Abstract
Introduction
MET amplification is a potentially actionable resistance mechanism in ALK-rearranged (ALK+) lung cancer. Studies describing treatment outcomes of this molecular subgroup are lacking.
Methods
We assembled a cohort of patients with ALK+ lung cancer and acquired MET amplification (identified by tissue or plasma) who received regimens targeting both ALK and MET. Efficacy and safety were assessed using the Response Evaluation Criteria in Solid Tumors version 1.1 and Common Terminology Criteria for Adverse Events version 4.03, respectively.
Results
A total of 12 patients were included in the series. MET amplification was detected after a median of 1.5 (range 1–5) lines of therapy. Four distinct regimens were implemented to address MET amplification: crizotinib (n = 2), lorlatinib plus crizotinib (n = 6), alectinib plus capmatinib (n = 3), and alectinib plus crizotinib (n = 1). Partial responses were observed in five (42%) of 12 patients, including patients who received crizotinib (n = one of two), lorlatinib plus crizotinib (n = three of six), and alectinib plus capmatinib (n = one of three). Primary progression was observed in four patients (33%). Grades 1 to 2 peripheral edema, occurring in seven (58%) patients, was found with both crizotinib and capmatinib. One patient required dose reduction of capmatinib plus alectinib for persistent grade 2 edema. Across the regimens, one patient discontinued therapy for toxicity, specifically neurocognitive toxicity from lorlatinib plus crizotinib. At progression on ALK+ MET therapy, potential resistance mechanisms included MET copy number changes and ALK kinase domain mutations.
Conclusions
Combined ALK and MET inhibition is associated with moderate antitumor activity in patients with ALK+ NSCLC with concurrent MET amplification. Prospective studies are indicated to confirm activity and identify individuals most likely to benefit from the treatment.
Keywords: ALK, MET amplification, Capmatinib, Crizotinib, Alectinib, Lorlatinib
Introduction
ALK-rearranged (i.e., ALK+) NSCLC is associated with sensitivity to ALK tyrosine kinase inhibitors (TKIs).1,2 Despite this initial sensitivity to ALK-targeted therapies, many ALK+ NSCLCs will eventually develop ALK-independent resistance mechanisms that limit the efficacy of ALK TKIs.3,4 Of the diverse bypass pathways implicated in ALK-independent resistance, MET activation is among the most common genetic mechanisms, with studies reporting this resistance mechanism in approximately 15% to 20% of biopsies from patients relapsing on next-generation ALK TKIs, including alectinib and lorlatinib.3 With the paradigm shift favoring next-generation ALK TKIs without MET activity over the ALK/MET TKI crizotinib for initial treatment of advanced ALK+ NSCLC, the relevance of MET bypass signaling as a mechanism of resistance to ALK-targeted therapy is anticipated to increase.3
Although MET activation represents a potentially actionable bypass signaling mechanism in ALK+ NSCLC,3,5 prospective clinical trials exploring efficacy of MET-directed therapies in ALK+ NSCLC are lacking. Currently, evidence of efficacy of this approach is limited to case reports. In EGFR-mutant NSCLC, adding a MET TKI to osimertinib yields partial responses in 30% to 50% of MET-amplified EGFR+ NSCLCs in clinical trials,6,7 suggesting that tumors with this resistance mechanism can benefit from inhibiting MET signaling. Here, we present outcomes of 12 patients with MET-amplified ALK+ NSCLC who received therapies targeting both ALK and MET.
Materials and Methods
Data Collection and Molecular Testing
Using an institutional database, we identified patients with ALK+ NSCLC with acquired MET amplification who had received regimens targeting both ALK and MET. MET amplification was identified by tissue or plasma testing. In plasma, MET amplification was defined as absolute MET plasma copy number more than or equal to 2.1 without evidence of aneuploidy/polysomy using the Guardant360 assay.8 In tissue, MET amplification was defined as MET to centromere 7 (MET:CEP7) ratio of more than or equal to 2.2 as calculated by fluorescence in situ hybridization or was determined on the basis of copy number as previously described using the FoundationOne assay.9 The FoundationOne, Guardant360, and SNaPshot NGS assay results were also reviewed to identify ALK kinase domain mutations and co-alterations involving other genes for patients in this series.10
Patients received one of four regimens (crizotinib, lorlatinib + crizotinib, alectinib + capmatinib, or alectinib + crizotinib) on the basis of treating physician discretion and ease of access to the various regimens. Six patients (50%) received combinations targeting both ALK and MET through institutional review board–approved protocols, including a prospective clinical trial of lorlatinib plus crizotinib (n = 3, NCT04292119, see Supplementary Fig. 1 A/B for the study schema) that has since closed to enrollment, the lorlatinib expanded-access program (n = 1, NCT03178071) through which Pfizer granted special permission to add crizotinib, and single-patient INDs (n = 2 alectinib + capmatinib). Of note, NCT04292119 has closed to enrollment as a result of slow accrual; data from all patients who enrolled to the MET amplification arm of the study are presented herein. As part of the consent process for these protocols, the option to receive standard-of-care therapies (i.e., chemotherapy) was included on the consent form. Outside of clinical trials, patients received crizotinib (n = 2) or were treated with off-label (n = 4) combinations, as described previously. Apart from patients treated on NCT04292119 where dosing was specified (lorlatinib 50 mg + crizotinib 250 mg twice daily), dosing for the combination regimens was at the discretion of the treating physician given absence of prospective data to use for guidance. Of the four patients who received combination therapy outside of institutional review board–approved protocols, two had previously received chemotherapy and the remaining two patients elected to receive the investigational combinations in lieu of chemotherapy.
