% to 44% are achieved after preoperative simultaneous chemoradiotherapy in defined patient groups or after chemotherapy in patients with stage IIIA tumors.
In patient with stage III NSCLC, curative resection can be performed in the context of multimodal therapy. Five-year survival rates of 40% to 44% are achieved after preoperative simultaneous chemoradiotherapy in defined patient groups (12) or after chemotherapy in patients with stage IIIA tumors (e6). One promising approach is curative surgery after combined pretreatment with chemotherapy and nivolumab (13), although approval by the European Medicines Agency (EMA) is still pending.
Adjuvant drug therapy
Adjuvant chemotherapy after resection of NSCLC improved overall survival at 5 years by 5% to 10% in several randomized controlled trials and meta-analyses (e7, e8). Allowing for patient-related factors, adjuvant drug therapy is indicated from stage pT2b or when lymph node metastasis has been detected. The standard is 4 cycles of cisplatin-based combination therapy (14); evidence for the value of carboplatin is poor (table 3).
Table 3. Adjuvant and consolidation drug therapy in curative treatment algorithms.
| Protocol | Median [95% CI; months] | Risk reduction (HR) | Reference |
| Cisplatin/vinorelbine vs. observation after surgery | Survival: 65.7 [47.9; 88.5] vs. 43.7 [35.7; 52.3] | 0.8 | (14) |
| Atezolizumab vs. observation after surgery and chemotherapy | Disease-free survival: n. a. [36.1; n.a.] vs. 35.3 [29.0; n. a.] | 0.66 | (15) |
| Osimertinib vs. placebo after surgery (and chemotherapy) | Disease-free survival: n. a. [38.8; n. a.] vs. 29.6 [16.6; 24.5] | 0.17 | (16) |
| Durvalumab vs. placebo (after radiotherapy) | Survival: n. a. [34.7; n. a.] vs. 28.7 [22.9; n. a.] | 0.68 | (19) |
95% CI, 95% confidence interval; HR, hazard ratio; n. a., not attained at the time of publication; vs., versus
In patients in whom the PD-L1 biomarker is expressed in tumor tissue (>50% of cases) and who are eligible for therapy, 1 year of treatment with atezolizumab reduces risk in relation to the endpoint “disease-free survival” by 34% (15). This treatment is ruled out for individuals whose NSCLC has an EGFR mutation or an ALK (anaplastic lymphoma kinase) fusion (table 2). In Europe, approval is restricted to NSCLC with high PD-L1 expression (tumor proportion score” [TPS] > 50%). If an EGFR mutation of exon 19 (in-frame deletion) or 21 (p.L858R) is detected in tumor tissue, 3 years’ oral treatment with osimertinib, an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI), following or instead of adjuvant chemotherapy reduces risk in relation to the endpoint “disease-free survival” by 83% (16). These approvals require testing for selected predictive biomarkers after curative resection of NSCLC (Figure 1, Table 2).
Curative and palliative radiotherapy
Curative and palliative radiotherapy.
Modern radiotherapy offers curative treatment in early-stage disease and also – within multimodal treatment protocols – in locally advanced lung cancer.
Modern radiotherapy is a core treatment modality in the management of lung cancer. In patients with locally advanced SCLC, clinical stage “limited disease” (classification of the Veterans Administration Lung Study Group [VALG]), so long as the patient is eligible for treatment, simultaneous chemoradiotherapy is the treatment of choice. Hyperfractionated accelerated radiotherapy is directed at the intrathoracic region of tumor spread up to a total dose of at least 45 Gy. Alternatively, conventional fractionated irradiation up to 60–66 Gy can be given. Due to the survival benefit shown in meta-analyses, patients in clinical stages I to III should be offered prophylactic cranial irradiation in addition (after completion of chemotherapy) (7). Hippocampus-sparing techniques can be used to reduce late neurotoxic sequelae (e9).
For patients with stage I or stage II NSCLC, especially where the tumor is functionally inoperable or surgery has been ruled out by individualized risk assessment, stereotactic ablative radiotherapy offers a curative treatment option with a high rate of local tumor control.
For stage III NSCLC, simultaneous chemoradiotherapy with a total dose equivalent to 60–70 Gy in conventional fractionation, combined with systemically adequate cisplatin-based combination therapy, represents a curative treatment approach, even when resection is technically or functionally impossible (12, 17, 18). With determination of target volumes through precise pretherapeutic diagnostic studies using FDG-PET/CT plus T and M stage defined by EBUS (18), and with the advent of intensity-modulated radiation therapy (IMRT) technique (e10), modern radiotherapy of NSCLC has become more accurate, individualized, and gentle. For example, IMRT halved the risk of severe pneumonitis and reduced the cardiac volume exposed to a dose above 40 Gy by 40% (e10).
