Abstract
Background
Our recent study demonstrated that selective aryl hydrocarbon receptor modulators (SAhRMs), such as 1,4-dihydroxy-2-napthoic acid (DHNA) act as antidepressants in female mice. Given that some effects of certain SAhRMs are known to also be mediated via estrogen receptor signaling, this study examined whether the effects of SAhRMs on mood, emotional state, and cognition are sex-dependent.
Methods
C57BL/6N mice were fed with vehicle or 20 mg/kg DHNA for three weeks prior to four weeks of unpredictable chronic mild stress (UCMS). Mice were examined for depression-like behaviors (sucrose preference, forced swim test (FST), splash test, tape groom test), emotional state (open-field test, light/dark test, marble burying, novelty-induced hypophagia, elevated-plus maze), and cognition (object location recognition, novel object recognition, Morris water maze).
Results
In females, UCMS decreased sucrose preference and increased FST immobility time; both effects were prevented by DHNA. In males, UCMS increased FST immobility time, and increased the latency to groom in the splash test. These effects were not mitigated by DHNA. However, in males, UCMS induced an increase in novelty-induced locomotion, an increase in the time spent in the light compartment in the L/D test, and an increase in the time spent with an object in a novel location. These effects were prevented by DHNA.
Conclusions
Our findings indicate that DHNA has high potential to act as antidepressants in females. However, given classical interpretation, DHNA did not appear to act as an antidepressant in males. Nonetheless, our findings indicate that DHNA can mitigate stress effects and reactivity in males.
Keywords: Stress; Depression; Anxiety; Cognition; Aryl hydrocarbon receptor (AhR); 1,4-dihydroxy-2-naphthoic acid (DHNA)
Introduction
Major depressive disorder (MDD) is a severe and debilitating disorder that affects approximately 280 million people worldwide (WHO, 2022). Depression reduces an individual’s life quality, work productivity, family and social functioning, and it is a leading cause of suicide (Ferrari et al., 2013, Hawton et al., 2013, O’Rourke et al., 2022). Although multiple pharmacological and behavioral treatments are currently available (McIntyre et al., 2014, Kautzky et al., 2019), about two-thirds of patients need to evaluate multiple medications, a process that takes months, before they experience symptom relief (van Bronswijk et al., 2019). Moreover, some do not respond to, or experience remission with currently available medications. Thus, although there are options for treating depression, there is still a critical need for improving our understanding of the underlying causes of depression and to provide improved, more precise, medications for individuals who do not respond well to presently available MDD treatments.
Our recent study demonstrated that selective aryl hydrocarbon receptor (AhR) modulators (SAhRMs) have a potential to act as an antidepressant (Madison et al., 2022). The AhR is a ligand-activated transcription factor that binds diverse ligands, including phytochemicals, endogenous biochemicals, microbial metabolites, and pharmaceuticals. Many of these SAhRMs exhibit AhR-dependent health benefits (Safe et al., 2018, Safe et al., 2020, Stockinger et al., 2021). Previously, we examined female mice, given that the prevalence of MDD is almost double in females as compared to males (Albert, 2015). We examined 3,3’-diindolylmethane (DIM) and 1,4-dihydroxy-2-naphthoic acid (DHNA), two SAhRMs that exhibit significant AhR agonist activity in multiple cell types (Cheng et al., 2017, Safe et al., 2018, Safe et al., 2020, Stockinger et al., 2021, Vermillion Maier et al., 2021). We employed unpredictable chronic mild stress (UCMS), an established rodent model of depression with high translational potential and relevance to human depression (Willner, 2017). UCMS induces a decrease in sucrose preference, which serves as a rodent model to measure anhedonia (Strekalova et al., 2006). Anhedonia is the decreased ability to experience pleasure (Ribot, 1896) and is a core feature of MDD (Kennedy, 2008). Female mice that were administered with DIM or DHNA before being subjected to UCMS did not exhibit UCMS-induced anhedonia-like. Female mice that were administered these compounds after the start of UCMS initially exhibited anhedonia-like behaviors, but recovered upon receiving the ligands (Madison et al., 2022). Moreover, 3,7-dihydroxy-2-naphthoic acid (3,7-DHNA), a structurally similar analog of DHNA that is predominantly inactive at the AhR (Cheng et al., 2017), did not prevent UCMS-induced anhedonia-like behaviors, suggesting a possible role for AhR ligands in protecting against the effects of UCMS.
Many AhR ligands, such as the polyphenolics, bind to both the AhR and the estrogen receptor (ER) (Parkin and Malejka-Giganti, 2004, Wihlén et al., 2009, Marques et al., 2014). Furthermore, ER-beta and AhR are highly co-localized in cortical tissue, and ERs exhibit reciprocal interactions with AhR, particularly in the presence of AhR agonists (Wormke et al., 2003, Matthews et al., 2005, Matthews and Gustafsson, 2006, Kajta et al., 2009, Tarnow et al., 2019). Thus, we hypothesize that the antidepressant effects of SAhRMs are to some degree sex-dependent. Therefore, in this study we compared the effects of DHNA to prevent UCMS-induced depression-like behaviors between males and females. Mice were examined for sucrose preference, as an indicator for anhedonia (Strekalova et al., 2006), and immobility in the forced swim test (FST) as an indicator for despair (i.e. feeling of futility or hopelessness) (Porsolt et al., 2001). Additionally, we used the splash test and the tape groom test, which are associated with levels of self-care and motivational behavior (Isingrini et al., 2010, Bouguiyoud et al., 2021). Furthermore, similar to our previous study, using a battery of behavioral tests, we also examined the effects of DHNA on emotional state and cognition.
2. Methods
2.1. Animals
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. In this study, 56 female and 64 male mice were used. C57BL/6N mice were purchased from Envigo Laboratories (Houston, Texas, USA) and housed four per cage with food and water ad libitum in a temperature-controlled (21 +/− 2°C, humidity 45%) vivarium with a 12-hour light/12-hour dark cycle (light on at 7:30 AM). Mice arrived in the vivarium at 6–7 weeks old and were acclimated for at least 6 days before beginning the experiment. Unless specifically mentioned, all tests were conducted in rooms containing low illumination (60 W white light) and 40 dB white noise generator.
2.2. SAhRM
1,4-dihydroxy-2-naphthoic acid (DHNA, 97%) was purchased from Sigma-Aldrich (St. Louis, MO). Mice were fed with vehicle or 20 mg/kg DHNA dissolved in corn oil and mixed into melted peanut butter (Madison et al., 2022). Mice were placed in individual cages for approximately 10–30 minutes until they finished eating and were then returned to their home cage. This method was chosen to avoid the stress of daily gavage injection (Gonzales et al., 2014).
2.3. Unpredictable chronic mild stress (UCMS)
The UCMS protocol is described in detail in our recent publication (Madison et al., 2022). Briefly, this protocol does not include food or water deprivation. Seven stressors were administered weekly over the course of 4–5 weeks - restrainer, wet cage, cage replacement, tilted cage, dampened bedding, empty cage, and light/dark disruption. The order of appearance and daily schedule of each stressor was altered weekly.
2.4. Depression-like behaviors:
2.4.1. Sucrose preference
Each week, for 2 consecutive days, animals were individually housed for 4 hours and had access to one bottle of water and one bottle of 3% sucrose. The bottles were placed side by side, were freely available, and their positions were switched daily to account for a side preference. Sucrose preference was calculated as [milliliters sucrose solution consumed]/[milliliters sucrose solution + milliliters water consumed]. For each mouse, data was averaged across the 2 consecutive days.
