Abstract
Background
Synovial Sarcoma (SS) is a rare soft tissue sarcoma. Mean time to get a SS diagnosis from the onset of symptoms is 10 years, furthermore, SS is associated with late metastasis. Surgery is the main treatment option, whose quality deeply affects SS outcomes, and it can be associated to preoperative or post-operative radiotherapy. Chemotherapy is considered very effective in Children, while in adults its efficacy is still under debate. The aim of this study was to investigate the oncologic results in SS treatment and to identify the risk factors for local and systemic control of the disease.
Methods
From 1994 to 2018, 211 patients affected by SS were treated in 3 Referral Centres of Orthopaedic Oncology. One hundred seventy-seven patients were included in the study, the median follow-up length was 96 months (5–374).
Results
Overall Survival on the Kaplan Meier Analyses was 80%, 70% and 56% at 5, 10 and 20 years. In multivariate analyses, OS correlated with tumour size and negative surgical margins. Chemotherapy use wasn't associated with better survival although patients who underwent CT had bigger and more aggressive tumours.
Conclusions
Our findings suggests that surgery with negative margins is the most important factor in Synovial Sarcoma. Adjuvant treatments as chemotherapy and radiation therapy didn't change the disease's course.
Keywords: Synovial sarcoma, Soft tissue sarcoma, Orthopedic oncology, Radiotherapy, Chemotherapy
1. Introduction
Synovial Sarcoma (SS) is a rare tumour, representing approximatively 5–10% of all soft tissue sarcomas (STS). It can affect patients of any ages, even though ninety percent of SS arise in people younger than 60 years old, with a peak incidence around 35 years of age.1, 2, 3, 4, 5 Synovial Sarcoma was firstly described in 1865 as a tumor originating from synovial membrane cells while, at present, the mesenchymal stem cell is indicated as the progenitor cell by most of the authors.2,4, 5, 6, 7 Despite the name, only less than 10% of SS arise within a joint capsule, while the vast majority arise in the extremities near tendineous insertions or bursae, with potential occurrence in any site.4 In most patients, SS grows slowly with an indolent course, without apparent local aggressiveness, although associated with metastatic potential and tendency to distant relapse, mainly to the lung and lymph nodes.8, 9, 10 Synovial Sarcoma has still an unfavourable prognosis and, during the last decades, very little has changed in outcome despite advancement in surgery, radiotherapy and chemotherapy.11,12 The cornerstone of treatment remains surgery, commonly associated with radiotherapy,13,14 with excellent local control rate.15,16 Usually, preoperative RT is preferred because of lower dose and volume of irradiation.9 Although SS have been described as one of the most chemo-sensitive STS,17 the efficacy of chemotherapy is still under debate.7,9,18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 A longer disease-free survival seems to be associated to the use of chemotherapy but there is little evidence about any advantage on overall survival.18,29, 30, 31, 32 Major prognostic factors predicting survival in SS are age, stage, size, site (extremities VS trunk) and local recurrence,3,10,22,24,33, 34, 35, 36, 37 the latter being object of debates in recent years.22,38 Except for the rare monophasic epithelioid-cell subtype which is associated with worse survival, no differences in outcome among different histologic subtypes was demonstrated,3 although for some authors, monophasic subtype is associated with worse prognosis.34,39,40 The aim of this observational retrospective multicentric study was to investigate the oncologic results of multimodal treatment of synovial sarcoma and to identify the risk factors for local and systemic control of the disease.
2. Materials and methods
From 1994 to 2018, 211 patients affected by SS were treated in 3 Referral Centres of Orthopaedic Oncology: Careggi University Hospital in Florence (Italy), Cisanello University Hospital in Pisa (Italy) and Ege University in Izmir (Turkey). Only patients with a minimum follow-up of 24 months or with an event occurred within 24 months (death, local recurrence, metastasis) were included in the study. One hundred seventy-seven patients were included in the study. Patients’ data were retrieved from electronic medical record and imaging studies, or phone interviews. A needle biopsy was performed in every patient who underwent planned excision. Multidisciplinary meetings in both hospitals were responsible of decision making around indication of adjuvant or neo-adjuvant therapies for each patient involved. Generally, patients with deep-seated and bigger than 5 cm tumours received both chemo and radiotherapy. Chemotherapy regimen was based on Adriamycin and Ifosfamide. Radiation dose was 1,8Gy/day with total 50,4Gy. The surveillance protocol applied in all the involved centres consisted in follow-up visits every 3 months for the first 2 years after surgery, then every 4 months for the 3rd year, then every 6 months for the 4th and 5th year, after which patients were seen on an annual basis until at least 10 years. Imaging at every visit included local MRI or ultrasound and chest CT scans or radiographs.
The median age of the patients was 55.7 years (7–94), the median follow-up period was 96 months (5–374). In Table 1 there are listed all patients’ characteristics. Most tumours were in the extremities (97%), 57% percent of patients underwent radiotherapy (adjuvant or neo-adjuvant) while 55% received chemotherapy. Nine patients (5%) had positive (intralesional) margins on pathologic examination, while 24% of them had intralesional or marginal pathological results (Table 1).