Oncologic equivalence of curative chemoradiotherapy and trimodality therapy in stage IIIA disease is likely, but has not been formally demonstrated. Therefore, where pneumonectomy is necessary for technical reasons, or where other risks are present, the indication for surgery should be carefully evaluated on an individual basis. In patients with stage III NSCLC, 1 year of consolidation treatment with the ICI durvalumab improves overall survival after definitive chemoradiotherapy by 32% (table 3) (19, e11). The EMA has restricted this treatment to patients whose tumors express the biomarker PD-L1 (TPS ≥ 1%, more than 50% of cases) (table 2).
Radotherapy for stage I and II NSCLC.
For patients with stage I or stage II NSCLC, especially where the tumor is functionally inoperable or surgery has been ruled out by individualized risk assessment, stereotactic ablative radiotherapy offers a curative treatment option with a high rate of local tumor control.
Even for metastatic lung cancer, radiotherapy is extremely important. Metastases are often localized to the brain and skeletal system. Irradiation of a limited number of cerebral metastases (high evidence: 1–4 metastases; low evidence: 5–10 metastases) is now preferentially performed as stereotactic radiosurgery, which carries lower neurocognitive toxicity than whole-brain irradiation (e12). For metastases more than 2 cm in diameter, and for multiple metastases, fractionation schedules giving 3–5 fractions are an alternative to single-fraction irradiation. Another situation where radiotherapy is indicated is after neurosurgical resection of brain metastases, when additive radiotherapy should be administered to the metastasis bed and any unresected metastases.
Indications for radiotherapy of bone metastases may include a risk of spinal cord compression, risk of instability or fracture, and pain control; the decision requires close coordination with other specialists, especially spine surgeons. Radiotherapy of spinal metastases can be hypofractionated (1–10 fractions) (e13). In addition, with modern techniques (stereotaxy, image-guided radiotherapy [IGRT]/intensity-modulated radiotherapy [IMRT]), radiotherapy can be used as an adjunct to systemic therapy for local ablation in patients with limited metastasis. Initial studies indicate there may be an additional benefit (20).
Drug therapy for metastatic lung cancer
Although considerable advances have been made in treatment, metastatic lung cancer still continues regularly to lead to death. When discussing therapeutic options with patients, therefore, the option of taking a purely symptom-oriented approach should be included. It should be noted that the approval process for currently available systemic therapies took systematic account of endpoints regularly reported by patients, such as overall state of health, symptom burden, and symptom severity. The great majority of new treatments have demonstrated benefits in terms of symptom control or prolongation of the interval to worsening of these parameters.
Treatment of metastatic small-cell lung cancer
Immune checkpoint inhibitors.
Used within curative and palliative treatment protocols, immune checkpoint inhibitors improve the long-term survival rates of patients with lung cancer, in some cases quite considerably.
Well over 70 % of SCLCs are diagnosed in a metastatic stage, IVA or IVB, or the clinical stage “extensive disease” (VALG classification). Based on the overall survival benefit (risk reduction 30% and 27%, respectively) demonstrated in two randomized phase III trials, the combination of chemotherapy with carboplatin/cisplatin and etoposide with atezolizumab or durvalumab is the treatment of choice (21, 22). Unfortunately, this treatment achieves progression-free survival of more than 2 years in only about 10% of patients, who so far are not identifiable by predictive biomarkers. For treatment after progression, topotecan or anthracycline combinations are well established (23). Patients with a remission duration of more than 3 months after first-line chemotherapy may benefit from re-exposure to platinum and etoposide (e14).
Drug therapy for metastatic lung cancer.
Although considerable progress has been made in treatment, metastatic lung cancer still continues regularly to lead to death. When discussing therapeutic options with patients, therefore, the option of taking a purely symptom-oriented approach should be included.
Today, personalized selection of the optimal drug therapy for metastatic NSCLC follows algorithms based on biomarkers (figure 1). Parameters include histomorphology (squamous cell carcinoma versus “nonsquamous” histology), expression of the biomarker PD-L1, and detection or exclusion of the presence of genomic biomarkers (Table 2, Figure 1, Figure 2).