Medical records were reviewed to gather information on demographics, treatment histories, and safety/tolerability of regimens administered. Adverse events were documented at each clinic visit for patients treated on clinical trials and single-patient IND programs, whereas these events were captured retrospectively for the remaining patients. This study was approved by the Partners Institutional Review Board. All patients included in this study provided written informed consent for molecular analysis and treatment with the regimens described.
Response and Safety Assessment
Response to dual ALK/MET inhibition was assessed by a board-certified thoracic radiologist (SRD) using Response Evaluation Criteria in Solid Tumors (RECIST) version (v.)1.1. Adverse events were graded according to Common Terminology Criteria for Adverse Events v.4.03.
Results
Study Population
The study included 12 patients with MET-amplified ALK+ NSCLC. Baseline clinicopathologic characteristics for the study cohort are summarized in Table 1. Patients were diagnosed with MET-amplified ALK+ NSCLC through analysis of tissue (n = 10) or plasma (n = 2) after progression on ALK-targeted therapy. Most patients had no smoking history (n = nine, 75%) and had lung adenocarcinoma (n = 11, 92%). The median number of previous lines of treatment was 1.5 (range 1–5). The median number of previous ALK TKIs was 1 (range 1–3). None of the patients had received crizotinib before developing MET-amplified NSCLC. Nevertheless, all patients had received a second-generation ALK TKI; five (42%) patients were treated with lorlatinib before detection of MET amplification. Five (42%) patients had received chemotherapy.
Table 1.
Clinicopathologic Characteristics of 12 Patients With MET-Amplified ALK+ NSCLC
| Characteristics | N = 12 |
|---|---|
| Age at baseline (y) | |
| Median | 40 |
| Range | 27–79 |
| Sex, n (%) | |
| Female | 5 (42) |
| Male | 7 (58) |
| Ethnicity, n (%) | |
| White | 10 (83) |
| Asian | 1 (8) |
| Hispanic | 1 (8) |
| Histology, n (%) | |
| Adenocarcinoma | 11 (92) |
| Poorly differentiated carcinoma | 1 (8) |
| EML4-ALK fusion variant, n (%) | |
| Unknown/not applicablea | 3 (25) |
| Variant 1 | 6 (50) |
| Variant 3 | 1 (8) |
| Variant 5 | 1 (8) |
| Variant 8 | 1 (8) |
| Smoking history, n (%) | |
| Never | 9 (75) |
| Everb | 3 (25) |
| Previous TKI lines, n (%) | |
| 1 | 7 (58) |
| ≥2 | 5 (42) |
| Previous crizotinib, n (%) | |
| Yes | 0 (0) |
| No | 12 (100) |
| Previous chemotherapy for metastatic disease, n (%) | |
| Yes | 5 (42) |
| No | 7 (58) |
TKI, tyrosine kinase inhibitor.
Fusion variant was not known as a result of testing strategy (fluorescence in situ hybridization, n = 2) or not applicable owing to non-EML4 fusion partner (n = 1).
Between 1 and 4 pack years.
EML4-ALK Fusion Variant and Concurrent Alterations
An EML4-ALK fusion was detected in nine tumors (75%) (Table 1). One tumor had a HIP1-ALK fusion. The fusion partner was not known for the two remaining tumors, as fluorescence in situ hybridization was used to identify the ALK rearrangement. Among nine specimens where the specific EML4-ALK fusion variant was known, the following fusion variants were identified: variant 1 (n = 6), variant 3 (n = 1), variant 5 (n = 1), and variant 8 (n = 1). None of the tissue or plasma specimens corresponding to initial detection of MET amplification contained concurrent ALK kinase domain mutations. TP53 mutations were identified in nine (75%) of 12 biopsies obtained before initiating dual ALK/MET therapy (Table 2).
Table 2.