Table 1.
Patient demographics.
| Florence + Pisa | Izmir | Total | Significance | |
|---|---|---|---|---|
|
Age |
55,7 (SD 17) |
33,4 (SD 16) |
49 (SD19) |
p<0.001 |
| Site | ||||
| Upper extremities | 33 | 11 | 44 | p = 0.9 |
| Lower extremities | 94 | 34 | 128 | |
| Trunk |
4 |
1 |
5 |
|
| Gender | ||||
| M | 62 | 22 | 84 | p = 0.5 |
| F |
69 |
24 |
93 |
|
| Clinical Scenario | ||||
| Planned Excision | 64 | 31 | 95 | |
| Re-Excision | 43 | 5 | 48 | |
| Excision of a Recurrence |
34 |
10 |
34 |
|
|
Pre-operative Metastasis |
12 (9.1%) |
7 (15%) |
46 (26%) |
p = 0.9 |
| Dimensions | ||||
| <5 cm | 71 | 10 | 81 |
p<0.001 |
| >5 cm < 10 cm | 43 | 29 | 72 | |
| >10 cm |
17 |
7 |
24 |
|
| Neo adjuvant RT | ||||
| yes | 27 (21%) | 20 (43%) | 47 |
p:0.004 |
| no |
104 |
26 |
130 |
|
| Adjuvant RT | ||||
| yes | 40 (30%) | 32 (69%) | 72 |
p<0.001 |
| no |
91 |
14 |
105 |
|
| Neo Adjuvant CT | ||||
| yes | 34 (26%) | 32 (43%) | 54 |
p<0.001 |
| no |
97 |
14 |
123 |
|
| Adjuvant CT | ||||
| yes | 38 (29%) | 37 (80%) | 75 |
p<0.001 |
| no |
93 |
9 |
102 |
|
| Margins | ||||
| wide + radical | 118 | 16 | 134 | p<0.001 |
| intralesional + marginal | 13 | 28 | 43 | |
2.1. Statistical analysis
Patients’ characteristics are presented by frequencies and percentages for categorical variables, median and range for continuous variables. The Kaplan Meier curves were calculated to estimate overall sarcoma-specific survival (OS), local recurrence (LR)-free survival and distant metastasis (DM)-free survival. Cox proportional Hazard Model was used for multivariate analyses. Local recurrence-free survival and DM-free survival time intervals were defined as the time between surgery and the first LR or DM, respectively, or last follow up available. Similarly, OS interval was defined as the time between surgery and death or last follow-up, considering what event came firstly. Patients who died of other causes were censored. Differences in survival rates were assessed by the log-rank test. P values < 0.05 were considered significant. All analysis was completed using the Statistical Package for Social Science (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBMCorp.).
3. Results
3.1. Patients demographics
One-hundred and seventy-seven patients were included in this study, 93 females and 84 males with a mean age of 49 years old. Eighty-one patients had tumours less than 5 cm in max diameter size, 72 between 5 and 10 cm, while 24 more than 10 cm. One-hundred and twenty-eight patients were affected in their lower extremities, 44 in upper extremities and 5 in trunk. Ninety-five patients had their first tumour excision in one of our tertiary centres (planned excision), 48 came to our attention for a re-excision after a first unplanned tumour excision, while 34 underwent excision of a local recurrence. Forty-six patients (26%) were metastatic at diagnosis, 102 received radiotherapy (adjuvant or neo-adjuvant) while 98 patients received chemotherapy. Considering surgical margins, 134 patients had wide or radical surgical margins after the operation in one of our centres, while 43 had intralesional or marginal surgical margins. Patients treated in Izmir had a significant lower mean age compared to who have been treated in Florence or Pisa (33.4 vs 55.7, p < 0.001) and had significantly bigger tumour sizes. Patients in Izmir received radio or chemotherapy in much higher rates than who was referred to Pisa or Florence (Patients demographics are resumed in Table 1). Mean follow-up length was 96 months (5–374).