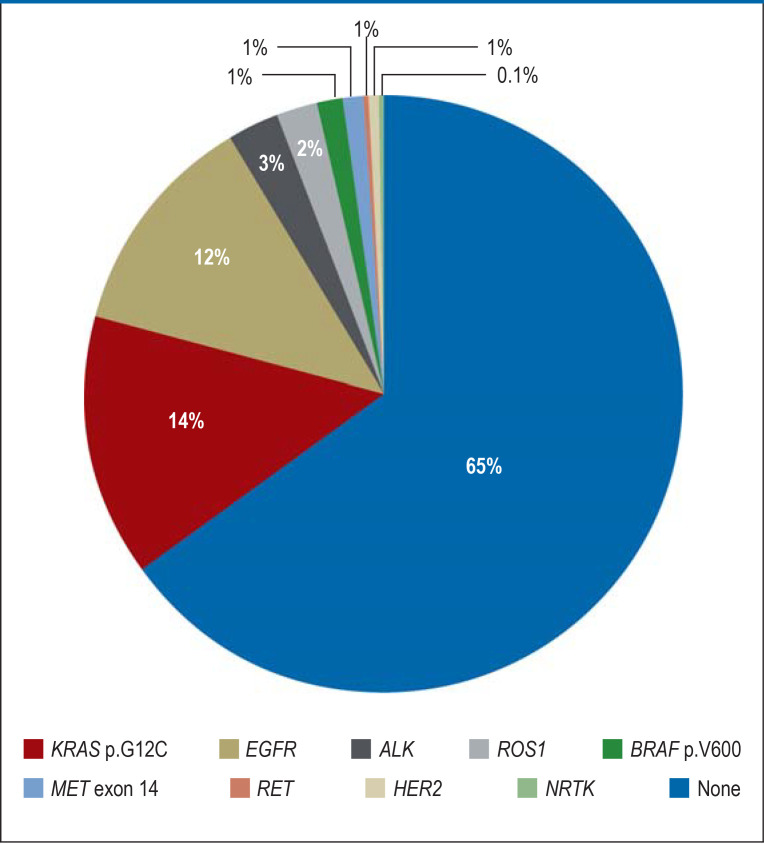
Figure 2.
Relative prevalence of genomic biomarkers relevant to therapy in patients with “nonsquamous” stage IV non-small-cell lung cancer (Lung Cancer Center, West German Cancer Center [Department of Medicine, University of Essen], 2021).
ALK, anaplastic lymphoma kinase; BRAF, B-rat fibrosarcoma; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; KRAS, Kirsten rat sarcoma viral oncogene; MET, MET proto-oncogene/receptor tyrosine kinase; NRTK, neurotrophic tyrosine kinase; RET, RET proto-oncogene; ROS1, ROS proto-oncogene 1
Personalized first-line treatment of non-small-cell lung cancer
The most important biomarker to guide treatment of patients with squamous cell carcinoma of the lung (about 30% of cases of NSCLC in Germany) is PD-L1. Genomic biomarkers with relevance for treatment (table 2) are very rarely detected in squamous cell carcinoma of the lung. As part of a patient-oriented approach, more common biomarkers, for example EGFR and Kirsten rat sarcoma viral oncogene (KRAS), should also be tested for in squamous cell carcinoma, since their presence cannot be ruled out, especially in the case of patients with little history of smoking or with mixed histology (figure 1).
If high PD-L1 expression (TPS ≥ 50%) is shown in the tumor tissue, immunotherapy alone using atezolizumab, cemiplimab, or pembrolizumab can be given. Compared with platinum-based chemotherapy, this treatment reduces risk in relation to overall survival by 38% to 41% (e15, e16, 24). For squamous cell carcinoma with low or no expression of PD-L1 (TPS 0–49%), immunotherapy is supplemented with platinum-based chemotherapy. The EMA has approved three alternative protocols (table 4): the combination of pembrolizumab with at least 4 cycles of chemotherapy with carboplatin/(nab-)paclitaxel (25) followed by maintenance therapy with pembrolizumab (risk reduction related to overall survival 29%); the combination of nivolumab plus ipilimumab with 2 cycles of carboplatin/paclitaxel followed by maintenance therapy with nivolumab and ipilimumab (risk reduction related to overall survival 38%) (26); and the combination of durvalumab plus tremelimumab with 4 cycles of platinum-based chemotherapy (no statistically significant risk reduction in the squamous cell carcinoma subgroup) (e37). Whether patients with squamous cell carcinoma with high PD-L1 expression (TPS ≥ 50%) benefit from having chemotherapy added to ICI is the subject of intense debate. Retrospective analyses suggest an additional benefit in cases with certain constellations of clinical factors, but evidence from comparative prospective studies is lacking.