Treatment Outcomes on ALK Plus MET-Directed Therapy and Longitudinal Biopsy Findings
| Patient | ALK/MET Therapy | Best Response Time on Treatment |
Pre-ALK/MET-Targeted Therapy Biopsy Findings |
Post-ALK/MET-Targeted Therapy Biopsy Findings |
|---|---|---|---|---|
| 1 | Crizotinib 250 mg BID | PD <1 mo |
MET/CEP7 ≥ 25, TP53 R273C, TP53 Q192∗, SETD2 V2280fs∗89 | N/A |
| 2 | Crizotinib 250 mg BID | PR (−38%) 3.5 mo |
MET/CEP7 ≥ 25, TSC2 D1612N, TP53 A161T | MET/CEP7 > 25, insufficient tissue for NGS |
| 3 | Lorlatinib 75 mg QDa + crizotinib 250 mg BID | PR (−30%) 3 mo |
MET/CEP7 5.7, ATM S378G, MDM4 splice region variant, ARID1A D1193N, PIK3CA E453K | MET/CEP7 > 25, ATM S378G, MDM4 splice variant, ARID1A D1193N, PIK3CA E453K |
| 4 | Lorlatinib 50 mg QD + crizotinib 250 mg BID | PD <1 mo |
MET/CEP7 2.4, NF1 G2379R, TP53 V274G, MYC gain | MET/CEP7 > 25, NF1 G2379R, TP53 V274G, NOTCHL1 S2364G, MYC gain |
| 5 | Lorlatinib 50 mg QDa + crizotinib 250 mg BID | PR (−60%) 11 mob |
MET/CEP7 ≥ 25, APC Y1642_V1644del | N/A |
| 6 | Lorlatinib 50 mg QD + crizotinib 250 mg BID | PD <1 mo |
MET/CEP7 5.5, TP53 R273C | N/A |
| 7 | Lorlatinib 50 mg QD + crizotinib 250 mg BID | PR (−51%) 6 mo |
MET amplification (2.5), TP53 E346∗, MYC amplification (3.8) by plasma | MET amplification (6.8), TP53 E346∗, MYC amplification (19), MET L329fs (0.01) by plasma |
| 8 | Lorlatinib 50 mg QD + crizotinib 250 mg BID | PD <1 mo |
MET amplification (5.4), TP53 C135F (4.6), BRCA2 D3188fs (2.4), APC M314T (0.6), STK11 A43V (0.3) | MET amplification (20.6), BRCA2 D3188fs (9.1), TP53 C135F (25.6), ST7-MET (0.7), APC M314T (0.4), CDK12 P670A (0.3) |
| 9 | Alectinib 600 mg BIDa + capmatinib 400 mg BID | SD (non-CR/non-PD) 9 mo |
MET/CEP7 ≥ 25, SMARCA4 P47T, EGFR P596L | (1) MET/CEP7 0.9, SMARCA4 P47T, EGFR P596Lc (2) MET/CEP7 2.3, ALK G1202R, SMARCA4 P47T, EGFR P596Lc |
| 10 | Alectinib 600 mg BID + capmatinib 400 mg BIDa | SD (−8%) 10 mo |
MET/CEP7 ≥ 25, TP53 E180∗, APC E1156K | MET/CEP7 1.0, NF1 H1748Y, BRCA1 C328Y, TP53 E171K, PIK3CA E545K, MYC S160L, MYC S206L |
| 11 | Alectinib 600 mg BID + capmatinib 300 mg BID | PR (−70%) 7 mo |
MET/CEP7 7.7, TP53 N131Y, SMARCA4 D1183N | N/A |
| 12 | Alectinib 600 mg BID + crizotinib 200 mg BID | SD (−26%) 6 mo |
MET amplification by NGS, TP53 E285K | MET amplification by NGS, TP53 E285K |
QD, daily; BID, twice daily; PD, progressive disease; PR, partial response; SD, stable disease; CR, complete response per RECIST v.1.1; N/A, not applicable, as no biopsy was performed; NGS, next-generation sequencing; CEP7, centromeric probe 7; RECIST, Response Evaluation Criteria in Solid Tumors.
Lorlatinib initially given at 50 mg and then escalated to 75 mg after 2 weeks for patient 3, lorlatinib reduced to 25 mg for neurocognitive toxicity for patient 5, alectinib escalated to 900 mg BID for patient 9 for brain progression, at which time capmatinib reduced to 300 mg BID, capmatinib reduced to 200 and 300 mg BID from 400 mg BID for patient 10 for pyrexia.
Treatment stopped for toxicity despite ongoing partial response.
Patient underwent resection and molecular analysis of two separate enlarging brain metastases.
Response to ALK/MET Therapy and Findings From Longitudinal Biopsies
We used RECIST v.1.1 to retrospectively evaluate the efficacy of dual ALK- plus MET-directed therapy (Table 2). As summarized in Table 2, regimens administered to the 12 patients included crizotinib (n = 2), lorlatinib plus crizotinib (n = 6), alectinib plus crizotinib (n = 1), and alectinib plus capmatinib (n = 3). Seven patients had longitudinal biopsies (i.e., repeat molecular analysis after progression on ALK- + MET-directed therapy). Findings from longitudinal tissue and plasma analysis are captured in Table 2. Outcomes of two patients (patients #1 and #3 in Table 2) have previously been reported.3
Crizotinib
Two patients received crizotinib 250 mg twice daily as monotherapy to address MET amplification (Table 2). As previously reported,3 patient #1 experienced a partial response before developing progressive disease within 3.5 months of commencing crizotinib, at which time plasma was found to have persistent MET amplification with multiple new ALK kinase domain mutations; tissue analysis noted stable MET amplification (MET/CEP7 ratio ≥ 25 in tissue pre- and post-crizotinib). Patient #2 experienced symptomatic benefit from crizotinib but had primary radiographic progression of disease prompting treatment discontinuation after 1 month. A post-crizotinib biopsy was not pursued for patient #2.