2.4.2. Forced swim test (FST)
A 35 cm height × 20 cm diameter cylindrical tank was filled with water to a depth of 15 cm, which was allowed to adjust to room temperature (23–25° C). The temperature was monitored for consistency throughout the testing. A single animal was placed in the cylinder for 6 minutes and recorded by a ceiling-mounted video camera. Videos were scored by two experimenters and the average immobility time from the two experimenters was calculated for each animal.
2.4.3. Splash test
Each mouse was placed in an empty home cage (free of bedding). The mouse’s dorsal coat was sprayed with a 10% sucrose solution and was observed for 5 minutes for latency to begin grooming and time spent grooming.
2.4.4. Tape groom test
A mouse was placed in an empty home cage (free of bedding). A small piece (1 cm × 1 cm) of laboratory tape was placed on the mouse’s back. The mouse was observed for latency to begin grooming the tape.
2.5. Emotional State
This battery of tests was conducted as described in our previous study (Madison et al., 2022). The difference between each behavior after and before being subjected to UCMS was calculated for each mouse.
2.5.1. Novelty-induced locomotion and open field test (OFT)
These tests were conducted in an automated optical beam activity monitor [40 × 40 × 30.5 (height) cm]. Subjects were placed in the center of the box to begin a 10-minute test session. The computerized integration of the data was used to score locomotion (total distance traveled in cm) and time spent in the center and in the periphery.
2.5.2. Light/dark (L/D) test
This test is assessed in the same activity boxes as the OFT, split into an 18×18 cm dark chamber and a light zone. A mouse was placed in the middle of the light chamber and recorded for 10 minutes. The computerized integration of the data was used to score time spent in the light and dark zones.
2.5.3. Elevated plus maze (EPM)
The EPM apparatus consists of four arms (87 mm wide, 155 mm long) elevated 63.8 cm above the ground, with two arms enclosed on two sides by 16.3 cm high opaque walls (Hofford et al., 2009). Mice were placed in the center of the maze facing toward an enclosed arm and recorded for 7 minutes by an overhead camera. Time spent in the open arms (defined as all four legs having crossed the entrance line to one of the open arms), and total crosses of the middle of the apparatus were scored for the last 5 minutes of each video.
2.5.4. Novelty-induced hypophagia (NIH)
In their home cage, mice were introduced to diluted condensed milk for 30 minutes daily for 3 consecutive days. Carnation sweetened condensed milk, diluted 1:3 in water, was provided in plastic serological pipettes (10 mL) with attached sippers and rubber stoppers that are mounted to the wire cage lid. On day 4, mice were tested individually in their home cages with low illumination level (50 lux). Each mouse was removed from the cage while the pipette is installed on the cage lid. Testing began immediately upon returning to the home cage. On day 5, mice were tested in a novel cage free of bedding and in bright illumination level (1200 lux). On both testing days, mice were recorded for the latency to the first sip of milk.
2.5.5. Marble Bury (MB) test
Each mouse was placed in a large cage (40 × 24 × 20 cm) filled with bedding 5 cm deep and containing 20 blue marbles (positioned in 4 × 5 grid) for 30 min. Marbles were counted as buried if 2/3 or more was covered by bedding. Number of buried marbles was recorded.
2.6. Cognition
2.6.1. Object Location Recognition (OLR) Test
This test is assessed in the same activity boxes as the OFT. The procedure is divided into three stages: habituation, familiarization, and test phases. In the habituation phase, mice are allowed to explore the box (with no objects) for 10 minutes to acclimate to the apparatus and reduce the animals’ fear of a new environment. The habituation phase is repeated for three consecutive days. On the fourth day, the familiarization and test phases begin. Firstly, two plastic toy soldiers are fixed to Plexiglas pedestals using wall-safe gum-tack. The mice are allowed to freely explore the box for 5 minutes. Then, after an interval of 30 minutes, mice are returned to the box in which one of the original objects changed location (“novel”) and the other object remained in the original position (“familiar”), and mice are allowed to freely explore the box again for 5 minutes. The mice are removed from the behavior boxes and placed in their home cages between phases and after testing. The boxes, pedestals, and objects are cleaned using 70% ethanol followed by water between each phase of testing. The increase in exploration of the object in the novel location was calculated for each mouse using the formula: [(time spent with the object in the novel location in round 2/time spent with the object in the original location in round 1) × 100].
2.6.2. Novel Object Recognition (NOR) Test.
Twenty-four hours after the OLR test, the NOR test is carried out, including the familiarization phase and the test phase, with an interval of 30 min between two phases. Firstly, two plastic toy soldiers are fixed to plexiglas pedestals using wall-safe gum-tack. The mice are allowed to freely explore the box for 5 minutes. In the test phase, mice are returned to the box in which one of the toy soldiers is replaced by a small metal bucket. Mice are allowed to freely explore the box for 5 minutes. The mice are removed from the behavior boxes and placed in their home cages between phases and after testing. The boxes, pedestals, and objects are cleaned using 70% ethanol followed by water between each phase of the test. The increase in exploration of the novel object was calculated for each mouse using the formula: [(time spent with the novel object in round 2/time spent with the familiar object in that location in round 1) × 100].
2.6.3. Morris water maze (MWM)
As described in our previous study (Madison et al., 2022), a 36” diameter pool was used as the maze with a 5 in2 clear Plexiglas platform placed in the eastern quarter, 7 inches from the edge of the pool. The room featured distinct visual cues on the northern, eastern, and western walls, and the experimenter stood in a marked position in the southern side of the room. The time to reach the platform was recorded on six consecutive days. On days 1–5, mice were subjected to 4 daily trial sessions; the start position was rotated across days and sessions, and the time to reach the platform over the 4 daily sessions was averaged. On day 6, the challenge day, a previously unused start position was chosen and the time to reach the platform was recorded.
2.7. Experimental Design
The study includes four experimental groups: 1) NL/NS – unstressed mice receiving vehicle; 2) DHNA/NS - unstressed mice receiving DHNA; 3) NL/UCMS – UCMS-exposed mice receiving vehicle; and 4) DHNA/UCMS - UCMS-exposed mice receiving DHNA. Sample size was 13–18 per experimental group, with two exceptions: 1) for the splash and tape groom tests n=7–8 per experimental group for the females, and 2) for the OLR and NOR tests n=7–8 per experimental group for both males and females. As demonstrated in Fig 1, following a habituation period, mice were administered daily with vehicle or DHNA for the entire duration of the experiment. During the third week of ligand administration, mice were tested for their emotional state. Then, mice either remained unstressed or were subjected to UCMS for 4 weeks. Sucrose preference tests were conducted once weekly at the end of each week, starting the second week of UCMS and continuing for a week after the cessation of UCMS. Following the cessation of UCMS, mice were retested for effects their emotional state and cognition. Then, mice were examined in the FST, splash test and groom test.
Fig 1. Experimental design.