Table 4. Chemoimmunotherapy protocols for patients with metastatic squamous cell carcinoma and “nonsquamous” non-small-cell lung cancer (after exclusion of EGFR and ALK aberrations).
| Protocol | Median survival [95% CI](months) | Risk reduction (HR) | Reference |
| Squamous cell carcinoma of the lung (PD-L1 TPS 0–100%) | |||
| Platinum/paclitaxel/nivolumab/Ipilimumab vs. platinum/paclitaxel | 14.5 [13.1; 19.4] vs. 9.1 [7.2; 11.6] | 0.62 | (26) |
| Platinum/(nab-)paclitaxel/pembrolizumab vs. platinum/(nab-)paclitaxel/placebo | 17.1 [14.4; 19.9] vs. 11.6 [10.1; 13.7] | 0.71 | (e34) |
| Platinum/gemcitabine or nab-paclitaxel/durvalumab/ tremelimumab vs. platinum/gemcitabine or nab-paclitaxel | 10.4 [8.4; 12.7] vs. 10.5 [8.0; 11.7] | Not significant | (e37) |
| “Nonsquamous” lung cancer (PD-L1 TPS 0–100%) | |||
| Carboplatin/nab-paclitaxel/atezolizumab vs. carboplatin/nab-paclitaxel | 18.6 [16.0; 21.2] vs. 13.9 [12.0; 18.7] | 0.79 | (e35) |
| Carboplatin/paclitaxel/bevacizumab/atezolizumab vs. carboplatinum/paclitaxel/bevacizumab | 19.5 [17.0; 22.2] vs. 14.7 [12.9; 17.1] | 0.8 | (e36) |
| Platinum/pemetrexed/nivolumab/ipilimumab vs. platinum/pemetrexed | 17.0 [14.0; n. e.] vs. 11.9 [9.9; 14.1] | 0.69 | (26) |
| Platinum/pemetrexed/pembrolizumab vs. platinum/pemetrexed/placebo | 22.0 [19.5; 24.5] vs. 10.6 [8.7; 13.6] | 0.56 | (40) |
| Platinum/pemetrexed or nab-paclitaxel/durvalumab/ tremelimumab vs. platinum/pemetrexed or nab-paclitaxel | 17.2 [14.9; 21.8] vs. 13.1 [10.6; 15.1] | 0.7 | (e37) |
| Non–small-cell lung cancer (PD-L1 TPS 50–100%) | |||
| Atezolizumab vs. platinum combinations | 20.2 [n. r.] vs. 13.1 [n. r.] | 0.59 | (e15) |
| Cemiplimab vs. platinum combinations | n. a. [17.9; n. a.] vs. 14.2 [11.2; 17.5] | 0.57 | (e16) |
| Pembrolizumab vs. platinum combinations | 26.6 [18.3; 40.4] vs. 13.4 [9.1; 18.3] | 0.62 | (24) |
95% CI, 95% confidence interval; ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; HR, hazard ratio;
n. r., not reported; n. a., not attained at the time of publication; PD-L1, programmed cell death 1 ligand-1; TPS, tumor proportion score;
ROS1, ROS proto-oncogene 1; vs., versus
Personalized first-line treatment of non-small-cell lung cancer.
The most important biomarker to guide treatment of patients with squamous cell carcinoma of the lung (about 30% of cases of NSCLC in Germany) is programmed cell death 1 ligand-1 (PD-L1).
Most “nonsquamous” NSCLC—about 60%—are pulmonary adenocarcinomas. In particular, with adenocarcinomas recurrent somatic genetic aberrations are found, which are predictive biomarkers for targeted systemic treatment with small molecule inhibitors and, in the future, also antibody–toxin conjugates (figure 2). European approvals are available for targeted first-line treatment of metastatic NSCLC on detection of oncogenic mutations of EGFR or B-rat fibrosarcoma (BRAF p.V600X) as well as fusion oncogenes involving ALK, neurotrophic tyrosine kinase (NTRK) 1–3, the RET proto-oncogene (RET), and the ROS proto-oncogene 1 (ROS1) (table 2).
In the field of precision oncology for lung cancer, EGFR TKIs have set the pace. Three generations of agents have now been approved for first-line treatment. In randomized controlled trials, the first-generation (erlotinib, gefitinib) and second-generation drugs (afatinib, dacomitinib) almost doubled median progression-free survival and improved patient-reported outcomes compared with platinum-based chemotherapies (27, 28, e17, e18). In a phase III study, the third-generation mutation-specific EGFR TKI osimertinib demonstrated a 20% risk reduction in relation to the endpoint “overall survival” compared with first-generation inhibitors (29). Because of this efficacy and its favorable safety profile, osimertinib is now widely used.