Lorlatinib Plus Crizotinib
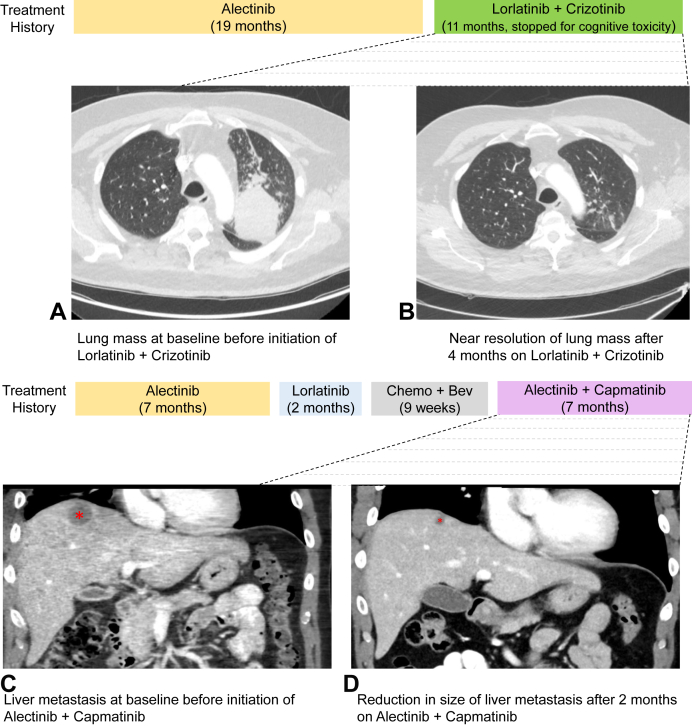
All six patients who received lorlatinib plus crizotinib initiated dosing at lorlatinib 50 mg once daily plus crizotinib 250 mg twice daily to prioritize activity against MET, given concerns about toxicity of full-dose lorlatinib (Table 3). One patient escalated lorlatinib to 75 mg after 2 weeks of dosing to maximize anti-ALK activity. Three of six patients who were treated with this regimen experienced a partial response. Two of the patients with partial response developed progressive disease within 6 months on treatment, both of whom underwent repeat molecular testing. In one case, analysis of longitudinal axillary node specimens revealed an increase in MET/CEP7 ratio from 5.7 to more than or equal to 25 without additional identified resistance mechanisms (patient #3, Table 2). The other patient’s plasma was found to have an increase in MET copies from 2.5 to 6.8 and a new MET L329fs mutation (0.01%) of indeterminate functional consequence after exposure to lorlatinib plus crizotinib (patient #7, Table 2). The third patient discontinued treatment for toxicity (see Safety of ALK/MET Therapy) despite ongoing response at 11 months (patient #5; Table 2 and Fig. 1A and B).
Table 3.
Safety Outcomes on ALK Plus MET-Directed Therapy
| Patient | Prior ALK TKIs | ALK/MET Therapy | Duration of Treatment | Dose Hold | Dose Reductiona | Treatment-Related Adverse Eventsb |
|---|---|---|---|---|---|---|
| 1 | Ceritinib | Crizotinib 250 mg BID | <1 mo | No | No | None reported |
| 2 | Alectinib | Crizotinib 250 mg BID | 3.5 mo | No | No | Peripheral edema (grade 1) |
| 3 | Ceritinib, alectinib, lorlatinib | Lorlatinib 75 mg QDc + crizotinib 250 mg BID | 3 mo | No | No | Nausea (grade 1), lightheadedness (grade 1) |
| 4 | Alectinib, lorlatinib | Lorlatinib 50 mg QD + crizotinib 250 mg BID | <1 mo | No | No | None reported |
| 5 | Alectinib | Lorlatinib 50 mg QDa + crizotinib 250 mg BID | 11 mo | Yes | Yes | Irritability (grade 2), anxiety (grade 2), confusion (grade 2), peripheral edema (grade 1), weight gain (grade 1) |
| 6 | Alectinib, brigatinib, lorlatinib | Lorlatinib 50 mg QD + crizotinib 250 mg BID | <1 mo | No | No | None reported |
| 7 | Alectinib, lorlatinib | Lorlatinib 50 mg QD + crizotinib 250 mg BID | 6 mo | No | No | Peripheral edema (grade 1), weight gain (grade 1), CPK elevation (grade 1), diarrhea (grade 1) |
| 8 | Alectinib | Lorlatinib 50 mg QD + crizotinib 250 mg BID | <1 mo | No | No | Weight gain (grade 2), peripheral edema (grade 2), speech change (grade 1) |
| 9 | Alectinib | Alectinib 600 mg BIDa + capmatinib 400 mg BID | 9 mo | No | Yes | Peripheral edema (grade 2), transaminase elevation (grade 1), constipation (grade 1), dry mouth (grade 1), thrombocytopenia (grade 1), nausea (grade 1), muscle soreness (grade 1) |
| 10 | Alectinib | Alectinib 600 mg BID + capmatinib 400 mg BIDa | 10 mo | Yes | Yes | Headache (grade 1), bilirubin increase (grade 1), fatigue (grade 1), pyrexia (grade 1), rash (grade 1), transaminase elevation (grade 1), peripheral edema (grade 2), joint stiffness (grade 1) |
| 11 | Alectinib, lorlatinib | Alectinib 600 mg BID + capmatinib 300 mg BID | 7 mo | Yes | No | Weight gain (grade 1), fatigue (grade 1), nausea (grade 1), peripheral edema (grade 2) |
| 12 | Alectinib | Alectinib 600 mg BID + crizotinib 200 mg BID | 6 mo | No | No | Bradycardia (grade 1), creatinine elevation (grade 1) |
QD, daily; BID, twice daily; CPK, creatine phosphokinase; RECIST, Response Evaluation Criteria in Solid Tumors; TKI, tyrosine kinase inhibitor.