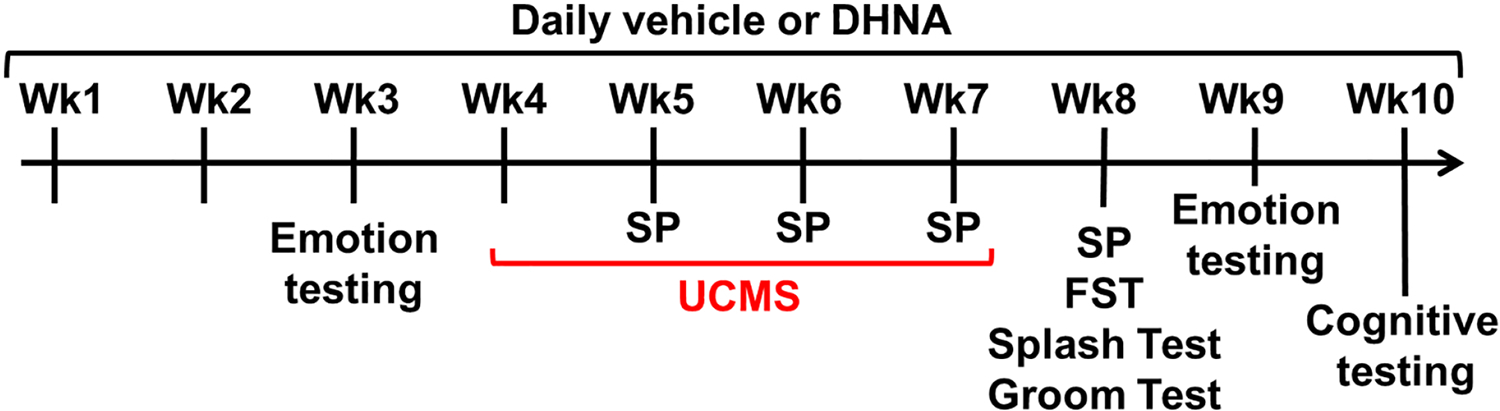
Wk = Week; Emotion testing = testing in the novelty-induced locomotion (NIL), open field test (OFT), light/dark (L/D) test, marble burying (MB), novelty-induced hypophagia (NIH) and elevated plus maze (EPM) paradigms; UCMS = Unpredictable chronic mild stress; SP = sucrose preference test; FST = forced swim test; Cognitive testing = testing in the object location recognition (OLR) test, novel object recognition (NOR) test, and the Morris water maze (MWM).
2.8. Data Analysis
For each behavioral test, data was analyzed using multivariate analysis of variance (MANOVA, IBM SPSE Statistics 25) for the between-group factors of sex, stress level (unstressed, UCMS), treatment (vehicle, DHNA) and within-group factors of week or day as applicable. Separate analysis was also conducted for each sex. Post hoc contrasts between each treatment group were conducted using the Bonferroni procedure. Differences with p-values of less than 0.05 were deemed statistically significant. Results are presented as mean ± SEM.
3. Results
3.1. Effects on weight
Males:
MANOVA analysis revealed a significant main effect of days (F(57, 3420)=101.906, p<0.0001), and significant interactions between DHNA and UCMS (F(1, 60)=4.113, p<0.05), day and DHNA (F(57, 3420)=1.486, p<0.05), and day and UCMS (F(57, 3420)=4.108, p<0.0001). Gain of weight did not significantly differ between the NL/NS and DHNA/NS groups. Overall, the NL/UCMS group gained significantly more weight than the DHNA/UCMS group (Fig 2A). However, the NL/UCMS did not significantly differ from the NL/NS group. Similarly, the DHNA/UCMS did not significantly differ from the DHNA/NS group.
Fig 2. Effects of DHNA and UCMS on body weight gain.
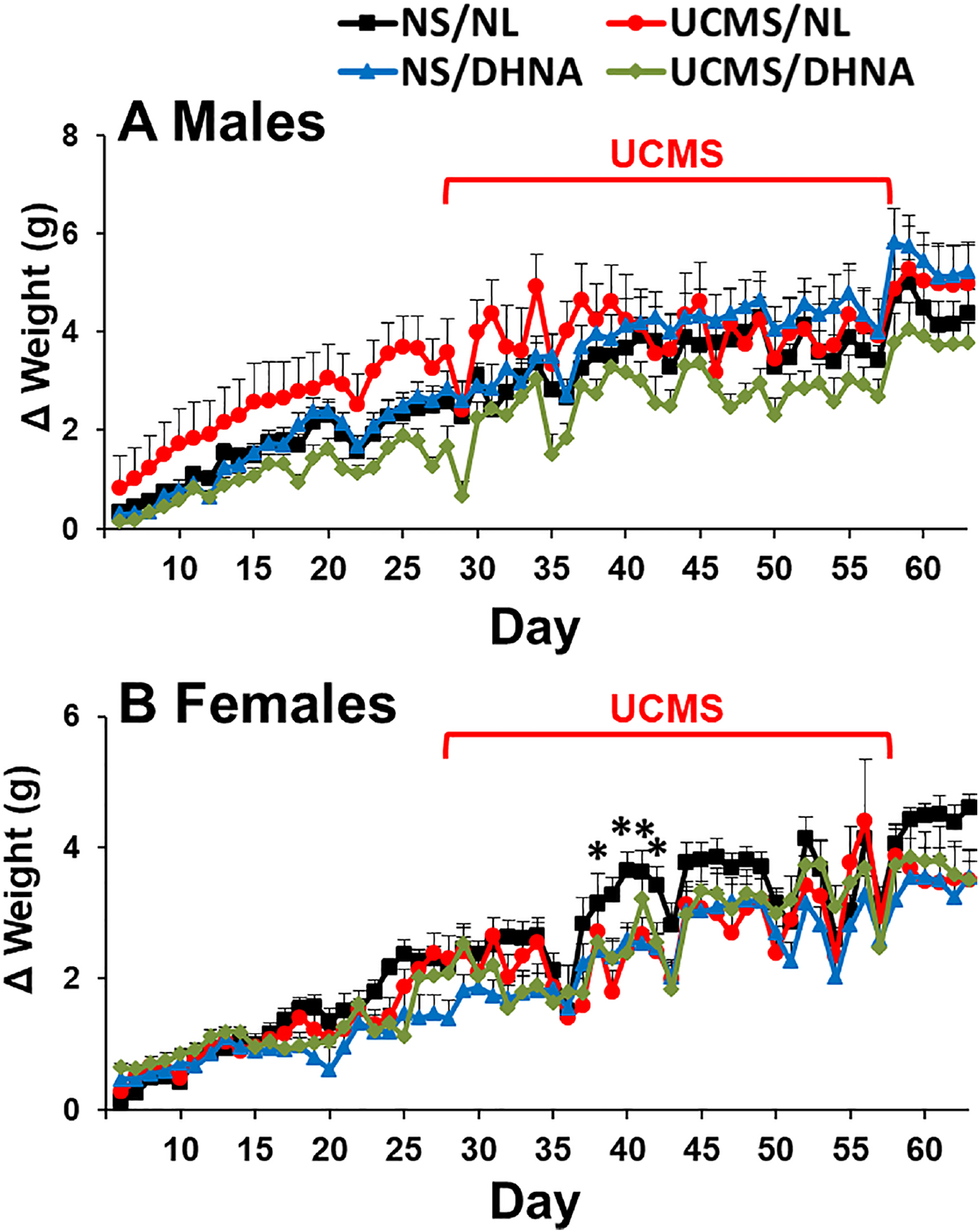
Male (A) and female (B) mice received vehicle or DHNA, and then were unstressed or subjected to UCMS for 4 weeks. (*) Bonferroni post hoc contrast indicates a significant difference from the other groups (p < 0.05). Results are presented as mean + SEM.
Females:
MANOVA analysis revealed a significant main effect of days (F(57, 2850)=89.377, p<0.0001), and significant interactions between day and DHNA (F(57, 2850)=1.569, p<0.01) and day and UCMS (F(57, 2850)=2.064, p<0.0001). Bonferroni post hoc contrast revealed that on experimental days 39–42 (second week of UCMS), the control unstressed group (NL/NS) gained significantly more weight as compared to the other groups (Fig 2B).