3.2. Treatment outcomes
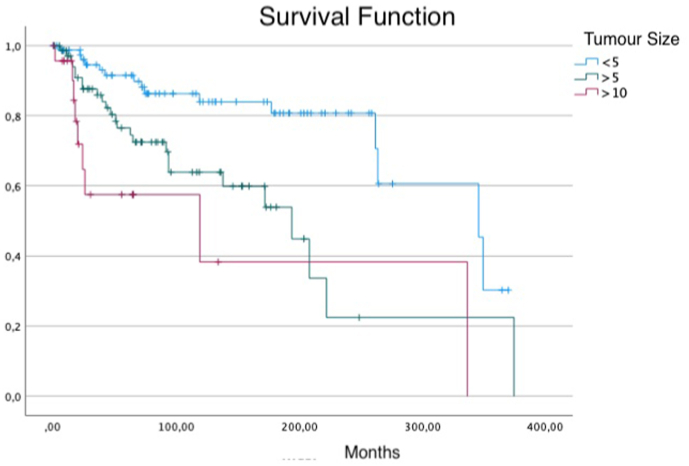
At follow-up, 52 patients had died and 125 were alive, 35 after treatment for relapse and 88 continuously disease free. There have been 37 local recurrences (21%) and 65 metastasis (36%) excluding patients who had a preoperative metastasis or who was treated for a local recurrence excision at presentation. Thirty-six of the Sixty-five patient who had post-operative metastasis received adjuvant chemotherapy after surgery. Twenty-one out of 37 patients who had a local recurrence received adjuvant radiotherapy while 16 received adjuvant chemotherapy. Mean time for the occurrence of a local recurrence was 40,5 months (range 1–146). Ten patients with an upper extremity tumour experienced a local recurrence compared to 25 with a lower extremity tumour and 2 with a tumour located in the trunk. Overall Survival on the Kaplan Meier Analyses was 80%, 70% and 56% at 5, 10 and 20 years respectively. Analysing prognostic factors as tumor presentation (planned excision, re-excision, excision of a recurrence), surgical margins, site, size, use of adjuvant therapies, age, no correlation with local control was observed. Metastasis free survival (MFS) was correlated with clinical scenario (p:0.016), with 72% and 50% (5- and 10-years) survival in patients who underwent a planned excision, 86 and 80% in who underwent a re-excision and 75 and 58% after an excision of a recurrence. Patients who underwent a planned excision had significantly bigger tumours. Furthermore, in univariate analysis, surgical margins had a significant impact on MFS with a 79-68% and 69-40% survival at 5 and 10 years in patients with wide or radical and intralesional or marginal resections respectively. Tumour size was a predictive factor of MFS (tumour <5 cm 90-81% at 5 and 10 years; tumour >5 cm 69-49%; tumour >10 cm 49-21%; p < 0.001), while patients who received chemotherapy had worse prognosis (p < 0.001), although a selection bias must be taken in account in these subgroups of patients. In multivariate analyses (Table 3), MFS was correlated to dimensions and the occurrence of a local recurrence. In fact, patients with bigger than 5 cm tumours had an HR to develop a distant recurrence of 2.9 (95 CI: 1.4–6.2, P:0.005) and patients with bigger than 10 cm tumours had an HR of 10.2 (95 CI: 3.8–27.2, p < 0.001), while who experienced a local recurrence had a HR of 2.5 (95 CI: 1.5–4.9). Clinical Scenario, Surgical Margins, operative site, and adjuvant therapies didn't significantly impact the MFS on multivariate analyses, only surgical margins were close to statistical significance level. The same prognostic factors had a significant impact in univariate analyses on overall survival (Data summarized in Table 2 and Fig. 1, Fig. 2, Fig. 3). In multivariate analyses, performed excluding patients who had metastasis at diagnosis, Overall Survival (OS) correlated with tumour size: tumours >5 cm had 2.8 (95 CI: 1.3–5.8) Hazard Ratio (HR) for death linked to cancer (p < 0.008), >10 cm had 3.2 HR (95 CI: 1.2–9.5; p:0.03). Surgical margins impacted significantly on OS, with a HR of 2.4 (95 CI: 1.2–4.9; p:0.01) while having a first local recurrence resulted in higher risk of death caused by the tumour, with 2.9 HR (95 CI: 1.5–5.9; p:0.05). Not receiving chemotherapy was associated with better survival (HR: 0.4; 95 CI: 0.2–0.8; p:0.02), while, considering only patients who had tumours bigger than 10 cm, chemotherapy didn't show any impact on overall survival.
Table 3.
Multivariate analysis of metastasis free survival predictive factors.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Clinical Scenario | |||
| Planned excision | 1 | ||
| Re-excision | 0.8 | 0.3-1.5 | p:0.3 |
| Excision of a recurrence |
0.3 |
0.6-2.5 |
p:0.5 |
| Surgical Margins | |||
| wide + radical | 1 | 1 | p:0.08 |
| Intralesional + marginal |
1.7 |
0.9-3.3 |
|
| Site | |||
| Upper extremities | 1 | ||
| Lower extremities | 1.4 | 0.7-2.9 | p:0.3 |
| trunk |
0.6 |
0.07–5 |
p:0.7 |
| Dimensions | |||
| <5 cm | 1 | 1 | |
| >5 < 10 cm | 2.9 | 1.4–6.2 | p:0.005 |
| >10 cm |
10,2 |
3.8–27.2 |
p<0.001 |
| Radiotherapy | |||
| yes | 1 | p:0.1 |
|
| no |
1.6 |
0.8-2.9 |
|
| Chemotherapy | |||
| yes | 1 | p:0.1 |
|
| no |
0.6 |
0.3-1.2 |
|
| Local Recurrence | |||
| yes | 2.5 | p:0.004 | |
| no | 1 | 1.5-4.9 | |
Table 2.