Lorlatinib reduced to 25 mg for neurocognitive toxicity for patient 5. Alectinib escalated to 900 mg BID for patient 9 for brain progression, at which time capmatinib reduced to 300 mg BID, capmatinib reduced to 200 and 300 mg BID from 400 mg BID for patient 10 for pyrexia.
Excluding asymptomatic lipid elevation.
Lorlatinib initially given at 50 mg and then escalated to 75 mg after 2 weeks for patient 3.
Figure 1.
Responses to two different ALK plus MET combination regimens. Longitudinal axial computed tomography images reveal (A) baseline lung mass immediately before initiating lorlatinib plus crizotinib and (B) significant decrease in the lung mass on combination therapy. Longitudinal computed tomography coronal images reveal (C) baseline liver mass immediately before initiating alectinib plus capmatinib and (D) significant decrease in the size of the liver lesion as denoted by an asterisk on combination therapy. Treatment histories are presented above the images for each patient. Bev, bevacizumab.
Three patients experienced primary progression of disease (i.e., refractory disease), two of whom were referred for repeat molecular analysis (Table 2). At progression, one patient with refractory disease underwent analysis of an enlarging pleural effusion. Compared with pleural fluid collected at progression on lorlatinib approximately 1 month earlier (immediately before addition of crizotinib), there was an increase in MET copies from 2.4 to more than or equal to 25 without new genetic alterations (patient #4, Table 2). Another patient with refractory disease had plasma collected before and after progression which had an increase in MET copies from 5.4 to 20.6, including a new ST7-MET fusion at 0.7% allelic frequency (patient #8, Table 2). The third patient with refractory disease had a lung biopsy with a MET gene to copy number ratio of 5.5 after nonresponse to lorlatinib and did not undergo repeat molecular testing after primary progression on lorlatinib plus crizotinib (patient #6, Table 2).
Thus, of the four patients who had serial biopsies on this regimen, MET copy number was observed to increase on crizotinib in all cases and two patients were found to have additional MET alterations in plasma.
Alectinib Plus Capmatinib
Of the three patients who received alectinib plus capmatinib, two were initially treated with alectinib 600 mg twice daily plus capmatinib 400 mg twice daily. The remaining patient received alectinib 600 mg twice daily in combination with capmatinib 300 mg twice daily out of concern for toxicity given considerable baseline fatigue. All three patients who received alectinib plus capmatinib experienced either disease stabilization or a partial response to the regimen (patient #9, patient #10, and patient #11, Table 2). Two patients had stable disease lasting more than 6 months, and the final patient had a partial response lasting 7 months (Table 2 and Fig. 1C and D). In the two cases where repeat tissue molecular analysis was performed at progression on alectinib plus capmatinib, the number of MET copies in the post-combination therapy specimen was lower than that in the pretreatment specimen. For example, one patient had a pretreatment lung biopsy with high-level MET amplification (MET/CEP7 ratio ≥ 25) and a post-treatment biopsy of a solitary adrenal site of progression that did not reveal MET amplification or new ALK mutations (patient #10, Table 2). The second patient commenced treatment with alectinib plus capmatinib in the setting of central nervous system (CNS)-only progression, at which time high-level MET amplification (MET/CEP7 ratio ≥ 25) was detected in a resected CNS lesion. After more than 9 months of intracranial disease stabilization on ALK/MET combination therapy, the patient underwent resection of two growing CNS metastases with molecular analysis revealing MET/CEP7 ratio of 0.9 in one brain lesion and MET/CEP7 ratio of 2.3 with concurrent ALK G1202R in a separate metastasis (patient #9, Table 2).
Alectinib Plus Crizotinib
One patient received alectinib 600 mg twice daily plus crizotinib 200 mg twice daily after progression on first-line alectinib. The lower dose of crizotinib was selected to minimize overlapping toxicity, namely muscle enzyme and creatinine elevation, given baseline renal involvement of tumor. Notably, MET amplification was detected through biopsy of an enlarging liver metastasis that was analyzed with the FoundationOne assay and copy number was not specified. On combination therapy, the patient experienced stable disease per RECIST v.1.1 lasting approximately 6 months (patient #12, Table 2). At progression, a biopsy of a new cutaneous nodule revealed persistent MET amplification (by NGS, copy number not specified) and absence of ALK or MET mutations.