3.2. Antidepressant-like effects
Sucrose preference:
MANOVA analysis revealed a significant main effect of week (F(3, 333)=8.361, p<0.0001) and significant interactions between week and sex (F(3, 333)=3.652, p<0.05), sex and UCMS (F(1, 111)=16.062, p<0.0001), week, sex and UCMS (F(3, 333)=3.788, p<0.05), sex and DHNA (F(1, 111)=14.313, p<0.0001), week, sex and DHNA (F(3, 333)=6.210, p<0.001), and UCMS and DHNA (F(1, 111)=14.823, p<0.0001). For the females, significant main effects of UCMS (F(1, 51)= 9.133, p<0.01), DHNA (F(1, 51)= 10.239, p<0.01) and significant interactions between week and DHNA (F(3, 153)=4.735, p<0.01), UCMS and DHNA (F(1, 51)= 16.124, p<0.0001), and week, UCMS and DHNA (F(3, 153)=4.053, p<0.01) were observed. Bonferroni post hoc contrast revealed that, as expected, UCMS significantly decreased sucrose preference and this was prevented by DHNA treatment (Fig 3B). For the males, significant main effects of week (F(3, 180)= 9.230, p<0.0001), UCMS (F(1, 60)= 7.778, p<0.01) and DHNA (F(1, 60)= 5.472, p<0.05) and significant interactions between week and DHNA (F(3, 180)= 3.204, p<0.05) and week and UCMS (F(3, 180)=3.213, p<0.05) were observed. UCMS did not reduce sucrose preference in male mice (Fig 3A). Surprisingly, DHNA had a significant effect of reducing sucrose preference in males, and this effect was prevented by UCMS (Fig 3A).
Fig 3. DHNA prevented UCMS-induced depression-like behavior in female, but not male, mice.
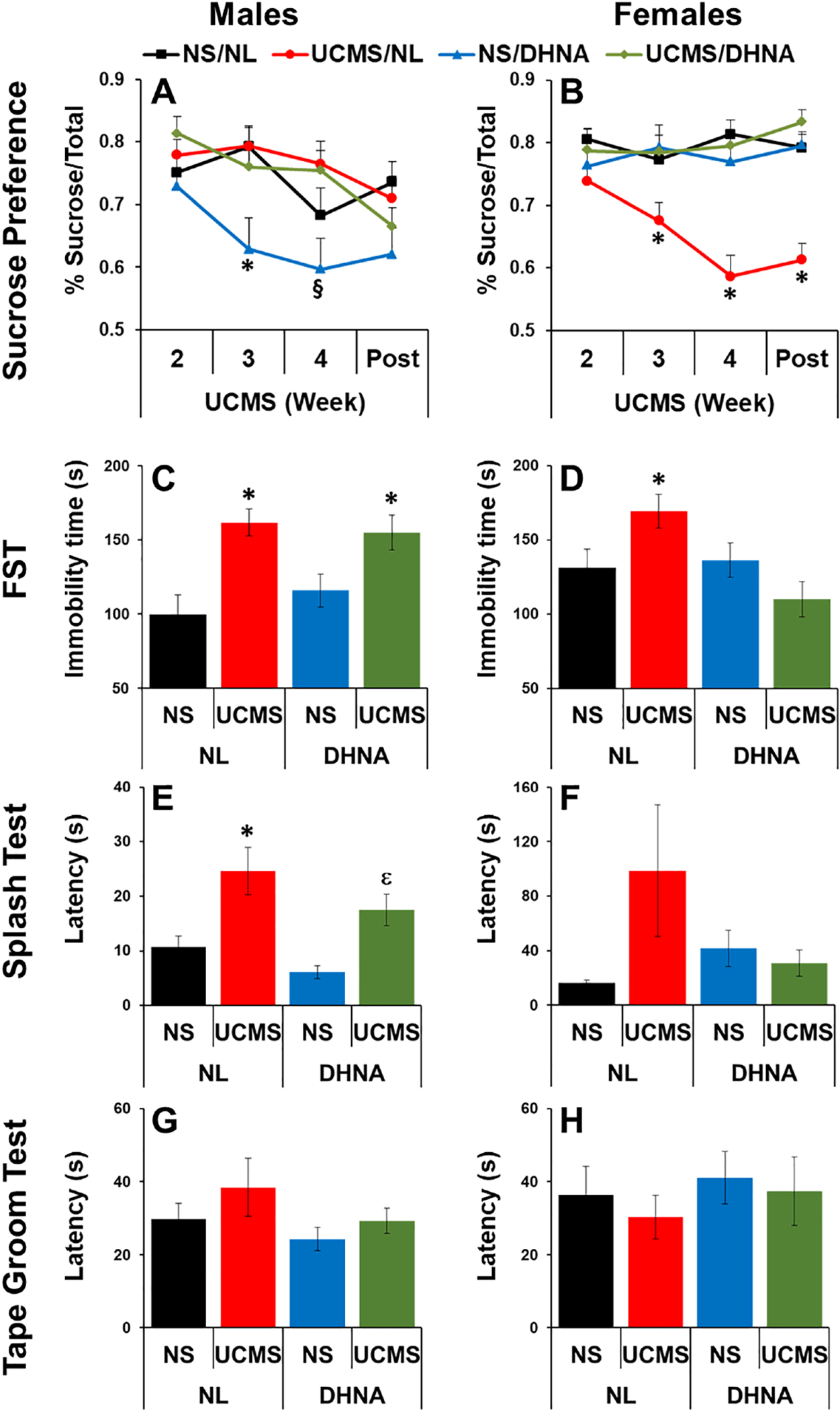
Male (A, C, E, G) and female (B, D, F, H) mice received vehicle or DHNA, and then were unstressed or subjected to UCMS for 4 weeks. Sucrose preference was monitored weekly starting at the end of the second week of UCMS, and continuing until 1 week post UCMS (A, B). Then mice were examined for FST (C, D), splash test (E, F), and tape groom test (G, H). (*)Bonferroni post hoc contrast indicates a significant difference from unstressed mice (p < 0.05); (§) Bonferroni post hoc contrast indicates a significant difference from DHNA/UCMS (p < 0.05). (ε)Bonferroni post hoc contrast indicates a significant difference from DHNA/NS (p < 0.05). Results are presented as mean + SEM.
FST:
MANOVA analysis revealed a significant main effect of UCMS (F(1, 110)=14.94, p<0.0001), and significant interactions between UCMS and DHNA (F(1, 110)=8.86, p<0.01), sex and UCMS (F(1, 110)=9.31, p<0.01), and sex and DHNA treatment (F(1, 110)=4.74, p<0.05). For the females, a significant main effect of DHNA (F(1, 50)=10.21, p<0.01), and a significant interaction between UCMS and DHNA (F(1, 50)=14.36, p<0.0001) were observed. Bonferroni post hoc contrast revealed that UCMS significantly increased immobility time, and this was prevented by DHNA treatment (Fig 3D). For the males, a significant main effect of UCMS (F(1, 60)= 19.86, p<0.0001) was observed. Similarly to females, UCMS significantly increased immobility time. However, in males this effect was not prevented by DHNA treatment (Fig 3C).
Splash and tape groom tests:
In the splash test, MANOVA analysis revealed significant main effects of sex (F(1, 87)=14.198, p<0.0001), and UCMS (F(1, 87)=7.992, p<0.01), and significant interactions between UCMS and DHNA (F(1, 87)=7.836, p<0.01), and sex, UCMS, and DHNA treatment (F(1, 87)=7.005, p<0.01). For the females, some trends were observed, but they did not reach statistical significance (Fig 3F). For the males, significant main effects of UCMS (F(1, 60)=20.226, p<0.0001) and DHNA (F(1, 60)= 4.331, p<0.05) were observed. Bonferroni post hoc contrast revealed that UCMS significantly increased the latency to groom. However, this effect was not prevented by DHNA treatment (Fig 3E). In the tape groom test, no significant effects were observed for the females and males (Figs 3G and 3H).