Multivariate analysis of overall survival predictive factors.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Clinical Scenario | |||
| Planned excision | 1 | 0.2-1.3 | |
| Re-excision | 0.5 | p:0.2 | |
| Excision of a recurrence |
1.1 |
0.5-2.5 |
p:0.8 |
| Surgical Margins | |||
| wide + radical | 1 | 1 |
p:0.01 |
| Intralesional + marginal |
2.4 |
1.2–4.9 |
|
| Site | |||
| Upper extremities | 1 | ||
| Lower extremities | 1.4 | 0.6-2.5 | p:0.9 |
| trunk |
1.3 |
0.3–3.1 |
p:0.8 |
| Dimensions | |||
| <5 cm | 1 | 1 | |
| >5 < 10 cm | 2.8 | 1.3–5.8 | p:0.008 |
| >10 cm |
3.2 |
1.1–9.5 |
p:0.03 |
| Radiotherapy | |||
| yes | 1 | 0.4-1.8 |
p:0.9 |
| no |
0.9 |
||
| Chemotherapy | |||
| yes | 1 | 0.2-0.8 |
p:0.02 |
| no |
0.4 |
||
| Local Recurrence | |||
| yes | 2.9 | 1.5–5.9 | p:0.003 |
| no | 1 | ||
Fig. 1.
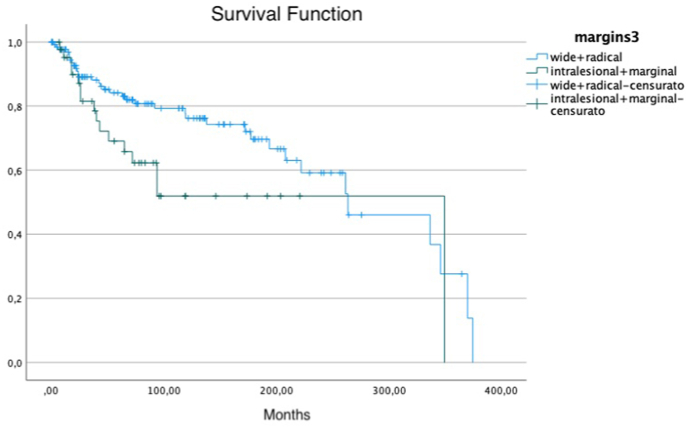
Margins on Overall Survival, wide or radical margin compared to intralesional or marginal margin.
Fig. 2.
Tumour size on Overall Survival. Survival in patients with tumours less than <5 cm compared to patients with tumour within 5 and 10 cm and to patients with tumours bigger than 10 cm.
Fig. 3.
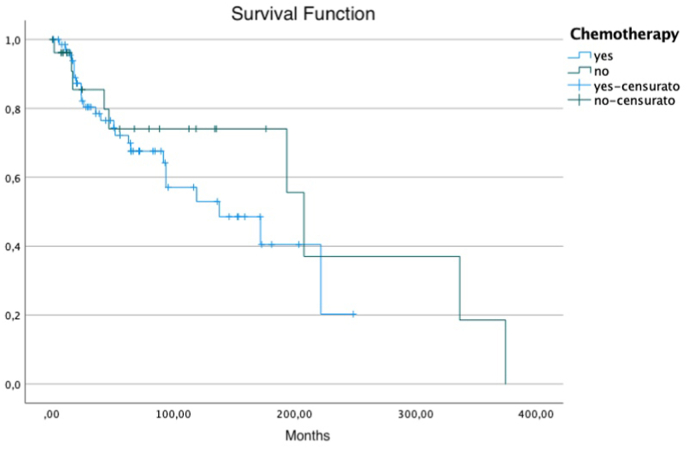
Impact of chemotherapy use on Overall Survival in patients who had tumours bigger than 5 cm only.
4. Discussion
Synovial Sarcoma (SS) is a soft tissue sarcoma histotype that despite all advancement in multimodal treatment and excellent rates of local control has still an unfavourable prognosis. The role of surgical margins and chemotherapy on overall survival of patients affected by SS is still under debate. This study aimed to investigate, with a large cohort of patients treated in 3 different centres, prognostic factors in SS, focusing on the impact of surgical margins and chemotherapy on local and systemic control of the disease. With numbers available, no one of the analyzed prognostic factors significantly correlated with local control of the disease. However, surgical margins continue to represent a major issue in SS treatment as in our study positive margins impacted negatively on MFS and OS. On the other hand, the use of chemotherapy was not associated with prolonged survival, even though a selection bias should be considered, as perioperative CT was usually reserved to locally advanced disease.