Safety of ALK/MET Therapy
Crizotinib
Neither of the two patients who were treated crizotinib 250 mg twice-daily monotherapy required dose reduction or dose interruption. The only adverse event noted was grade 1 peripheral edema (Table 3).
Lorlatinib Plus Crizotinib
Four of six patients treated with lorlatinib plus crizotinib developed nonlaboratory treatment-related adverse events (Table 3). The remaining two patients were on treatment for less than 1 month before discontinuing treatment for progression. One patient discontinued treatment for neurocognitive toxicity, as described in subsequent texts. Three patients (50%) developed peripheral edema and weight gain, all of which were grade 1 to 2 events that did not require dose interruption. Two of the three patients received diuretics to address the edema. Two patients (33%) experienced neurocognitive adverse events on lorlatinib 50 mg plus crizotinib 250 mg twice daily, neither of whom had received lorlatinib before combination therapy. Specifically, one patient experienced grade 1 speech changes but did not require dose adjustments as she discontinued therapy within 1 month of initial dosing in the setting of disease progression. The second patient experienced grade 2 irritability, anxiety, and confusion necessitating a 3-week dose interruption followed by dose reduction of lorlatinib from 50 mg to 25 mg with sustained dosing of crizotinib at 250 mg twice daily. The patient ultimately discontinued treatment for persistent neurocognitive toxicity at lorlatinib 25 mg despite ongoing partial response. Additional adverse events observed across patients included grade 1 diarrhea and grade 1 creatinine phosphokinase elevation in a patient who also had grade 1 peripheral edema and grade 1 weight gain and grade 1 nausea and lightheadedness in a separate patient.
Alectinib-Based Combinations
All four patients who received alectinib-based combinations (n = 1 alectinib + crizotinib; n = 3 alectinib + capmatinib) experienced a treatment-related adverse event (Table 3). Two patients required dose interruption for toxicity (n = 1 grade 2 peripheral edema and n = 1 grade 1 pyrexia, respectively), one of whom ultimately required reduction of the capmatinib dose without change in alectinib dosing. No patient discontinued treatment for toxicity. All three patients who received capmatinib in combination with alectinib developed grade 2 peripheral edema for which diuretics were prescribed. Two of these patients also had grade 1 muscle or grade 1 joint adverse events and grade 1 transaminase level elevation. One patient who received alectinib plus capmatinib developed persistent grade 1 pyrexia necessitating dose interruption, reduction of capmatinib dose, and implementation of hypersensitivity medications (steroids, acetaminophen, antihistamines) before dosing. The patient’s capmatinib dose was eventually re-escalated to 300 mg twice daily from 200 mg twice daily and maintained at this dose without further need of steroids or acetaminophen. Finally, the patient who received alectinib plus crizotinib developed grade 1 asymptomatic bradycardia in the setting of concurrent metoprolol use and grade 1 creatinine elevation, neither of which required interruption or adjustment of alectinib or crizotinib dosing.
Discussion
Despite the recognition of MET amplification as a recurrent driver of off-target resistance to ALK TKI therapy, robust studies evaluating the sensitivity of this subset of ALK+ NSCLC to MET-directed approaches are lacking. Here, we summarize efficacy and safety outcomes of 12 patients with MET-amplified ALK+ NSCLC who received therapies targeting ALK and MET. Our series suggests that combined ALK and MET inhibition may be active in ALK+ NSCLC harboring concurrent MET amplification, as partial responses were observed in five of 12 patients. Across the various regimens administered, peripheral edema was the most common side effect.
As a standalone oncogenic driver, MET amplification confers sensitivity to MET therapeutic targeting, particularly in the context of high-level amplification.11,12 Similarly, in MET-amplified EGFR-mutant NSCLC, sensitivity to MET TKIs is most impressive when cohorts are enriched for tumors with higher MET copies. In our series, the level of MET amplification did not seem to affect responses, as primary progression was observed among patients with tumors harboring high-level MET amplification and partial responses were found in the presence of lower-level MET amplification. This discordance may have resulted from multiple factors, including the small cohort size, variety of regimens and doses used, and potential molecular heterogeneity of these ALK+ NSCLCs. This molecular heterogeneity might have also accounted for the four (33%) tumors with primary progression on MET-directed therapy in our study. Notably, 75% of MET-amplified tumors in our study harbored TP53 mutations; TP53 mutations have been linked to genetic instability and earlier development of resistance to targeted therapy in oncogene-driven lung cancer.13 Thus, additional larger studies are warranted to fully explore the relationship between MET copies and response to MET-targeted therapy in ALK+ NSCLC. Ideally, such studies should also evaluate how co-alterations in other genes might modulate sensitivity to MET targeting.