3.3. Effects on emotional state
Novelty-induced locomotion:
MANOVA analysis revealed a significant main effect of UCMS (F(1, 111)=9.231, p<0.01) and DHNA (F(1, 111)=4.1087, p<0.05) were observed. In females, a significant main effect of UCMS was observed (F(1, 52)= 6.274, p<0.05), but DHNA did not significantly prevent this effect (Fig 4B). For the males, a significant main of DHNA treatment was observed (F(1, 59)= 5.359, p<0.05). Bonferroni post-hoc analysis revealed that DHNA significantly prevented UCMS-induced increase of novelty-induced locomotion (Fig 4A).
Fig 4. DHNA prevented UCMS-induced stress-response in males.
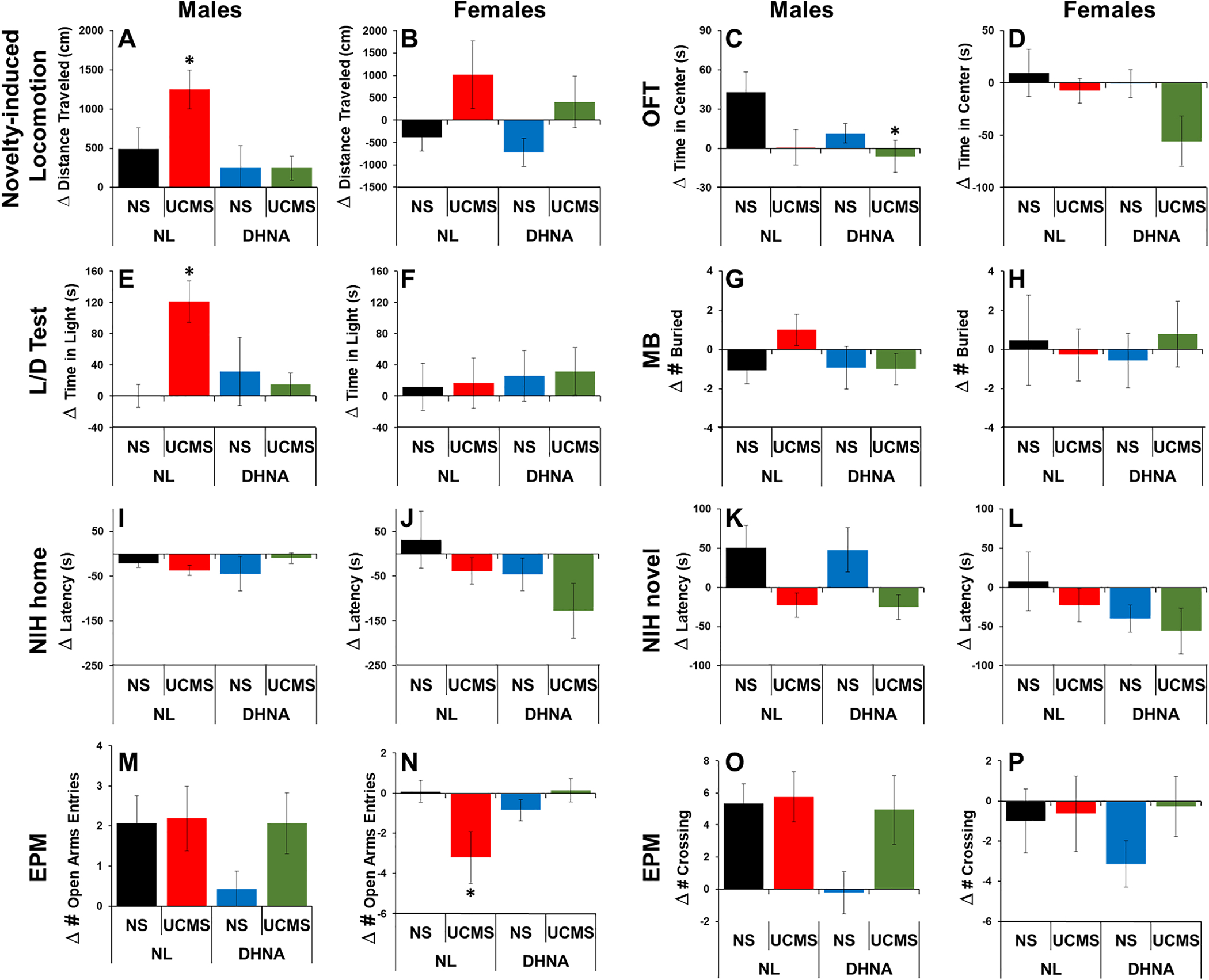
Male (A, C, E, G, I, K, M, O) and female (B, D, F, H, J, L, N, P) were examined for their baseline emotional state. Then, they received vehicle or DHNA, were unstressed or subjected to UCMS for 4 weeks, and retested. Data is presented as the difference between the re-test and baseline for novelty-induced locomotion (A, B); time spent in the center in the OFT (C, D); time spent in the light compartment in the L/D test (E, F); number of buried marbles (G, H); latency for the first sip in the NIH test in their home cages (I, J) and novel environment (K, L); and time spent in the open arm (M, N) and total crosses (O, P) in the EPM. (*) Bonferroni post hoc contrast indicates a significant difference from the other groups (p < 0.05). Results are presented as mean + SEM.
OFT:
MANOVA analysis revealed significant main effects of sex (F(1, 112)=5.707, p<0.05), UCMS (F(1, 112)=9.258, p<0.01), and DHNA (F(1, 112)=4.967, p<0.05) were observed. When analyzed separately by sex, a significant main effect of UCMS was observed for both males (F(1, 60)= 5.359, p<0.05) and females (F(1, 52)= 4.097, p<0.05). Similar to our previous studies, no significant differences were observed between the various experimental group for the females (Fig 4D). In males, only the UCMS/DHNA group differed significantly from NL/NS group (Fig 4C).
L/D Test:
Similar to our previous studies, no significant effects were observed for the females (Fig 4F). For the males, MANOVA analysis revealed significant main effects of UCMS (F(1, 60)= 6.833, p<0.01) and a significant interaction between UCMS and DHNA (F(1, 60)= 11.82, p<0.001) were observed. Bonferroni post-hoc analysis revealed the mice subjected to UCMS spend significantly more time in the light than controls, and DHNA treatment prevented this UCMS-induced effect (Fig 4E).
Marble burying test:
No significant effects were observed for the females or males (Figs 4G and 4H).
Novelty-induced hypophagia:
No significant effects were observed for the females and males for the home environment (Figs 4J and 4I). At the novel environment, MANOVA analysis revealed significant main effects of sex (F(1, 112)=4.908, p<0.05) and UCMS (F(1, 112)=6.443, p<0.05) were observed. No significant differences were observed for the females (Fig 4L). A significant main effect of UCMS was observed in males (F(1, 60)= 7.903, p<0.01). UCMS significantly reduces the latency to consume food, but this effect was not prevented by DHNA treatment (Fig 4K).
Elevated-plus maze:
MANOVA analysis revealed a significant main effect of sex on number of open arm entries (F(1, 111)=25.890, p<0.0001), time spent in open arms (F(1, 111)=21.630, p<0.0001), and number of middle crosses (F(1, 111)=14.005, p<0.0001). For the number open arm entries, there was a significant interaction between sex and DHNA (F(1, 111)=4.027, p<0.05). In females, there was a significant interaction between UCMS and DHNA (F(1, 51)=7.453, p<0.01) for the number of open arm entries. UCMS reduced the number of open arm entries and DHNA prevented this effect (Fig 4N). No significant differences were found in the time spent in the open arms or in general activity (Figs 4P). In males, DHNA appears to reduce the number of open arm entries (Figs 4M), time spent in open arms, and general activity (Figs 4O), and these effects appear to be reversed by UCMS. However, this did not reach statistical significance.