The approvals of the ALK inhibitors alectinib, brigatinib, ceritinib, crizotinib, and lorlatinib are based on randomized phase III trials that demonstrated their superiority to standard chemotherapy or the first-generation inhibitor crizotinib for the surrogate endpoint “progression-free survival” (30, e19– e21). European approvals for inhibitors of the NTRK (entrectinib, larotrectinib), RET (pralsetinib, selpercatinib), or ROS1 kinases (crizotinib, entrectinib), and for dual inhibition of oncogenic BRAF V600 mutants by the combination of dabrafenib/trametinib have been based on prospective, noncomparative studies because of the rarity of these aberrations and the high clinical efficacy of these therapies (31– 34, e22– e24). The probability of efficacy is notably higher than that of chemotherapy in historical comparator patient populations.
Genomic biomarkers.
Genomic biomarkers guide molecular targeted drug therapy particularly in some patients with pulmonary adenocarcinoma.
However, the majority of patients with NSCLC of “nonsquamous” histology do not exhibit any of the aforementioned genomic biomarkers in their tumor tissue (figure 2). If high PD-L1 expression (TPS ≥ 50%) is present, immunotherapy alone with atezolizumab, cemiplimab, or pembrolizumab can be given, as in squamous cell carcinoma (24, e15, e16). If PD-L1 is not expressed or is expressed at low levels (TPS 0–49%), various combinations of ICI with chemotherapeutics (table 4) are approved for first-line treatment. No direct comparison of the different immunochemotherapy regimens with each other is available, nor has any direct comparison with ICI alone in patients with high PD-L1 expression been carried out. Thus, the choice of therapy is based on the preferences of the patient and his or her physician.
Second-line treatment of non-small-cell lung cancer
Second-line therapy of non-small-cell lung cancer.
For patients with progression after pretreatment with platinum-based chemotherapy and an immune checkpoint inhibitor (ICI), some biomarker-guided targeted therapy options have been approved on the basis of nonrandomized trials.
For patients with progression after pretreatment with platinum-based chemotherapy and an ICI, some biomarker-guided targeted therapy options have been approved on the basis of nonrandomized trials (figure 2). It is therefore justified to test for these markers in the primary diagnostic workup (table 2). EMA-approved drugs currently include sotorasib for tumors showing the KRAS p.G12C mutation (approximately 14% of adenocarcinomas) and capmatinib and tepotinib for those showing MET proto-oncogene/receptor tyrosine kinase (MET) exon 14 mutation (1% to 2% of adenocarcinomas) (35, 36, e25). For patients with insertion mutations in exon 20 of EGFR (approximately 1% of adenocarcinomas) and progression after chemotherapy, the antibody amivantamab is approved (e26). For NSCLC with oncogenic mutations of HER2, the antibody–toxin conjugate trastuzumab–deruxtecan and the TKI poziotinib have already been approved in the United States (e27, e28).
Trastuzumab–deruxtecan and poziotinib.
For NSCLC with oncogenic mutations of HER2/ERBB2 (human epidermal growth factor receptor 2/erb-b2 receptor tyrosine kinase 2), the antibody–toxin conjugate trastuzumab–deruxtecan and the tyrosine kinase inhibitor poziotinib have already been approved in the USA.
A special situation exists when acquired resistance develops during first-line molecular targeted therapy. In this case, it is worth obtaining a new tumor biopsy or – in certain circumstances – a liquid biopsy in order to identify, through further molecular pathological diagnostic studies, resistance mechanisms that could be the target of second-line therapies (37). Clinically relevant examples are second-line treatment with osimertinib in EGFR p.T790M-associated acquired resistance to first-generation EGFR TKIs (38), and targeted treatment sequences of ALK inhibitors, guided by molecular resistance mechanisms (e29).
Most patients with metastatic NSCLC today continue to show no genomic biomarkers (figure 2) that would allow targeted first- or second-line therapy. Their first-line treatment typically includes combined ICI and platinum-based chemotherapy (table 4). The standard second-line treatment for these individuals is monochemotherapy with, for example, docetaxel or pemetrexed (e30). Based on two randomized phase III trials that show an improvement in overall survival (risk reduction 14% and 25%, respectively), docetaxel can be combined with the antiangiogenic antibody ramucirumab (e31) or, for adenocarcinomas, the antiangiogenic TKI nintedanib (e32).
In summary, personalized medicine today offers very good options for patients at all stages of lung cancer. Quality-controlled access is through lung cancer centers certified by the German Cancer Society. However, it should be pointed out that FDG-PET/CT scanning, thoracic surgery in lung cancer centers with externally monitored treatment outcomes (e33), and comprehensive pretreatment biomarker analysis (for metastatic stages) are still not carried out as part of the staging process in all individuals eligible for treatment (39). Thus, comprehensive education of patients and their relatives as well as their primary care physicians could contribute significantly to improving the overall prognosis of lung cancer patients in Germany.