The role of surgical margins in overall survival both in STS and SS has been long debated. While on one side, it has been generally accepted that a negative margin is paramount to have good oncological outcomes, in recent years many authors haven't found a clear correlation between adequate surgical margins and overall survival.41 Indeed, Brecht et al.,38 in a cohort of 150 patients younger than 21 years old with localized SS, did not find any difference in survival between patients with negative or positive surgical margins. Similarly, Guillou et al.33 did not observe a different outcome on overall survival comparing patients with microscopically positive or negative margins. In addition, Shi et al.35 did not find any correlation between surgical margin quality (wide + radical VS marginal) and local recurrence rate or overall survival. Even De Silva ey al36 found out similar results in his series. On the other hand, Italiano et al.,22 in a multivariate analysis of a 237 patients' cohort with localized SS observed a significant worse OS in patients without a R0 surgical margin. Accordingly, Krieg10 reported a significant association between negative surgical margins and better overall survival in a setting where most patients had been treated incorrectly. Patients with wide resection had better prognosis if compared to patients who had intralesional or marginal resection, even when radiotherapy was associated. Finally, Trassard et al.,37 in a 128 patients' cohort found out that surgery with microscopically negative margins was associated with better disease specific survival on univariate analysis, even though this correlation was not confirmed on multivariate survival analysis. These conflicting results could be due to the different surgical margins classifications that are currently used, some considering only the quality margins, while others based on the distance between the closest margin and the tumour. In this study, using qualitative margins MSTS classification,67 we found a strong correlation between adequate surgery and overall survival. Thus, it seems crucial to focus on surgery planning in order to obtain negative surgical margins.
The impact of local recurrence on distant metastasis and mortality is a long-debated topic. Some authors suggest that there is no causal correlation between LR and systemic control as they just reflect the inner biological aggressiveness of the neoplasm.41, 42, 43, 44, 45, 46, 47, 48, 49 Conversely, other authors suggest that LR have a direct impact on distant metastasis and overall survival.50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 It is indeed very hard to solve this enigma, since in STS research most studies are retrospective, and with this methodology it's hard to detect a direct cause-effect relationship between a first local recurrence and patients' survival. Our results are in favour of the latter hypothesis, since in our study group after a first local recurrence there was an Odds Ratio to specific death of 2.9. STS capability to give metastasis is unique and relative to every single tumour and it has been postulated that tumour aggressiveness is quite entirely dependent on its inherent biology. In our opinion, even if there's no direct evidence, it could be assumed that after one or more recurrences, changes in tumour DNA could result in a higher aggressiveness and metastatic potential. Indeed, Bianchi et al.34 observed that locally recurrent SS were associated with worse overall survival, suggesting a progression into a more aggressive tumour in LR.
The role of chemotherapy in multimodal treatment of synovial sarcoma have been deeply investigated. Better results with chemotherapy were observed in paediatric patients than in SS of the adult.3,9,12, 13, 14, 15,29 Most authors recommend using chemotherapy only in large (>5 cm) and deep seated localized and metastatic SS.18,29, 30, 31, 32 Our results showed that who received chemotherapy didn't have a clear beneficial effect on overall survival although there's an obvious selection bias since who received chemotherapy had bigger tumour size and more aggressive ones compared to who didn't receive it.
5. Study limitations
The present study has several limitations: firstly, this is a retrospective observational multicentric study. Given the rarity of the disease, a multicentric study allows a higher number of cases, although data collection and results can be influenced by including three different tertiary centres with possible different therapeutic strategies. Another limitation is represented by the differences in population characteristics and type of treatments in our centres. In Turkey, younger patients with bigger tumours have been treated, receiving more often chemotherapy and radiotherapy compared to patients treated in Italy. This could be considered as a confounding factor but including patients with different characteristics helped us to define the independent role of prognostic factors to affect the outcome. Nevertheless, we do believe that, despite some important limitations, the current study has given solid and coherent results.
6. Conclusions
Our findings suggests that surgery with negative margins is paramount and the most important factor in multimodal treatment of Synovial Sarcoma.
Author contributions
Conceptualization Federico Sacchetti, Andac Alsina, Dundar Sabah and Rodolfo Capanna. methodology, Federico Sacchetti and Andac Alsina; formal analysis Federico Sacchetti, Domenico Andrea Campanacci and Francesco Muratori; investigation Federico Sacchetti, Andac Alsina; data curation Federico Sacchetti, Andac Alsina and Huseyin Kaya; writing—original draft preparation Federico Sacchetti and Andac Alsina.; writing—review and editing Francesco Muratori, Domenico Andrea Campanacci and Guido Scoccianti.; supervision Dundar Sabah and Rodolfo Capanna. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional ethical committee approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Declaration of competing interest
Each author certifies that he or she has no commercial associations(eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc that might pose a conflict of interest in connection with the submitted article.
Acknowledgement
we would like to thank Dr Michael Cresci and Dr Ginevra Bayon for their help in data extraction and database curation.
Contributor Information
Federico Sacchetti, Email: federico.sacchetti1989@gmail.com, sacchettif@aou-careggi.toscana.it.
Andac Celasun Alsina, Email: andacalsina@gmail.com.