3.4. Effects on cognition
OLR:
MANOVA analysis revealed significant main effects of sex (F(1, 54)=23.444, p<0.0001), UCMS (F(1, 54)=9.245, p<0.01), and DHNA (F(1, 54)=6.273, p<0.05), and significant interactions between UCMS and DHNA (F(1, 54)=10.329, p<0.01), sex and UCMS (F(1, 54)=10.569, p<0.01), sex and DHNA (F(1, 54)=5.914, p<0.05), and sex, UCMS, and DHNA treatment (F(1, 54)=5.931, p<0.05). For the females, no significant effects were observed (Fig 5B). For the males, significant main effects of UCMS (F(1, 27)=11.095, p<0.01), DHNA (F(1, 27)=6.831, p<0.05), and a significant interaction between UCMS and DHNA (F(1, 27)=8.945, p<0.01) were observed. In both males and females, DHNA did not have a negative impact on cognitive memory of object location. In the males, UCMS significantly increased time exploring the object in the new location, and this effect was prevented by DHNA (Fig 5A).
Fig 5. DHNA prevented UCMS-induced stress-response in males.
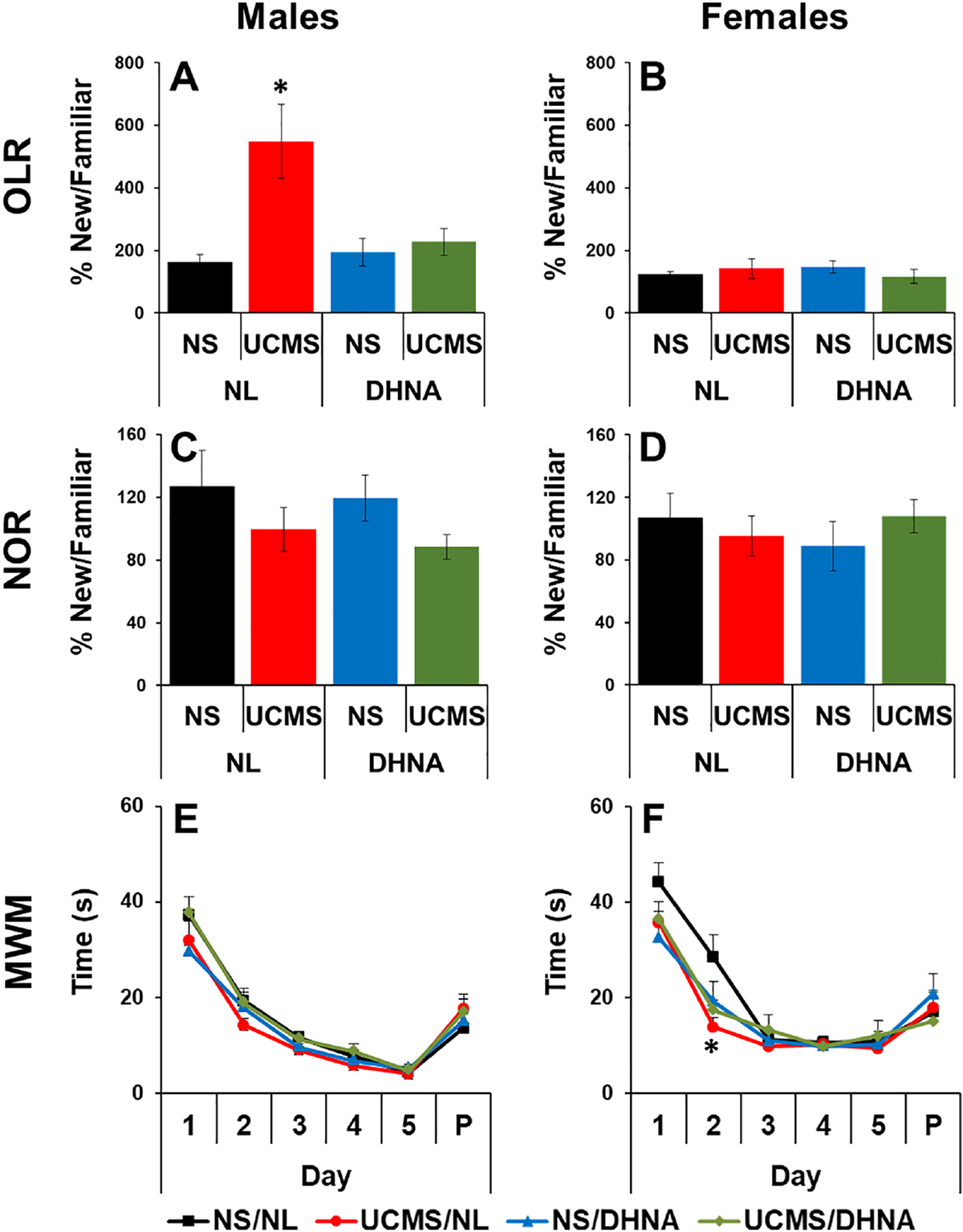
Male (A, C, E) and female (B, D, F) mice received vehicle or DHNA, and then were unstressed or subjected to UCMS for 4 weeks. Then, mice were examined for time spent with an object in a novel location in the OLR test (A, B); time spent with a novel object in the NOR test (C, D); and time to find the platform in the MWM (E, F); (*) Bonferroni post hoc contrast indicates a significant difference from unstressed mice (p < 0.05). Results are presented as mean + SEM.
NOR:
In both males and females, DHNA did not have a negative impact on cognitive recognition of a novel object. No significant differences were observed for both males and females (Fig 5D). In the males, a trend for UCMS to reduce time spent with the novel object was noted, but it did not reach statistical significance (Fig 5C).
MWM:
MANOVA analysis revealed a significant main effect of day (F(5, 555)=142.611, p<0.0001), and significant interactions between UCMS and DHNA (F(1, 111)=6.491, p<0.05), and day, UCMS, and DHNA treatment (F(5, 555)=3.068, p<0.01). In the females, UCMS significantly enhanced spatial learning only on day 2 (Fig 5F). In both males and females, DHNA did not have negative impact on spatial learning (Figs 5E and 5F).
4. Discussion
In this study, DHNA prevented both UCMS-induced decrease in sucrose preference and UCMS-induced increase in immobility in the FST test in females. In other words, our study demonstrated that, in females, DHNA has a potential to act as an effective antidepressant, as it prevents the development of both anhedonia- and despair-like behaviors. Additionally, DHNA prevented UCMS-induced decrease in number of open arm entries in the EPM. Thus, this study further validates and expands on the results of our earlier study (Madison et al., 2022). In contrast, these effects were not observed in males. UCMS did not result in anhedonia-like behaviors in males. This is not entirely surprising, given that other studies reported strain and sex differences in the sensitivity to the effects of UCMS on sucrose preference (Konkle et al., 2003, Grønli et al., 2005, Sachs et al., 2014, Marco et al., 2017). However, in males, UCMS induced an increase in immobility in the FST test, but this was not prevented by DHNA. Moreover, UCMS induced an increase in the latency for males to groom in the splash test, which is generally interpreted to mean a lower motivational drive for self-care (Isingrini et al., 2010, Bouguiyoud et al., 2021), and this was also not prevented by DHNA. Therefore, given classical interpretation of these behaviors, DHNA appears to be a potential antidepressant in females, but not males.