Summary.
In summary, personalized medicine today offers very good options for patients at all stages of lung cancer. Quality-controlled access is through lung cancer centers certified by the German Cancer Society.
Further information on CME.
Access to the CME certification program is only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 27 April 2024. Submissions by letter, e-mail, or fax cannot be accepted.
Once a new CME module comes online, it remains available for 12 months. Results can be accessed 4 weeks after you start work on a module. Please note the closing date for each module, which can be found at cme.aerzteblatt.de
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN), which is found on the CME card (8027XXXXXXXXXXX). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results.
CME credit for this unit can be obtained via cme.aerzteblatt.de until 27 April 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following is by far the most common cause of death from cancer?
Ovarian cancer
Bile duct cancer
Leiomyosarcoma
Colorectal cancer
Lung cancer
Question 2
What proportion of primary diagnoses are non-small-cell lung cancer?
55%
65%
75%
85%
95%
Question 3
Which therapeutic agents can be used to treat non-small-cell lung cancer showing an oncogenic RET translocation?
Afatinib, osimertinib
Pralsetinib, selpercatinib
Dabrafenib, trametinib
Crizotinib, entrectinib
Durvalumab, pembrolizumab
Question 4
In a case of suspected lung cancer, which investigation is set out in the guidelines as mandatory for determining N and M stage?
FDG-PET/CT
Lung X-ray
Contrast-enhanced ultrasonography of the lungs
CT angiography of the lungs
Ultrasound-guided forceps biopsy
Question 5
According to new studies, what minimal extent of resection appears to be adequate for non-small-cell lung cancers with a radiologic tumor diameter of 2 cm or less?
Anatomic segmental resection
Bilobectomy
Lobectomy
Sleeve lobectomy
Pneumonectomy
Question 6
Which of the following statements about curative resection of non-small-cell lung cancer is true?
Systematic lymph node dissection is not required if preoperative lymph node staging by EBUS and FDG-PET/CT is negative.
The VIOLET trial demonstrated that open surgery is superior to a minimally invasive procedure in terms of postoperative recovery, morbidity, safety, and oncologic efficacy.
Robot-assisted surgical procedures currently have no place in the curative resection of non-small-cell lung cancer.
In curatively resected stage IB, II, and III(op) non-small-cell lung cancer, ALK, EGFR, and PD-L1 are predictive biomarkers for adjuvant drug therapy.
Prior to curative resection, preoperative histologic confirmation is mandatory for peripherally located non-small-cell lung cancer with a radiologic tumor diameter of 2 cm or less.
Question 7
Which of the following statements about the definitive treatment of small-cell lung cancer, clinical stage “limited disease,” is true?
Prophylactic cranial irradiation is performed at the same time as chemotherapy.
Standard thoracic radiotherapy is hypofractionated.
Chemotherapy given at the same time as radiotherapy is carried out with adriamycin.
Conventional fractionated thoracic radiotherapy is carried out up to total doses of 60–66 Gy.
Hyperfractionated accelerated radiotherapy of intrathoracic tumors is less effective than conventional fractionated radiotherapy.
Question 8
Which treatment offers a survival benefit and should, unless contraindicated, be offered to patients with clinical stage I to III small-cell lung cancer?
Ozone therapy
Prophylactic cranial irradiation
Low-dose whole-body irradiation for 12 weeks
Autologous blood therapy
Mistletoe therapy
Question 9
Which of the following statements about adjuvant drug therapy for non-small-cell lung cancer is true?
Adjuvant chemotherapy is usually based on carboplatin.
If specific EGFR mutations (exon 19 or p.L858R deletion) are shown, adjuvant therapy with osimertinib may be indicated.
Adjuvant cisplatin-based chemotherapy increases the 5-year overall survival rate by 70%.
Adjuvant immune checkpoint inhibition with atezolizumab is indicated only in patients with PD-L1-negative tumors.
In a patient with postoperative stage IA disease, adjuvant chemotherapy is always recommended.
Question 10
What is the most important biomarker to guide treatment when squamous cell carcinoma of the lung has been diagnosed?
RET
BRAF
PD-L1
NTRK1–3
HER2
► Access to the CME certification program is possible only over the internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Kersti Wagstaff.
Footnotes
Conflict of interest statement
MS has received financial support through Essen University Hospital from AstraZeneca and Bristol Myers-Squibb. He has received consulting fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, Merck Serono, Novartis, Roche, Sanofi, and Takeda. He has received fees for educational events from Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Novartis. He has had travel expenses reimbursed by BMS, Boehringer Ingelheim, Janssen, and Novartis.