The size of our series, variation in dosing of the MET inhibitors, and the retrospective nature of the analysis also limited any formal comparison of the four therapeutic regimens. Still, we observed potential differences between the regimens that should be followed-up with larger studies. For example, the durability of disease control with the combination of capmatinib plus alectinib was particularly encouraging given the CNS penetration of both drugs. Nevertheless, the combination was associated with toxicity—namely grade 2 peripheral edema—necessitating diuretics, dose interruption, and dose reduction. Peripheral edema is a class toxicity of MET inhibitors that was found with crizotinib as well, indicating a need for refinement of the dosing strategy for these combinations. Notably, a different class of toxicity—neurocognitive toxicity—limited the ability to continuously dose lorlatinib in a patient who had a durable partial response to lorlatinib plus crizotinib. In addition to potential differences in the toxicity profiles of the various regimens, we saw differences in the molecular alterations that emerged at resistance to the different regimens. Specifically, we detected ALK mutations in tumors of patients receiving crizotinib and alectinib that were not observed when lorlatinib was used. With respect to MET-dependent resistance mechanisms, we observed increase in MET copies on crizotinib compared with decrease on capmatinib. This raises the possibility that the more potent MET TKI (capmatinib) may better suppress outgrowth of MET-amplified populations. Nevertheless, as this finding is counterintuitive to current understanding of crizotinib activity,11 larger translational studies are needed to formally validate and fully reconcile the conflicting trajectories of MET copies observed with the two MET-targeting drugs in our small series. Notably, we did not detect MET exon 14 skipping in either pre- or post-treatment specimens.
Our study has limitations, several of which have been discussed previously. These limitations include the retrospective nature of the analysis, inconsistent capturing of adverse events in clinic notes particularly for patients who received therapies off-label or through commercial access, variety of ALK/MET regimens used, variation in dosing within specific regimens given lack of prospective trials, and differences in disease sites where biopsy was done before commencing ALK/MET therapy versus after progression on ALK/MET therapy. Many of these limitations arise from the study’s focus on a molecularly defined subpopulation of a rare subset of NSCLC. Indeed, because of the overall rarity of MET-amplified ALK+ NSCLC in the overall population of patients with NSCLC, our attempt to conduct a prospective single-institution clinical trial of lorlatinib plus crizotinib was unsuccessful because of poor accrual, as was the case with a multicenter NCI prospective study (NCT03737994) that recruited patients with MET-amplified ALK+ NSCLC. In addition, patients in this study were able to access these investigational combinations through off-label insurance approval, expanded-access programs, and single-patient IND studies; these options may not be available to patients residing outside of the United States, patients without insurance, and patients with prohibitive co-pays.
In summary, we observed antitumor activity of several distinct ALK/MET regimens in MET-amplified ALK+ NSCLC and have identified potential mechanisms of resistance to dual ALK plus MET inhibition. The variable duration of disease control observed with the heterogeneous regimens in our small series highlights the need for future, larger studies that focus on efficacy of particular regimens in addition to characterizing the molecular determinants of response and resistance to the specific ALK- and MET-based combinations.
CRediT Authorship Contribution Statement
Ibiayi Dagogo-Jack: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing.
Lesli A. Kiedrowski: Data curation, Formal analysis, Investigation, Methodology, Writing—review and editing.
Rebecca S. Heist: Resources, Data curation, Writing—review and editing.
Jessica J. Lin: Resources, Data curation, Writing—review and editing.
Catherine B. Meador: Data curation, Writing—review and editing.
Elizabeth A. Krueger: Resources, Data curation, Writing—review and editing.
Andrew Do: Data curation, Project administration, Writing—review and editing.
Jennifer Peterson: Data curation, Project administration, Writing—review and editing.
Lecia V. Sequist: Resources, Data curation, Writing—review and editing.
Justin F. Gainor: Resources, Data curation, Writing—review and editing.
Jochen K. Lennerz: Data curation, Formal analysis, Methodology, Visualization, Writing—original draft.
Subba R. Digumarthy: Data curation, Formal analysis, Investigation, Writing—review and editing.
Acknowledgments
This work was supported by an institutional research grant from the Massachusetts General Hospital Center for Diversity and Inclusion (Dr. Dagogo-Jack), a National Cancer Institute Career Development Award (K12CA087723-16 to Dr. Dagogo-Jack), a grant from LUNGevity/ALK-Positive (Dr. Dagogo-Jack), philanthropic funding (Be A Piece of the Solution), U.S. National Institutes of Health (NIH) grant R37 CA225655 (to Dr. Lennerz), and an NIH grant 5R01 CA164273 (to Dr. Lin). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or any other organization. The authors thank the patients who provided tissue and plasma specimens for this analysis, the patients who participated in the clinical trials referenced in the text, and the clinical trials staff at the Massachusetts General Hospital.