Nonetheless, DHNA had significant effects in males in reducing some stress-related effects and stress reactivity. UCMS induced higher locomotion in a novel environment; this effect was more pronounced in males compared to females. Additionally, in males, but not females, UCMS induced increases in time spent in the light compartment in the L/D test. These UCMS-mediated effects can be interpreted as reduced anxiety; however UCMS increased depression-like behaviors, and increased anxiety-like behaviors in both the OFT and NIH tests. Thus, these behaviors more likely represent an increase in seeking and/or reward of novel experiences or an increase in escape or risk-taking behaviors (Arrant et al., 2013). Furthermore, in the OLR test, UCMS induced longer exploration time of an object in a new location in males but not females. This could be explained as enhanced spatial memory, although we did not observe UCMS induced alterations to spatial memory in the MWM. Thus, this UCMS effect might be due to an increase in spatial memory when seeking novel experiences and locations. Notably, in males, these effects were prevented by DHNA treatment. Thus, although in males DHNA did not improve mood in a classical way, it still appears to mitigate certain stress reactivity triggered by exposure to UCMS.
Presently, the mechanism by which DHNA acts is unknown. Brain derived neurotrophic factor (BDNF) was suggested to play an important role in the pathology of MDD. BDNF levels were demonstrated to be reduced in the hippocampus of individuals suffering from depression, which correlates with atrophy and a lower hippocampal volume observed among depressed patients (Duman and Monteggia, 2006), and antidepressant treatments increase BDNF levels (Chen et al., 2001). Similarly, in rodents UCMS was demonstrated to reduce BDNF levels and the expression of neuronal PAS domain protein 4 (NPAS4) in the hippocampus (Zhang et al., 2014). Notably, the binding of the transcription factors NPAS4 together with the aryl hydrocarbon receptor nuclear translocator 2 (ARNT2) to promoter I was demonstrated to be important in facilitating the transcription of BDNF (Pruunsild et al., 2011). Furthermore, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an environmental toxicant and potent AhR ligand, suppresses NMDA-induced BDNF expression and enhances NMDA excitotoxicity (Lin et al., 2009). This neurotoxicity is likely mediated via AhR given that another AhR agonist, beta-naphthoflavone, was also demonstrated to cause neuronal apoptosis, and this is likely to involve ER signaling because apoptosis was enhanced by an ER antagonist and a selective ER modulator (Kajta et al., 2009). In contrast to toxic ligands of AhR, SAhRMs like DIM were demonstrated to have neuroprotective and anti-apoptotic properties that are likely mediated by decreased AhR and ARNT expression and increased ER-beta expression (Rzemieniec et al., 2016). Thus, SAhRM-mediated hippocampal neuroprotective effects via AhR and ER signaling may explain the sex-dependent beneficial effect of DHNA on mood observed in this study.
Effects of UCMS on the emotional states, observed mainly in males, may be due to effects of stress on the amygdala. In contrast to its effects on the hippocampus, stress was shown to increase levels of BDNF in the amygdala (Lakshminarasimhan and Chattarji, 2012). However, the effects of AhR ligands on the amygdala are largely unknown. Exposure to TCDD in utero disrupts dendritic branch growth in both the hippocampus and amygdala (Kimura et al., 2015). However, further studies are required to examine the cellular and molecular mechanisms by which SAhRMs affect various brain areas, and on the role of ER signaling, to improve our understanding on the differential effects in females and males to alter mood and stress reactivity.
Additionally, in this study DHNA by itself affected sucrose preference in males, but not females. This is not likely a general effect on food consumption, given that no effect on body weight gain was observed in the DHNA/NS groups as compared to the control NL/NS group. In males, DHNA resulted in an anhedonic phenotype, or a reduction in sucrose preference. However, DHNA did not cause an increase in FST immobility time or behavioral despair. Similarly, acyloxyacyl hydrolase (AOAH)-deficient mice exhibited reduced sucrose preference, but no increase in FST immobility time (Aguiniga et al., 2019). The effects in the AOAH knockout mice were demonstrated to be dependent on AhR stimulating the expression of corticotropin-releasing factor (CRF) in the hypothalamic paraventricular nucleus (PVN). CRF is known to regulate stress response via the hypothalamic-pituitary-adrenal (HPA) axis. Thus, it is possible that DHNA increases HPA activity in the PVN resulting in an anhedonic phenotype. However, in the AOAH knockout study, only female mice were examined (Aguiniga et al., 2019). In contrast, in our study, this effect was observed only in males, but not females. Moreover, surprisingly this effect of DHNA was reversed by exposure to stress, or UCMS. An increase in risk-taking or seeking reward and novel experiences induced in males by UCMS could potentially explain the increased sucrose preference, a novel and rewarding taste, in the DHNA/UCMS group as compared with the DHNA/NS group. In line with these interpretations, the trends observed in male’s behaviors in the EPM could be explained by a reduction of risk-taking and sensation-seeking caused by DHNA that was reversed by exposure to UCMS.
5. Conclusions
Our findings suggest that SAhRMs, such as DHNA, can potentially be an effective antidepressant in females. Additionally, although given classical interpretation, DHNA did not appear to be a potential antidepressant in males, it demonstrated a potential to mitigate certain stress effects and stress reactivity in males. In order to fully understand the therapeutic potential of DHNA and other AhR ligands, future studies need to determine the underlying molecular mechanisms including the effects of SAhRMs on gene expression in various brain areas, in unstressed and stressed conditions, and in different sexes. In addition, our future studies will utilize AhR knockout mice to further define the selectivity of AhR ligands and define which responses are AhR-dependent.
Highlights.
Dietary AhR ligand DHNA is a potential antidepressant in females but not males
Behavioral anhedonia and despair are prevented by DHNA in female but not male mice
DHNA reduced stress-related effects and reactivity in male mice
Spatial learning is not affected by DHNA in male or female mice
DHNA may affect emotional state in unstressed male mice
Acknowledgements
This work was supported by a Seed Grant from Texas A&M University (SE) and supported in part by NIH grant R35 CA197707 (RSC) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention (RSC).