KD has received consultancy fees from Olympus. He has received fees for educational events from Broncus Medical. He has had travel expenses and conference fees reimbursed by Storz. He is a member of the Advisory Board of Olympus and Fujifilm.
MSt has received financial support from the University Hospital of Essen from AstraZeneca. He has received consulting fees from Sanofi-Aventis, Bristol-Meyers Squibb, Janssen-Cilag, and AstraZeneca.
The other authors declare that they have no conflicts of interest.
References
- 1.Robert Koch Institut Krebs in Deutschland für 2017/2018. www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2021/krebs_in_deutschland_2021.pdf?__blob=publicationFile (last accessed on 3 August 2022) [Google Scholar]
- 2.Wengler A, Rommel A, Plaß D, et al. Years of life lost to death—a comprehensive analysis of mortality in Germany conducted as part of the BURDEN 2020 project. Dtsch Arztebl Int. 2021;118:137–144. doi: 10.3238/arztebl.m2021.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368:1980–1991. doi: 10.1056/NEJMoa1209120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–513. doi: 10.1056/NEJMoa1911793. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649. doi: 10.1056/NEJMoa1916623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF) Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms, Langversion 2.1 - Dezember 2022, AWMF-Registrierungsnummer: 020/007OL. www.leitlinienprogramm-onkologie.de/leitlinien/lungenkarzinom (last accessed on 5 December 2022) [Google Scholar]
- 8.Crombag LMM, Dooms C, Stigt JA, et al. Systematic and combined endosonographic staging of lung cancer (SCORE study) Eur Respir J. 2019;53 doi: 10.1183/13993003.00800-2018. 1800800. [DOI] [PubMed] [Google Scholar]
- 9.Krebs MG, Malapelle U, André F, et al. Practical considerations for the use of circulating tumor DNA in the treatment of patients with cancer A narrative review. JAMA Oncol. 2022;8:1830–1839. doi: 10.1001/jamaoncol.2022.4457. [DOI] [PubMed] [Google Scholar]
- 10.Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 11.Lim E, Batchelor T, Shackcloth M, et al. Study protocol for VIdeo assisted thoracoscopic lobectomy versus conventional Open LobEcTomy for lung cancer, a UK multicentre randomised controlled trial with an internal pilot (the VIOLET study) BMJ Open. 2019;9 e029507 doi: 10.1136/bmjopen-2019-029507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberhardt WE, Pöttgen C, Gauler TC, et al. Phase III study of surgery versus definitive concurrent chemoradiotherapy boost in patients with resectable stage IIIA(N2) and selected IIIB non-small-cell lung cancer after induction chemotherapy and concurrent chemoradiotherapy (ESPATUE) J Clin Oncol. 2015;33:4194–4201. doi: 10.1200/JCO.2015.62.6812. [DOI] [PubMed] [Google Scholar]
- 13.Forde PM, Spicer J, Lu S, et al. Neoadjuvant Nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 15.Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398:1344–1357. doi: 10.1016/S0140-6736(21)02098-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi: 10.1056/NEJMoa2027071. [DOI] [PubMed] [Google Scholar]
- 17.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without Cetuximab for unresectable stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestle U, Schimek-Jasch T, Kremp S, et al. Imaging-based target volume reduction in chemoradiotherapy for locally advanced non-small-cell lung cancer (PET-Plan): a multicentre, open-label, randomised, controlled trial. Lancet Oncol. 2020;21:581–592. doi: 10.1016/S1470-2045(20)30013-9. [DOI] [PubMed] [Google Scholar]
- 19.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 20.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn L, Mansfield AS, Szczęsna A, et al. First-line Atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 22.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394:1929–1939. doi: 10.1016/S0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 23.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 24.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Five-year outcomes with Pembrolizumab versus chemotherapy for metastatic non-small-cell lung cancer with PD-L1 tumor proportion score ≥ 50. J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 26.Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 27.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 28.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 29.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with Osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 30.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 31.Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drilon A, Oxnard GR, Tan DSW, et al. Efficacy of Selpercatinib in RET fusion-positive non-small-cell lung cancer. N Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 35.Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS pG12C mutation. N Engl J Med. 2021;384:2371–2381. doi: 10.1056/NEJMoa2103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 37.Scheffler M, Wiesweg M, Michels S, et al. Rebiopsy in advanced non-small cell lung cancer, clinical relevance and prognostic implications. Lung Cancer. 2022;168:10–20. doi: 10.1016/j.lungcan.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP registry (AIO-TRK-0315) Lung Cancer. 2021;152:174–184. doi: 10.1016/j.lungcan.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez-Abreu D, Powell SF, Hochmair MJ, et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: protocol-specified final analysis from KEYNOTE-189. Ann Oncol. 2021;32:881–895. doi: 10.1016/j.annonc.2021.04.008. [DOI] [PubMed] [Google Scholar]