References
- 1.Corey R.M., Swett K., Ward W.G. Epidemiology and survivorship of soft tissue sarcomas in adults: a national cancer database report. Cancer Med. 2014;3:1404–1415. doi: 10.1002/cam4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Necochea-Campion R., Zuckerman L.M., Mirshahidi H.R., Khosrowpour S., Chen C.S., Mirshahidi S. Metastatic biomarkers in synovial sarcoma. Biomark Res. 2017;5:1–8. doi: 10.1186/s40364-017-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sultan I., Rodriguez-Galindo C., Saab R., Yasir S., Casanova M., Ferrari A. Comparing children and adults with synovial sarcoma in the surveillance, epidemiology, and end results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115:3537–3547. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- 4.Fisher C. Synovial sarcoma. Ann Diagn Pathol. 1998;2:401–421. doi: 10.1016/s1092-9134(98)80042-7. [DOI] [PubMed] [Google Scholar]
- 5.Thway K., Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014;18:369–380. doi: 10.1016/j.anndiagpath.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Kimura T., Wang L., Tabu K., et al. Identification and analysis of CXCR4-positive synovial sarcoma-initiating cells. Oncogene. 2016;35:3932–3943. doi: 10.1038/onc.2015.461. Nature Publishing Group. [DOI] [PubMed] [Google Scholar]
- 7.Haldar M., Randall R.L., Capecchi M.R. Synovial sarcoma: from genetics to genetic-based animal modeling. Clin Orthop Relat Res. 2008;466:2156–2167. doi: 10.1007/s11999-008-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canter R.J., Qin L.X., Maki R.G., Brennan M.F., Ladanyi M., Singer S. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14:8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheer M., Dantonello T., Hallmen E., et al. Synovial sarcoma recurrence in children and young adults. Ann Surg Oncol. 2016;23:618–626. doi: 10.1245/s10434-016-5535-2. [DOI] [PubMed] [Google Scholar]
- 10.Krieg A.H., Hefti F., Speth B.M., et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22:458–467. doi: 10.1093/annonc/mdq394. Elsevier Masson SAS. [DOI] [PubMed] [Google Scholar]
- 11.Wang S., Song R., Sun T., et al. Survival changes in patients with synovial sarcoma, 1983-2012. J Cancer. 2017;8:1759–1768. doi: 10.7150/jca.17349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan B., Stiller C., Grimer R., Dennis N., Broggio J., Francis M. Outcome and the effect of age and socioeconomic status in 1318 patients with synovial sarcoma in the English National Cancer Registry: 1985–2009. Clin Sarcoma Res. BioMed Central. 2016;6:1–9. doi: 10.1186/s13569-016-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okcu M.F., Munsell M., Treuner J., et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21:1602–1611. doi: 10.1200/JCO.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari A., de Salvo G.L., Brennan B., et al. Synovial sarcoma in children and adolescents: the European pediatric soft tissue sarcoma study group prospective trial (EpSSG NRSTS 2005) Ann Oncol. 2015;26:567–572. doi: 10.1093/annonc/mdu562. Elsevier Masson SAS. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A.A., Al-Hussaini H., Hogg D., et al. Clinical features, treatment, and outcome in 102 adult and pediatric patients with localized high-grade synovial sarcoma. Sarcoma. 2011;Volume 2011:7. doi: 10.1155/2011/231789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song S., Park J., Kim H.J., et al. Effects of adjuvant radiotherapy in patients with synovial sarcoma. Am. J. Clin. Oncol.: Cancer Clin Trials. 2017;40:306–311. doi: 10.1097/COC.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 17.Vlenterie M., Litière S., Rizzo E., et al. Outcome of chemotherapy in advanced synovial sarcoma patients: review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; Setting a new landmark for studies in this entity. Eur J Cancer. 2016;58:62–72. doi: 10.1016/j.ejca.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Woll P.J., Reichardt P., le Cesne A., et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–1054. doi: 10.1016/S1470-2045(12)70346-7. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- 19.Vining C.C., Sinnamon A.J., Ecker B.L., et al. Adjuvant chemotherapy in resectable synovial sarcoma. J Surg Oncol. 2017;116:550–558. doi: 10.1002/jso.24688. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Yang Y., Wang C., Shi Y. Adjuvant chemotherapy decreases and postpones distant metastasis in extremity stage IIB/III synovial sarcoma patients. J Surg Oncol. 2012;106:162–168. doi: 10.1002/jso.23061. [DOI] [PubMed] [Google Scholar]
- 21.DeLaney T.F., Spiro I.J., Suit H.D., et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–1127. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 22.Italiano A., Penel N., Robin Y.M., et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20:425–430. doi: 10.1093/annonc/mdn678. Elsevier Masson SAS. [DOI] [PubMed] [Google Scholar]