Role of Funding Source
This work was supported by a Seed Grant from Texas A&M University (SE) and supported in part by NIH grant R35 CA197707 (RSC) and funds from the Allen Endowed Chair in Nutrition & Chronic Disease Prevention (RSC). The funding source had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest: The authors have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiniga LM, Yang W, Yaggie RE, Schaeffer AJ and Klumpp DJ, 2019. Acyloxyacyl hydrolase modulates depressive-like behaviors through aryl hydrocarbon receptor. Am J Physiol Regul Integr Comp Physiol. 317, R289–R300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR, 2015. Why is depression more prevalent in women? J Psychiatry Neurosci. 40, 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrant AE, Schramm-Sapyta NL and Kuhn CM, 2013. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav Brain Res. 256, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguiyoud N, Roullet F, Bronchti G, Frasnelli J and Al Aïn S, 2021. Anxiety and Depression Assessments in a Mouse Model of Congenital Blindness. Front Neurosci. 15, 807434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF and Young LT, 2001. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 50, 260–265. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Jin U-H, Davidson LA, Chapkin RS, Jayaraman A, Tamamis P, Orr A, Allred C, Denison MS, Soshilov A, Weaver E and Safe S, 2017. Editor’s Highlight: Microbial-Derived 1,4-Dihydroxy-2-naphthoic Acid and Related Compounds as Aryl Hydrocarbon Receptor Agonists/Antagonists: Structure-Activity Relationships and Receptor Modeling. Toxicological sciences : an official journal of the Society of Toxicology. 155, 458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS and Monteggia LM, 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 59, 1116–1127. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T and Whiteford HA, 2013. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Medicine. 10, e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales C, Zaleska MM, Riddell DR, Atchison KP, Robshaw A, Zhou H and Sukoff Rizzo SJ, 2014. Alternative method of oral administration by peanut butter pellet formulation results in target engagement of BACE1 and attenuation of gavage-induced stress responses in mice. Pharmacology Biochemistry and Behavior. 126, 28–35. [DOI] [PubMed] [Google Scholar]
- Grønli J, Murison R, Fiske E, Bjorvatn B, Sørensen E, Portas CM and Ursin R, 2005. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 84, 571–577. [DOI] [PubMed] [Google Scholar]
- Hawton K, Casanas ICC, Haw C and Saunders K, 2013. Risk factors for suicide in individuals with depression: a systematic review. Journal of Affective Disorders. 147, 17–28. [DOI] [PubMed] [Google Scholar]
- Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ and Eitan S, 2009. Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal. Behav Pharmacol. 20, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isingrini E, Camus V, Le Guisquet AM, Pingaud M, Devers S and Belzung C, 2010. Association between repeated unpredictable chronic mild stress (UCMS) procedures with a high fat diet: a model of fluoxetine resistance in mice. PLoS One. 5, e10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajta M, Wójtowicz AK, Maćkowiak M and Lasoń W, 2009. Aryl hydrocarbon receptor-mediated apoptosis of neuronal cells: a possible interaction with estrogen receptor signaling. Neuroscience. 158, 811–822. [DOI] [PubMed] [Google Scholar]
- Kautzky A, Dold M, Bartova L, Spies M, Kranz GS, Souery D, Montgomery S, Mendlewicz J, Zohar J, Fabbri C, Serretti A, Lanzenberger R, Dikeos D, Rujescu D and Kasper S, 2019. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatrica Scandinavica. 139, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, 2008. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin Neurosci. 10, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Kubo K, Matsuyoshi C, Benner S, Hosokawa M, Endo T, Ling W, Kohda M, Yokoyama K, Nakajima K, Kakeyama M and Tohyama C, 2015. Developmental origin of abnormal dendritic growth in the mouse brain induced by in utero disruption of aryl hydrocarbon receptor signaling. Neurotoxicol Teratol. 52, 42–50. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z and Bielajew C, 2003. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 992, 227–238. [DOI] [PubMed] [Google Scholar]
- Lakshminarasimhan H and Chattarji S, 2012. Stress leads to contrasting effects on the levels of brain derived neurotrophic factor in the hippocampus and amygdala. PloS one. 7, e30481–e30481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Chen CC, Chou CM, Wang CY, Hung CC, Chen JY, Chang HW, Chen YC, Yeh GC and Lee YH, 2009. Knockdown of the aryl hydrocarbon receptor attenuates excitotoxicity and enhances NMDA-induced BDNF expression in cortical neurons. J Neurochem. 111, 777–789. [DOI] [PubMed] [Google Scholar]
- Madison CA, Kuempel J, Albrecht GL, Hillbrick L, Jayaraman A, Safe S, Chapkin RS and Eitan S, 2022. 3,3’-Diindolylmethane and 1,4-dihydroxy-2-naphthoic acid prevent chronic mild stress induced depressive-like behaviors in female mice. J Affect Disord. 309, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Ballesta JA, Irala C, Hernández MD, Serrano ME, Mela V, López-Gallardo M and Viveros MP, 2017. Sex-dependent influence of chronic mild stress (CMS) on voluntary alcohol consumption; study of neurobiological consequences. Pharmacol Biochem Behav. 152, 68–80. [DOI] [PubMed] [Google Scholar]
- Marques M, Laflamme L, Benassou I, Cissokho C, Guillemette B and Gaudreau L, 2014. Low levels of 3,3’-diindolylmethane activate estrogen receptor α and induce proliferation of breast cancer cells in the absence of estradiol. BMC Cancer. 14, 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J and Gustafsson JA, 2006. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 4, e016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Wihlén B, Thomsen J and Gustafsson JA, 2005. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Biol. 25, 5317–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M and Miguelez M, 2014. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. Journal of Affective Disorders. 156, 1–7. [DOI] [PubMed] [Google Scholar]
- O’Rourke MC, Jamil RT and Siddiqui W, 2022. Suicide Screening and Prevention. StatPearls Publishing LLC, Treasure Island (FL). [PubMed] [Google Scholar]
- Parkin DR and Malejka-Giganti D, 2004. Differences in the hepatic P450-dependent metabolism of estrogen and tamoxifen in response to treatment of rats with 3,3’-diindolylmethane and its parent compound indole-3-carbinol. Cancer Detect Prev. 28, 72–79. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C and Roux S, 2001. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. Chapter 8, Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Pruunsild P, Sepp M, Orav E, Koppel I and Timmusk T, 2011. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 31, 3295–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot T-A, 1896. La psychologie des sentiments. Felix Alcan, Paris. [Google Scholar]
- Rzemieniec J, Litwa E, Wnuk A, Lason W, Krzeptowski W and Kajta M, 2016. Selective Aryl Hydrocarbon Receptor Modulator 3,3’-Diindolylmethane Impairs AhR and ARNT Signaling and Protects Mouse Neuronal Cells Against Hypoxia. Mol Neurobiol. 53, 5591–5606. [DOI] [PubMed] [Google Scholar]
- Sachs BD, Ni JR and Caron MG, 2014. Sex differences in response to chronic mild stress and congenital serotonin deficiency. Psychoneuroendocrinology. 40, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Han H, Goldsby J, Mohankumar K and Chapkin RS, 2018. Aryl Hydrocarbon Receptor (AhR) Ligands as Selective AhR Modulators: Genomic Studies. Curr Opin Toxicol. 11–12, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Jin UH, Park H, Chapkin RS and Jayaraman A, 2020. Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). Int J Mol Sci. 21, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Shah K and Wincent E, 2021. AHR in the intestinal microenvironment: safeguarding barrier function. Nat Rev Gastroenterol Hepatol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O and Bartsch D, 2006. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 17, 271–287. [DOI] [PubMed] [Google Scholar]
- Tarnow P, Tralau T and Luch A, 2019. Chemical activation of estrogen and aryl hydrocarbon receptor signaling pathways and their interaction in toxicology and metabolism. Expert Opinion on Drug Metabolism & Toxicology. 15, 219–229. [DOI] [PubMed] [Google Scholar]
- van Bronswijk S, Moopen N, Beijers L, Ruhe HG and Peeters F, 2019. Effectiveness of psychotherapy for treatment-resistant depression: a meta-analysis and meta-regression. Psychological Medicine. 49, 366–379. [DOI] [PubMed] [Google Scholar]
- Vermillion Maier ML, Siddens LK, Uesugi SL, Choi J, Leonard SW, Pennington JM, Tilton SC, Smith JN, Ho E, Chow HHS, Nguyen BD, Kolluri SK and Williams DE, 2021. 3,3’-Diindolylmethane (BioResponse DIM(®)) Exhibits Significant Metabolism Following Oral Dosing in Humans. Drug Metab Dispos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2022. World Health Organization: Depression. https://www.who.int/news-room/fact-sheets/detail/depression (accessed 21 September 2022).
- Wihlén B, Ahmed S, Inzunza J and Matthews J, 2009. Estrogen receptor subtype- and promoter-specific modulation of aryl hydrocarbon receptor-dependent transcription. Mol Cancer Res. 7, 977–986. [DOI] [PubMed] [Google Scholar]
- Willner P, 2017. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 6, 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R and Safe S, 2003. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol Cell Biol. 23, 1843–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Fei P, Mu J, Li W and Song J, 2014. Hippocampal expression of aryl hydrocarbon receptor nuclear translocator 2 and neuronal PAS domain protein 4 in a rat model of depression. Neurol Sci. 35, 277–282. [DOI] [PubMed] [Google Scholar]