Footnotes
Disclosure: Dr. Dagogo-Jack has received honoraria from Foundation Medicine, Creative Education Concepts, OncLive, ASCO Post, DAVA Oncology, Medscape, Research to Practice, Total Health, Aptitude Health, and American Lung Association; consulting fees from AstraZeneca, Boehringer Ingelheim, Bayer, BostonGene, Bristol-Myers Squibb, Catalyst, Genentech, Janssen, Merus, Novocure, Pfizer, Sanofi-Genzyme, Syros, and Xcovery; research support from Array, Genentech, Novartis, Pfizer, and Guardant Health; and travel support from Array and Pfizer. Mrs. Kiedrowski is an employee and shareholder of Guardant Health. Dr. Lin has served as a compensated consultant for Genentech, C4 Therapeutics, Blueprint Medicines, Nuvalent, Bayer, Elevation Oncology, Novartis, Mirati Therapeutics, Regeneron, AnHeart Therapeutics, and Turning Point Therapeutics; received honorarium and travel support from Pfizer; received institutional research funds from Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, Bayer, Elevation Oncology, Roche, Linnaeus, Nuvalent, and Novartis; and received CME funding from OncLive, MedStar Health, and Northwell Health. Dr. Heist received consulting fees from AbbVie, Claim Therapeutics, Daiichi Sankyo, EMD Serono, Eli Lilly, Novartis, Regeneron, and Sanofi and has research support (to institution, not to self) from AbbVie, Agios, Corvus, Daiichi Sankyo, Erasca, Eli Lilly, Mirati, Novartis, and Turning Point. Mrs. Krueger has received consulting fees from Pfizer. Dr. Sequist received consulting fees from AstraZeneca, Genentech, Janssen, Takeda, and Pfizer and research support from Boehringer Ingelheim, Novartis, AstraZeneca, and Delfi Diagnostics. Dr. Gainor has served as a compensated consultant or received honoraria from Bristol-Myers Squibb, Genentech/Roche, Takeda, Loxo/Eli Lilly, Blueprint, AstraZeneca, Gilead, Moderna, AstraZeneca, Curie Therapeutics, Mirati, Nuvalent, Pfizer, Novartis, Merck, iTeos, Karyopharm, Silverback Therapeutics, and GlydeBio; research support from Novartis, Genentech/Roche, and Takeda; institutional research support from Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array BioPharma, Merck, Adaptimmune, Novartis, and Alexo; holds equity in AI Proteins; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. Dr. Digumarthy provides independent image analysis for hospital-contracted clinical research trials programs for Merck, Pfizer, Bristol-Myers Squibb, Novartis, Roche, Polaris, Cascadian, AbbVie, Gradalis, Bayer, Zai Laboratories, Biengen, Riverain, Resonance, AstraZeneca, and Analise; has research grants from Lunit Inc., GE, Qure AI, and Vuno; and receives honorarium from Siemens and book royalties from Elsevier. The remaining authors declare no conflict of interest.
Cite this article as: Dagogo-Jack I, Kiedrowski LA, Heist RS, et al. Efficacy and tolerability of ALK/MET combinations in patients with ALK-rearranged lung cancer with acquired MET amplification: a retrospective analysis. JTO Clin Res Rep. 2023;4:100534.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100534.
Supplementary Data
References
- 1.Gainor J.F., Dardaei L., Yoda S., et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6:1118–1133. doi: 10.1158/2159-8290.CD-16-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin J.J., Riely G.J., Shaw A.T. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017;7:137–155. doi: 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagogo-Jack I., Yoda S., Lennerz J.K., et al. MET alterations are a recurring and actionable resistance mechanism in ALK-positive lung cancer. Clin Cancer Res. 2020 doi: 10.1158/1078-0432.CCR-19-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiba-Ishii A., Johnson T.W., Dagogo-Jack I., et al. Analysis of lorlatinib analogs reveals a roadmap for targeting diverse compound resistance mutations in ALK-positive lung cancer. Nat Cancer. 2022;3:710–722. doi: 10.1038/s43018-022-00399-6. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 2021;16 12:2091–2108. doi: 10.1016/j.jtho.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Sequist L.V., Lee J.S., Han J.Y., et al. TATTON Phase Ib expansion cohort: osimertinib plus savolitinib for patients with EGFR-mutant, MET-amplified NSCLC after progression on prior epidermal growth factor (EGFR) tyrosine kinase inhibitors (TKI) Lancet Oncol. 2020;21:373–386. doi: 10.1016/S1470-2045(19)30785-5. [DOI] [PubMed] [Google Scholar]
- 7.Mazieres J., Kim T.M., Lim B.K., et al. LBA52 - tepotinib + osimertinib for EGFRm NSCLC with MET amplification (METamp) after progression on first-line (1L) osimertinib: initial results from the Insight 2 study. Ann Oncol. 2022;33(suppl 7):S808–S869. [Google Scholar]
- 8.Odegaard J.I., Vincent J.J., Mortimer S., et al. Validation of a plasma-based comprehensive cancer genotyping assay utilizing orthogonal tissue- and plasma-based methodologies. Clin Cancer Res. 2018;24:3539–3549. doi: 10.1158/1078-0432.CCR-17-3831. [DOI] [PubMed] [Google Scholar]
- 9.Frampton G.M., Fichtenholtz A., Otto G.A., et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–1031. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Z., Liebers M., Zhelyazkova B., et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 11.Camidge D., GA O., Clark J., et al. Crizotinib in patients with MET-amplified non-small cell lung cancer: updated safety and efficacy findings from a phase 1 trial. J Clin Oncol. 2018:9062. [Google Scholar]
- 12.Wolf J., Seto T., Han J.Y., et al. Capmatinib in. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 13.Vokes N.I., Chambers E., Nguyen T., et al. Concurrent TP53 mutations facilitate resistance evolution in EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2022;17:779–792. doi: 10.1016/j.jtho.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.