- 23.Frustaci S., de Paoli A., Bidoli E., et al. Ifosfamide in the adjuvant therapy of soft tissue sarcomas. Oncology. 2003;65:80–84. doi: 10.1159/000073366. [DOI] [PubMed] [Google Scholar]
- 24.el Beaino M., Araujo D.M., Gopalakrishnan V., Lazar A.J., Lin P.P. Prognosis of T1 synovial sarcoma depends upon surgery by oncologic surgeons. J Surg Oncol. 2016;114:490–494. doi: 10.1002/jso.24306. [DOI] [PubMed] [Google Scholar]
- 25.Andrassy R.J., Okcu M.F., Despa S., Raney R.B. Synovial sarcoma in children: surgical lessons from a single institution and review of the literature. J Am Coll Surg. 2001;192:305–313. doi: 10.1016/s1072-7515(00)00806-1. [DOI] [PubMed] [Google Scholar]
- 26.Palmerini E., Staals E.L., Alberghini M., et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115:2988–2998. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh R., Mankin H.J., Singer S. Synovial sarcoma: the importance of size and location for survival. Clin Orthop Relat Res. 2004:155–161. [PubMed] [Google Scholar]
- 28.Riedel R.F., Jones R.L., Italiano A., et al. Systemic anti-cancer therapy in synovial sarcoma: a systematic review. Cancers. 2018;10:1–19. doi: 10.3390/cancers10110417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frustaci S., Gherlinzoni F., de Paoli A., et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19:1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 30.Tierney J.F. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Lancet. 1997;350:1647–1654. [PubMed] [Google Scholar]
- 31.Lewis J.J., Antonescu C.R., Leung D.H.Y., et al. Synovial sarcoma: a multivariate analysis of prognostic factors in 112 patients with primary localized tumors of the extremity. J Clin Oncol. 2000;18:2087–2094. doi: 10.1200/JCO.2000.18.10.2087. [DOI] [PubMed] [Google Scholar]
- 32.Pervaiz N., Colterjohn N., Farrokhyar F., Tozer R., Figueredo A., Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 33.Guillou L., Benhattar J., Bonichon F., et al. Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol. 2004;22:4040–4050. doi: 10.1200/JCO.2004.11.093. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi G., Sambri A., Righi A., Dei Tos A.P., Picci P., Donati D. Histology and grading are important prognostic factors in synovial sarcoma. Eur J Surg Oncol. 2017;43:1733–1739. doi: 10.1016/j.ejso.2017.05.020. Elsevier Ltd. [DOI] [PubMed] [Google Scholar]
- 35.Shi W., Indelicato D.J., Morris C.G., Scarborough M.T., Gibbs C.P., Zlotecki R.A. Long-term treatment outcomes for patients with synovial sarcoma: a 40-year experience at the university of Florida. Am. J. Clin. Oncol.: Cancer Clin Trials. 2013;36:83–88. doi: 10.1097/COC.0b013e31823fe450. [DOI] [PubMed] [Google Scholar]
- 36.de Silva M.V.C., McMahon A.D., Reid R. Prognostic factors associated with local recurrence, metastases, and tumor-related death in patients with synovial sarcoma. Am. J. Clin. Oncol.: Cancer Clin Trials. 2004;27:113–121. doi: 10.1097/01.coc.0000047129.97604.d6. [DOI] [PubMed] [Google Scholar]
- 37.Trassard M., le Doussal V., Hacène K., et al. Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–534. doi: 10.1200/JCO.2001.19.2.525. [DOI] [PubMed] [Google Scholar]
- 38.Brecht I.B., Ferrari A. Int-Veen C et al. Grossly-resected synovial sarcoma treated by the German and Italian pediatric soft tissue sarcoma cooperative groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer. 2006;46:11–17. doi: 10.1002/pbc.20502. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari A., Gronchi A., Casanova M., et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101:627–634. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 40.Singer B.S., Baldini E.H., Demetri G.D., Fletcher J.A., Corson J.M. Synovial sarcoma: prognostic significance of tumor size, margin of resection, and mitotic activity for survival by. J Clin Oncol. 1996;14:1201–1208. doi: 10.1200/JCO.1996.14.4.1201. [DOI] [PubMed] [Google Scholar]
- 41.Trovik C.S., Bauer H.C.F., Alvegård T.A., et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur J Cancer. 2000;36:710–716. doi: 10.1016/s0959-8049(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 42.Ueda T., Yoshikawa H., Mori S., et al. Influence of local recurrence on the prognosis of soft-tissue sarcomas. J Bone Joint Surg-Series B. 1997;79:553–557. doi: 10.1302/0301-620x.79b4.7487. [DOI] [PubMed] [Google Scholar]
- 43.Brennan M.F. The enigma of local recurrence. The Society of Surgical Oncology. Ann Surg Oncol. 1997;4:1–12. doi: 10.1007/BF02316804. http://www.ncbi.nlm.nih.gov/pubmed/8985511 [DOI] [PubMed] [Google Scholar]
- 44.Gronchi A., Miceli R., Fiore M., et al. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14:1583–1590. doi: 10.1245/s10434-006-9325-0. [DOI] [PubMed] [Google Scholar]