- E1.Gu P, Zhao YZ, Jiang LY, Zhang W, Xin Y, Han BH. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: a systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–1396. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- E2.Theegarten D, Hager T. [Pathology of lung cancer] Radiologe. 2016;56:777–785. doi: 10.1007/s00117-016-0154-2. [DOI] [PubMed] [Google Scholar]
- E3.Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:295–301. doi: 10.1016/j.prro.2017.04.014. [DOI] [PubMed] [Google Scholar]
- E4.Lim EKS, Batchelor TJP, Dunning J, et al. Video-assisted thoracoscopic versus open lobectomy in patients with early-stage lung cancer: one-year results from a randomized controlled trial (VIOLET) J Clin Oncol. 2021;39 8504 [Google Scholar]
- E5.Jin R, Zheng Y, Yuan Y, et al. Robotic-assisted versus video-assisted thoracoscopic lobectomy: short-term results of a randomized clinical trial (RVlob Trial) Ann Surg. 2022;275:295–302. doi: 10.1097/SLA.0000000000004922. [DOI] [PubMed] [Google Scholar]
- E6.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015;386:1049–1056. doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- E7.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- E8.Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol. 2010;5:220–228. doi: 10.1097/JTO.0b013e3181c814e7. [DOI] [PubMed] [Google Scholar]
- E9.Rodríguez de Dios N, Couñago F, Murcia-Mejía M, et al. Randomized phase III trial of prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer (PREMER): A GICOR-GOECP-SEOR Study. J Clin Oncol. 2021;39:3118–3127. doi: 10.1200/JCO.21.00639. [DOI] [PubMed] [Google Scholar]
- E10.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40:1301–1311. doi: 10.1200/JCO.21.01308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Gondi V, Bauman G, Bradfield L, et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12:265–282. doi: 10.1016/j.prro.2022.02.003. [DOI] [PubMed] [Google Scholar]
- E13.Oldenburger E, Brown S, Willmann J, et al. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother Oncol. 2022;173:240–253. doi: 10.1016/j.radonc.2022.06.002. [DOI] [PubMed] [Google Scholar]
- E14.Baize N, Monnet I, Greillier L, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2020;21:1224–1233. doi: 10.1016/S1470-2045(20)30461-7. [DOI] [PubMed] [Google Scholar]
- E15.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- E16.Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- E17.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- E18.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- E19.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018;379:2027–2039. doi: 10.1056/NEJMoa1810171. [DOI] [PubMed] [Google Scholar]
- E20.Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017;389:917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- E21.Shaw AT, Bauer TM, de Marinis F, et al. First-line Lorlatinib or Crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383:2018–2029. doi: 10.1056/NEJMoa2027187. [DOI] [PubMed] [Google Scholar]
- E22.Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271–282. doi: 10.1016/S1470-2045(19)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Drilon A, Siena S, Ou SI, et al. Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor Entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1) Cancer Discov. 2017;7:400–409. doi: 10.1158/2159-8290.CD-16-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Gainor JF, Curigliano G, Kim DW, et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021;22:959–969. doi: 10.1016/S1470-2045(21)00247-3. [DOI] [PubMed] [Google Scholar]
- E25.Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET Exon 14 skipping mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E26.Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39:3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E27.Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant non-small-cell lung cancer. N Engl J Med. 2022;386:241–251. doi: 10.1056/NEJMoa2112431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for patients with HER2 Exon 20 Mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40:702–709. doi: 10.1200/JCO.21.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E29.Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- E31.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- E32.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- E33.Baum P, Lenzi J, Diers J, et al. Risk-adjusted mortality rates as a quality proxy outperform volume in surgical oncology-a new perspective on hospital centralization using national population-based data. J Clin Oncol. 2022;40:1041–1050. doi: 10.1200/JCO.21.01488. [DOI] [PubMed] [Google Scholar]
- E34.Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of Pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- E35.West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- E36.Socinski MA, Nishio M, Jotte RM, et al. IMpower150 final overall survival analyses for Atezolizumab plus Bevacizumab and chemotherapy in first-line metastatic nonsquamous NSCLC. J Thorac Oncol. 2021;16:1909–1924. doi: 10.1016/j.jtho.2021.07.009. [DOI] [PubMed] [Google Scholar]
- E37.Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without Tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: The phase III POSEIDON study. J Clin Oncol. 2023;41:1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]