- 45.Gustafson P., Rooser B., Rydholm A. Local recurrence is of minor importance for metastases in soft tissue sarcoma. Acta Orthop Scand Suppl. 1991;62:68. doi: 10.1002/1097-0142(19910415)67:8<2083::aid-cncr2820670813>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 46.Harati K., Kolbenschlag J., Bohm J., et al. Long-term outcomes of patients with soft tissue sarcoma of the chest wall: analysis of the prognostic significance of microscopic margins. Oncol Lett. 2018;15:2179–2187. doi: 10.3892/ol.2017.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potter D.A., Kinsella T., Glatstein E., et al. High‐grade soft tissue sarcomas of the extremities. Cancer. 1986;58:190–205. doi: 10.1002/1097-0142(19860701)58:1<190::aid-cncr2820580133>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Sugiura H., Tsukushi S., Yoshida M., Nishida Y. What is the success of repeat surgical treatment of a local recurrence after initial wide resection of soft tissue sarcomas? Clin Orthop Relat Res. 2018;476:1791–1800. doi: 10.1007/s11999.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stojadinovic A., Jaques D.P., Leung D.H.Y., Healey J.H., Brennan M.F. Amputation for recurrent soft tissue sarcoma of the extremity: indications and outcome. Ann Surg Oncol. 2001;8:509–518. doi: 10.1007/s10434-001-0509-3. [DOI] [PubMed] [Google Scholar]
- 50.Abatzoglou S., Turcotte R.E., Adoubali A., Isler M.H., Roberge D. Local recurrence after initial multidisciplinary management of soft tissue sarcoma: is there a way out? Clin Orthop Relat Res. 2010;468:3012–3018. doi: 10.1007/s11999-010-1481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bianchi G., Sambri A., Cammelli S., et al. Impact of residual disease after “unplanned excision” of primary localized adult soft tissue sarcoma of the extremities: evaluation of 452 cases at a single Institution. Musculoskelet Surg. 2017;101:243–248. doi: 10.1007/s12306-017-0475-y. Springer Milan. [DOI] [PubMed] [Google Scholar]
- 52.DeLaney T.F., Kepka L., Goldberg S.I., et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 53.Gustafson P., Dreinh E., Rydholm A. Metastasis free recurrence survival after local reccurrence of soft-tissue sarcoma. J Bone Joint Surg. 1993;75B:658–660. doi: 10.1302/0301-620X.75B4.8331127. [DOI] [PubMed] [Google Scholar]
- 54.Karakousis C.P. Local recurrence and survival in soft-tissue sarcomas. Ann Surg Oncol. 1996;3:255–260. doi: 10.1007/BF02306280. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto S., Ahmed A.R., Kawaguchi N., Manabe J., Matsushita Y. Results of surgery for malignant fibrous histiocytomas of soft tissue. Int J Clin Oncol. 2003;8:104–109. doi: 10.1007/s101470300018. [DOI] [PubMed] [Google Scholar]
- 56.Espat N.J., Lewis J.J. The biological significance of failure at the primary site on ultimate survival in soft tissue sarcoma. Semin Radiat Oncol. 1999;9:369–377. doi: 10.1016/s1053-4296(99)80031-9. [DOI] [PubMed] [Google Scholar]
- 57.Novais E.N., Demiralp B., Alderete J., Larson M.C., Rose P.S., Sim F.H. Do surgical margin and local recurrence influence survival in soft tissue sarcomas? Clin Orthop Relat Res. 2010;468:3003–3011. doi: 10.1007/s11999-010-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stotter A.T., A'Hern R.P., Fisher C., Mott A.F., Fallowfield M.E., Westbury G. The influence of local recurrence of extremity soft tissue sarcoma on metastasis and survival. Cancer. 1990;65:1119–1129. doi: 10.1002/1097-0142(19900301)65:5<1119::aid-cncr2820650515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 59.Weitz J., Antonescu C.R., Brennan M.F. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21:2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Billingsley K.G., Lewis J.J., Leung D.H.Y., et al. Multifactorial analysis of the survival of patients with distant metastasis arising from primary extremity sarcoma. Cancer. 1999;85–2:389–395. [PubMed] [Google Scholar]
- 61.Collin Godbold, Hajdu B. Localized extremity soft tissue sarcoma: an analysis of factors affecting survival. J Clin Oncol. 1987;5:601–612. doi: 10.1200/JCO.1987.5.4.601. [DOI] [PubMed] [Google Scholar]
- 62.Daigeler A., Zmarsly I., Hirsch T., et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer. 2014;110:1456–1464. doi: 10.1038/bjc.2014.21. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis J.J., Leung D., Heslin M., Woodruff J.M., Brennan M.F. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. doi: 10.1200/JCO.1997.15.2.646. [DOI] [PubMed] [Google Scholar]
- 64.Pisters P.W., Leung D.H., Woodruff J., Shi W., Brennan M.F. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 65.Emrich L.J., Ruka W., Driscoll D.L., Karakousis C.P. The effect of local recurrence on survival time in adult high-grade soft tissue sarcomas. J Clin Epidemiol. 1989;42:105–110. doi: 10.1016/0895-4356(89)90083-8. [DOI] [PubMed] [Google Scholar]
- 66.Gronchi A., Casali P.G., Mariani L., et al. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: a series of patients treated at a single institution. J Clin Oncol. 2005;23:96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 67.Enneking W.F., Spanier S.S., Malawer M.M. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer. 1981;47(5):1005–1022. doi: 10.1002/1097-0142(19810301)47:5<1005. [DOI] [PubMed] [Google Scholar]